Abstract
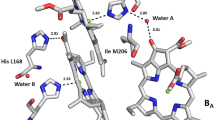
Methods of photoinduced Fourier transform infrared (FTIR) difference spectroscopy and circular dichroism were employed for studying features of pigment-protein interactions caused by replacement of isoleucine L177 by histidine in the reaction center (RC) of the site-directed mutant I(L177)H of Rhodobacter sphaeroides. A functional state of pigments in the photochemically active cofactor branch was evaluated with the method of photo-accumulation of reduced bacteriopheophytin H −A . The results are compared with those obtained for wild-type RCs. It was shown that the dimeric nature of the radical cation of the primary electron donor P was preserved in the mutant RCs, with an asymmetric charge distribution between the bacteriochlorophylls PA and PB in the P+ state. However, the dimers P in the wild-type and mutant RCs are not structurally identical due probably to molecular rearrangements of the PA and PB macrocycles and/or alterations in their nearest amino acid environment induced by the mutation. Analysis of the electronic absorption and FTIR difference P+Q−/PQ spectra suggests the 173-ester group of the bacteriochlorophyll PA to be involved in covalent interaction with the I(L177)H RC protein. Incorporation of histidine into the L177 position does not modify the interaction between the primary electron acceptor bacteriochlorophyll BA and the bacteriopheophytin HA. Structural changes are observed in the monomer bacteriochlorophyll BB binding site in the inactive chromophore branch of the mutant RCs.
Similar content being viewed by others
Abbreviations
- BChl:
-
bacteriochlorophyll
- BPheo:
-
bacteriopheophytin
- CD:
-
circular dichroism
- FTIR:
-
Fourier transform infrared spectroscopy
- LDAO:
-
N,N-dimethyldodecylamine-N-oxide
- P:
-
primary electron donor
- RC:
-
reaction center
References
Shuvalov, V. A. (1990) Primary Light Energy Transformation in Photosynthesis [in Russian], Nauka, Moscow.
Ermler, U., Fritzsch, G., Buchanan, S. K., and Michel, H. (1994) Structure, 2, 925–936.
Straley, S. C., Parson, W. W., Mauzerall, D. C., and Clayton, R. K. (1973) Biochim. Biophys. Acta, 305, 597–609.
Van der Rest, M., and Gingras, G. (1974) J. Biol. Chem., 249, 6446–6453.
Fufina, T. Y., Vasilieva, L. G., Khatypov, R. A., Shkuropatov, A. Ya., and Shuvalov, V. A. (2007) FEBS Lett., 581, 5769–5773.
Fiedor, L., Rosenbach-Belkin, V., Sai, M., and Scherz, A. (1996) Plant. Physiol. Biochem., 34, 393–398.
Rau, H. K., Snigula, H., Struck, A., Robert, B., Scheer, H., and Haehnel, W. (2001) Eur. J. Biochem., 268, 3284–3295.
Khatypov, R. A., Vasilieva, L. G., Fufina, T. Yu., Bolgarina, T. I., and Shuvalov, V. A. (2005) Biochemistry (Moscow), 70, 1256–1261.
Philipson, K. D., and Sauer, K. (1973) Biochemistry, 12, 535–539.
Mantele, W. (1993) in The Photosynthetic Reaction Center (Deisenhofer, H., and Norris, J. R., eds.) Vol. II, Academic Press, San Diego, pp. 239–283.
Jones, M. R., Visschers, R. W., van Grondelle, R., and Hunter, C. N. (1992) Biochemistry, 31, 4458–4465.
Shuvalov, V. A., and Asadov, A. A. (1979) Biochim. Biophys. Acta, 545, 296–308.
Breeze, R. H., and Ke, B. (1972) Analyt. Biochem., 50, 281–303.
Okamura, M. Y., Isaacson, R. A., and Feher, G. (1979) Biochim. Biophys. Acta, 546, 394–417.
Shuvalov, V. A., Shkuropatov, A. Ya., Kulakova, S. M., Ismailov, V. A., and Shkuropatova, V. A. (1986) Biochim. Biophys. Acta, 849, 337–346.
