-
PDF
- Split View
-
Views
-
Cite
Cite
Lei Chen, Mingpeng Wang, Jin Hou, Liangyu Liu, Jiafang Fu, Yu Shen, Zhaojie Zhang, Xiaoming Bao, Regulation of Saccharomyces cerevisiaeMEF1 by Hda1p affects salt resistance of bdf1Δ mutant, FEMS Yeast Research, Volume 14, Issue 4, June 2014, Pages 575–585, https://doi.org/10.1111/1567-1364.12144
Close - Share Icon Share
Abstract
Bromodomain factor 1 (Bdf1p) is a transcriptional regulator. The absence of Bdf1p causes salt sensitivity with abnormal nucleus and mitochondrial dysfunction. In this study, we reported that the salt sensitivity, mitochondrial dysfunction, and nuclear instability of bdf1Δ mutant were suppressed by HDA1 deletion or MEF1 overexpression. Hda1p overexpression inhibited the relieving effects of low-copy overexpression of MEF1. Further analysis showed that Bdf1p regulated HDA1 transcription positively by binding to its promoter at −201 to +6 bp, whereas Hda1p modulated MEF1 expression negatively by binding to its promoter at −201 to +6 bp. These results suggested that Bdf1p likely regulated MEF1 expression negatively by regulating HDA1 positively. Mitochondrial proteomics analysis showed that the expression levels of six mitochondrial proteins were significantly changed by MEF1 overexpression. Among the six genes, over-expression of PDB1, ILV5, or ATP2 partially recovered the salt stress sensitivity of bdf1Δ. However, none of these mitochondrial proteins could recover mitochondrial respiration indicating that the individual functional proteins could not replace Mef1p activity. It indicated that positive regulation of MEF1 was important in recovering the salt sensitivity of bdf1Δ mutant.
Overexpression of the mitochondrial elongation factor gene, MEF1, recovers the salt resistance of a Bdf1 bromodomain protein mutant; positive regulation of MEF1, via the histone deacetylase gene, HDA1 may be important for recovering the loss of respiratory function of the BDF1 deletion strain.
Introduction
Organisms employ various regulation mechanisms in responding to environmental stresses to improve their survival. Saccharomyces cerevisiae is a model microorganism used to study stress response mechanisms. High concentration of salt, a common and important stress, can injure cells through Na+ toxicity and hyperosmolality. In our previous study, we reported that the deletion of the bromodomain factor 1 gene (BDF1) caused growth defect, mitochondrial dysfunction, and apoptotic cell death under salt stress condition (Liu et al., 2009;). Transcriptome analysis showed that under salt stress, seven genes involved in the mitochondrial function were downregulated in bdf1Δ mutant. Among the seven genes, mitochondrial translation elongation factor MEF1 showed the most obvious decrease (more than 3.7-fold; Liu et al., 2007).
Bromodomain factor 1 (Bdf1p) is a transcription factor that contains two copies of bromodomain. It preferentially binds to the acetylated histone H4 tail, and histone acetylation levels affect Bdf1p-involved regulation of gene expression. Bdf1p also binds directly or indirectly to promoters to regulate gene transcription via the formation of basal transcription factor IID complexes (TFIID; Matangkasombut & Buratowski, 2003; Huisinga & Pugh, 2004; Martinez-Campa et al., 2004; Zhang et al., 2005). In S. cerevisiae, histone acetylation is regulated by two types of functionally antagonistic enzymes, acetyl transferases and deacetylases, which are responsible for the acetylation and deacetylation of histones, respectively. Each enzyme has several homologs that modify different amino acid residues of histones. Combined with other factors, these enzymes also regulate gene expression at the epigenetic level (Camblong et al., 2007; Lin et al., 2008).
Hda1p is one of the important deacetylases in yeast. The Hda1p dimer interacts with Hda2p–Hda3p to compose the histone deacetylase complex, HDA1 (Lee et al., 2009). This complex reduces histone acetylation and leads to chromosome compaction and repression of gene expression. Deletion of HDA1 increases histone acetylation (Wu et al., 2001a). Hda1p shares a similar sequence with another deacetylase, Rpd3p. However, their deacetylation preferences are distinct (Rundlett et al., 1996). Hda1p also deacetylates subtelomeric domains of chromosomes (10–25 kilobases from the telomeres) that contain normally repressed genes involved in hexose transport, carbon source utilization, and stress response (Robyr et al., 2007).
MEF1 encodes the yeast mitochondrial translation elongation factor, which regulates protein synthesis in mitochondria (Vambutas et al., 1991). MEF1 deletion strain is unable to utilize nonfermentable carbon source, and its mitochondrial genome is unstable (Vambutas et al., 1991).
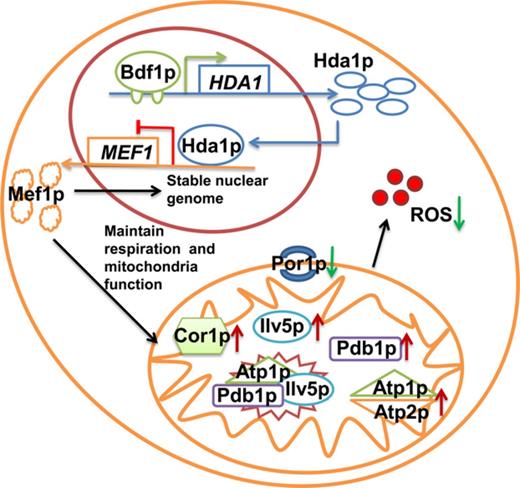
In previous study, we found autophagy stimulated by Hal2p played the role in recovering mitochondrial functions and Na+ sensitivity of bdf1Δ (Chen et al., 2013). In this study, we observed another two elements HDA1 and MEF1 which were related to the survival of bdf1Δ mutant under the condition of salt stress. HDA1 deletion and overexpression of MEF1 caused an increase in salt resistance of bdf1Δ mutant by our further results. MEF1 affected the mitochondrial proteins translation and finally the function. Our results suggest that Bdf1p likely regulates MEF1 expression negatively by positively regulating HDA1. Moreover, mitochondrial function is essential for yeast resistance to salt stress.
