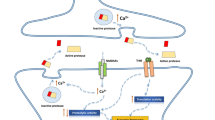
Abstract
Investigation of biochemical mechanisms underlying the long-term storage of information in nervous system is one of main problems of modern neurobiology. As a molecular basis of long-term memory, long-term changes in kinase activities, increase in the level and changes in the subunit composition of receptors in synaptic membranes, local activity of prion-like proteins, and epigenetic modifications of chromatin have been proposed. Perhaps a combination of all or of some of these factors underlies the storage of long-term memory in the brain. Many recent studies have shown an exclusively important role of atypical protein kinases (PKCζ, PKMζ, and PKCι/λ) in processes of learning, consolidation and maintenance of memory. The present review is devoted to consideration of mechanisms of transcriptional and translational control of atypical protein kinases and their roles in induction and maintenance of long-term synaptic plasticity and memory in vertebrates and invertebrates.
Similar content being viewed by others
Abbreviations
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolpropionic acid receptor
- aPKC:
-
atypical protein kinase C
- CaMKII:
-
Ca2+/calmodulin-dependent protein kinase II
- cAMP:
-
cyclic adenosine monophosphate
- CBP:
-
CREB-binding protein
- CRE:
-
cAMP response-element
- CREB:
-
a protein binding cAMP-sensitive element (CRE-binding protein)
- DAG:
-
diacylglycerol
- DNMT:
-
DNA methyltransferase
- DTEs:
-
dendritic targeting elements
- E-LTP/L-LTP:
-
early/late longterm potentiation
- ERK:
-
extracellular signal-regulated kinase
- GluA:
-
subunit of glutamate receptor AMPA
- GluA1AMPA/GluA2-AMPA:
-
glutamate AMPA-receptor containing GluA1/GluA2 subunit
- HDAC:
-
histone deacetylase
- LTP:
-
longterm potentiation
- mTOR:
-
mammalian target of rapamycin
- PICK1:
-
protein interacting with C kinase
- PI3K:
-
phosphoinositide-3-kinase
- PIN1:
-
peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
- PKA:
-
protein kinase A
- PKC:
-
protein kinase C
- PKCλ:
-
protein kinase Cλ
- PKMζ:
-
protein kinase Mζ
- PS:
-
phosphatidylserine
- shRNA:
-
short hairpin RNA
- TSA:
-
trichostatin A
- uORF:
-
upstream open reading frame
- UTRs:
-
untranslated regions
- ZIP:
-
Zeta Inhibitory Peptide
References
Sacktor, T. C. (2012) Memory maintenance by PKMζ–an evolutionary perspective, Mol. Brain, 5, 31.
Morris, M. J., and Monteggia, L. M. (2014) Role of DNA methylation and the DNA methyltransferases in learning and memory, Dialogues Clin. Neurosci., 16, 359–371.
Ling, D. S., Benardo, L. S., Serrano, P. A., Blace, N., Kelly, M. T., Crary, J. F., and Sacktor, T. C. (2002) Protein kinase M? is necessary and sufficient for LTP maintenance, Nat. Neurosci., 5, 295–296.
Serrano, P., Yudong, Y., and Sacktor, T. C. (2005) Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation, J. Neurosci., 25, 1979–1984.
Yao, Y., Kelly, M. T., Sajikumar, S., Serrano, P., Tian, D., Bergold, P. J., Frey, J. U., and Sacktor, T. C. (2008) PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors, J. Neurosci., 28, 7820–7827.
Tsokas, P., Hsieh, C., Yao, Y., Lesburgueres, E., Wallace, E. J., Tcherepanov, A., Jothianandan, D., Hartley, B. R., Pan, L., Rivard, B., Farese, R. V., Sajan, M. P., Bergold, P. J., Hernandez, A. I., Cottrell, J. E., Shouval, H. Z., Fenton, A. A., and Sacktor, T. C. (2016) Compensation for PKMζ in long-term potentiation and spatial long-term memory in mutant mice, eLife, 5, e14846.
