-
PDF
- Split View
-
Views
-
Cite
Cite
Pan‐Pan Jiang, Stephanie Bedhomme, N.G. Prasad, Adam Chippindale, SPERM COMPETITION AND MATE HARM UNRESPONSIVE TO MALE‐LIMITED SELECTION IN DROSOPHILA: AN EVOLVING GENETIC ARCHITECTURE UNDER DOMESTICATION, Evolution, Volume 65, Issue 9, 1 September 2011, Pages 2448–2460, https://doi.org/10.1111/j.1558-5646.2011.01328.x
Close - Share Icon Share
Abstract
Earlier research by W.R. Rice showed that experimentally limiting gene expression to males in Drosophila melanogaster leads to the rapid evolution of higher fitness. Using a similar male‐limited (ML) selection protocol, we confirmed that result and showed that eliminating intralocus sexual conflict results in a comprehensive remodeling of the sexually dimorphic phenotype. However, despite starting from laboratory‐evolved descendants of the same founder population used in earlier work, we found no evidence for the increased performance in sperm competition or increased postmating harm to females previously demonstrated. We employed females with both ancestral population genotypes and those of the special “clone generator” females used in ML selection. Despite strong differences in sperm storage or usage patterns between these females, there was no detectable adaptation by males to the specific female stock used in the selection protocol. The lack of evolution of postcopulatory traits suggests either that requisite genetic variation was eliminated by long‐term domestication of the base population, or that complex male‐by‐male‐by‐female interactions made these traits unavailable to selection. The different evolutionary outcomes produced by two very similar experiments done at different time points underscores the potential for cryptic adaptation in the laboratory to qualitatively affect inferences made using quantitative genetic methodologies.
Most sexual species are promiscuous, with male and female interests rarely aligning perfectly (Bateman 1948; Trivers 1972; Dawkins 1976). As a result, individuals may be selected to maximize their own fitness through means that are suboptimal, or even manifestly detrimental, to their mates (reviewed in Arnqvist and Rowe 2005; Wedell et al. 2006). Hence, different strategies employed by the sexes can create sexual conflict, the “differences in the evolutionary interests between males and females” discussed by Parker (1979). In the past several decades, the potential importance of sexual conflict in shaping genome organization and the evolution of reproductive isolation has been recognized (Arnqvist and Rowe 2005; Bedhomme and Chippindale 2007; Bonduriansky and Chenoweth 2009).
Sexual conflict is manifested through two distinct mechanisms: inter‐ and intralocus conflict. Interlocus conflict, the variety emphasized by Parker (1979), results from direct antagonistic interaction between the sexes stimulating selection upon different loci in each sex. Numerous examples of apparently antagonistic coevolution have been catalogued across taxonomically widespread species (see Arnqvist and Rowe 2005 for review). The second form, intralocus sexual conflict, occurs when male and female optima differ for the same trait but selection is constrained by genetic correlation between the sexes. Intralocus conflict promotes the evolution of sex chromosomes and sex‐limitation of genes to ameliorate the effects of gender load—the reduction of the average fitness of one sex resulting from sharing of the gene pool with the other (Rice 1984; Rice and Chippindale 2002). Recognition of the potential importance of intralocus sexual conflict and its empirical exploration has been a relatively recent phenomenon, but a growing list of examples is accruing from diverse taxa (e.g., Bonduriansky and Chenoweth 2009; Cox and Calsbeek 2009).
Drosophila melanogaster has been central to the study of sexual conflict because of its promiscuous mating system, rapid generation time, the accessibility of genetic approaches, and the ability to measure fitness components with confidence. Males perform elaborate pre‐copulatory displays such as stereotyped courtship dances involving licking, wing‐vibration, wing‐scissoring, and mounting attempts (Sturtevant 1915; Greenspan and Ferveur 2000). Males are often persistent in their courtship attempts, and while this increases mating success, it also tends to decrease their mate's survival and fecundity (Fowler and Partridge 1989; Chapman et al. 1995; Kuijper et al. 2006). For example, one study with the LHM laboratory population of D. melanogaster estimated that females lose over 20% of their potential lifetime fecundity due to antagonistic male courtship and remating effects (Rice et al. 2006).
Interlocus conflict may also involve postcopulatory components. In D. melanogaster, as with many other insect species, mated females can store and use sperm long after mating (Lefevre and Jonsson 1962; Birkhead and Möller 1993). The efficient retention of sperm in the female reproductive tract is partially mediated by accessory gland proteins delivered in the seminal fluid (e.g., Neubaum and Wolfner 1999). But sperm storage also creates an arena for male–male competition after mating, with potentially harmful side effects for females. For example, Chapman et al. (1995) demonstrated that the life span of females was sharply reduced by receipt of accessory gland proteins (ACPs). Drosophila ACPs appear to be evolving rapidly (Aguade et al. 1992; Begun et al. 2000; Betran and Long 2003), which is consistent with selection for male–male competition and coevolution between the sexes. In postcopulatory competition, a male can achieve high fitness in two ways. If he is the first to mate with a female, he can play “defense” by inducing fidelity in his mate (mating defense) and preventing sperm displacement, should remating occur (sperm defense). But if he is the second to mate with a female, he can play “offence” by convincing females to remate (mating offence) and afterwards supplanting sperm from the first male (sperm offence).
Considering the importance of sperm competition to male fitness in Drosophila, relatively low levels of additive genetic variation (VA) segregating in populations might be expected. Nonetheless, theory suggests that polymorphism for genes related to sperm competition success can be maintained, even under a single‐locus population genetic model (Prout and Clark 1996). And high levels of VA have been documented in several Drosophila populations (e.g., Prout and Bundgaard 1977; Clark et al. 1995, Hughes 1997, Civetta and Clark 2000; Bjork et al. 2007). Rice (1996, 1998) documented the rapid evolution of sperm offence and defense characters in D. melanogaster under a male‐limited (ML) selection protocol (further discussion below). Despite the apparent availability of additive variation, a laboratory evolution experiment by Bjork et al. (2007) did not yield a response to selection for either the defense or offence components of male sperm competition. Notably, Bjork et al. (2007) began with a population descended from the same base population used by Rice, and that we report on in the present study.