Materials and methods
Yeast cells were routinely grown in yeast peptone dextrose (YPD) media (1% yeast extract, 2% peptone, 2% glucose) or in a synthetic complete medium [0.17% yeast nitrogen base, 0.5% (NH4)2SO4, 2% glucose] and supplemented with all amino acids, except those that maintain plasmids (uracil or leucine).
Strains and plasmid construction
Serial strains were constructed to study the compensation function of MEF1 and HDA1 overexpression in bdf1Δ mutants under salt stress, including bY (bdf1Δ, harboring the YCplac111 plasmid), bp (bdf1Δ, harboring the pYX242 plasmid as an empty vector), bdf1Δ [MEF1L] (bdf1Δ, harboring the YCplac111-MEF1 plasmid), bdf1Δ [MEF1 H] (bdf1Δ, harboring the pYX242-MEF1 plasmid), bdf1Δ [MEF1 L][HDA1 H] (bdf1Δ, harboring the YCplac111-MEF1 and pXM204-HDA1 plasmids), and bdf1Δ [MEF1 H][HDA1 H] (bdf1Δ, harboring the pYX242-MEF1 and pXM204-HDA1 plasmids; Table 1). All of the strains were derived from W303-1A (MATa ade 2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1) background.
Strains and plasmids used in this study
| Strains and Plasmids | Genotype/properties* | Reference or source |
| WT (W303-1A) | MATa, leu2-3/112 ura3-1 trp1-1 his3-11/15 ade2-1 can1-100 GAL SUC2 mal0 | Ferreira et al. (2001) |
| bdf1Δ (BLZ-A) | W303-1A derivative; BDF1::kanMX4 | Liu et al. (2007) |
| BDF1-Flag | W303-1A derivative; BDF1::BDF1-Flag | This study |
| HDA1-Flag | W303-1A derivative; HDA1::HDA1-Flag | This study |
| hda1Δ | W303-1A derivative; HDA1::URA3 | This study |
| bdf1Δhda1Δ | W303-1A derivative; BDF1::kanMX4HDA1::URA3 | This study |
| bp | bdf1Δ derivative; [pYX242] | This study |
| bY | bdf1Δ derivative; [YCplac111] | This study |
| bdf1Δ [MEF1 L]† | bdf1Δ derivative; [YCplac111-MEF1] | This study |
| bdf1Δ [MEF1 L] [HDA1 H] | bdf1Δ derivative; [YCplac111-MEF1] [pXM204-HDA1] | This study |
| bdf1Δ [MEF1 H]† | bdf1Δ derivative; [pYX242-MEF1] | This study |
| bdf1Δ [MEF1 H] [HDA1 H] | bdf1Δ derivative; [pYX242-MEF1] [pXM204-HDA1] | This study |
| pdb1Δ | W303-1A derivative; PDB1::URA3 | This study |
| ilv5Δ | W303-1A derivative; ILV51::URA3 | This study |
| atp1Δ | W303-1A derivative; ATP1:: URA3 | This study |
| atp2Δ | W303-1A derivative; ATP2:: URA3 | This study |
| cor1Δ | W303-1A derivative; COR1:: URA3 | This study |
| por1Δ | W303-1A derivative; POR1:: URA3 | This study |
| bdf1Δ [PDB1] | bdf1Δ derivative; [pYX242-PDB1] | This study |
| bdf1Δ [ILV5] | bdf1Δ derivative; [pYX242-ILV5] | This study |
| bdf1Δ [ATP1] | bdf1Δ derivative; [pYX242-ATP1] | This study |
| bdf1Δ [ATP2] | bdf1Δ derivative; [pYX242-ATP2] | This study |
| bdf1Δ [COR1] | bdf1Δ derivative; [pYX242-COR1] | This study |
| WT [POR1] | W303-1A derivative; [pYX242-POR1] | This study |
| pYX242 | A 2 μmulticopy plasmid containing a LEU2 selection marker and a TPI promoter | McGuire & Mangroo (2007) |
| pXM204 | A 2 μmulticopy plasmid, YEp24 (ATCC 37051) derivative; containing a URA3 selection marker and a PGK1 promoter | This study |
| YCplac111 | A centromere lower-copy plasmid containing a LEU2 selection marker | Swart et al. (1995) |
| Strains and Plasmids | Genotype/properties* | Reference or source |
| WT (W303-1A) | MATa, leu2-3/112 ura3-1 trp1-1 his3-11/15 ade2-1 can1-100 GAL SUC2 mal0 | Ferreira et al. (2001) |
| bdf1Δ (BLZ-A) | W303-1A derivative; BDF1::kanMX4 | Liu et al. (2007) |
| BDF1-Flag | W303-1A derivative; BDF1::BDF1-Flag | This study |
| HDA1-Flag | W303-1A derivative; HDA1::HDA1-Flag | This study |
| hda1Δ | W303-1A derivative; HDA1::URA3 | This study |
| bdf1Δhda1Δ | W303-1A derivative; BDF1::kanMX4HDA1::URA3 | This study |
| bp | bdf1Δ derivative; [pYX242] | This study |
| bY | bdf1Δ derivative; [YCplac111] | This study |
| bdf1Δ [MEF1 L]† | bdf1Δ derivative; [YCplac111-MEF1] | This study |
| bdf1Δ [MEF1 L] [HDA1 H] | bdf1Δ derivative; [YCplac111-MEF1] [pXM204-HDA1] | This study |
| bdf1Δ [MEF1 H]† | bdf1Δ derivative; [pYX242-MEF1] | This study |
| bdf1Δ [MEF1 H] [HDA1 H] | bdf1Δ derivative; [pYX242-MEF1] [pXM204-HDA1] | This study |
| pdb1Δ | W303-1A derivative; PDB1::URA3 | This study |
| ilv5Δ | W303-1A derivative; ILV51::URA3 | This study |
| atp1Δ | W303-1A derivative; ATP1:: URA3 | This study |
| atp2Δ | W303-1A derivative; ATP2:: URA3 | This study |
| cor1Δ | W303-1A derivative; COR1:: URA3 | This study |
| por1Δ | W303-1A derivative; POR1:: URA3 | This study |
