Abstract
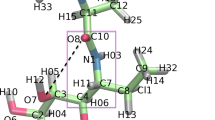
The ribosome is a molecular machine that synthesizes all cellular proteins via translation of genetic information encoded in polynucleotide chain of messenger RNA. Transition between different stages of the ribosome working cycle is strictly coordinated by changes in structure and mutual position both of subunits of the ribosome and its ligands. Therein, information regarding structural transformations is transmitted between functional centers of the ribosome through specific signals. Usually, functional centers of ribosomes are located at a distance reaching up to several tens of angstroms, and it is believed that such signals are transduced allosterically. In our study, we attempted to answer the question of how allosteric signal can be transmitted from one of the so-called sensory elements of ribosomal tunnel (RT) to the peptidyl transferase center (PTC). A segment of RT wall from the E. coli ribosome composed of nucleotide residues A2058, A2059, m2A2503, G2061, A2062, and C2063 of its 23S rRNA was examined by molecular dynamics simulations. It was found that a potential signal transduction pathway A2058-C2063 acted as a dynamic ensemble of interdependent conformational states, wherein cascade-like changes can occur. It was assumed that structural rearrangement in the A2058-C2063 RT segment results in reversible inactivation of PTC due to a strong stacking contact between functionally important U2585 residue of the PTC and nucleotide residue C2063. A potential role for the observed conformational transition in the A2058-C2063 segment for regulating ribosome activity is discussed.
Similar content being viewed by others
References
Polacek, N., Patzke, S., Nierhaus, K. H., and Barta, A. (2000) Periodic conformational changes in rRNA: moni-toring the dynamics of translating ribosomes, Mol. Cell, 6, 159–171.
Zhou, J., Lancaster, L., Donohue, J. P., and Noller, H. F. (2013) Crystal structures of EF-G–ribosome complexes trapped in intermediate states of translocation, Science, 340, 1236086.
Pulk, A., and Cate, J. H. D. (2013) Control of ribosomal subunit rotation by elongation factor G, Science, 340, 1235970.
Ogle, J. M., Brodersen, D. E., Clemons, W. M., Tarry, M. J., Carter, A. P., and Ramakrishnan, V. (2001) Recognition of cognate transfer RNA by the 30S ribosomal subunit, Science, 292, 897–902.
Munro, J. B., Sanbonmatsu, K. Y., Spahn, C. M., and Blanchard, S. C. (2009) Navigating the ribosome’s metastable energy landscape, Trends Biochem. Sci., 34, 390–400.
Steitz, T. A. (2008) A structural understanding of the dynamic ribosome machine, Nat. Rev. Mol. Cell Biol., 9, 242–253.
Rheinberger, H.-J., and Nierhaus, K. H. (1986) Allosteric interactions between the ribosomal transfer RNA-binding sites A and E, J. Biol. Chem., 261, 9133–9139.
Bogdanov, A. A., Dontsova, O. A., Dokudovskaya, S. S., and Lavrik, I. N. (1995) Structure and function of 5S rRNA in the ribosome, Biochem. Cell Biol., 73, 869–876.
Chan, Y.-L., Dresios, J., and Wool, I. G. (2006) A pathway for the transmission of allosteric signals in the ribosome through a network of RNA tertiary interactions, J. Mol. Biol., 355, 1014–1025.
Blaha, G., Gurel, G., Schroeder, S. J., Moore, P. B., and Steitz, T. A. (2008) Mutations outside the anisomycin-binding site can make ribosomes drug-resistant, J. Mol. Biol., 379, 505–519.
Davidovich, C., Bashan, A., Auerbach-Nevo, T., Yaggie, R. D., Gontarek, R., and Yonath, A. (2007) Induced-fit tightens pleuromutilins binding to ribosomes and remote interactions enable their selectivity, Proc. Natl. Acad. Sci. USA, 104, 4291–4296.
Wang, L., Pulk, A., Wasserman, M. R., Feldman, M. B., Altman, R. B., Cate J. H., and Blanchard, S. C. (2012) Allosteric control of the ribosome by small-molecule antibiotics, Nat. Struct. Mol. Biol., 19, 957–963.
Sothiselvam, S., Liu, B., Han, W., Ramu, H., Klepacki, D., Atkinson, G. C., Brauer, A., Remm, M., Tenson, T., Schulten, K., Vazquez-Laslop, N., and Mankin, A. S. (2014) Macrolide antibiotics allosterically predispose the ribosome for translation arrest, Proc. Natl. Acad. Sci. USA, 111, 9804–9809.