Rice (1996, 1998) used chromosomal aberrations and visible markers carried by “clone generator” (CG) females, to artificially restrict selection to experimental males. CG females carry a compound X (DX), a Y chromosome, and a marked translocation of the two major autosomes. By picking only males of the appropriate genotype, and discarding all other flies, hemiclones of all major chromosomes [cI(X), cII, cIII] can be passed down in patrilines. Because CG females supplied a Y chromosome and translocated autosomes anew to sons each generation, genes passed forward in the experiment were effectively ML in expression. Experimental elimination of selection for female function should theoretically allow the accumulation of sexually antagonistic male‐benefit/female‐detriment alleles that were polymorphic in the founding population. Improvements in male fitness would thus reflect the removal of intralocus conflict via release from counter‐selection in females. However, increased fitness of males could also result from evolved specialization to breeding with the CG females, which were not part of the control treatment; these females are phenotypically distinct and very frail as a result of the marker mutations and chromosome rearrangements they carry.
Rice (1996) showed elevated net fitness, sperm offence and defense in ML lines when they were tested with CG females. In these males, evolved haploid genotypes were partnered with the translocation derived from CG females, as they would be during a normal bout of selection. Later work using hybrid males expressing a “double dose” of ML genes (Rice 1998) showed that the advantage in sperm defense of the ML males was not repeatable when the males interacted with effectively wild‐type females. This suggested that part of the fitness increase resulted from adaptation to the reproductive phenotype of the CG females. But Rice (1998) showed that experimental males retained a net fitness advantage over control males, even when not tested with CG stock females, suggesting that male performance had improved in part because of a release from counter‐selection in females, and a resulting reduction in gender load. In the same study, it was established that experimental males had higher remating rates, and achieved elevated sperm offence abilities when tested with wild‐type females. Rice's results therefore pointed to a combination of specialization by males on the “target” female stock used to create them, and a generic increase in male vigor upon release from counter selection in females. Rice's work also demonstrated markedly higher mortality rates of females exposed to even a single mating with ML‐evolved males, suggesting that a consequence of the ML selection protocol was increased mate harm; this could have been caused by the production of more, or more harmful, ejaculate proteins, which induce increased mortality in females (Chapman et al. 1995).
We undertook a study of ML evolution in our laboratory using a protocol very similar to Rice (1996, 1998) to identify the phenotypic basis of sexual conflict. Sex‐limited selection has the potential to concentrate and exaggerate sexually antagonistic traits, making many aspects of the fitness phenotype measurable. We wanted to tease apart the roles of interlocus sexual conflict (i.e., female‐specific adaptation for manipulation of female fertility and mate harm) from intralocus sexual conflict (i.e., the general improvement in fitness from reduced gender load). In an earlier, role‐reversed experiment, Rice (1992) observed a decline in fitness of males carrying sexually antagonistic female‐benefit genes, thereby showing the potential for intralocus sexual conflict to shape the evolution of both sexes. But in the later work (Rice 1998), female productivity was unaffected by expression of ML chromosomes, although their development time increased, potentially reflecting the effects of sexually antagonistic alleles.
So far, our study of ML evolution has revealed a burst of adaptation of precopulatory traits when selection for female‐specific function was eliminated. Evolved changes included increased developmental time, reduced growth rate and body size (Prasad et al. 2007), changes in wing shape and symmetry (Abbott et al. 2010), and aspects of mate‐interaction or courtship behavior (Bedhomme et al. 2008). Where the phenotype was shared between the sexes, these changes were generally masculinizing: tending to move the population means in the “male direction” of extant sexual dimorphism, presumably closer to the male optimal phenotype and further from the female optimal phenotype. Importantly, when females carried ML‐evolved chromosomes they also exhibited changes that made them more male‐like in body mass, wing size and shape, and development time, and their fitness was reduced. Hence our work has supported the view that intralocus sexual conflict helped sustain variation in fitness‐related characters in the ancestor (LHM) population.
Here, we extend our analysis of ML evolution to characters related to postcopulatory fitness via sperm competition. As noted above, these characters responded rapidly to selection in the work of Rice (1996, 1998) and females were affected strongly and negatively by interaction with ML males. Therefore, as a further measure of postcopulatory male adaptation, we measured the longevity of females exposed to ML males.
Variation in genes coding for uniquely male traits such as testis, sperm, and ejaculate proteins should not be directly shaped by intralocus sexual conflict, because females do not express them. For example, in D. melanogaster, many genes involved in spermatogenesis are located on the Y chromosome (Gatti and Pimpinelli 1992; Carvalho et al. 2000, 2001) and therefore ML in nature. On these grounds we suspected that interlocus sexual conflict, manifested as female‐specific adaptation, would be an important contributor to the selection response for postcopulatory traits. We therefore assessed the degree to which the increase in ML fitness was the result of specific adaptation to the CG females with which they had evolved. As we report here, however, characters related to sperm competition did not respond to ML selection, irrespective of the genetic background employed or the selective history of the females in which competition was arranged.
Methods and Materials
EXPERIMENTAL EVOLUTION OF ML LINES
The evolution of ML lines and their matched controls (C) is described in detail by Prasad et al. (2007), and follows closely the protocol outlined by Rice (1996) on a larger scale. Briefly, four large subpopulations were derived from a long‐term, laboratory‐adapted population (LHM, as described by Chippindale et al. 2001). This population is descended from the LH population used by Rice (1996, 1998), but it had been maintained for over 150 generations on a strictly defined moderate (the “M” in LHM) density protocol in vials, at a population size of 1800–1900 adults per generation. The LH population used to initiate LHM was a large bottle‐maintained population (20 bottles, mixed every two weeks) with unregulated and fluctuating densities. Both the shorter total period of time under domestication and the more variable maintenance environment predict higher levels of genetic polymorphism in LH relative to LHM. The application of the ML and C protocols, in vials and at moderate density, represented a close match to the LHM stock culture practice. The four LHM subpopulations were maintained in isolation from each other for 10 generations. From each subpopulation, a selected population (ML1‐4) and control population (C1‐4), matched by a subscript number, was created.
To initiate the ML lines, haploid genomes were sampled from each replicate subpopulation by crossing ∼1000 males to CG females carrying a compound X (C(1)DX, y, f), a Y chromosome (from LHM base population) and a homozygous‐viable translocation of the two major autosomes (T(2:3)rdgc st in ri pp bw). These markers and chromosome rearrangements, along with the absence of molecular recombination in male D. melanogaster, allow for the clonal transmission of an ML genomic haplotype (cI (X), cII, and cIII chromosomes, which constitute approximately 99.5% of the haploid genome) from father to son. Each generation, only males carrying a new, maternally derived translocation and a paternally transmitted wild‐type haplotype (originally sampled from the LHM population) were selected to mate with virgin CG females; all females, and males carrying paternally derived copies of the translocation, were discarded. Because all other chromosomes were used as transmission vehicles and immediately discarded, only selection for male fitness variation among originating haplotypes was allowed. As a corollary, by following this protocol, female selection pressures were removed because ML haplotypes were never expressed in females. This protocol will allow adaptation of male functions through the removal of intralocus conflict, if sexually antagonistic genes are polymorphic in the founding population.