| bdf1Δ [PDB1] | bdf1Δ derivative; [pYX242-PDB1] | This study |
| bdf1Δ [ILV5] | bdf1Δ derivative; [pYX242-ILV5] | This study |
| bdf1Δ [ATP1] | bdf1Δ derivative; [pYX242-ATP1] | This study |
| bdf1Δ [ATP2] | bdf1Δ derivative; [pYX242-ATP2] | This study |
| bdf1Δ [COR1] | bdf1Δ derivative; [pYX242-COR1] | This study |
| WT [POR1] | W303-1A derivative; [pYX242-POR1] | This study |
| pYX242 | A 2 μmulticopy plasmid containing a LEU2 selection marker and a TPI promoter | McGuire & Mangroo (2007) |
| pXM204 | A 2 μmulticopy plasmid, YEp24 (ATCC 37051) derivative; containing a URA3 selection marker and a PGK1 promoter | This study |
| YCplac111 | A centromere lower-copy plasmid containing a LEU2 selection marker | Swart et al. (1995) |
Genotypes inside the bracket represent the plasmid, whereas those outside the bracket represent the genome.
L, low-copy plasmid; H, multicopy plasmid.
Strains and plasmids used in this study
| Strains and Plasmids | Genotype/properties* | Reference or source |
| WT (W303-1A) | MATa, leu2-3/112 ura3-1 trp1-1 his3-11/15 ade2-1 can1-100 GAL SUC2 mal0 | Ferreira et al. (2001) |
| bdf1Δ (BLZ-A) | W303-1A derivative; BDF1::kanMX4 | Liu et al. (2007) |
| BDF1-Flag | W303-1A derivative; BDF1::BDF1-Flag | This study |
| HDA1-Flag | W303-1A derivative; HDA1::HDA1-Flag | This study |
| hda1Δ | W303-1A derivative; HDA1::URA3 | This study |
| bdf1Δhda1Δ | W303-1A derivative; BDF1::kanMX4HDA1::URA3 | This study |
| bp | bdf1Δ derivative; [pYX242] | This study |
| bY | bdf1Δ derivative; [YCplac111] | This study |
| bdf1Δ [MEF1 L]† | bdf1Δ derivative; [YCplac111-MEF1] | This study |
| bdf1Δ [MEF1 L] [HDA1 H] | bdf1Δ derivative; [YCplac111-MEF1] [pXM204-HDA1] | This study |
| bdf1Δ [MEF1 H]† | bdf1Δ derivative; [pYX242-MEF1] | This study |
| bdf1Δ [MEF1 H] [HDA1 H] | bdf1Δ derivative; [pYX242-MEF1] [pXM204-HDA1] | This study |
| pdb1Δ | W303-1A derivative; PDB1::URA3 | This study |
| ilv5Δ | W303-1A derivative; ILV51::URA3 | This study |
| atp1Δ | W303-1A derivative; ATP1:: URA3 | This study |
| atp2Δ | W303-1A derivative; ATP2:: URA3 | This study |
| cor1Δ | W303-1A derivative; COR1:: URA3 | This study |
| por1Δ | W303-1A derivative; POR1:: URA3 | This study |
| bdf1Δ [PDB1] | bdf1Δ derivative; [pYX242-PDB1] | This study |
| bdf1Δ [ILV5] | bdf1Δ derivative; [pYX242-ILV5] | This study |
| bdf1Δ [ATP1] | bdf1Δ derivative; [pYX242-ATP1] | This study |
| bdf1Δ [ATP2] | bdf1Δ derivative; [pYX242-ATP2] | This study |
| bdf1Δ [COR1] | bdf1Δ derivative; [pYX242-COR1] | This study |
| WT [POR1] | W303-1A derivative; [pYX242-POR1] | This study |
| pYX242 | A 2 μmulticopy plasmid containing a LEU2 selection marker and a TPI promoter | McGuire & Mangroo (2007) |
| pXM204 | A 2 μmulticopy plasmid, YEp24 (ATCC 37051) derivative; containing a URA3 selection marker and a PGK1 promoter | This study |
| YCplac111 | A centromere lower-copy plasmid containing a LEU2 selection marker | Swart et al. (1995) |
| Strains and Plasmids | Genotype/properties* | Reference or source |
| WT (W303-1A) | MATa, leu2-3/112 ura3-1 trp1-1 his3-11/15 ade2-1 can1-100 GAL SUC2 mal0 | Ferreira et al. (2001) |
| bdf1Δ (BLZ-A) | W303-1A derivative; BDF1::kanMX4 | Liu et al. (2007) |
| BDF1-Flag | W303-1A derivative; BDF1::BDF1-Flag | This study |
| HDA1-Flag | W303-1A derivative; HDA1::HDA1-Flag | This study |
| hda1Δ | W303-1A derivative; HDA1::URA3 | This study |
| bdf1Δhda1Δ | W303-1A derivative; BDF1::kanMX4HDA1::URA3 | This study |
| bp | bdf1Δ derivative; [pYX242] | This study |
| bY | bdf1Δ derivative; [YCplac111] | This study |
| bdf1Δ [MEF1 L]† | bdf1Δ derivative; [YCplac111-MEF1] | This study |
| bdf1Δ [MEF1 L] [HDA1 H] | bdf1Δ derivative; [YCplac111-MEF1] [pXM204-HDA1] | This study |
| bdf1Δ [MEF1 H]† | bdf1Δ derivative; [pYX242-MEF1] | This study |
| bdf1Δ [MEF1 H] [HDA1 H] | bdf1Δ derivative; [pYX242-MEF1] [pXM204-HDA1] | This study |
| pdb1Δ | W303-1A derivative; PDB1::URA3 | This study |
| ilv5Δ | W303-1A derivative; ILV51::URA3 | This study |
| atp1Δ | W303-1A derivative; ATP1:: URA3 | This study |
| atp2Δ | W303-1A derivative; ATP2:: URA3 | This study |
| cor1Δ | W303-1A derivative; COR1:: URA3 | This study |
| por1Δ | W303-1A derivative; POR1:: URA3 | This study |
| bdf1Δ [PDB1] | bdf1Δ derivative; [pYX242-PDB1] | This study |
| bdf1Δ [ILV5] | bdf1Δ derivative; [pYX242-ILV5] | This study |
| bdf1Δ [ATP1] | bdf1Δ derivative; [pYX242-ATP1] | This study |
