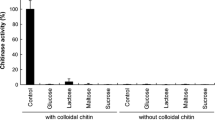
Abstract
The paper reports on the isolation of an extracellular chitinase produced by the alkaliphilic Bacillus mannanilyticus IB-OR17 B1 strain grown in media containing crab shell and bee chitin at a pH of 8–11. The enzyme was 860-fold purified by ultrafiltration and chitin sorption. The molecular weight of the purified chitinase was shown by denaturing electrophoresis to be 56 kDa. The enzyme showed maximum activity at a pH of 7.5–8.0 and 65°C and was stable within a pH range of 3.5–10.5 and temperature range of 75–85°C. With colloidal chitin as substrate, the kinetic characteristics of the chitinase were determined as follows: KM ~ 1.32 mg/mL and Vmax ~ 5.05 μM min–1. N-acetyl-D-glucosamine and its dimer were the main products of enzymatic chitin cleavage, while the trisaccharide was detected just in minor quantities. The chitinase actively hydrolyzed p-nitrophenyl-GlcNAc2 according to the exo-mechanism of substrate hydrolysis characteristic of chitobiosidases.
Similar content being viewed by others
References
Horikoshi, K., Microbiol. Mol. Biol. Rev., 1999, vol. 63, no. 2, pp. 735–750.
Preiss, L., Hicks, D.B., Suzuki, S., Meier, T., and Krulwich, T.A., Front. Bioeng. Biotechnol., 2015, vol. 3, pp. 1–10. doi 10.3389/fbioe.2015.00075
Sarethy, I.P., Saxena, Y., Kapoor, A., Sharma, M., Sharma, S.K., Gupta, V., and Gupta, S., J. Ind. Microbiol. Biotechnol., 2011, vol. 38, no. 7, pp. 769–790.
Horikoshi, K. and Akiba, T., Alkalophilic Microorganisms: A New Microbial World, Heidelberg: Springer-Verlag KG,1982.
Tsujibo, H., Kubota, T., Yamamoto, M., Myiamoto, K., and Inamori, Y., Appl. Environ. Microbiol., 2003, vol. 69, no. 2, pp. 894–900.
Bhushan, B., J. Appl. Microbiol., 2000, vol. 88, no. 5, pp. 800–808.
Loni, P.P. and Bajekal, S.S., Indian J. Fundam. Appl. Life Sci., 2011, vol. 1, no. 3, pp. 161–165.
Loni, P.P., Patil, J.U., Phugare, S.S., and Bajekal, S.S., J. Basic. Microbiol., 2014, vol. 54, no. 10, pp. 1080–1089.
Rajan, L.A., Dharini, J., Singh, K.H.P., Sivvaswaamy, S.N., Sheela, J.S., and Sundar, N., Biotechnology, 2010, vol. 9, no. 2, pp. 229–233.
Songsiriritthigul, C., Lapboonrueng, S., Pechsrichuang, P., Pesatcha, P., and Yamabhai, M., Bioresource Technol., 2010, vol. 101, no. 11, pp. 4096–4103.
Wang, S.-L., Liang, T.-W., and Yen, Y.-H., Carbohydr. Res., 2011, vol. 84, no. 2, pp. 732–742.
Liang, T.-W., Lo, B.-C., and Wang, S.-L., Mar. Drugs, 2015, vol. 13, no. 8, pp. 4576–4593.
Bansode, V.B. and Bajekal, S.S., Indian J. Biotechnol., 2006, vol. 5, no. 3, pp. 357–363.
Prasanna, L., Eijsink, V.G.H., Meadow, R., and Gaseidnes, S., Appl. Microbiol. Biotechnol., 2013, vol. 97, no. 4, pp. 1601–1611.
Kuzu, S.B., Guvenmez, H.K., and Denizci, A.A., Biotechnol. Res. Int., 2012, vol. 2012. doi 10.1155/2012/135498
Meruvu, H. and Donthireddy, S.R.R., Appl. Biochem. Biotechnol., 2014, vol. 172, no. 1, pp. 196–205.
