Abstract
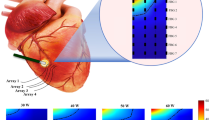
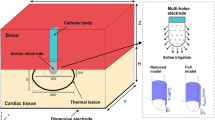
This paper presents an in vitro temperature mapping study of bovine cardiac tissue during radiofrequency ablation. The objectives were to: (i) develop a technique for measuring the spatial and temporal temperature distribution in the tissue and in the blood during ablation, and (ii) use the temperature measurements to characterize the effects of fluid flow on lesion dimensions, ablation efficiency, and temperature distributions. In vitro ablation (20 W, 60 s) of bovine cardiac tissue was performed. The tissue was placed in a saline–dextrose solution maintained at 37±0.5° The solution also irrigated the tissue surface and simulated blood flow velocities of (i) 30, (ii) 55, and (iii) 85 mm/s. Thermocouple measurements were recorded from 25 and 2 locations in the tissue and in the fluid, respectively. The lowest flow resulted in the largest lesion, the maximum tissue, fluid, and electrode temperature increases, and the highest ablation efficiency. The lesions were 5.8±0.81, 4.8±0.84, and 4.4±1.25 mm deep, and 9.3±1.07, 7.9±1.48 and 7.8±1.27 wide for flows (i)–(iii), respectively. The blood and tissue temperature distributions were asymmetric around the ablating electrode axis with higher temperatures on the outflow than on the inflow side. The experimental measurements were used to validate a numeric model of ablation in an accompanying paper. © 2000 Biomedical Engineering Society.
PAC00: 8754Br, 8719Hh, 8719Uv, 8750Jk
Similar content being viewed by others
REFERENCES
Avital, B., K. Mughal, and J. Hare. The effects of thoracic electrode-tissue contact on radiofrequency lesion generation. PACE20:2899–2910, 1997.
Calkins, H., E. Prystowsky, M. Carlson, L. S. Klein, J. P. Saul, and P. C. Gillette. Temperature monitoring during radiofrequency catheter ablation procedures using a closedloop control. Circulation90:1279–1286, 1994.
Cunningham, D. High-energy catheter ablation of cardiac arrhythmias: An outmoded technique in the 1990s. Clin. Cardiol.14:595–602, 1991.
Duck, F. A. In: Physical Properties of Tissue: A Comprehensive Reference Book. San Diego, CA: Academic, 1990.
Evans, G. T., and W. H. Huang. Comparison of direct current and radiofrequency energy for catheter ablation of the atrioventricular junction: Results of a a prospective multiple center study. Circulation82(4):III–719, 1990.
Guyton, A. C. In: Textbook of Medical Physiology. Philadelphia, PA: Saunders, 1981.
Haines, D. E. The biophysics of radiofrequency catheter ablation in the heart: The importance of temperature monitoring. PACE16:586–591, 1993.
Haines, D. E., and D. D. Watson. Tissue heating during radiofrequency catheter ablation: A thermodynamic model and observations in isolated perfused and superfused canine right and left ventricular free wall. PACE12:962–976, 1989.
Haines, D. E., D. D. Watson, and A. F. Verow. Electrode radius predicts lesion radius during radiofrequency energy heating. Circ. Res.67:124–129, 1980.
Hoyt, R. H., S. K. Huang, F. I. Marcus, and R. S. Odell. Factors influencing trans-catheter radiofrequency ablation of the myocardium. J. Appl. Cardiol.1:469–486, 1986.
Huang, S. K. S., A. R. Graham, M. A. Lee, M. E. Ring, and G. D. Gorman. Comparison of catheter ablation using radio frequency energy versus direct current energy: Biophysical, electrophysiological, and pathological observations. J. Am. Coll. Cardiol.18:1091–1097, 1991.
Incropera, F. P., and D. P. DeWitt. Fundamentals of Heat Transfer. New York: Wiley, 1981.