Creighton, T. E. (1994) Protein Structure and Molecular Properties, W. H. Freeman and Co., New York.
Williams, J. C., Alden, R. G., Murchison, H. A., Peloquin, J. M., Woodbury, N. W., and Allen, J. P. (1992) Biochemistry, 31, 11029–11037.
Sauer, K., Dratz, E. A., and Coyne, L. (1968) Proc. Natl. Acad. Sci. USA, 61, 17–24.
Reed, D. W., and Ke, B. (1973) J. Biol. Chem., 248, 3041–3045.
Struck, A., Cmiel, E., Katheder, I., and Scheer, H. (1990) FEBS Lett., 268, 180–184.
Mar, T., and Gingras, G. (1995) Biochemistry, 34, 9071–9078.
Clayton, R. (1980) Photosynthesis: Physical Mechanisms and Chemical Patterns, Cambridge University Press, Cambridge.
Xie, X., and Simon, J. D. (1991) Biochim. Biophys. Acta, 1057, 132–139.
Breton, J., Nabedryk, E., and Parson, W. W. (1992) Biochemistry, 31, 7503–7510.
Reimers, J. R., and Hush, N. S. (2003) J. Chem. Phys., 119, 3262–3277.
Nabedryk, E., Allen, J. P., Taguchi, A. K. W., Williams, J. C., Woodbury, N. W., and Breton, J. (1993) Biochemistry, 32, 13879–13885.
Nabedryk, E., Schulz, C., Muh, F., Lubitz, W., and Breton, J. (2000) Photochem. Photobiol., 71, 582–588.
Vasilieva, L. G., Fufina, T. Y., Khatypov, R. A., Proskuryakov, I. I., Zabelin, A. A., Shkuropatov, A. Y., and Shuvalov, V. A. (2007) in 7th Int. Conf. on Tetrapyrrole Photoreceptors in Photosynthetic Organisms, Kyoto, Japan, Book of Abstracts, p. 93.
Johnson, E. T., Muh, F., Nabedryk, E., Williams, J. C., Allen, J. P., Lubitz, W., Breton, J., and Parson, W. W. (2002) J. Phys. Chem. B, 106, 11859–11869.
Leonhard, M., Wollenweber, A., Berger, G., Kleo, J., Nabedryk, E., Breton, J., and Mantele, W. (1989) in Techniques and New Developments in Photosynthesis Research (Barber, J., and Malkin, R., eds.) Vol. 168, NATO ASI Series, Plenum, New York, pp. 115–118.
Struck, A., Cmiel, E., Katheder, I., Schafer, W., and Scheer, H. (1992) Biochim. Biophys. Acta, 1101, 321–328.
Tsuchiya, T., Suzuki, T., Yamada, T., Shimada, H., Masuda, T., Ohta, H., and Takamiya, K. (2003) Plant Cell Physiol., 44, 96–101.
Arkus, K. J. A., Cahoon, E. B., and Jez, J. M. (2005) Arch. Biochem. Biophys., 438, 146–155.
Michalski, T. J., Hunt, J. E., Bradshaw, C., Wagner, A. M., Norris, J. R., and Katz, J. J. (1987) J. Am. Chem. Soc., 110, 5888–5891.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A. A. Zabelin, T. Y. Fufina, L. G. Vasilieva, V. A. Shkuropatova, M. G. Zvereva, A. Y. Shkuropatov, V. A. Shuvalov, 2009, published in Biokhimiya, 2009, Vol. 74, No. 1, pp. 86–94.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM08-149, December 14, 2008.
Rights and permissions
About this article
Cite this article
Zabelin, A.A., Fufina, T.Y., Vasilieva, L.G. et al. Mutant reaction centers of Rhodobacter sphaeroides I(L177)H with strongly bound bacteriochlorophyll a: Structural properties and pigment-protein interactions. Biochemistry Moscow 74, 68–74 (2009). https://doi.org/10.1134/S0006297909010106
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297909010106