| bdf1Δ [ATP2] | bdf1Δ derivative; [pYX242-ATP2] | This study |
| bdf1Δ [COR1] | bdf1Δ derivative; [pYX242-COR1] | This study |
| WT [POR1] | W303-1A derivative; [pYX242-POR1] | This study |
| pYX242 | A 2 μmulticopy plasmid containing a LEU2 selection marker and a TPI promoter | McGuire & Mangroo (2007) |
| pXM204 | A 2 μmulticopy plasmid, YEp24 (ATCC 37051) derivative; containing a URA3 selection marker and a PGK1 promoter | This study |
| YCplac111 | A centromere lower-copy plasmid containing a LEU2 selection marker | Swart et al. (1995) |
Genotypes inside the bracket represent the plasmid, whereas those outside the bracket represent the genome.
L, low-copy plasmid; H, multicopy plasmid.
An hda1::URA3 disruption cassette was used to generate the hda1Δ and bdf1Δhda1Δ mutants. The pdb1Δ, ilv5Δ, atp1Δ, atp2Δ, cor1Δ, and por1Δ mutants were generated by replacing the respective open reading frames with a URA3 disruption cassette, following a previously described method (Guldener et al., 1996). The bdf1Δ mutants were generated by replacing the respective open reading frames with a loxP-kanMX-loxp disruption cassette (Supporting Information, Table S1).
Plasmid construction
The gene was amplified from chromosomal DNA derived from W303-1A, digested with the proper enzyme, and ligated to a plasmid. The plasmids used in this study included two 2 μ plasmids, pYX242 (McGuire & Mangroo, 2007) with a LEU2 marker, and pXM204 with a URA3 marker, and one centromeric plasmid, YCplac111 (Swart et al., 1995) with a LEU2 marker. Three recombinant plasmids were constructed in this study: pYX242-MEF1, a 2 μ plasmid containing TPI promoter-MEF1-PolyA terminators; YCplac111-MEF1, a centromeric plasmid containing TEF1 promoter-MEF1-PGK1 terminator; and pXM204-HDA1, a 2 μ plasmid containing PGK promoter-HDA1-PGK1 terminators (Table 1).
Spot assays
Cells were cultured in YPD liquid medium overnight and harvested by centrifugation. The cells were washed twice with water and re-suspended in water. The cell density was normalized to OD 600 = 1.0. A 10-fold serial dilution of the culture was prepared, and 4 μL of each dilution was spotted onto an appropriate solid medium.
RNA extraction and quantitative PCR
Cells were cultivated by inoculating a preculture in 100 mL fresh YPD to an OD600 = 0.2 and grown to the mid-log phase (OD600 = 0.7). The cultures were divided into two 50 mL samples. Next, 10 mL of YPD media containing 3 M NaCl was added to one sample (final concentration of 0.5 M NaCl), and the same volume of sterile water was added to the other sample. After 45 min of treatment, both cultures were collected by centrifugation and immediately frozen in liquid nitrogen. The total yeast RNA was isolated using UNIQ-10 spin column Trizol Total RNA purification kits (BBI) in accordance with the manufacturer's instructions. An aliquot of 5 μg total RNA was treated with RNA-free DNase I for 30 min at 37 °C. Then, 2 μL of treated RNA was employed to synthesize the first cDNA strand in a 20 μL reverse transcription (RT) volume. For PCR amplification, 1 μL of the RT reaction products was used, utilizing the SYBR Green I monitoring method. Fold changes in gene expression were calculated using the 2−△△CT method (Chung et al., 2002).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as previously described (Dedon et al., 1991; Braunstein et al., 1993). Cultures were grown in 100 mL YPD medium at 30 °C to an OD600 of 1.0. The cells were cross-linked with 1% formaldehyde for 60 min. The cross-linking was quenched with 125 mM glycine for 5 min. The cells were subsequently harvested by centrifugation for 5 min at 2000 g and converted to spheroplasts, as previously described (Braunstein et al., 1993). The spheroplasts were washed sequentially in 5 mL ice-cold PBS, 5 mL buffer I (0.25% Triton X-100, 10 mM EDTA, 0.5 mM EGTA, 10 mM HEPES at pH 6.5, 0.8 μg mL−1 pepstatin A, 0.6 μg mL−1 leupeptin, and 0.5 mM PMSF), and 5 mL buffer II (200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 10 mM HEPES, 0.8 μg mL−1 pepstatin A at pH 6.5, 0.6 μg mL−1 leupeptin, and 0.5 mM PMSF). Next, the spheroplasts were centrifuged at 2000 g at 4 °C for 5 min to pellet the cells. Subsequent procedures were described in the manufacturer's protocol for the EZ-CHIP™ ChIP Kit (Catalog#17-37; Millipore). The condition of sonication was as follows: pulse on for 5 s, pulse off for 45 s, for 24 cycles.