Drier, E. A., Tello, M. K., Cowan, M., Wu, P., Blace, N., Sacktor, T. C., and Jin, J. C. (2002) Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster, Nat. Neurosci., 5, 316–324.
Cai, D., Pearce, K., Chen, S., and Glanzman, D. L. (2011) Protein kinase M maintains long-term sensitization and long-term facilitation in aplysia, J. Neurosci., 31, 6421–6431.
Chen, S., Cai, D., Pearce, K., Sun, P. Y., Roberts, A. C., and Glanzman, D. L. (2014) Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia, Elife, 3, e03896.
Balaban, P. M., Roshchin, M., Timoshenko, A. Kh., Zuzina, A. B., Lemak, M., Ierusalimsky, V. N., Aseyev, N. A., and Malyshev, A. Y. (2015) Homolog of protein kinase Mzeta maintains context aversive memory and underlying long-term facilitation in terrestrial snail Helix, Front. Cell Neurosci., 9, 222.
Pastalkova, E., Serrano, P., Pinkhasova, D., Wallace, E., Fenton, A. A., and Sacktor, T. C. (2006) Storage of spatial information by the maintenance mechanism of LTP, Science, 313, 1141–1144.
Serrano, P., Friedman, E. L., Kenney, J., Taubenfeld, S. M., Zimmerman, J. M., Hanna, J., Alberini, C., Kelley, A. E., Maren, S., Rudy, J. W., Yin, J. C., Sacktor, T. C., and Fenton, A. A. (2008) PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories, PLoS Biol., 6, 2698–2706.
Migues, P. V., Hardt, O., Wu, D. C., Gamache, K., Sacktor, T. C., Wang, Y. T., and Nader, K. (2010) PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking, Nat. Neurosci., 13, 630–634.
Evuarherhe, O., Barker, G. R., Savalli, G., Warburton, E. C., and Brown, M. W. (2014) Early memory formation disrupted by atypical PKC inhibitor ZIP in the medial prefrontal cortex but not hippocampus, Hippocampus, 24, 934–942.
Shema, R., Sacktor, T. C., and Dudai, Y. (2007) Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM, Science, 317, 951–953.
Shema, R., Hazvi, S., Sacktor, T. C., and Dudai, Y. (2009) Boundary conditions for the maintenance of memory by PKMzeta in neocortex, Learn. Mem., 16, 122–128.
Nishizuka, Y. (1988) The molecular heterogeneity of protein kinase C and its implications for cellular regulation, Nature, 334, 661–665.
Newton, A. C. (1997) Regulation of protein kinase C, Curr. Opin. Cell Biol., 9, 161–167.
Newton, A. C. (2003) Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm, Biochem. J., 370, 361–371.
Ono, Y., Fujii, T., Ogita, K., Kikkawa, U., Igarashi, K., and Nishizuka, Y. (1989) Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties, PNAS, 86, 3099–3103.
Nakanishi, H., Brewer, K. A., and Exton, J. H. (1993) Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3, 4, 5-trisphosphate, J. Biol. Chem., 268, 13–16.
Sacktor, T. C., Osten, P., Valsamis, H., Jiang, X., Naik, M. U., and Sublette, E. (1993) Persistent activation of the zeta isoform of protein kinase C in the maintenance of longterm potentiation, PNAS, 90, 8342–8346.
Hernandez, A. I., Blace, N., Crary, J. F., Serrano, P. A., Leitges, M., Libien, J. M., Weinstein, G., Tcherapanov, A., and Sacktor, T. C. (2003) Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory, J. Biol. Chem., 278, 40305–40316.
Bougie, J. K., Lim, T., Farah, C. A., Manjunath, V., Nagakura, I., Ferraro, G. B., and Sossin, W. S. (2009) The atypical protein kinase C in Aplysia can form a protein kinase M by cleavage, J. Neurochem., 109, 1129–1143.
