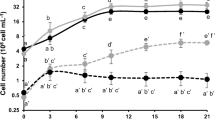
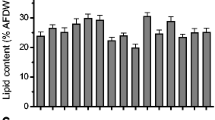
Abstract
A search for strains capable of the simultaneous production of high amounts of several biologically valuable compounds and/or high biomass productivity has been carried out. The growth characteristics and biochemical composition of 12 microalgal and cyanobacterial strains from the IPPAS Collection were studied at the exponential and stationary growth phases. All of the strains had high growth rates (a doubling time of 6–22 h). The strains Cyanobacterium sp. IPPAS B-1200, Chlorella sp. IPPAS C-1210, Nannochloris sp. IPPAS C-1509, Cyanidium caldarium IPPAS P-510, and Vischeria sp. IPPAS H-242 demonstrated the highest biotechnological potential and can be used for the production of various types of biofuel, pigments, and feed and food additives, including those with a high content of eicosapentaenoic acid (20 : 5 Δ5, 8,11, 14, 17).






Similar content being viewed by others
REFERENCES
Falkowski, P.G. and Raven, J.A., Aquatic Photosynthesis, 2nd ed., Princeton, New Jersey: Princeton Univ. Press, 2007.
Chisti, Y., Constraints to commercialization of algal fuels, J. Biotechnol., 2013, no. 3, p. 201.
Spolaore, P., Joannis-Cassan, C., Duran, E., and Isambert, A., Commercial applications of microalgae, J. Biosci. Bioeng., 2006, no. 2, pp. 87–96. https://doi.org/10.1263/jbb.101.87
Becker, W., Microalgae in human and animal nutrition, in Handbook of Microalgal Culture, Richmond, A., Ed., Blackwell: Oxford, 2004, pp. 312–351.
Hudek, K., Davis, L.C., Ibbini, J., and Erickson, L., Commercial products from algae, in Algal Biorefneries, Bajpai, R., Prokop, A., and Zappi, M., Eds., Springer: Dordrecht, 2014, pp. 275‒296.
Pacheco, M.M., Hoeltz, M., Moraes, M.S.A., and Schneider, R.C.S., Microalgae: cultivation techniques and wastewater phycoremediation, J. Environ. Sci. Health A, 2015, vol. 50, no. 6, pp. 585–601. https://doi.org/10.1080/10934529.2015.994951
Solovchenko, A.E., Semenova, L.R., Selyakh, I.O., et al., Assessment of a new Chlorella vulgaris (Chlorophyta) IPPAS C-2015 strain for application in poultry wastewater bioremediation, Biotekhnologia, 2016, vol. 32, no. 2, pp. 72‒81. https://doi.org/10.21519/0234-2758-2016-2-72-81
Guiry, M.D., How many species of algae are there?, J. Phycol., 2012, vol. 48, pp. 1057–1063. https://doi.org/10.1111/j.1529-8817.2012.01222.x
Levine, I.A., Algae: a way of life and health, in Microalgae in Health and Disease Prevention, Levine, I.A. and Fleurence, J., Eds., Academic Press, 2018, pp. 1–10. https://doi.org/10.1016/B978-0-12-811405-6.00001-3
Gifuni, I., Pollio, A., Safi, C., et al., Current bottlenecks and challenges of the microalgal biorefinery, Trends Biotechnol., 2019, vol. 37, no. 3, pp. 242–252. https://doi.org/10.1016/j.tibtech.2018.09.006
Petkov, G., Ivanova, A., Iliev, I., and Vaseva, I., A critical look at the microalgae biodiesel, Eur. J. Lipid Sci. Technol., 2012, vol. 114, pp. 103–111. https://doi.org/10.1002/ejlt.201100234
Deprá, M.C., Santos, A.M., Severo, I.A., et al., Microalgal biorefineries for bioenergy production: can we move from concept to industrial reality?, BioEnergy Res., 2018, vol. 11, no. 4, pp. 727–747. https://doi.org/10.1007/s12155-018-9934-z