Bhattacharya, S., Das, A., Samadder, S., and Rajan, S.S., 3 Biotech., 2016, vol. 6, no. 1, p. 87. doi 10.1007/s13205-016-0406-x
Melentiev, A.I., Galimzianova, N.F., Gilvanova, E.A., Schelchkova, E.A., Kuzmina, L.Yu., Boyko, T.F., Usanov, N.G., and Aktuganov, G.E., Adv. Microbiol., 2014, vol. 4, no. 8, pp. 455–464.
Nogi, Y., Takami, H., and Horikoshi, K., Int. J. Syst. Evol. Microbiol., 2005, vol. 55, no. 6, pp. 2309–2315.
Akino, T., Nakamura, N., and Horikoshi, K., Appl. Microbiol. Biotechnol., 1987, vol. 26, no. 4, pp. 323–327.
Aktuganov, G.E., Galimzyanova, N.F., Teregulova, G.A., and Melent’ev, A.I., Appl. Biochem. Microbiol., 2016, vol. 52, no. 5, pp. 531–536.
Aktuganov, G.E., Melent’ev, A.I., Kuz’mina, L.Yu., Galimzyanova, N.F., and Shirokov, A.V., Microbiology (Moscow), 2003, vol. 72, no. 3, pp. 313–317.
Aktuganov, G.E., Melent’ev, A.I., Galimzyanova, N.F., and Shirokov, A.V., Microbiology (Moscow), 2008, vol. 77, no. 6, pp. 700–709.
Lee, Y.-S., Park, I.-H., Yoo, J.-S., Chung S.-Y., Lee, Y.-C., Cho, Y.-S., Ahn, S.-C., Kim, C.-M., and Choi, Y.-L., Bioresource Technol., 2007, vol. 98, no. 14, pp. 2734–2741.
Waghmare, S.R. and Ghosh, J.S., Carbohydr. Res., 2010, vol. 345, no. 18, pp. 2630–2635.
Yuli, P.E., Suhartono, M.T., Rukayadi, Y., Hwang, J.K., and Pyun, Y.R., Enzyme Microb. Technol., 2004, vol. 35, nos. (2–3), pp. 147–153.
Pradeep, G.C., Choi, Y.H., Choi, Y.S., Suh, S.E., Seong, J.H., Cho, S.S., Bae, M.-S., and Yoo, J.C., Process Biochem., 2014, vol. 49, no. 2, pp. 223–229.
Sorokin, D.Y., Tourova, T.P., Sukhacheva, M.V., Mardanov, A.V., and Ravin, N.V., Extremophiles, 2012, vol. 16, no. 6, pp. 883–894.
Sorokin, D.Y., Rakitin, A.L., Gumerov, V.M., Beletsky, A.V., Sinninghe, Damste, J.S., Mardanov, A.V., and Ravin, N.V., Front. Microbiol., 2016, vol. 7, p. 407. doi 10.3389/fmicb.2016.00407
Sampei, Z., Nagao, Y., Fukazawa, T., Fukagawa, S., Matsuo, T., Endo, K., Yatsunami, R., and Nakamura, S., Nucleic Acids Symp. Ser., 2004, vol. 48, no. 1, pp. 167–168.
Uni, F., Lee, S., Yatsuanmi, R., Fukui, T., and Nakamura, S., Biosci. Biotechnol. Biochem., 2012, vol. 76, no. 3, pp. 530–535.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.E. Aktuganov, N.F. Galimzianova, E.A. Gilvanova, L.Yu. Kuzmina, T.F. Boyko, V.R. Safina, A.I. Melentiev, 2018, published in Prikladnaya Biokhimiya i Mikrobiologiya, 2018, Vol. 54, No. 5, pp. 506–512.
Rights and permissions
About this article
Cite this article
Aktuganov, G.E., Galimzianova, N.F., Gilvanova, E.A. et al. Characterization of Chitinase Produced by the Alkaliphilic Bacillus mannanilyticus IB-OR17 B1 Strain. Appl Biochem Microbiol 54, 505–511 (2018). https://doi.org/10.1134/S0003683818050022
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683818050022