To reduce the impact of linkage, which could retard adaptation through hitchhiking and background selection, we allowed recombination among a fraction of the ML‐selected individuals. Each generation, 4% of the ML males were passed through a series of crosses so that ML haplotypes were allowed to recombine in females for one generation, allowing novel collections of male‐benefit alleles to be generated. These recombined ML haplotypes were then reintroduced into the large evolving population from which they were taken.
C lines experienced the same culture protocol as ML lines, however transmission of the genome was normal, through both males and females. Egg (and resultant adult) densities were regulated by manually trimming out excess eggs from control populations to account for the absence of aneuploidy in wild‐type flies compared to flies produced by the CG protocol. After adjusting for the fact that only 50% of the eggs produced in the ML culture protocol hatch (half are unbalanced for X‐chromosome count) larval densities were approximately 150 per 25 × 95 mm rearing vial. All flies were raised on molasses‐cornmeal‐yeast medium at 25°C and 50% relative humidity in a 12:12 h light/dark cycle.
DERIVING EXPERIMENTAL ASSAY MALES
During selection, ML males were heterozygous for the autosomal translocation to ensure proper transmission of ML hemiclones from father to son. This translocation contains only recessive markers with no visible heterozygous phenotype, and the population of translocation‐carrying flies was extensively backcrossed to LHM prior to initiating selection to make it genetically variable and hardier. However the potential exists for male adaptations that are specific to fixed features (e.g., breakpoints) of this genetic construct to evolve via epistasis. To eliminate this complication, males were passed through a series of crosses so that they expressed an entire ML haploid genome opposite an otherwise random (LHM) background. At generation 65 of experimental evolution, 150 haploid genomes per population (ML1‐4 and C1‐4) were captured by crossing males with CG females bearing a dominant eye‐color marker (bwD). F1 males were then mated to females carrying a compound X [C(1)DX, y, f], a Y chromosome (from LHM base population) and two sets of LHM autosomes. Red‐eyed male progeny from this cross expressed the genomes of interest, whereas brown‐eyed males and all females were discarded.
DEFENSE AND OFFENCE ASSAYS
In all assays, the densities and age of flies, food composition, amount of yeast, timing of matings, and oviposition times were all matched to the conditions experienced by ML and C lines during their biweekly selection.
When measuring defense (sperm defense and mating defense) and offence (sperm offence and mating offence), ML and C males competed with a marker stock. The competitor males were homozygous for the recessive eye‐color marker, scarlet that had been backcrossed into LHM for over 10 generations initially, and backcrossed once per year, approximately every 26 generations; these were called LHST. Because scarlet is quite benign, naturally occurring in the LH founder stock at low but appreciable frequency, it provided a vigorous marked replica of the founder population for fitness comparison.
The females used were from two different populations, to test whether higher ML fitness is due to overall “better” male traits, or adaptation to a particular female type. The first were the CGs that the ML males had encountered for the entirety of their selection treatment. As noted before, CG females are homozygous for a translocation, which contains several recessive markers, including the scarlet (st) allele. The second type of female was drawn from the same LHST population as the competitor males. Offspring of either female fathered by ML or C males were red‐eyed (st/wild type), whereas those fathered by a LHST male were scarlet‐eyed (st/st). Offence and defense abilities of ML males were assayed with both CG and LHST females. Control (C) males were similarly assayed.
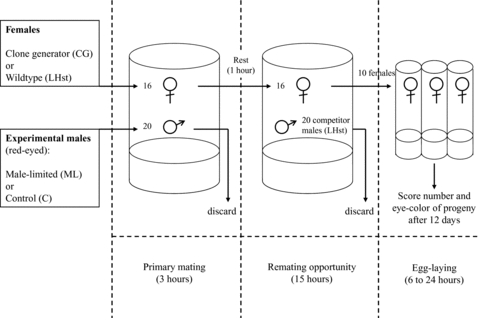
In the defense assay, 16 virgin females (CG or LHST) were first mated to 20 experimental males (ML or C) for 3 h on 5 mg of live yeast, to mimic usual selection conditions (Fig. 1). Almost all virgin females were observed to mate within the first hour after introduction. Next, the males were removed under light CO2 anesthesia, the females were allowed 1 h to recover from CO2, and 20 competitor males (LHST) were then added. Competitor males were allowed to potentially remate the females for 15 h. In total, females experienced 18 h of interaction with males, which was the normal interaction time allowed during biweekly selection. The competitor males were then removed and 10 females were transferred to individual laying tubes after being lightly anesthetized with CO2. Egg laying times varied from 6 to 24 h, depending on how long it took the females to lay a relatively equal number of eggs, thus controlling for density effects on juvenile survival to avoid confounding viability differences with fertilization differences. Offspring emerging from these tubes were counted and scored for eye color 12 days after eggs were laid.
Schematic of defense experiment, showing the number and selection history of experimental (ML or C) and competitor animals (LHST scarlet‐eyed males) along the timeline for the experiment. Females were scarlet‐eyed to allow paternity scoring; the phenotype is recessive. In the offence experiments, experimental males were introduced second, after females mated with LHST males.
Defense fitness can be partitioned into two components: mating defense and sperm defense. Mating defense is calculated as the proportion of females that remated with a competitor male, which is evidenced by the production of at least one progeny with scarlet eyes. Low proportions of remating females indicate high mating defense abilities. Sperm defense is calculated as the fraction of any individual female's offspring sired by the first, red‐eyed experimental male when she subsequently remated with a competitor, scarlet‐eyed male. High fractions of red‐eyed progeny indicate high sperm defense abilities. In order not to confound sperm defense with mating defense, only females that produced at least one offspring from the second, competitor male were included in the sperm defense analysis. Because sperm precedence is very high in D. melanogaster (Clark et al. 1995), the chances of excluding females that did remate but produced no offspring with second male are low.
The offence assay protocol was identical except for the order in which experimental and competitor males were combined. That is, females encountered competitor males first, and experimental males second. As with defense, offence can be partitioned into two components: mating offence and sperm offence. In this case, high proportions of remating females indicate high mating offence abilities, and, similar to defense measures, high fractions of red‐eyed progeny indicate high sperm offence abilities. Again, only females that produced at least one offspring sired by the second male were included in the sperm offence analysis.