Bastiaens, L., Van Roy, S., Thomassen, G., and Elst, K., Biorefinery of algae: technical and economic considerations, in Microalgae-Based Biofuels and Bioproducts, Gonzalez-Fernandez, C. and Muñoz, R., Eds., Woodhead Publishing, 2017, pp. 327–345. https://doi.org/10.1016/B978-0-08-101023-5.00014-5
Khozin-Goldberg, I. and Cohen, Z., Unraveling algal lipid metabolism: recent advances in gene identification, Biochimie, 2011, vol. no. 1, pp. 91–100. https://doi.org/10.1016/j.biochi.2010.07.020
D'Alessandro, E.B. and Antoniosi Filho, N.R., Concepts and studies on lipid and pigments of microalgae: a review, Renew. Sust. Energ. Rev., 2016, vol. 58, pp. 832–841. https://doi.org/10.1016/j.rser.2015.12.162
Heydarizadeh, P., Poirier, I., Loizeau, D., et al., Plastids of marine phytoplankton produce bioactive pigments and lipids, Mar. Drugs, 2013, vol. 11, no. 9, pp. 3425–3471. https://doi.org/10.3390/md11093425
Gacheva, G.V. and Gigova, L.G., Biological activity of microalgae can be enhanced by manipulating the cultivation temperature and irradiance, Centr. Eur. J. Biol., 2014, vol. 9, no. 12, pp. 1168–1181. https://doi.org/10.2478/s11535-014-0350-x
Abdullaev, A.A. and Semenenko, V.E., The intense culture of Dunaliella salina Teod. and its physiological characteristics, Fiziol. Rast., 1974, vol. 21, no. 6, pp. 1145–1153.
Sarsekeyeva, F.K., Usserbaeva, A.A., Zayadan, B.K., et al., Isolation and characterization of a new cyanobacterial strain with a unique fatty acid composition, Adv. Microbiol., 2014, vol. 4, no. 15, pp. 1033–1043. https://doi.org/10.4236/aim.2014.415114
Hase, E., Morimura, Y., and Tamiya, H., Some data on the growth physiology of Chlorella studied by the technique of synchronous culture, Arch. Biochem. Biophys., 1957, vol. 69, pp. 149–165. https://doi.org/10.1016/0003-9861(57)90482-4
Stanier, R.Y., Kunisawa, R., Mandel, M., and Cohen-Bazire, G., Purification and properties of unicellular blue-green algae (order Chroococcales), Bacteriol. Rev., 1971, vol. 35, pp. 171–205.
Vladimirova, M.G., Bartsevich, E.D., Zholdakov, I.A., et al., IPPAS— Collection of Microalgae of Timiryazev Institute of Plant Physiology, Acad. Sci. USSR, in Katalog kul’tur mikrovodoroslei v kollektsiyakh SSSR (Catalogue of Microalgal Cultures in the Collections of USSR), Semenenko, V.E., Ed., Moscow: Ross. Akad. Nauk, 1991, pp. 8–61.
Zavřel, T., Sinetova, M.A., and Červený, J., Measurement of chlorophyll a and carotenoids concentration in cyanobacteria, Bioprotocol, 2015, vol. 5, no. 9, e1467. https://doi.org/10.21769/BioProtoc.1467
Wellburn, A.R., The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution, J. Plant Physiol., 1994, vol. 144, no. 3, pp. 307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Ritchie, R.J., Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents, Photosynth. Res., 2006, vol. 89, no. 1, pp. 27‒41. https://doi.org/10.1007/s11120-006-9065-9
Zavrel, T., Očenášová, P., Sinetova, M.A., and Červený, J., Determination of storage (starch/glycogen) and total saccharides content in algae and cyanobacteria by a phenol-sulfuric acid method, Bioprotocol, 2018, vol. 8, no. 15, e2966. https://doi.org/10.21769/BioProtoc.2966
Rumin, J., Bonnefond, H., Saint-Jean, B., et al., The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae, Biotechnol. Biofuels, 2015, vol. 8, p. 42. https://doi.org/10.1186/s13068-015-0220-4
Fogg, G.E. and Thake, B., Algal Cultures and Phytoplankton Ecology, London: University of Wisconsin Press, 1987.