Marshall, B. S., Price, G., and Powell, C. T. (2000) Rat protein kinase C zeta gene contains alternative promoters for generation of dual transcripts with 5′-end heterogeneity, DNA Cell Biol., 19, 707–719.
Muslimov, I. A., Nimmrich, V., Hernandez, A. I., Tcherepanov, A., Sacktor, T. C., and Tiedge, H. (2004) Dendritic transport and localization of protein kinase Mzeta mRNA: implications for molecular memory consolidation, J. Biol. Chem., 279, 52613–52622.
Kelly, M. T., Crary, J. F., and Sacktor, T. C. (2007) Regulation of protein kinase Mzeta synthesis by multiple kinases in long-term potentiation, J. Neurosci., 27, 3439–3444.
Hernandez, A. I., Oxberry, W. C., Crary, J. F., Mirra, S. S., and Sacktor, T. C. (2013) Cellular and subcellular localization of PKMζ, Philos. Trans. R Soc. Lond. B Biol. Sci., 369, 20130140.
Yerusalimsky, V. N., Borodinova, A. A., and Balaban, P. M. (2015) Localization of atypical protein kinase Cζ in the nervous system of the terrestrial mollusk Helix, Neirokhimiya, 32, 295–301.
Neri, L. M., Martelli, A. M., Borgatti, P., Colamussi, M. L., Marchisio, M., and Capitani, S. (1999) Increase in nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol (3, 4, 5) trisphosphate synthesis precedes PKC-zeta translocation to the nucleus of NGF-treated PC12 cells, FASEB J., 13, 2299–2310.
Ko, H. G., Kim, J. I., Sim, S. E., Kim, T., Yoo, J., Choi, S. L., Baek, S. H., Yu, W. J., Yoon, J. B., Sacktor, T. C., and Kaang, B. K. (2016) The role of nuclear PKMζ in memory maintenance, Neurobiol. Learn. Mem., 135, 50–56.
Naik, M. U., Benedikz, E., Hernandez, I., Libien, J., Hrabe, J., Valsamis, M., Dow-Edwards, D., Osman, M., and Sacktor, T. C. (2000) Distribution of protein kinase Mzeta and the complete protein kinase C isoform family in rat brain, J. Comp. Neurol., 426, 243–258.
Jaken, S. (1996) Protein kinase C isozymes and substrates, Curr. Opin. Cell Biol., 8, 168–173.
Naz, F., Islam, A., Ahmad, F., and Hassan, M. I. (2015) Atypical PKC phosphorylates microtubule affinity-regulating kinase 4 in vitro, Mol. Cell Biochem., 410, 223–228.
Tobias, I. S., and Newton, A. C. (2016) Protein scaffolds control localized protein kinase C? activity, J. Biol. Chem., 291, 13809–13822.
Ren, S. Q., Yan, J. Z., Zhang, X. Y., Bu, Y. F., Pan, W. W., Yao, W., Tian, T., and Lu, W. (2013) PKC? is critical in AMPA receptor phosphorylation and synaptic incorporation during LTP, EMBO J., 32, 1365–1380.
Bliss, T. V., and Lomo, T. (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path, J. Physiol., 232, 331–356.
Jaafari, N., Henley, J. M., and Hanley, J. G. (2012) PICK1 mediates transient synaptic expression of GluA2-lacking AMPA receptors during glycine-induced AMPA receptor trafficking, J. Neurosci., 32, 11618–11630.
Osten, P., Valsamis, L., Harris, A., and Sacktor, T. C. (1996) Protein synthesis-dependent formation of protein kinase Mzeta in long-term potentiation, J. Neurosci., 16, 2444–2451.
Chen, C., Meng, S. Q., Xue, Y. X., Han, Y., Sun, C. Y., Deng, J. H., Chen, N., Bao, Y. P., Zhang, F. L., Cao, L. L., Zhu, W. G., Shi, J., Song, W. H., and Lu, L. (2016) Epigenetic modification of PKMζ rescues aging-related cognitive impairment, Sci. Rep., 6, 22096.
