Abstract
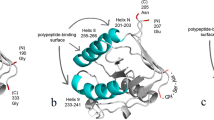
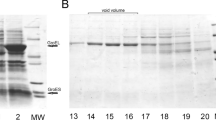
At biosynthetic production of hydrophobic recombinant proteins there is often a problem of their aggregation in so-called inclusion bodies, after which these proteins are difficult to renature. Obtaining such proteins in soluble forms is a formidable obstacle on the way of using them. One of possible solutions for this problem is the creation of fusion constructs with a leading protein. Previously, we have developed a system for biosynthetic production of insoluble hydrophobic proteins as a part of a fusion construct with a leader based on chaperone GroEL apical domain (GrAD, GroEL Apical Domain). Expressed as a part of a fusion with GrAD, two initially insoluble proteins were successfully obtained in soluble form. Still, such a system may have limitations in use, because supporting hydrophobic proteins in soluble state requires their interaction with GrAD substrate binding surface, which implies correct mutual orientation of proteins constituent parts of the construct, and for some target proteins such interaction may be sterically impeded. To enlarge the capability of using GrAD, the strategy of making permutations was used, which consists in linking original GrAD N- and C-termini with a linker and creating new N- and C-termini situated in closer proximity to the GrAD substrate binding surface. This work describes production and the study of physico-chemical properties of two permutated GrAD variants intended to be used as leaders in fusion constructs.






Similar content being viewed by others
REFERENCES
Terpe, K., Appl. Microbiol. Biotechnol., 2003, vol. 60, no. 5, pp. 523–533.
Riggs, P., La Vallie, E.R., and McCoy, J.M., Curr. Protoc. Mol. Biol., Frederick, M. and Ausubel, Al., Eds., 2001, chapter 16, unit 16.4A.
Waugh, D.S., Trends Biotechnol., 2005, vol. 23, no. 6, pp. 316–320.
Schmidt, S.R., Curr. Opin. Drug Discov. Dev., 2009, vol. 12, no. 2, pp. 284–295.
Esposito, D. and Chatterjee, D.K., Curr. Opin. Biotechnol., 2006, vol. 17, no. 4, pp. 353–358.
Panavas, T., Sanders, C., and Butt, T.R., Methods Mol. Biol. Clifton N.J., 2009, vol. 497, pp. 303–317.
Kapust, R.B., and Waugh, D.S., Protein Sci. Publ. Protein Soc.,1999, vol. 8, no. 8, pp. 1668–1674.
Huth, J.R., Bewley, C.A., Jackson, B.M., Hinnebusch, A.G., Clore, G.M., and Gronenborn, A.M., Protein Sci. Publ. Protein Soc., 1997, vol. 6, no. 11, pp. 2359–2364.
Walls, D. and Loughran, S.T., Methods Mol. Biol. Clifton N.J., 2011, vol. 681, pp. 151–175.
Zou, Z., Cao, L., Zhou, P., Su, Y., Sun, Y., and Li, W., J. Biotechnol., 2008, vol. 135, no. 4, pp. 333–339.
Santner, A.A., Croy, C.H., Vasanwala, F.H., Uversky, V.N., Van, Y.-Y.J., and Dunker, A.K., Biochemistry (Moscow), 2012, vol. 51, no. 37, pp. 7250–7262.
Kyratsous, C.A. and Panagiotidis, C.A., Methods Mol. Biol. Clifton NJ, 2012, vol. 824, pp. 109–129.
Furutani, M., Hata, J., Shomura, Y., Itami, K., Yoshida, T., Izumoto, Y., Togi, A., Ideno, A., Yasunaga, T., Miki, K., Maruyama, T., Protein Sci. Publ. Protein Soc., 2005, vol. 14, no. 2, pp. 341–350.
Fedorov, A.N. and Yurkova, M.S., NanoWorld J., 2018, vol. 4, no. 1, pp. 8–15.
Chatellier, J., Hill, F., Lund, P.A., and Fersht, A.R., Proc. Natl. Acad. Sci. U.S.A., 1998, vol. 95, no. 17, pp. 9861–9866.
Wang, Q., Buckle, A.M., and Fersht, A.R., J. Mol. Biol., 2000, vol. 298, no. 5, pp. 917–926.
Altamirano, M.M., Golbik, R., Zahn, R., Buckle, A.M., and Fersht, A.R., Proc. Natl. Acad. Sci. U.S.A., 1997, vol. 94, no. 8, pp. 3576–3578.