4′, 6-Diamidino-2-phenylindole staining
Yeast cells in the log phase were collected and washed twice with 1 × PBS buffer, re-suspended in 80% ethanol for 10 min, washed with 1 × PBS buffer, and incubated with 1 μg mL−1 of 4′, 6-diamidino-2-phenylindole (DAPI) for 15 min, away from the light. The stained cells were mounted on a coverslip and visualized via fluorescence microscopy, immediately.
Detection of reactive oxygen species
Yeast cells in the log phase were collected and washed with 1 × PBS buffer, re-suspended in 1 × PBS buffer, and incubated with 50 mM DHR for 20 min, away from the light (Petrangeli et al., 2009;). Reactive oxygen species (ROS) production was immediately detected via fluorescence microscopy.
Fluorescence microscopy
Fluorescence microscopy was performed using a fluorescence microscopy system (Nikon ECLIPSE 80i) equipped with a plan Apochromat 60× oil objective (numerical aperture, 1.40). Images were acquired and analysis was performed using the NIS-Elements AR 3.1 software.
Dimensional electrophoresis
The extraction of purified yeast mitochondrial protein was performed according to standard procedures (Meisinger et al., 2000). The purified proteins were stored on dry ice for delivery. Dimensional electrophoresis was performed by the Beijing Protein Innovation with electrophoresis apparatus Ettan DALT twelve System (GE) and protein isoelectric system Ettan IPGphor3 (GE). Mitochondrial protein samples (200 μg) were dissolved in 20 mM Tris-HCl (pH 8.2), 8 M urea, 2 M thiourea, and 4% CHAPS. The samples were separated on the same 2D gel in the first dimension (pH 3–10 gradients) and SDS polyacrylamide gel electrophoresis in the second dimension. Geldians were scanned by scanistor Powerlook 2100XL-USB (UMAX) and then analyzed using ImageMaster 2D Platinum 5.0 software to locate and measure different protein expressions in three replicates. The selected protein spots were subjected to in-gel trypsin digestion, peptide extraction, desalting, and MALDI-TOF analysis. The MASCOT and SGD database analyzed the peptides to identify the selected proteins.
Statistical analysis of data
Assuming equal variances, we used a two-tailed t-test to determine whether behavioral differences among the different strains were statistically significant.
Results
HDA1 deletion increased salt stress resistance of the bdf1Δ mutant
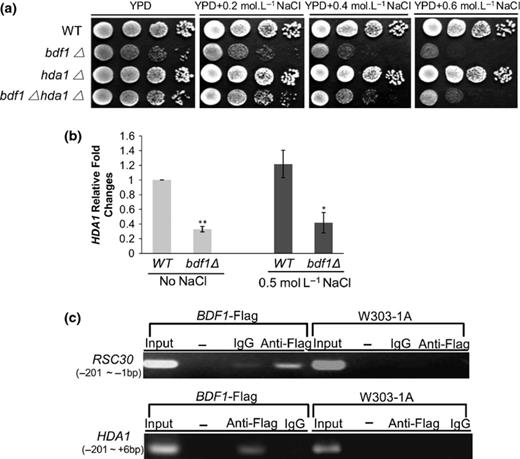
In consideration of the possible antagonistic action of Hda1p to Bdf1p in histone acetylation (Robyr et al., 2007; Matangkasombut & Buratowski, 2003), the HDA1 gene was deleted in the bdf1Δ strain to see whether it could reduce the salt stress sensitivity. As shown in Fig. 1a, bdf1Δhda1Δ double deletion increased the salt stress resistance compared to bdf1Δ single deletion. Like wild type, the hda1Δ mutant showed no sensitivity to 0.6 M NaCl (Fig. 1a). Quantitative PCR (qPCR) analysis showed that the relative expression level of the HDA1 was significantly decreased in bdf1Δ, either in the presence or absence of salt stress (P < 0.05; Fig. 1b). This result suggests that Bdf1p may positively regulate the transcription of the HDA1. To further confirm this speculation, ChIP was performed to detect whether Bdf1p binds HDA1. The BDF1-Flag strain (Table 1), which exhibited the same NaCl resistance as the wild type, and the Flag antibody (anti-Flag) were used for Bdf1p precipitation. The promoter of RSC30, which Bdf1p directly binds to (Bianchi et al., 2004), was used as a positive control. Our results showed that Bdf1p bound to the HDA1 promoter at a region between −201 and +6 bp from the translation initiation site (Fig. 1c).
Bdf1p regulated its antagonistic action on Hda1p directly and positively. (a) HDA1 deletion increased the salt stress resistance in bdf1Δ. Yeast cells were grown to an exponential phase. An aliquot of 4 μL from a 10-fold serial dilution was spotted onto yeast peptone dextrose (YPD) or YPD plates supplemented with the indicated NaCl concentrations. Cells were incubated at 30 °C for 3 days. (b) Bdf1p regulated HDA1 gene transcription, positively. The mid-log phage cultures were inoculated for 45 min with and without 0.5 M NaCl treatment. The relative fold changes of HDA1 mRNA were calculated against the wild-type strain, without NaCl treatment. Error bars denote standard error (SE). *P < 0.05, **P < 0.01, vs. the WT strain under the same treatment (n = 3). (c) Bdf1p regulated HDA1 gene transcription, directly. Chromatins from the bromodomain factor 1 gene (BDF1)-Flag strain were precipitated with antibodies against Flag and IgG (negative control). Samples without precipitation (Input) and no DNA template (−) were also tested. The HDA1 promoter regions probed by ChIP corresponded to the nucleotides between −201 and 6 bp from the translation initiation site. RSC30 acted as the positive control. WT, wild type.