Ling, D. S., Benardo, L. S., and Sacktor, T. C. (2006) Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors, Hippocampus, 16, 443–452.
Schuette, S. R., Fernandez-Fernandez, D., Lamla, T., Rosenbrock, H., and Hobson, S. (2016) Overexpression of protein kinase M? in the hippocampus enhances long-term potentiation and long-term contextual but not cued fear memory in rats, J. Neurosci., 36, 4313–4324.
Lee, S. H., Liu, L., Wang, Y. T., and Sheng, M. (2002) Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD, Neuron, 36, 661–674.
Sacktor, T. C. (2011) How does PKMzeta maintain longterm memory? Nat. Rev. Neurosci., 12, 9–15.
Shema, R., Haramati, S., Ron, S., Hazvi, S., Chen, A., Cacktor, T. C., and Dudai, Y. (2011) Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex, Science, 331, 1207–1210.
Kwapis, J. L., Jarome, T. J., Lonergan, M. E., and Helmstetter, F. J. (2009) Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus, Behav. Neurosci., 123, 844–850.
Zuzina, A. B., and Balaban, P. M. (2015) Extinction and reconsolidation of memory, Zh. Vyssh. Nerv. Deyat. im. Pavlova, 65, 564–576.
Kwapis, J. L., Jarome, T. J., Gilmartin, M. R., and Helmstetter, F. J. (2012) Intra-amygdala infusion of the protein kinase Mzeta inhibitor ZIP disrupts foreground context fear memory, Neurobiol. Learn. Mem., 98, 148–153.
Deng, Z., Lubinski, A. J., and Page, T. L. (2015) Zeta inhibitory peptide (ZIP) erases long-term memories in a cockroach, Neurobiol. Learn. Mem., 118, 89–95.
Li, L., You, L., Sunyer, B., Patil, S., Hoger, H., Pollak, A., Stork, O., and Lubec, G. (2014) Hippocampal protein kinase C family members in spatial memory retrieval in the mouse, Behav. Brain Res., 258, 202–207.
Lee, A. M., Kanter, B. R., Wang, D., Lim, J. P., Zou, M. E., Qiu, C., McMahon, T., Dadgar, J., Fischbach-Weiss, S. C., and Messing, R. O. (2013) Prkcz null mice show normal learning and memory, Nature, 493, 416–419.
Volk, L. J., Bachman, J. L., Johnson, R., Yu, Y., and Huganir, R. L. (2013) PKM-ζ is not required for hippocampal synaptic plasticity, learning and memory, Nature, 493, 420–423.
Palida, S. F., Butko, M. T., Ngo, J. T., Mackey, M. R., Gross, L. A., Ellisman, M. H., and Tsien, R. Y. (2015) PKMζ, but not PKCλ, is rapidly synthesized and degraded at the neuronal synapse, J. Neurosci., 35, 7736–7749.
Bal, N., Susorov, D., Chesnokova, E., Kasyanov, A., Mikhailova, T., Alkalaeva, E., Balaban, P., and Kolosov, P. (2016) uORFs located in the 5′-UTR of PKMζ mRNA regulate its translation, Front. Mol. Neurosci., 9, 103.
Westmark, P. R., Westmark, C. J., Wang, S., Levenson, J., O’Riordan, K. J., Burger, C., and Malter, J. S. (2010) Pin1 and PKMzeta sequentially control dendritic protein synthesis, Sci. Signal., 3, ra18.
Smet-Nocca, C., Launay, H., Wieruszeski, J. M., Lippens, G., and Landrieu, I. (2013) Unraveling a phosphorylation event in a folded protein by NMR spectroscopy: phosphorylation of the Pin1 WW domain by PKA, J. Biomol. NMR, 55, 323–337.