Tsoglin, L.N. and Pronina, N.A., Biotekhnologiya mikrovodoroslei (Biotechnology of Microalgae), Moscow: Nauchnyi Mir, 2012.
Ogbonna, J.C., Yada, H., and Tanaka, H., Kinetic study on light-limited batch cultivation of photosynthetic cells, J. Ferm. Bioeng., 1995, vol. 80, no. 3, pp. 259–264. https://doi.org/10.1016/0922-338X(95)90826-L
Sinetova, M.A., Cerveny, J., Zavrel, T., and Nedbal, L., On the dynamics and constraints of batch culture growth of the cyanobacterium Cyanothece sp. ATCC 51142, J. Biotechnol., 2012, vol. 162, no. 1, pp. 148‒155.
Graziani, G., Schiavo, S., Nicolai, M.A., et al., Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria,Food Funct., 2013, vol. 4, no. 1, pp. 144–152. https://doi.org/10.1039/C2FO30198A
Gao, B., Yang, J., Lei, X., et al., Characterization of cell structural change, growth, lipid accumulation, and pigment profile of a novel oleaginous microalga, Vischeria stellata (Eustigmatophyceae), cultured with different initial nitrate supplies, J. Appl. Phycol., 2016, vol. 28, pp. 821–830. https://doi.org/10.1007/s10811-015-0626-1
Rampen, S.W., Datema, M., Rodrigo-Gámiz, M., Schouten, S., et al., Sources and proxy potential of long chain alkyl diols in lacustrine environments, Geochem. Cosmochem. Acta, 2014, vol. 144, pp. 59–71. https://doi.org/10.1016/j.gca.2014.08.033
Slocombe, S.P., Zhang, Q., Ross, M., et al., Unlocking nature’s treasure-chest: screening for oleaginous algae, Sci. Rep., 2015, vol. 5, p. 9844. https://doi.org/10.1038/srep09844
Chisti, Y., Biodiesel from microalgae, Biotechnol. Adv., 2007, vol. 25, pp. 294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Yu, J., Liberton, M., Cliften, P.F., et al., Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2, Sci. Rep., 2015, vol. 5, p. 8132. https://doi.org/10.1038/srep08132
Weissman, J.C., Likhogrud, M., and Thomas, D.C., High-light selection produces a fast-growing Picochlorum celery,Algal Res., 2018, vol. 36, pp. 17–28. https://doi.org/10.1016/j.algal.2018.09.024
Tumolo, T. and Lanfer-Marquez, U.M., Copper chlorophyllin: A food colorant with bioactive properties?, Food Res. Int., 2012, vol. 46, no. 2, pp. 451–459. https://doi.org/10.1016/j.foodres.2011.10.031
Young, R.W. and Beregi, J.S., Use of chlorophyllin in the care of geriatric patients, J. Am. Geriatr. Soc., 1980, vol. 28, no. 1, pp. 46–47. https://doi.org/10.1111/j.1532-5415.1980.tb00124.x
Sorensen, L., Hantke, A., and Eriksen, N.T., Purification of the photosynthetic pigment C-phycocyanin from heterotrophic Galdieria sulphuraria,J. Sci. Food Agricult., 2013, vol. 93, no. 12, pp. 2933–2938. https://doi.org/10.1002/jsfa.6116
Piorreck, M., Baasch, K.-H., and Pohl, P., Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes, Phytochemistry, 1984, vol. 23, no. 2, pp. 207–216. https://doi.org/10.1016/S0031-9422(00)80304-0
Gonzalez, Lopez, C.V. and Garcia, M., d.C.C., Fernandez F.G.A., et al. Protein measurements of microalgal and cyanobacterial biomass, Biores. Techn., 2010, vol. 101, no. 19, pp. 7587–7591. https://doi.org/10.1016/j.biortech.2010.04.077
Stepanchenko, N.S., Novikova, G.V., and Moshkov, I.E., Protein quantification, Russ. J. Plant Physiol., 2011, vol. 58, no. 4, pp. 737–742. https://doi.org/10.1134/S1021443711040182