Overexpression of MEF1 suppressed the salt sensitivity of bdf1Δ
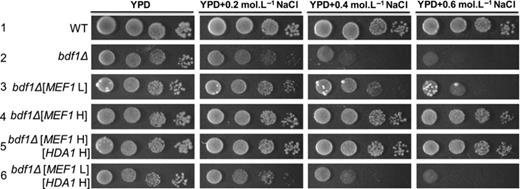
Our laboratory showed previously (Liu et al., 2007) that in the bdf1Δ strain, MEF1 was significantly downregulated under NaCl stress. To further understand the role of MEF1 in BDF1-involved salt stress response, MEF1was overexpressed in bdf1Δ using either the low-copy plasmid YCplac111, or the multi-copy plasmid pYX242. The results showed that MEF1 overexpression via the multi-copy plasmid completely suppressed the salt stress sensitivity of bdf1Δ, while overexpression via low-copy plasmid partially recovered the salt sensitivity of bdf1Δ (Fig. 2, Lines 3 and 4). The results demonstrated that the salt resistance of bdf1Δ has a positive correlation with MEF1 copy number (Fig. 2, Lines 2–4).
Mef1p suppressed the salt sensitivity of bdf1Δ in a dosage-dependent manner. Yeast cells were grown to an exponential phase. An aliquot of 4 μL from a 10-fold serial dilution was spotted onto yeast peptone dextrose (YPD) or YPD plates supplemented with the indicated NaCl concentrations. Cells were incubated at 30 °C for 3 days. WT, wild type.
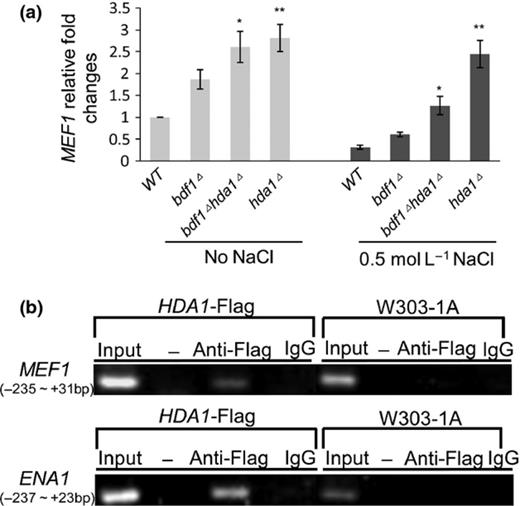
MEF1 expression was negatively regulated by Hda1p
MEF1overexpression or HDA1 deletion improved the salt resistance of bdf1Δ, and Hda1p usually represses gene expression (Lee et al., 2009). Therefore, we tested the regulatory relationship between HDA1 and MEF1 at the level of transcription to find out whether Hda1p also repressed the expression of MEF1. The relative expression levels of MEF1 were detected by qPCR. The results showed that deletion of HDA1 increased MEF1 expression either in wild type (P < 0.01) or bdf1Δ (P < 0.05; Fig. 3a). Deletion of BDF1 increased MEF1 expression levels (P < 0.05 with and P < 0.01 without NaCl treatment), suggesting that Bdf1p regulated MEF1 transcription negatively. We further investigated whether HDA1 overexpression could repress the phenotypes caused by MEF1overexpression. A 2 μ plasmid containing HDA1 was transferred into low-copy plasmid strain (bdf1Δ [MEF1L]). The growth phenotype showed a negative effect of Hda1p on Mef1p that the growth of the bdf1Δ [MEF1L] was repressed by HDA1overexpression (Fig. 2, Lines 3 and 6). However, upon overexpression of MEF1 in the multi-copy plasmid, repression did not occur (Fig. 2, Lines 4 and 5). This result revealed that HDA1 overexpression could offset the compensation effects of MEF1 in salt stress resistance of the bdf1Δ strain when MEF1 expression levels were not sufficiently high.
Mef1p expression was regulated by Hda1p, directly and negatively. (a) Hda1p regulated MEF1 gene transcription, negatively. The mid-log phase cultures were inoculated for 45 min with and without 0.5 M NaCl treatment. The relative fold changes of MEF1 mRNA were calculated against wild-type levels, without NaCl treatment. Error bars denote SE. *P < 0.05, vs. the bdf1Δ strain under the same treatment, **P < 0.01 vs. the WT strain under the same treatment, n = 3. (b) Bdf1p regulated HDA1 gene transcription, directly. Chromatins from the HDA1-Flag strain were precipitated with antibodies against Flag (anti-Flag) and IgG (negative control). Samples without precipitation (Input) and without DNA template (−) were also tested. The MEF1 promoter regions probed by ChIP corresponded to the nucleotides between −235 and +31 bp from the translation initiation site. ENA1 acted as a positive control. WT, wild type.
ChIP was performed to detect how Hda1p regulated MEF1 gene expression using the HDA1-Flag strain (Table 1), which exhibited the same NaCl resistance as the wild type. The promoter of ENA1, to which Hda1p directly binds (Wu et al., 2001b), was used as a positive control. The ChIP results confirmed that Hda1p bound to the MEF1 promoter at a region between −235 and +31 bp from the translation initiation site (Fig. 3b), suggesting that Hda1p regulates the transcription of the MEF1 by directly binding to its promoter.
MEF1 overexpression improved nuclear stability and respiration of bdf1Δ
Mitochondrial defects generally affect genome stability (Towpik, 2005; Veatch et al., 2009). The chromatin fragmentation was also investigated to monitor the genome stability. The bdf1Δ strain displayed dispersive nuclear or chromatin fragments, whereas wild type showed intact nucleus (Fig. 4c; Lines 1 and 2).