This assay was repeated on three consecutive days, with two to five vials for each male × female × offence/defense combination (average of 12.6 replicates for each assay combination). All flies (males and females) used for the experiment were 12 days from egg (two‐ to three‐day old as adults). Females from each vial were individually cultured in test tubes and allowed to lay eggs for two days. In total, 4040 test tubes of offspring were scored. However, this sample size was reduced to 3199 because females that produced no offspring were removed from the analysis.
MALE IMPACT UPON FEMALE LONGEVITY AND OFFSPRING PRODUCTION
Because of the scale of the experiments, measurements of male harm to mates were made in two experiments, each involving half of the replicate populations. Replicate populations ML/C 1 and 2 were tested at generation 69 and populations 3 and 4 were tested at generation 75. Measurements of longevity and female productivity were made to assess male impact on female fitness.
Longevity data were collected from females that interacted with males continuously throughout their lives from three or four days posteclosion and for females that interacted with males for 18 h, with the male subsequently removed under light CO2 anesthesia. Each vial was stocked with 20 males from either the ML or C selection treatment, and 16 females of one of two types: (1) LHST females from our standard competitor stock, which are nearly as vigorous as wild‐type females and genetically similar to the ancestral LHM stock, and (2) the CG females used in the ML selection treatment. For each male (ML or C) × female (LHST or CG) × interaction time (continuous or 18 h) combination in the factorial design, 200 males and 160 females (10 replicate vials) were employed. Food was refreshed every other day by transfer without anesthesia, and observations of mortality were made daily until all females were dead. Across the experiment, after losses and immediately dead animals (generally CG females) were discounted, data from 3911 females were used to estimate male effects on female longevity.
Female offspring production measurements were derived from a second set of experimental flies collected at the same time as the longevity flies, with male interaction also beginning at three to four days from eclosion. Ten vials with 20 males:16 virgin females were combined in each vial for an 18‐h period. Under these conditions virtually all females will mate at least once. The flies were subsequently knocked out with gentle CO2 anesthesia and a sample of the females was split into four vials, each with three females, to lay eggs for a 24‐h period; females were subsequently discarded. The female age and egg‐laying period were chosen to reflect the normal culturing protocol of the ML and C populations and hence should be a good approximation of adult fitness. Progeny were counted 12–14 days later, after all darkened pupae were observed to have eclosed to adulthood. CG females produce 50% hatch rates, and so an adjustment for built‐in aneuploid mortality was applied to progeny counts.
STATISTICAL ANALYSIS
Mating (offence and defense) rates were calculated as the proportion of females that did or did not remate, respectively, per vial. Sperm competition (offence and defense) was calculated as the average proportion of offspring sired by the tester male (ML or C) across test tubes per vial.
A three‐way analysis of variance (ANOVA) was performed using selection (ML or C), population replicate (1–4), and female (CG or LHST) and their two‐ and three‐way interactions as the factors, to test for genetic differentiation between ML and C males for defense (sperm and mating) and offence (sperm and mating). Population replicate was modeled as a random effect. All data were analyzed at the vial level. Indeed, females experiencing the same environment (vial) are not independent, so we used the average of progeny paternity across all test tubes drawn from a vial and weighted each datapoint by the number of observations contributing to each mean value. ANOVAs were initially performed with “day” as an additional factor but because this effect was never significant, the analyses we present here use results pooled for all three days.
For the mate harm data (longevity and fecundity) two experiments separated by six generations of selection were performed. In the first experiment (block), replicate populations one and two were tested, and in the second, populations three and four were tested. Population means were used as data for the analysis. We note that the block effect was confounded with replicate number in the analysis, but the data were qualitatively similar and the statistical conclusions unaffected by separate analysis of each block. We therefore opted to analyze these data in one model with experimental generation as a fixed factor, along with male selection history, female genotype, and duration of exposure to males.
Results
DEFENSE
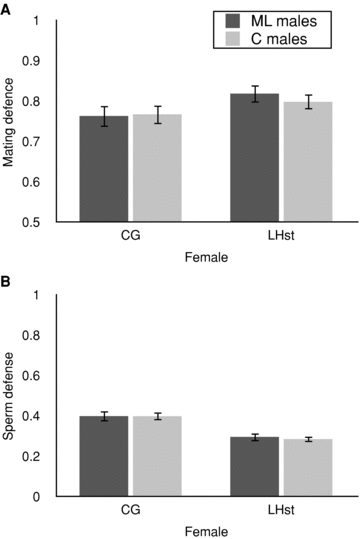
Figure 2A shows that 81% of the LHST females and 75% of the CG females remated, as estimated by the proportion of females producing some or all scarlet‐eyed progeny. As is typical of last male precedence in this species, the majority of families (78% of LHST and 60% of CG females that remated) were composed of predominantly the second male's offspring (i.e., scarlet‐eyed). Selection treatment had no impact on this measure of mating defense: overall, analysis of variance (Table 1; F1,182= 0.11; P= 0.74) did not detect significant differences in ML versus C males for mating defense. Sperm defense ability also did not differ between ML or C male selection treatments (Table 1; F1,181= 0.08; P= 0.78). There were no significant differences between replicate populations, or any replicate interaction factors. The lack of a significant interaction between selection and replicate indicates that there was no measurable inconsistency among replicates of the experimental and control selection treatments on offence or defense.
Defense ability. (A) Mating defense. Measured as the proportion of females (± SE) producing some or all progeny from the P2 (second) males, indicating remating. The higher the score, the lower the mating defense, as more females had evidently remated. (B) Sperm defense. Measured as the mean proportion of progeny sired by the first male (± SE) for females that produced progeny from both P1 and P2 males. The higher the score, the higher the sperm defense. Dark bars represent ML‐evolved male; light bars represent control males.