Halim, R., Danquah, M.K., and Webley, P.A., Extraction of oil from microalgae for biodiesel production: a review, Biotechnol. Adv., 2012, vol. 30, no. 3, pp. 709–732. https://doi.org/10.1016/j.biotechadv.2012.01.001
Suzuki, E. and Suzuki, R., Variation of storage polysaccharides in phototrophic microorganisms, J. Appl. Glycosci., 2013, vol. 60, no. 1, pp. 21‒27. https://doi.org/10.5458/jag.jag.JAG-2012_016
Zhang, J., Wan, L., Xia, S., Li, A., et al., Morphological and spectrometric analyses of lipids accumulation in a novel oleaginous microalga, Eustigmatos cf. polyphem (Eustigmatophyceae), Bioprocess Biosyst. Eng., 2013, vol. 36, no. 8, pp. 1125–1130. https://doi.org/10.1007/s00449-012-0866-2
Evstigneeva, R.P., Serebryannikova, G.A., Zvonkova, E.N., et al., Khimiya biologicheski aktivnykh prirodnykh soedinenii (Chemistry of Biologicaly Active Natural Compounds), Moscow: Khimiya, 1976.
John, R.P., Anisha, G.S., Nampoothiri, K.M., and Pandey, A., Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol., 2011, vol. 102, no. 1, pp. 186–193. https://doi.org/10.1016/j.biortech.2010.06.139
Ho, S.H., Huang, S.W., Chen, C.Y., Hasunuma, T., et al., Bioethanol production using carbohydrate-rich microalgae biomass as feedstock, Bioresour. Technol., 2013, vol. 135, pp. 191–198. https://doi.org/10.1016/j.biortech.2012.10.015
Sarsekeyeva, F., Zayadan, B., Usserbaeva, A., Bedbenov, V., et al., Cyanofuels: biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res., 2015, pp. 1‒12. https://doi.org/10.1007/s11120-015-0103-3
Guschina, I.A. and Harwood, J.L., Lipids and lipid metabolism in eukaryotic algae, Prog. Lipid Res., 2006, vol. 45, no. 2, pp. 160–186. https://doi.org/10.1016/j.plipres.2006.01.001
Dunstan, G.A., Volkman, J.K., Barrett, S.M., and Garland, C.D., Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture, J. Appl. Phycol., 1993, vol. 5, no. 1, pp. 71‒83.
Lenihan-Geels, G., Bishop, K., and Ferguson, L., Alternative sources of omega-3 fats: can we find a sustainable substitute for fish?, Nutrients, 2013, vol. 5, no. 4, p. 1301. https://doi.org/10.3390/nu5041301
Funding
This work was supported by a grant from the Russian Science Foundation (no. 14-14-00904).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or humans performed by any of the authors.
Additional information
Translated by I. Gordon
Abbreviations: ARA—arachidonic acid; Car—content of total carotenoids; dw—dry weight; Chl a(b)—chlorophyll a(b) content; EPA—eicosapentaenoic acid; EPS—exopolysaccharide(s); FA—fatty acid; FAME—fatty acid methyl ester; FFA—free fatty acid; OD750—optical density at a wavelength of 750 nm; Pf—final productivity; PUFA—polyunsaturated fatty acid; SDS—sodium dodecyl sulfate; Td—biomass doubling time; TAG—triacylglycerol; TLs—total lipids; UI—unsaturation index; μ—specific growth rate; μmax—maximal specific growth rate at the exponential growth phase.
Rights and permissions
About this article
Cite this article
Sinetova, M.A., Sidorov, R.A., Starikov, A.Y. et al. Assessment of the Biotechnological Potential of Cyanobacterial and Microalgal Strains from IPPAS Culture Collection. Appl Biochem Microbiol 56, 794–808 (2020). https://doi.org/10.1134/S0003683820070030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683820070030