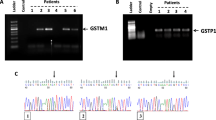
Abstract
The genes of the glutathione S-transferase (GST) family encode enzymes that appear to be critical in cellular protection against the cytotoxic effects, whereas p53 is a tumor suppressor gene. Despite a large number of studies on germline polymorphisms of GSTM1, GSTT1 and p53 genes, there have been very few reports on genotyping of these genes in human malignant tumor cells. In this study, we investigated GSTM1, GSTT1 and p53 codon 72 polymorphisms in a variety of human tumor cell lines originating from different organs to clarify tissuespedic polymorphic frequency of these genes in human solid tumors. The GSTM1 and GSTT1 genetic polymorphisms were evaluated using multiplex PCR techniques and PCR-RFLP analysis was conducted to identify p53 codon 72 genotypes. Gene expression of GSTM1 or GSTT1 was detected by RT-PCR in the cells with respective present genotype for each. Polymorphisms of p53 codon 72 detected by PCR-RFLP were also confirmed using SSCP and sequence analyses. GSTM1 and GSTT1 genotypes were various in 104 cell lines examined. Null GSTM1 genotype was dominant in small cell lung, kidney and ovarian carcinoma cells, whereas null GSTT1 genotype was dominant in cervical and endometrial Carcinoma cells. GSTM1 and GSTT1 genotypes in ovarian carcinoma cells were quite similar to those in small cell lung carcinoma cells. Polymorphic frequency of p53 codon 72 was also various among the cells, however, the Pro allele was found in only 1 of 6 kidney, 14 cervical and 4 endometrial carcinoma cell lines. There was a significant difference in GSTM1 and p53 genotypes between 34 small cell and 24 non small cell lung carcinoma cells (P < 0.01). Combined study on the distribution of GSTM1, GSTT1 and p53 genotypes revealed that null GSTM1 genotype was associated with the Arg allele of p53 codon 72 in 58 lung carcinoma cells and null GSTT1 genotype was associated with the Pro/Pro homozygote in 104 tumor cell lines examined. This is the first study examining GSTM1, GSTT1 and p53 codon 72 polymorphisms in a variety of human solid tumor cells and suggesting that polymorphic frequency of these genes may be tissue- and organ-specific. The molecular interaction between GST gene defects and p53 codon 72 genotype in the development of human malignant tumors should be further investigated.
Similar content being viewed by others
References
Tew KD: Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 54: 4313–4320, 1994.
Singhal SS, Zimniak P, Awasthi S, et al.: Several closely related glutathione S-transferase isoenzymes catalysing conjugation of 4-hydroxynonenal are differentially expressed in human tissues. Arch Biochem Biophys. 311: 242–250, 1994.
Baez S, Segura-Aguilar J, Widersten M, et al.: Glutathione transferases catalyse the detoxification of oxidised metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 324: 25–28, 1997.
Wiencke JK, Kelsey KT, Lamela RA, et al.: Human glutathione S-transferase deficiency as a marker of susceptibility to epoxide-induced cytogenetic damage. Cancer Res. 50: 1585–1590, 1990.
Schroeder KR, Wibel FA, Reich S, et al.: Glutathione S-transferase (GST) θ polymorphism influences background SCE rate. Arch Toxicol. 69: 505–507, 1995.
Nazar-Stewart V, Motulsky AG, Eaton DL, et al.: The glutathione S-transferase mu polymorphism as a marker for susceptibility to lung carcinoma. Cancer Res. 53: 2313–2318, 1993.
Hirvonen A, Husgafvel-Pursiainen K, Anttila S, et al.: The GSTM1 null genotype as a potential risk modifier for squamous cell carcinoma of the lung. Carcinogenesis (Lond). 14: 1479–1481, 1993.
Kihara M, Kihara M and Noda E Lung cancer risk of GSTM1 null genotype is dependent on the extent of tobacco smoke exposure. Carcinogenesis (Lond). 15: 415–418, 1994.
Cheng L, Sturgis EM, Eicher SA, et al.: Glutathione-S-transferase polymorphisms and risk of squamous-cell carcinoma of the head and neck. Int J Cancer. 84: 220–224, 1999.
Jourenkova N, Reininen M, Bouchardy C, et al.: Larynx cancer risk in relation to glutathione S transferase M1 and T1 genotypes and tobacco smoking. Cancer Epidemiol Biomarkers Prevent. 7: 19–23, 1998.
Ambrosone CB, Sweeney C, Coles BF, et al.: Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res. 61: 7130–7135, 2001.
Howells REJ, Redman CWE, Dhar KK, et al.: Association of glutathione S-transferase GSTM1 and GSTT1 null genotypes with clinical outcome in epithelial ovarian cancer. Clin Cancer Res. 4: 2439–2445, 1998.
Wang L, Groves MJ, Hepburn MD, et al.: Glutathione S-transferase enzyme expression in hematopoietic cell lines implies a differential protective role for T1 and A1 isoenzymes in erythroid and for M1 in lymphoid lineages. Haematologica 85: 573–579, 2000.