MEF1 overexpression recovered the respiration deficiency and nuclear stability of bdf1Δ. (a) MEF1 overexpression recovered the growth of bdf1Δ on a nonfermentable carbon source, glycerol. Yeast cells were grown to an exponential phase. An aliquot of 4 μL from a 10-fold serial dilution was spotted onto a YPG plate. Cells were incubated at 30 °C for 3 days. (b) MEF1 overexpression in bdf1Δ significantly decreased ROS levels. Yeast cells were treated with and without 0.5 M NaCl for 45 min after being grown to an exponential phase. DHR was used to monitor the production of ROS (top lane). (c) MEF1 overexpression improved nucleic genomic stability. Yeast cells were treated with and without 0.5 M NaCl for 45 min after growth to an exponential phase. DAPI staining was used to visualize the position of the chromatin within the nuclei. The numbers representing the strains are as follows: 1, WT; 2, bdf1Δ; 3, bdf1Δ [MEF1 L]; 4, bdf1Δ [MEF1 H]; 5, bdf1Δ [MEF1 L] [HDA1 H]; 6, bdf1Δ [MEF1 H] [HDA1 H] (Table 1). WT, wild type.
Although MEF1 expression in the low-copy plasmid strain (bdf1Δ [MEF1L]) partially inhibited the salt stress sensitivity of bdf1Δ (Fig. 2, Line 4), it did not recover the growth phenotype on the glycerol; it showed the distributed nuclear fragments and high ROS accumulation especially under salt stress (Fig. 4a–c Line 3). Compared with bdf1Δ [MEF1L], further introduction of HDA1 resulted in poorer growth on salt stress, more accumulation of ROS, and more scattered nuclear fragments (Fig. 4a–c, Line 5; Fig. 2, Line 6). MEF1 overexpression in the multi-copy plasmid strain (bdf1Δ [MEF1 H]) completely recovered the growth, not only on the salt plate but also on the glycerol plate (Fig. 2, Line 5; Fig. 3a, Line 4). Moreover, MEF1 overexpression (bdf1Δ [MEF1 H]) decreased the ROS level and recovered the nuclear morphology, either in the presence or absence of salt stress (Fig. 3b and c, Line 4). Nonetheless, the introduction of HDA1 did not change the growth phenotype on glycerol (Fig. 4a, Line 6). This indicated that high copies of MEF1 were necessary to restore the mitochondrial respiratory function and to enhance the nucleic genome stability.
Mitochondrial proteins were affected under salt stress
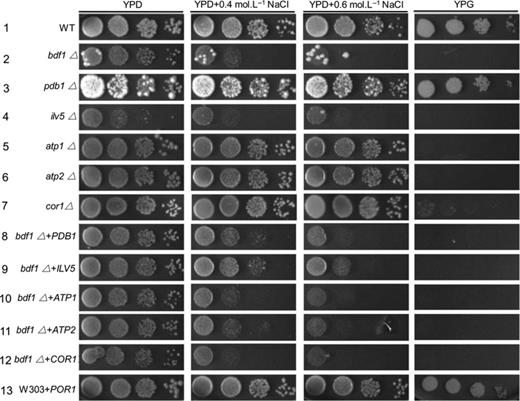
MEF1 overexpression recovered the mitochondrial dysfunction of bdf1Δ (Fig. 4a and b). To better understand its mechanism, mitochondrial proteomics analysis was performed in the MEF1 overexpression strain (bdf1Δ [MEF1 H]) and in the bdf1Δ strain, under 0.5 M NaCl conditions. Among the 47 protein spots that showed significant expression changes in the 2D polyacrylamide gel electrophoresis (Fig. 5), 28 spots changed more than threefold. Finally, 13 proteins, including six mitochondrial proteins and seven cytoplasmic proteins, were identified by mass spectrometry (Tables 2 and 3). Genetic manipulation was performed on the six mitochondrial proteins (Pdb1p, Ilv5p, Atp1p, Atp2p, Cor1p, and Por1p) to test their contributions to salt stress response. The five upregulated genes (ILV5, ATP1, ATP2, COR1, and PDB1) were knocked out in wild type. The deleted strains grew normal on plates with or without NaCl (Fig. 6), except ilv5Δ (Fig. 6, Line 4). This result indicated that ILV5 is critical for growth even under normal conditions. Meanwhile, the other deletion strains, except pdb1Δ, showed no growth on the glycerol plate (Fig. 6, Lines 3–7), demonstrating that ILV5, ATP1, ATP2, COR1, but not PDB1, were involved in respiration. The downregulated gene, POR1, was overexpressed in wild type and did not exhibit any growth defect on either salt or glycerol plates (Fig. 6, Line 13).