Results of three‐way ANOVA using male selection (ML or C), females (CG and LHST), and population replicate (1–4) as factors affecting defense and offence. Mating defense refers to a P1 (first) male's ability to deter copulation with subsequent males (remating) whereas sperm defense refers to the proportion of sperm retained by the female from the P1 male when she did remate. Mating offence represents P2 (second) male success in remating, whereas sperm offence is a measure of the proportion of sperm retained from the P2 male.
| Male Role | Component | Effect | F ratio | Prob > F | dfnum | dfden |
| P1 (Defence) | Mating | Selection | 0.11 | 0.74 | 1 | 182 |
| Female | 3.24 | 0.07 | 1 | 182 | ||
| Population | 0.44 | 0.73 | 3 | 182 | ||
| Selection × female | 0.26 | 0.61 | 1 | 182 | ||
| Selection × population | 0.54 | 0.66 | 3 | 182 | ||
| Female × population | 0.50 | 0.68 | 3 | 182 | ||
| Selection × female × population | 0.52 | 0.67 | 3 | 182 | ||
| Sperm | Selection | 0.08 | 0.78 | 1 | 181 | |
| Female | 28.4 | <0.0001 | 1 | 181 | ||
| Population | 0.29 | 0.83 | 3 | 181 | ||
| Selection × female | 0.07 | 0.80 | 1 | 181 | ||
| Selection × population | 0.66 | 0.58 | 3 | 181 | ||
| Female × population | 0.66 | 0.58 | 3 | 181 | ||
| Selection × female × pop | 0.52 | 0.67 | 3 | 181 | ||
| P2 (Offence) | Mating | Selection | 1.80 | 0.18 | 1 | 190 |
| Female | 0.15 | 0.70 | 1 | 190 | ||
| Population | 1.20 | 0.31 | 3 | 190 | ||
| Selection × female | 1.93 | 0.17 | 1 | 190 | ||
| Selection × population | 1.64 | 0.18 | 3 | 190 | ||
| Female × population | 2.04 | 0.11 | 3 | 190 | ||
| Selection × female × population | 1.22 | 0.30 | 3 | 190 | ||
| Sperm | Selection | 0.31 | 0.58 | 1 | 190 | |
| Female | 8.83 | 0.003 | 1 | 190 | ||
| Population | 0.11 | 0.96 | 3 | 190 | ||
| Selection × female | 0.12 | 0.73 | 1 | 190 | ||
| Selection × population | 0.97 | 0.41 | 3 | 190 | ||
| Female × population | 2.01 | 0.11 | 3 | 190 | ||
| Selection × female × pop | 0.71 | 0.550 | 3 | 190 |
| Male Role | Component | Effect | F ratio | Prob > F | dfnum | dfden |
| P1 (Defence) | Mating | Selection | 0.11 | 0.74 | 1 | 182 |
| Female | 3.24 | 0.07 | 1 | 182 | ||
| Population | 0.44 | 0.73 | 3 | 182 | ||
| Selection × female | 0.26 | 0.61 | 1 | 182 | ||
| Selection × population | 0.54 | 0.66 | 3 | 182 | ||
| Female × population | 0.50 | 0.68 | 3 | 182 | ||
| Selection × female × population | 0.52 | 0.67 | 3 | 182 | ||
| Sperm | Selection | 0.08 | 0.78 | 1 | 181 | |
| Female | 28.4 | <0.0001 | 1 | 181 | ||
| Population | 0.29 | 0.83 | 3 | 181 | ||
| Selection × female | 0.07 | 0.80 | 1 | 181 | ||
| Selection × population | 0.66 | 0.58 | 3 | 181 | ||
| Female × population | 0.66 | 0.58 | 3 | 181 | ||
| Selection × female × pop | 0.52 | 0.67 | 3 | 181 | ||
| P2 (Offence) | Mating | Selection | 1.80 | 0.18 | 1 | 190 |
| Female | 0.15 | 0.70 | 1 | 190 | ||
| Population | 1.20 | 0.31 | 3 | 190 | ||
| Selection × female | 1.93 | 0.17 | 1 | 190 | ||
| Selection × population | 1.64 | 0.18 | 3 | 190 | ||
| Female × population | 2.04 | 0.11 | 3 | 190 | ||
| Selection × female × population | 1.22 | 0.30 | 3 | 190 | ||
| Sperm | Selection | 0.31 | 0.58 | 1 | 190 | |
| Female | 8.83 | 0.003 | 1 | 190 | ||
| Population | 0.11 | 0.96 | 3 | 190 | ||
| Selection × female | 0.12 | 0.73 | 1 | 190 | ||
| Selection × population | 0.97 | 0.41 | 3 | 190 | ||
| Female × population | 2.01 | 0.11 | 3 | 190 | ||
| Selection × female × pop | 0.71 | 0.550 | 3 | 190 |
Results of three‐way ANOVA using male selection (ML or C), females (CG and LHST), and population replicate (1–4) as factors affecting defense and offence. Mating defense refers to a P1 (first) male's ability to deter copulation with subsequent males (remating) whereas sperm defense refers to the proportion of sperm retained by the female from the P1 male when she did remate. Mating offence represents P2 (second) male success in remating, whereas sperm offence is a measure of the proportion of sperm retained from the P2 male.
| Male Role | Component | Effect | F ratio | Prob > F | dfnum | dfden |
| P1 (Defence) | Mating | Selection | 0.11 | 0.74 | 1 | 182 |
| Female | 3.24 | 0.07 | 1 | 182 | ||
| Population | 0.44 | 0.73 | 3 | 182 | ||
| Selection × female | 0.26 | 0.61 | 1 | 182 | ||
| Selection × population | 0.54 | 0.66 | 3 | 182 | ||
| Female × population | 0.50 | 0.68 | 3 | 182 | ||
| Selection × female × population | 0.52 | 0.67 | 3 | 182 | ||
| Sperm | Selection | 0.08 | 0.78 | 1 | 181 | |
| Female | 28.4 | <0.0001 | 1 | 181 | ||
| Population | 0.29 | 0.83 | 3 | 181 | ||
| Selection × female | 0.07 | 0.80 | 1 | 181 | ||
| Selection × population | 0.66 | 0.58 | 3 | 181 | ||
| Female × population | 0.66 | 0.58 | 3 | 181 | ||
| Selection × female × pop | 0.52 | 0.67 | 3 | 181 | ||
| P2 (Offence) | Mating | Selection | 1.80 | 0.18 | 1 | 190 |
| Female | 0.15 | 0.70 | 1 | 190 | ||
| Population | 1.20 | 0.31 | 3 | 190 | ||
| Selection × female | 1.93 | 0.17 | 1 | 190 | ||