Dulic V, Kaufmann WK, Wilson SJ, et al.: p53 dependent inhibition of cyclin-dependent kinase activities in human fibroblast during radiation-induced G1 arrest. Cell. 76: 1013–1023, 1994.
Woods DB and Vousden KH: Regulation of p53 function. Exp Cell Res. 264: 56–66, 2001.
Greenblatt MS, Bennett WP, Hollstein M, et al.: Mutations in the p53 gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 55: 4855–4878, 1994.
Ara S, Lee PSY, Hansen MF, et al.: Codon 72 polymorphism of the TP53 gene. Nucleic Acids Res. 18: 4961, 1990.
Kawajiri K, Nakachi K, Imai K, et al.: Germ lines polymorphisms of p53 and CYP1A1 genes involved in human lung cancer. Carcinogenesis (Lond). 14: 1085–1089, 1993.
To-Figueras J, Gene M, Gomez-Catalan J, et al.: Glutathione-S-transferase M1 and codon 72 p53 polymorphisms in a northwestern mediterranean population and their relation to lung cancer susceptibility. Cancer Epidemiol Biomarkers Prevent. 5: 337–342, 1996.
Fan R, Wu MT, Miller D, et al.: The p53 codon 72 polymorphism and lung cancer risk. Cancer Epidemiol Biomarkers Prevent. 9: 1037–1042, 2000.
Liu G, Miller DP, Zhou W, et al.: Differential association of the codon 72 p53 and GSTM1 polymorphisms on histological subtype of non-small cell lung carcinoma. Cancer Res. 61: 8718–8722, 2001.
Miller DP, Liu G, Vivo ID, et al.: Combinations of the variant genotypes of GSTP1, GSTM1, and p53 are associated with an increased lung cancer risk. Cancer Res. 62: 2819–2823, 2002.
Lee JM, Lee YC, Yang SY, et al.: Genetic polymorphisms of p53 and GSTP1, but not NAT2, are associated with susceptibility to squamous-cell carcinoma of the esophagus. Int J Cancer. 89: 458–464, 2000.
Chen CL, Liu Q and Relling MV: Simultaneous characterization of glutathione S-transferase M1 and T1 polymorphisms by polymerase chain reaction in american whites and blacks. Pharmacogenetics. 6: 187–191, 1996.
Ueda M, Gemmill RM, West J, et al.: Mutations of the β- and γ-catenin genes are uncommon in human lung, breast, kidney, cervical and ovarian carcinomas. Br J Cancer. 85 64–68, 2001.
Ueda M, Terai Y, Yamashita Y, et al.: Correlation between vascular endothelial growth factor-C expression and invasion phenotype in cervical carcinomas. Int J Cancer. 98: 335–343, 2002.
Ueda M, Yamashita Y, Takehara M, et al.: Survivin gene expression in endometriosis. J Clin Endocrinol Metab. 87: 3452–3459, 2002.
Meyer DJ, Christodoulides LG, Hong-Tan K, et al.: Isolation, properties and tissue distribution of rat glutathione transferase E. FEBS Lett. 173: 327–330, 1984.
Chen H, Sandler DP, Taylor JA, et al.: Increased risk for myelodysplastic syndromes in individuals with glutathione transferase theta 1 (GSTT1) gene defect. Lancet. 347: 295–297, 1996.
Elexpuru-Camiruaga J, Buxton N, Kandula V, et al.: Susceptibility to astrocytoma and meningioma. Influence of allelism at glutathione S-transferase GSTT1 and GSTM1 and cytochrome P450 CYP2D6 loci. Cancer Res. 55: 4237–4239, 1995.
Goodman MT, McDuffie K, Hernandez B, et al.: CYP1A1, GSTM1, and GSTT1 polymorphisms and the risk of cervical squamous intraepithelial lesions in a multiethnic population. Gynecol Oncol. 81: 263–269, 2001.
Storey A, Thomas M, Kalita A, et al.: Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature (Lond). 393: 229–234, 1998.
Marin MC, Jost CA, Brooks LA, et al.: A common polymorphism acts as an intragenic modifier of mutant p53 behaviour. Nat Genet. 25: 47–54, 2000.
Brooks LA, Tidy JA, Gusterson B, et al.: Preferential retention of codon 72 arginine p53 in squamous cell carcinoma of the vulva occurs in cancers positive and negative for human papillomavirus. Cancer Res. 60: 6875–6877, 2000.
Thomas M, Kalita A, Labrecque S, et al.: Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol. 19: 1092–1100, 1999.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueda, M., Hung, YC., Terai, Y. et al. Glutathione S-transferase GSTM1, GSTT1 and p53 codon 72 polymorphisms in human tumor cells. Hum Cell 16, 241–251 (2003). https://doi.org/10.1111/j.1749-0774.2003.tb00158.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1111/j.1749-0774.2003.tb00158.x