Wang, Q., Buckle, A.M., and Fersht, A.R., J. Mol. Biol., 2000, vol. 304, no. 5, pp. 873–881.
Sharapova, O.A., Yurkova, M.S., and Fedorov, A.N., Protein Eng. Des. Sel. PEDS, 2016, vol. 29, no. 2, pp. 57–64.
Bliven, S. and Prlić, A., PLoS Comput. Biol., 2012, vol. 8, no. 3. e1002445.
Jung, J. and Lee, B., Protein Sci. Publ. Protein Soc., 2001, vol. 10, no. 9, pp. 1881–1886.
Yu, Y. and Lutz, S., Trends Biotechnol., 2011, vol. 29, no. 1, pp. 18–25.
Goldenberg, D.P. and Creighton, T.E., J. Mol. Biol., 1983, vol. 165, no. 2, pp. 407–413.
Luger, K., Hommel, U., Herold, M., Hofsteenge, J., and Kirschner, K., Science, 1989, vol. 243, no. 4888, pp. 206–210.
Bulaj, G., Koehn, R.E., and Goldenberg, D.P., Protein Sci. Publ. Protein Soc., 2004, vol. 13, no. 5, pp. 1182–1196.
Capraro, D.T., Roy, M., Onuchic, J.N., and Jennings, P.A., Proc. Natl. Acad. Sci. U.S.A., 2008, vol. 105, no. 39, pp. 14844–14848.
Viguera, A.R., Serrano, L., and Wilmanns, M., Nat. Struct. Biol., 1996, vol. 3, no. 10, pp. 874–880.
Zhang, P. and Schachman, H.K., Protein Sci. Publ. Protein Soc., 1996, vol. 5, no. 7, pp. 1290–1300.
Topell, S., Hennecke, J., and Glockshuber, R., FEBS Lett., 1999, vol. 457, no. 2, pp. 283–289.
Cheltsov, A.V., Barber, M.J., and Ferreira, G.C., J. Biol. Chem., 2001, vol. 276, no. 22, pp. 19141–19149.
Whitehead, T.A., Bergeron, L.M., and Clark, D.S., Protein Eng. Des. Sel. PEDS, 2009, vol. 22, no. 10, pp. 607–613.
Qian, Z. and Lutz, S., J. Am. Chem. Soc., 2005, vol. 127, no. 39, pp. 13466–13467.
Hua, Q., Dementieva, I.S., Walsh, M.A., Hallenga, K., Weiss, M.A., and Joachimiak, A., J. Mol. Biol., 2001, vol. 306, no. 3, pp. 513–525.
Kurov, K.A., Savvin, O.I., Yurkova, M.S., Zenin, V.A., Nagibina, G.S., Melnik, B.S., and Fedorov, A.N., Biotekhnologiya, 2018, vol. 34, no. 6, pp. 43–50.
Uversky, V.N., J. Biol. Chem., 2016, vol. 291, no. 13, pp. 6681–6688.
Altamirano, M.M., Woolfson A., Donda A., Shamshiev, A., Briseño-Roa, L., Foster, N.W., Veprintsev, D.B., De Libero, G., Fersht, A.R. and Milstein C., Proc. Natl. Acad. Sci. U.S.A., 2001. vol. 98, no. 6, pp. 3288–3293.
Ben-Zvi, A.P., Chatellier, J., Fersht, A.R., and Goloubinoff, P., Proc. Natl. Acad. Sci. U.S.A., 1998, vol. 95, no. 26, pp. 15275–15280.
Karadimitris, A., Gadola, S., Altamirano, M., Brown, D., Woolfson, A., Klenerman, P., Chen, J.L., Koezuka, Y., Roberts, I.A., Price, D.A., Dusheiko, G., Milstein, C., Fersht, A., Luzzatto, L., Cerundolo, V. Proc. Natl. Acad. Sci. U.S.A., 2001, vol. 98, no. 6, pp. 3294–3298.
Priya, S., Sharma, S. K., Sood, V., Mattoo, R.U., Finka, A., Azem, A., De Los Rios, P., Goloubinoff, P., Proc. Natl. Acad. Sci. U.S.A., 2013, vol. 110, no. 18, pp. 7199–7204.
Funding
The work was supported by the Ministry of science and higher education of the Russian Federation, unique identifier RFMEFI57517X0151.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yurkova, M.S., Zenin, V.A., Nagibina, G.S. et al. Physico-Chemical Characterization of Permutated Variants of Chaperone GroEL Apical Domain. Appl Biochem Microbiol 55, 588–595 (2019). https://doi.org/10.1134/S0003683819130027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683819130027