| Selection × population | 1.64 | 0.18 | 3 | 190 | ||
| Female × population | 2.04 | 0.11 | 3 | 190 | ||
| Selection × female × population | 1.22 | 0.30 | 3 | 190 | ||
| Sperm | Selection | 0.31 | 0.58 | 1 | 190 | |
| Female | 8.83 | 0.003 | 1 | 190 | ||
| Population | 0.11 | 0.96 | 3 | 190 | ||
| Selection × female | 0.12 | 0.73 | 1 | 190 | ||
| Selection × population | 0.97 | 0.41 | 3 | 190 | ||
| Female × population | 2.01 | 0.11 | 3 | 190 | ||
| Selection × female × pop | 0.71 | 0.550 | 3 | 190 |
| Male Role | Component | Effect | F ratio | Prob > F | dfnum | dfden |
| P1 (Defence) | Mating | Selection | 0.11 | 0.74 | 1 | 182 |
| Female | 3.24 | 0.07 | 1 | 182 | ||
| Population | 0.44 | 0.73 | 3 | 182 | ||
| Selection × female | 0.26 | 0.61 | 1 | 182 | ||
| Selection × population | 0.54 | 0.66 | 3 | 182 | ||
| Female × population | 0.50 | 0.68 | 3 | 182 | ||
| Selection × female × population | 0.52 | 0.67 | 3 | 182 | ||
| Sperm | Selection | 0.08 | 0.78 | 1 | 181 | |
| Female | 28.4 | <0.0001 | 1 | 181 | ||
| Population | 0.29 | 0.83 | 3 | 181 | ||
| Selection × female | 0.07 | 0.80 | 1 | 181 | ||
| Selection × population | 0.66 | 0.58 | 3 | 181 | ||
| Female × population | 0.66 | 0.58 | 3 | 181 | ||
| Selection × female × pop | 0.52 | 0.67 | 3 | 181 | ||
| P2 (Offence) | Mating | Selection | 1.80 | 0.18 | 1 | 190 |
| Female | 0.15 | 0.70 | 1 | 190 | ||
| Population | 1.20 | 0.31 | 3 | 190 | ||
| Selection × female | 1.93 | 0.17 | 1 | 190 | ||
| Selection × population | 1.64 | 0.18 | 3 | 190 | ||
| Female × population | 2.04 | 0.11 | 3 | 190 | ||
| Selection × female × population | 1.22 | 0.30 | 3 | 190 | ||
| Sperm | Selection | 0.31 | 0.58 | 1 | 190 | |
| Female | 8.83 | 0.003 | 1 | 190 | ||
| Population | 0.11 | 0.96 | 3 | 190 | ||
| Selection × female | 0.12 | 0.73 | 1 | 190 | ||
| Selection × population | 0.97 | 0.41 | 3 | 190 | ||
| Female × population | 2.01 | 0.11 | 3 | 190 | ||
| Selection × female × pop | 0.71 | 0.550 | 3 | 190 |
OFFENCE
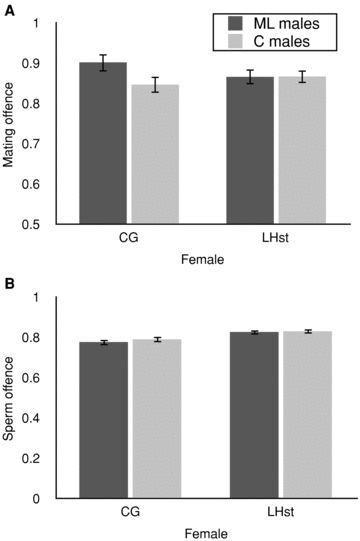
Both females remated at a slightly higher rate when wild‐type males of either selection treatment (rather than competitor, scarlet males) were on offence (86% for both females; Figs. 2A and 3A) perhaps reflecting a small effect of the scarlet marker on male performance or attractiveness in sexual selection. Again, due to last male precedence, the majority of families (90% of LHST and 83% of CG females that remated) were composed of predominantly the second male's offspring (i.e., red‐eyed). Male selection treatment had no impact on this measure of mating offence (Table 1; F1,190= 0.18; P = 0.18). Although ML males were slightly superior in sperm displacement when tested with CG females (Fig. 3B), overall sperm offence did not differ between selection treatments (Table 1; F1,190= 0.31; P= 0.58) and there was no interaction between selection treatment and female type (Table 1; F1,190= 0.12; P= 0.73).
Offence ability. (A) Mating offence, measured as the proportion of females (± SE) producing some progeny from the P2 (second) male. (B) Sperm offence. Measured as the mean proportion of progeny sired by the second male (± SE) for females that produced progeny from both P1 and P2 males. Dark bars represent ML‐evolved male; light bars represent control males.
FEMALE EFFECTS ON SPERM COMPETITION OUTCOMES
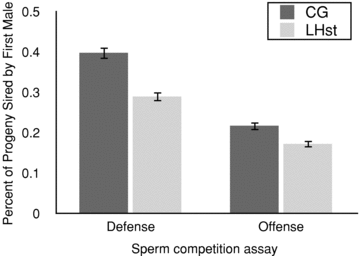
To address the specificity of male adaptation to female type under ML evolution, we employed females from both CG stock and from the scarlet marked copy of the LHM (LHST). As noted above, our results show that female type did not interact with selection treatment for either component of defense or offence (Table 1). However female type had a marked effect on defense. For mating defense, this effect was marginally nonsignificant (F1,182= 3.24; P= 0.07), but for sperm defense it was highly significant (F1,182= 27.4 P < 0.0001). CG females remated less frequently than scarlet females did, and retained or used more sperm from the first mate when they did remate (Fig. 4). In the offence assay, female type affected sperm offence (F1,190= 8.83; P= 0.003). Again, the CG females produced more offspring from their first mate, when they mated multiply.
Female genotype mediates sperm storage or utilization strategy. Clone generator (CG) females produce more progeny with the first male's sperm than wild‐type LHST females do. Results shown ± SE
These effects of female type provide insight into variation in sperm displacement, storage, or utilization strategies. Overall, compared to scarlet‐marked (effectively wild‐type) females, CG females use more of the first male's sperm than the second's (Fig. 4, offence assay, 26% more, P= 0.003; defense assay, 37% more, P < 0.0001; Fisher's combined probability test, χ2= 29.85, df = 4, PFisher < 0.001). Despite these differences between females, there was no evidence of specialization by ML males, as the male selection × female effect was not significant.