Sergiev, P. V., Bogdanov, A. A., Dahlberg, A. E., and Dontsova, O. (2000) Mutations at position A960 of E. coli 23S ribosomal RNA influence the structure of 5S ribosomal RNA and the peptidyltransferase region of 23S ribosomal RNA, J. Mol. Biol., 299, 379–389.
Sergiev, P. V., Lesnyak, D. V., Burakovsky, D. E., Kiparisov, S. V., Leonov, A. A., Bogdanov, A. A., Brimacombe, R., and Dontsova, O. A. (2005) Alteration in location of a conserved GTPase-associated center of the ribosome induced by mutagenesis influences the structure of peptidyltrans-ferase center and activity of elongation factor G, J. Biol. Chem., 280, 31882–31889.
Sergiev, P. V., Kiparisov, S. V., Burakovsky, D. E., Lesnyak, D. V., Leonov, A. A., Bogdanov, A. A., and Dontsova, O. A. (2005) The conserved A-site finger of the 23S rRNA: just one of the intersubunit bridges or a part of the allosteric communication pathway? J. Mol. Biol., 353, 116–123.
Burakovsky, D. E., Sergiev, P. V., Steblyanko, M. A., Konevega, A. L., Bogdanov, A. A., and Dontsova, O. A. (2011) The structure of helix 89 of 23S rRNA is important for peptidyl transferase function of Escherichia coli ribosome, FEBS Lett., 585, 3073–3078.
Monod, J., Wyman, J., and Changeux, J.-P. (1965) On the nature of allosteric transitions: a plausible model, J. Mol. Biol., 12, 88–118.
Goodey, N. M., and Benkovic, S. J. (2008) Allosteric regulation and catalysis emerge via a common route, Nat. Chem. Biol., 4, 474–482.
Sethi, A., Eargle, J., Black, A. A., and Luthey-Schulten, Z. (2009) Dynamical networks in tRNA:protein complexes, Proc. Natl. Acad. Sci. USA, 106, 6620–6625.
Williams, S. G., and Hall, K. B. (2014) Linkage and allostery in snRNP protein/RNA complexes, Biochemistry, 53, 3529–3539.
Wilson, D. N. (2009) The A-Z of bacterial translation inhibitors, Crit. Rev. Biochem. Mol. Biol., 44, 393–433.
Ban, N., Nissen, P., Hansen, J., Moore, P. B., and Steitz, T. A. (2000) The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution, Science, 289, 905–920.
Ito, K., and Chiba, S. (2013) Arrest peptides: cis-acting modulators of translation, Annu. Rev. Biochem., 82, 171–202.
Vazquez-Laslop, N., Ramu, H., and Mankin, A. S. (2011) Nascent peptide mediated ribosome stalling promoted by antibiotics, in Ribosomes Structure, Function and Dynamics (Rodnina, M. V., Wintermeyer, W., and Green, R., eds.) Springer, Vienna, pp. 377–392.
Seidelt, B., Innis, C. A., Wilson, D. N., Gartmann, M., Armache, J.-P., Villa, E., Trabuco, L. G., Becker, T., Mielke, T., Schulten, K., Steitz, T. A., and Beckmann, R. (2009) Structural insight into nascent polypeptide chainmediated translational stalling, Science, 326, 1412–1415.
Arenz, S., Ramu, H., Gupta, P., Berninghausen, O., Beckmann, R., Vazquez-Laslop, N., Mankin, A. S., and Wilson, D. N. (2014) Molecular basis for erythromycin-dependent ribosome stalling during translation of the ErmBL leader peptide, Nat. Commun., 5, 3501.
Kannan, K., and Mankin, A. S. (2011) Macrolide antibiotics in the ribosomal tunnel: species-specific binding and action, Ann. N.Y. Acad. Sci., 1241, 33–47.
Weisblum, B. (1995) Erythromycin resistance by ribosome modification, Antimicrob. Agents Chemother., 39, 577–585.
Sanbonmatsu, K. Y. (2012) Computational studies of molecular machines: the ribosome, Curr. Opin. Struct. Biol., 22, 168–174.
Trabuco, L. G., Harrison, C. B., Schreiner, E., and Schulten, K. (2010) Recognition of the regulatory nascent chain TnaC by the ribosome, Structure, 18, 627–637.
Gumbart, J., Schreiner, E., Wilson, D., Beckmann, R., and Schulten, K. (2012) Mechanism of SecM-mediated stalling in the ribosome, Biophys. J., 103, 331–341.