Jain, M. K., G. Tomassoni, R. E. Riley, and P. D. Wolf. Effect of skin electrode location on radiofrequency ablation lesions: An in vivo and a three-dimensional finite-element study. J. Cardiovas. Electrophys.9:1225–1235, 1998.
Jain, M. K., and P. D. Wolf. Finite-element analysis predicts dose–response relationship for constant power and temperature-controlled radiofrequency ablation. Proc. of the 19th Ann. Int. Conf. of the IEEE Eng. in Med. and Biol. Soc.:165–169, 1997.
Jain, M. K., and P. D. Wolf. Temperature-controlled and constant power radiofrequency ablation: What affects lesion growth? IEEE Trans. Biomed. Eng.46:1405–1412, 1999.
Labonte', S. Numerical model for radio-frequency ablation of the endocardium and its experimental validation. IEEE Trans. Biomed. Eng.41:108–115, 1994.
Langberg, J. J., M. C. Chin, M. Rosenqvist, J. Cockrell, N. Dullet, G. V. Hare, J. C. Griffin, and M. M. Scheinman. Catheter ablation of the atrioventricular junction with radiofrequency energy. Circulation80:1527–1535, 1989.
Langberg, J. J., M. A. Lee, M. C. Chin, and M. Rosenqvist. Radiofrequency catheter ablation: The effect of electrode size on lesion volume in vivo. PACE 13:1242–1248, 1990.
Lighthill, M. J. Physiological fluid dynamics: A survey. J. Fluid Mech.52:475, 1972.
McRury, I. D., and D. E. Haines. Ablation for the treatment of arrhythmias. Proc. IEEE84:404–416, 1996.
Nakagawa, H., W. S. Yamanashi, J. V. Pitha, M. Arruda, X. Wang, K. Otomo, K. J. Beckman, J. H. McClelland, R. Lazzara, and W. M. Jackman. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation91:2264–2273, 1991.
Nath, S., C. Lynch, J. G. Whayne, and D. E. Haines. Cellular electrophysiological effects of hyperthermia on isolated guinea pig papillary muscle. Circulation88:1826–1831, 1993.
Packer, D. L., and S. B. Johnson. Impact of ablation location on impedance pop occurrence with irrigated tip energy delivery in dogs. PACE20:1565, 1997.
Panescu, D., J. G. Whayne, S. D. Fleischman, M. S. Mirotznik, D. K. Swanson, and J. G. Webster. Threedimensional finite-element analysis of current density and temperature distributions during radio-frequency ablation. IEEE Trans. Biomed. Eng.42:879–890, 1995.
Petersen, H. H., A. Pietersen, and J. H. Svendsen. The relation of lesion size to the ablation site during radiofrequency ablation (abstract) PACE 20:1565, 1997.
Petersen, H. H., A. Pietersen, J. H. Svendsen, and S. Haunso. Lesion dimensions during temperature-controlled radiofrequency catheter ablation of left ventricular porcine myocardium: Impact of ablation site, electrode size, and convective Cooling. Circulation99:319–325, 1999.
Ruffy, R., M. A. Imran, D. J. Santel, and J. M. Wharton. Radiofrequency delivery through a cooled catheter tip allows the creation of larger endomyocardial lesions in ovine hearts. J. Cardiovas. Electrophys.6:1089–1096, 1995.
Willems, S., X. Chen, H. Kottkamp, G. Hindricks, W. Haverkamp, B. Rotman, M. Shenasa, G. Breithardt, and M. Borggrefe. Temperature-controlled radiofrequency ablation of manifest accessory pathways. Eur. Heart J.17:445–452, 1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jain, M.K., Wolf, P.D. In Vitro Temperature Map of Cardiac Ablation Demonstrates the Effect of Flow on Lesion Development. Annals of Biomedical Engineering 28, 1066–1074 (2000). https://doi.org/10.1114/1.1310218
Issue Date:
DOI: https://doi.org/10.1114/1.1310218