MALE IMPACT ON FEMALE LONGEVITY
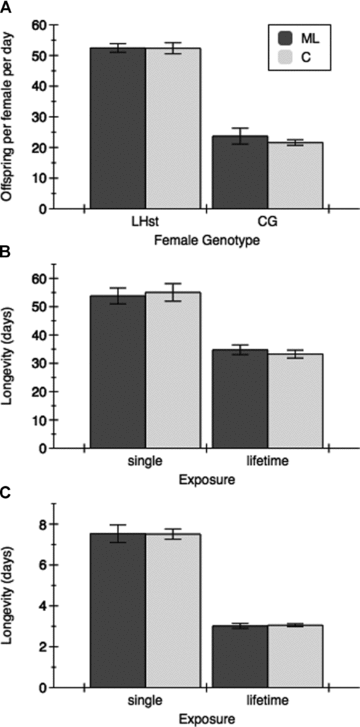
The selective history of a female's mate had no bearing on her life span, whether continuously housed or allowed a discrete interaction period. In fact, overall, life span of females encountering ML versus C males differed by only 0.06 h over a grand mean of 24.75 days (0.01%; P= 0.97) (Fig. 5). The lack of significance of this difference carried through all interactions throughout the four‐factor, fixed effect ANOVA, and through other potential analyses (e.g., with the two experiments analyzed separately and with replicate factored in). The three dominant effects apparent from the experiment were generation, exposure, and female type. Overall longevity was two days (8%) lower in the g75 experiment than in the g69 experiment for unknown reasons, genetic or environmental. As expected, CG females proved extremely frail and had an average life span of 5.3 ± 0.6 days, compared to LHST with a life span of 44.2 ± 3.0 days (F1,31= 740.4, P < 0.0001). Females housed with males throughout their lives had markedly shorter mean life span than females that had interacted with males for single 18 h period at an early age (18.5 ± 4.1 days vs. 31.0 ± 6.2 days; F1,31= 66.9, P < 0.0001). There was a stronger effect of male exposure on CG females than LHST females, reflected in a strong interaction term; CG females lived 2.5 times longer if males were removed after 18 h, whereas the life extension for the more robust LHST females was 1.6 times (F1,31= 25.9, P < 0.0001). A factor potentially contributing to this difference is that males were not refreshed throughout the experiment, and so may have declined in courtship effort with age or because of continued exposure to the same group of females.
Female offspring production and longevity in relation to male selection history. (A) Productivity of females (LHST or Clone Generator, adjusted for the 50% nonhatch rate of CG females) was unaffected by the selection history (ML or C) of males they interacted with prior to egg‐laying. CG females have markedly lower realized fertility. Average longevity of LHST females (B) or CG females (C) was not affected by the selection history of males. In both cases, continuous exposure to males throughout life strongly reduced the life span of females compared to an 18 h exposure early in life. Comparing panels (B) and (C), the frailty of CG females is apparent.
MATE HARM: FEMALE OFFSPRING PRODUCTION
There were no differences in female offspring production based upon male type: females produced an average of 37.5 offspring in the 24‐h laying period with ML‐mated females producing 1.1 offspring/female more than control‐mated females (F1,11= 0.66, n.s.). Females from the two test populations differed markedly in productivity, with LHST females producing 2.3× more offspring (52.4 ± 1.3 offspring/day vs. 22.7 ± 1.1 offspring/day; F1,11= 492.3, P < 0.0001). No significant interaction was detected between male and female genotypes (F1,11= 0.55, P≤ 0.46). Females in the g69 experiment, on average, produced 39.8 (±5.5) offspring, whereas at g75 they produced 35.3 (±5.9) offspring, a highly significant difference (F1,15= 12.0, P < 0.01). For both longevity and productivity measures of harm this factor is, of course, confounded with replicate effects because the experiment was conducted in two parts. However in neither case did “generation” interact with any other factors nor were any other interactions significant in the three‐way (factors: male selection, female genotype, generation) full‐factorial ANOVA.
Discussion
The evidence to date suggests that our ML males achieved higher fitness as a result of release from counter‐selection in females. Sexually dimorphic traits were masculinized by selection to varying degrees, and males became more efficient in courtship (Prasad et al. 2007; Bedhomme et al. 2008, Abbott et al. 2010). Here we rule out the contribution of postcopulatory sexual selection to the response: Irrespective of the female genotype employed, we found no evidence of a ML postcopulatory advantage over control males, or for the evolution of increased mate harm, despite substantial differences in female robustness and sperm storage or usage patterns. These results differ from those of Rice (1996, 1998). We explore several potential explanations, and ultimately conclude it is most likely that extended domestication under constant conditions had eroded the requisite variation for these males to evolve postcopulatory traits. This has important implications for the utility of model systems to explore fundamental aspects of evolutionary change.
BACKGROUND EFFECTS
We assayed ML males carrying an ML‐evolved or control genomic haplotype opposite an otherwise random (LHM) chromosomal background, whereas previous work on ML lines used males expressing the ML haplotype alongside the translocation used in their evolution (i.e., the t2;3 described as part of the CG female genotype; Rice 1996; Prasad et al. 2007) or hybrid males derived from two ML lines expressing a “double dose” of ML genes (Rice 1998). Hence it seemed possible that significant background‐by‐ML treatment interactions exist, with part of the previously measured ML fitness effects reflecting adaptation to the translocation. However, earlier control experiments indicated no effect of genetic background on ML fitness—the ML hemiclones performed equally well matched with an autosomal translocation, a newly backcrossed (presumed more genetically variable) autosomal translocation, or wild‐type background, relative to controls (N. G. Prasad et al., unpubl. data). In addition, background adaptation would not explain the decrease in fitness when females carry an ML haploid genome, or the evolution of a masculinized phenotype under ML selection (Prasad et al. 2007; Bedhomme et al. 2008; Abbott et al. 2010).
INTERSEXUAL COEVOLUTION
Specialized male–female interactions may be important in determining male fitness, much like signal–receiver interactions are important in communication. Specific male‐by‐female genotype interactions have been observed in D. melanogaster with both male and female fitness often dependent on the genotype of their mates (Clark and Begun 1998; Clark et al. 1999; Long et al. 2006; McGraw et al. 2009). As noted earlier, complex interactions may limit the availability of additive genetic variation to selection, as evidenced by Bjork et al. (2007) who were unable to evolve sperm competition characters with direct selection on the LHM population.
Nonetheless, we were surprised to find no evidence for adaptation of the ML males to the CG females in either elevated sperm competitive ability or in remating behavior for three reasons: first, CG females are phenotypically unusual, expressing mutations in body color (yellow, known to affect sexual selection [Drapeau et al. 2003]), wing‐veination, and bristle morphology. They are also extremely frail and have greatly reduced survivorship, making them difficult to work with even during routine selection on young animals. We therefore expected to see ML males evolve increased fitness with these females.