Shishkina, A., Makarov, G., Tereshchenkov, A., Korshunova, G., Sumbatyan, N., Golovin, A., Svetlov, M., and Bogdanov, A. (2013) Conjugates of amino acids and peptides with 5-O-mycaminosyltylonolide and their interaction with the ribosomal exit tunnel, Bioconj. Chem., 24, 1861–1869.
Jack, A., Dunkle, J. A., Xiong, L., Mankin A. S., and Cate, J. H. (2010) Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action, Proc. Natl. Acad. Sci. USA, 107, 17152–17157.
Cannone, J. J., Subramanian, S., Schnare, M. N., Collett, J. R., D’Souza, L. M., Du, Y., Feng, B., Lin, N., Madabusi, L. V., Muller, K. M., Pande, N., Shang, Z., Yu, N., and Gutell, R. R. (2002) The comparative RNA web (CRW) site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs, BMC Bioinformatics, 3, 2.
Byrd, R. H., Lu, P., and Nocedal, J. (1995) A limited memory algorithm for bound constrained optimization, SIAM J. Sci. Comput., 16, 1190–1208.
Bussi, G., Donadio, D., and Parrinello, M. (2007) Canonical sampling through velocity rescaling, J. Chem. Phys., 126, 014101.
Van der Spoel, D., Lindahl, E., Hess, B., Groenhof, G., Mark, A. E., and Berendsen, H. J. C. (2005) GROMACS: fast, flexible, free, J. Comput. Chem., 26, 1701–1718.
Hess, B., Kutzner, C., van der Spoel, D., and Lindahl, E. (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation, J. Chem. Theory Comput., 4, 435–447.
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., Di Nola, A., and Haak, J. R. (1984) Molecular dynamics with coupling to an external bath, J. Chem. Phys., 81, 3684–3690.
Darden, T., York, D., and Pedersen, L. (1993) Particle mesh Ewald: an N log(N) method for Ewald sums in large systems, J. Chem. Phys., 98, 10089–10092.
Jorgensen, W. L., Chandrasekhar, J., and Madura, J. D. (1983) Comparison of simple potential functions for simulating liquid water, J. Chem. Phys., 79, 926–935.
Reshetnikov, R. V., Sponer, J., Rassokhina, O. I., Kopylov, A. M., Tsvetkov, P. O., Makarov, A. A., and Golovin, A. V. (2011) Cation binding to 15-TBA quadruplex DNA is a multiple-pathway cation-dependent process, Nucleic Acids Res., 39, 9789–9802.
Athavale, S. S., Petrov, A. S., Hsiao, C., Watkins, D., Prickett, C. D., Gossett, J. J., Lie, L., Bowman, J. C., O’ Neill, E., Bernier, C. R., Hud, N. V., Wartell, R. M., Harvey, S. C., and Williams, L. D. (2012) RNA folding and catalysis mediated by iron(II), PloS One, 7, e38024.
Bonomi, M., Branduardi, D., Bussi, G., Camilloni, C., Provasi, D., Raiteri, P., Donadio, D., Marinelli, F., Pietrucci, F., Broglia, R. A., and Parrinello, M. (2009) PLUMED: a portable plugin for free-energy calculations with molecular dynamics, Comput. Phys. Commun., 180, 1961–1972.
Arenz, S., Meydan, S., Starosta, A. L., Berninghausen, O., Beckmann, R., Vazquez-Laslop, N., and Wilson, D. N. (2014) Drug sensing by the ribosome induces translational arrest via active site perturbation, Mol. Cell, 56, 446–452.
Hashem, Y., and Auffinger, P. (2009) A short guide for molecular dynamic simulation of RNA systems, Methods, 47, 187–197.
Laio, A., and Parrinello, M. (2002) Escaping free energy minima, Proc. Natl. Acad. Sci. USA, 99, 12562–12566.
Hansen, J., Ippolito, J., Ban, N., Nissen, P., Moore, P., and Steitz, T. (2002) The structures of four macrolide antibiotics bound to the large ribosomal subunit, Mol. Cell, 10, 117–128.
Hansen, J. L., Moore, P. B., and Steitz, T. A. (2003) Structures of five antibiotics bound at the peptidyl transferase center of the large ribosomal subunit, J. Mol. Biol., 330, 1061–1075.
Vazquez-Laslop, N., Ramu, H., Klepacki, D., Kannan, K., and Mankin, A. S. (2010) The key function of a conserved and modified rRNA residue in the ribosomal response to the nascent peptide, EMBO J., 29, 3108–3117.