Smith, L., Wang, Z., and Smith, J. B. (2003) Caspase processing activates atypical protein kinase C zeta by relieving autoinhibition and destabilizes the protein, Biochem. J., 375, 663–671.
Jalil, S. J., Sacktor, T. C., and Shouval, H. Z. (2015) Atypical PKCs in memory maintenance: the roles of feedback and redundancy, Learn. Mem., 22, 344–353.
Sacktor, T. C. (2010) PINing for things past, Sci. Signal., 3, pe9.
Penney, J., and Tsai, L. H. (2014) Histone deacetylases in memory and cognition, Sci. Signal., 7, re12.
Lattal, K. M., Barrett, R. M., and Wood, M. A. (2007) Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction, Behav. Neurosci., 121, 1125–1131.
Bahari-Javan, S., Maddalena, A., Kerimoglu, C., Wittnam, J., Held, T., Bahr, M., Burkhardt, S., Delalle, I., Kugler, S., Fischer, A., and Sananbenesi, F. (2012) HDAC1 regulates fear extinction in mice, J. Neurosci., 32, 5062–5073.
Franklin, A. V., Rusche, J. R., and McMahon, L. L. (2014) Increased long-term potentiation at medial-perforant path-dentate granule cell synapses induced by selective inhibition of histone deacetylase 3 requires Fragile X mental retardation protein, Neurobiol. Learn. Mem., 114, 193–197.
Levenson, J. M., O’Riordan, K. J., Brown, K. D., Trinh, M. A., Molfese, D. L., and Sweatt, J. D. (2004) Regulation of histone acetylation during memory formation in the hippocampus, J. Biol. Chem., 279, 40545–40559.
Vecsey, C. G., Hawk, J. D., Lattal, K. M., Stein, J. M., Fabian, S. A., Attner, M. A., Cabrera, S. M., McDonough, C. B., Brindle, P. K., Abel, T., and Wood, M. A. (2007) Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation, J. Neurosci., 27, 6128–6140.
Korzus, E., Rosenfeld, M. G., and Mayford, M. (2004) CBP histone acetyltransferase activity is a critical component of memory consolidation, Neuron, 42, 961–972.
Stefanko, D. P., Barrett, R. M., Ly, A. R., Reolon, G. K., and Wood, M. A. (2009) Modulation of long-term memory for object recognition via HDAC inhibition, Proc. Natl. Acad. Sci. USA, 106, 9447–9452.
Hawk, J. D., Florian, C., and Abel, T. (2011) Post-training intrahippocampal inhibition of class I histone deacetylases enhances long-term object-location memory, Learn. Mem., 18, 367–370.
Federman, N., Fustinana, M. S., and Romano, A. (2009) Histone acetylation is recruited in consolidation as a molecular feature of stronger memories, Learn. Mem., 16, 600–606.
Kim, J., Kwon, J. T., Kim, H. S., Josselyn, S. A., and Han, J. H. (2014) Memory recall and modifications by activating neurons with elevated CREB, Nat. Neurosci., 17, 65–72.
Miller, C. A., and Sweatt, J. D. (2007) Covalent modification of DNA regulates memory formation, Neuron, 53, 857–869.
Miller, C. A., Gavin, C. F., White, J. A., Parrish, R. R., Honasoge, A., Yancey, C. R., Rivera, I. M., Rubio, M. D., Rumbaugh, G., and Sweatt, J. D. (2010) Cortical DNA methylation maintains remote memory, Nat. Neurosci., 13, 664–666.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A. A. Borodinova, A. B. Zuzina, P. M. Balaban, 2017, published in Biokhimiya, 2017, Vol. 82, No. 3, pp. 372-388.
Rights and permissions
About this article
Cite this article
Borodinova, A.A., Zuzina, A.B. & Balaban, P.M. Role of atypical protein kinases in maintenance of long-term memory and synaptic plasticity. Biochemistry Moscow 82, 243–256 (2017). https://doi.org/10.1134/S0006297917030026
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917030026