Second, CG females were presumably engaged in a coevolutionary relationship with males in their own population. Before experiments, the CG stock was backcrossed to LHM, and was therefore genetically variable. With the various genetic aberrations noted above, sexual selection and mate interactions would likely steer this population down a different pathway from the wild‐type. Unlike controls, ML males would be able to track this coevolution and should be better adapted to the CG females than control males. This raises an intriguing possibility with respect to sperm characters, in particular. It is known that the Drosophila Y chromosome, while having few protein‐coding sequences, is nonetheless vital to sperm production, The CG populations used in this study were backcrossed to the LHM population and therefore began with males carrying a cross‐section of Y chromosomes from that population. Chippindale and Rice (2002) reported substantial epistatic interaction for male fitness between Y chromosomes and autosomes, whereas Jiang et al (2010) reported significant Y‐by‐autosomal background effects on genome‐wide gene expression in males. If the pool of Y chromosomes evolved in the CG stock, ML evolved males might be expected to adapt via changes in other, epistatically interacting, chromosomes in the genome. When measured without CG‐evolved Y chromosomes (as we did here) such an advantage would be lost. However, we do not think this a likely evolutionary scenario, as fitness was shown to improve and for reasons outlined above in the section on background adaptation: adaptation to CG‐evolved Y chromosomes would not explain the observed decrease in fitness when females carry an ML haploid genome, or the evolution of a masculinized phenotype under ML selection (Prasad et al. 2007; Bedhomme et al. 2008; Abbott et al. 2010).
Third, we have demonstrated that the marked LHST tester stock (effectively wild‐type) differs markedly from the CG females in terms of sperm storage or usage patterns. CG females were significantly more likely to use the first male's sperm than were wild‐type‐proxy scarlet‐eyed females (LHST). Differences in sperm‐usage patterns could be due to morphological differences between CG female and wild‐type population females in seminal receptacles or spermathecae, or differences in receipt of new sperm or “dumping” of old sperm upon remating (Snook and Hosken 2004). If sperm competition could evolve, we suggest that it should have under these circumstances.
MATE HARM AND SEMINAL FLUID EFFECTS
One of the most striking conclusions of earlier research with ML evolution (Rice 1996, 1998) was that mate harm can be selectively favored, presumably as an indirect result of male–male competition (Civetta and Clark 2000). Rice (1996) showed evidence of increased mate harm to CG females from a single copulation, which was interpreted as evidence for antagonistic coevolution. Rice (1998) argued that increased mortality of effectively LH wild‐type (carrying the benign pink marker) tester females under continuous exposure also reflected this process. However, his EA and EB (= our designation ML) had no opportunity to adapt to females from that population, so a more plausible explanation would be that the generically enhanced fitness of those males and their increased remating success explain the increase in mate harm. Further to this, Lew and Rice (2005) showed a positive relationship between male fitness and harm to mates using hemiclonal analysis of the LHM population. We have also seen an increase in male fitness and courtship efficiency (Prasad et al. 2007; Bedhomme et al. 2008). The experiments presented here, however, do not show evidence for increased harm to females from either one‐time exposure or continuous cohabitation with mates in the ML lines. Nor do they provide evidence for antagonistic coevolution through specific adaptation to CG females.
EVOLVED POPULATION DIFFERENCES
We derived our selection lines from a population designated LHM, as did Bjork et al. (2007) who selected directly upon sperm competition traits. This is a descendent of the LH population employed in Rice's original work, but differs in important ways. LH was kept in bottles with unregulated, changeable and often extreme densities, whereas the LHM population (Chippindale et al. 2001) was maintained on a strictly defined moderate (the “M” in LHM) density protocol in vials for over 150 generations before establishment of our ML and control populations. Perhaps selectable variation for sperm competition traits existed in the LH population, but was winnowed away in LHM by a more controlled selection environment. This would be plausible if trade‐offs between different sperm competition traits, or between sperm competition traits and other components of fitness, were only manifested under crowding and resource‐limitation (occurring in the original LH population). Moreover, the changes from the crowded culture of LH to novel moderate conditions in Rice's experiment should have created a variety of potential avenues for adaptation, including via sexual selection. Using hemiclonal analysis, Friberg et al. (2005) reported low but measurable heritability for sperm offence and defense (h2≤ 2%) in the LHM population maintained in their laboratory. Although Friberg et al. concluded that there was genetic variation available to support an evolutionary arms race, we suggest that additive genetic variation was probably declining in both laboratories in response to strong directional selection under constant environmental conditions, explaining their low heritability estimates and the lack of response shown herein.
Conclusions
Rice's 1996 study documented rapid increases in male fitness and harm to females under ML selection. Here we show that some of the specific mechanisms of adaptation were not retraced in a similar experiment conducted on descendents of the same base population. Our ML males did not achieve higher fitness through increased sperm defense, mating defense, sperm offence, or mating offence, and we found no evidence for the evolution of increased mate harm. Our data also suggest that males did not achieve higher fitness through evolution of a lock‐and‐key fit with CG females, despite strong differences in phenotype and sperm storage or usage strategies from wild‐type females. Our results therefore contribute to a growing picture of limited realized heritability of sperm competition traits in this species, at least in populations in the laboratory. We suggest that the loss of additive genetic variation for postcopulatory traits in males, or its preservation only in the sort of complex genetic interactions documented by Bjork et al. (2007) in the same base population diminished the evolvability of these characters.
Long‐term domestication appears to have had a strong impact upon the makeup of standing genetic variation in this laboratory population. Because quantitative genetic studies, including selection experiments of this sort, are driven mainly by extant standing genetic variation, they are bound to be sensitive to adaptation by the base population(s) employed. This differs from typical microbial selection experiments where populations are often initially invariant and new mutations supply the fuel for adaptation. In this particular case, what may be a fundamental feature of sexual populations—coevolution by mates—was observed in the early domestication of LH, but not observed after further adaptation to the laboratory in general, or the more controlled conditions of LHM culture, specifically. Our work may serve as an instructive example for those using laboratory populations for the investigation of evolutionary change. The base population itself is an evolving entity, and different selection responses might even be predicted. Here it appears that long‐term laboratory adaptation has resulted in a population relatively enriched for standing genetic variation sustained by trade‐offs and other sources of disruptive selection, and depleted for variation related to fitness in one sex alone. This may explain why our ML selection response was dominated by the resolution of intralocus sexual conflict, rather than changes in postcopulatory traits that are innately sex‐limited.
Associate Editor: T. Chapman
ACKNOWLEDGMENTS
We thank everyone in the Chippindale lab for their help. M. Reuter, W. Rice, and T. Chapman provided thoughtful comments on the submitted paper. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (to PPJ), a Lavoisier Fellowship from the French government (to SB), an NSERC Discovery Grant, and support from the Canadian Foundation for Innovation and Canada Research Chairs (to AKC).
LITERATURE CITED
Author notes
Current address: Evolutionary Systems Virology Group, Instituto de Biología Molecular y Celular de Plantas (CSIC‐UPV), Ingeniero Fausto Elio s/n, 46022 València, Spain
Current address: Indian Institute of Science Education and Research, Mohali, MGSIPA Complex, Sector 26 Chandigarh, 160 019, India