Leontis, N. B., Stombaugh, J., and Westhof, E. (2000) The non-Watson–Crick base pairs and their associated isostericity matrices, Nucleic Acids Res., 30, 3497–3531.
Hansen, J. L., Schmeing, T. M., Moore, P. B., and Steitz, T. A. (2002) Structural insights into peptide bond formation, Proc. Natl. Acad. Sci. USA, 99, 11670–11765.
Vazquez-Laslop, N., Thum, C., and Mankin, A. S. (2008) Molecular mechanism of drug-dependent ribosome stalling, Mol. Cell, 30, 190–202.
Youngman, E. M., Brunelle, J. L., Kochaniak, A. B., and Green, R. (2004) The active site of the ribosome is composed of two layers of conserved nucleotides with distinct roles in peptide bond formation and peptide release, Cell, 117, 589–599.
Nissen, P., Hansen, J., Ban, N., Moore, P. B., and Steitz, T. A. (2000) The structural basis of ribosome activity in peptide bond synthesis, Science, 289, 920–930.
Polikanov, Y. S., Steitz, T. A., and Innis, C. A. (2014) A proton wire to couple aminoacyl-tRNA accommodation and peptide-bond formation on the ribosome, Nat. Struct. Mol. Biol., 21, 787–793.
Sergiev, P. V., Lesnyak, D. V., Burakovsky, D. E., Svetlov, M., Kolb, V. A., Serebryakova, M. V., Demina, I. A., Govorun, V. M., Dontsova, O. A., and Bogdanov, A. A. (2012) Non-stressful death of 23S rRNA mutant G2061C defective in puromycin reaction, J. Mol. Biol., 416, 656–667.
Chirkova, A., Erlacher, M. D., Clementi, N., Zywicki, M., Aigner, M., and Polacek, N. (2010) The role of the universally conserved A2450–C2063 base pair in the ribosomal peptidyl transferase center, Nucleic Acids Res., 38, 4844–4855.
Leung, E. K., Suslov, N., Tuttle, N., Sengupta, R., and Piccirilli, J. A. (2011) The mechanism of peptidyl transfer catalysis by the ribosome, Annu. Rev. Biochem., 80, 527–555.
Schmeing, T. M., Huang, K. S., Strobel, S. A., and Steitz, T. A. (2005) An induced-fit mechanism to promote peptide bond formation and exclude hydrolysis of peptidyl-tRNA, Nature, 438, 520–524.
Bhushan, S., Hoffmann, T., Seidelt, B., Frauenfeld, J., Mielke, T., Berninghausen, O., Wilson, D. N., and Beckmann, R. (2011) SecM-stalled ribosomes adopt an altered geometry at the peptidyl transferase center, PLoS Biol., 18, e1000581.
Tsai, A., Kornberg, G., Johansson, M., Chen, J., and Puglisi, J. D. (2014) The dynamics of SecM-induced translational stalling, Cell Rep., 7, 1521–1533.
Sothiselvam, S., Liu, B., Han, W., Ramu, H., Klepacki, D., Atkinson, G. C., Brauer, A., Remm, M., Tenson, T., Schulten, K., Vazquez-Laslop, N., and Mankin, A. S. (2014) Macrolide antibiotics allosterically predispose the ribosome for translation arrest, Proc. Natl. Acad. Sci. USA, 111, 9804–9809.
Bischoff, L., Berninghausen, O., and Beckmann, R. (2014) Molecular basis for the ribosome functioning as an L-tryptophan sensor, Cell Rep., 9, 469–475.
Kannan, K., Kanabar, P., Schryerm, D., Florin, T., Oh, E., Bahroos, N., Tenson, T., Weissman, J. S., and Mankin, A. S. (2014) The general mode of translation inhibition by macrolide antibiotics, Proc. Natl. Acad. Sci. USA, 111, 15958–15963.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G. I. Makarov, A. V. Golovin, N. V. Sumbatyan, A. A. Bogdanov, 2015, published in Biokhimiya, 2015, Vol. 80, No. 8, pp. 1250–1261.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM15-063, July 5, 2015.
Rights and permissions
About this article
Cite this article
Makarov, G.I., Golovin, A.V., Sumbatyan, N.V. et al. Molecular dynamics investigation of a mechanism of allosteric signal transmission in ribosomes. Biochemistry Moscow 80, 1047–1056 (2015). https://doi.org/10.1134/S0006297915080106
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915080106