Mitochondrial proteins with fold change > 3, with MEF1 overexpression (bdf1Δ[MEF1 H]) vs. in the bdf1Δ strain, under 0.5 M NaCl for 45 min
| Spot ID | Ratio* | Protein | Gene | function |
| 329 | +5.40 | Ilv5p | YLR355C | Branched-chain amino acid biosynthesis and maintenance of mitochondrial DNA |
| 600† | ||||
| 602† | ||||
| 720† | Atp2p | YJR121W | Beta-subunit of the F1 sector of mitochondrial F1F0 ATP synthase | |
| 748 | +3.95 | Atp1p | YBL099W | Alpha-subunit of the F1 sector of mitochondrial F1F0 ATP synthase |
| 789 | +3.25 | |||
| 499 | +5.15 | Pdb1p | YBR221C | E1 beta-subunit of the pyruvate dehydrogenase (PDH) complex |
| 667 | +3.50 | Cor1p | YBL045C | Core subunit of the ubiquinol-cytochrome c reductase complex (bc1 complex) |
| 30 | −13.9 | Por1p | YNL055C | Mitochondrial porin maintains mitochondrial osmotic stability and mitochondrial membrane permeability |
| Spot ID | Ratio* | Protein | Gene | function |
| 329 | +5.40 | Ilv5p | YLR355C | Branched-chain amino acid biosynthesis and maintenance of mitochondrial DNA |
| 600† | ||||
| 602† | ||||
| 720† | Atp2p | YJR121W | Beta-subunit of the F1 sector of mitochondrial F1F0 ATP synthase | |
| 748 | +3.95 | Atp1p | YBL099W | Alpha-subunit of the F1 sector of mitochondrial F1F0 ATP synthase |
| 789 | +3.25 | |||
| 499 | +5.15 | Pdb1p | YBR221C | E1 beta-subunit of the pyruvate dehydrogenase (PDH) complex |
| 667 | +3.50 | Cor1p | YBL045C | Core subunit of the ubiquinol-cytochrome c reductase complex (bc1 complex) |
| 30 | −13.9 | Por1p | YNL055C | Mitochondrial porin maintains mitochondrial osmotic stability and mitochondrial membrane permeability |
Average fold change after cross-gel analysis of three independent 2D analyses.
Spots were only found on the gel from the bdf1Δ [MEF1 H] strain.
Mitochondrial proteins with fold change > 3, with MEF1 overexpression (bdf1Δ[MEF1 H]) vs. in the bdf1Δ strain, under 0.5 M NaCl for 45 min
| Spot ID | Ratio* | Protein | Gene | function |
| 329 | +5.40 | Ilv5p | YLR355C | Branched-chain amino acid biosynthesis and maintenance of mitochondrial DNA |
| 600† | ||||
| 602† | ||||
| 720† | Atp2p | YJR121W | Beta-subunit of the F1 sector of mitochondrial F1F0 ATP synthase | |
| 748 | +3.95 | Atp1p | YBL099W | Alpha-subunit of the F1 sector of mitochondrial F1F0 ATP synthase |
| 789 | +3.25 | |||
| 499 | +5.15 | Pdb1p | YBR221C | E1 beta-subunit of the pyruvate dehydrogenase (PDH) complex |
| 667 | +3.50 | Cor1p | YBL045C | Core subunit of the ubiquinol-cytochrome c reductase complex (bc1 complex) |
| 30 | −13.9 | Por1p | YNL055C | Mitochondrial porin maintains mitochondrial osmotic stability and mitochondrial membrane permeability |
| Spot ID | Ratio* | Protein | Gene | function |
| 329 | +5.40 | Ilv5p | YLR355C | Branched-chain amino acid biosynthesis and maintenance of mitochondrial DNA |
| 600† | ||||
| 602† | ||||
| 720† | Atp2p | YJR121W | Beta-subunit of the F1 sector of mitochondrial F1F0 ATP synthase | |
| 748 | +3.95 | Atp1p | YBL099W | Alpha-subunit of the F1 sector of mitochondrial F1F0 ATP synthase |
| 789 | +3.25 | |||
| 499 | +5.15 | Pdb1p | YBR221C | E1 beta-subunit of the pyruvate dehydrogenase (PDH) complex |
| 667 | +3.50 | Cor1p | YBL045C | Core subunit of the ubiquinol-cytochrome c reductase complex (bc1 complex) |
| 30 | −13.9 | Por1p | YNL055C | Mitochondrial porin maintains mitochondrial osmotic stability and mitochondrial membrane permeability |
Average fold change after cross-gel analysis of three independent 2D analyses.
Spots were only found on the gel from the bdf1Δ [MEF1 H] strain.
Cytoplastic proteins with fold change > 3, with MEF1 overexpression (bdf1Δ[MEF1 H]) vs. in the bdf1Δ strain, under 0.5 M NaCl for 45 min
| Spot ID | Ratio* | Protein | Gene | function |
| 8 | −6.15 | Ctk2p | YJL006C | Beta-subunit of C-terminal domain kinase I (CTDK-I) affect transcription, pre-mRNA 3′ end processing and translational fidelity |
| 210 | +3.10 | Dst1p | YGL043W | General transcription elongation factor TFIIS enables RNA polymerase II to read through blocks to elongation |
| 486 | −5.26 | Bmh2p | YDR099W | 14-3-3 protein controls proteome at post-transcriptional level |
| 567 | −3.20 | Erg26p | YGL001C | C-3 sterol dehydrogenase involved in ergosterol biosynthesis |
| 599 | −4.80 | Adh1p | YOL086C | Alcohol dehydrogenase required for the reduction of acetaldehyde to ethanol |
| 731 | −3.95 | Cys4p | YGR155W | Cystathionine beta-synthase, synthesis of cystathionine from serine and homocysteine and hydrogen sulfide generation |
| 765 | −4.83 | Yll058wp | Yll058wp | Putative protein important in sulfur metabolism |
| Spot ID | Ratio* | Protein | Gene | function |
| 8 | −6.15 | Ctk2p | YJL006C | Beta-subunit of C-terminal domain kinase I (CTDK-I) affect transcription, pre-mRNA 3′ end processing and translational fidelity |
| 210 | +3.10 | Dst1p | YGL043W | General transcription elongation factor TFIIS enables RNA polymerase II to read through blocks to elongation |
| 486 | −5.26 | Bmh2p | YDR099W | 14-3-3 protein controls proteome at post-transcriptional level |
| 567 | −3.20 | Erg26p | YGL001C | C-3 sterol dehydrogenase involved in ergosterol biosynthesis |
| 599 | −4.80 | Adh1p | YOL086C | Alcohol dehydrogenase required for the reduction of acetaldehyde to ethanol |
| 731 | −3.95 | Cys4p | YGR155W | Cystathionine beta-synthase, synthesis of cystathionine from serine and homocysteine and hydrogen sulfide generation |
| 765 | −4.83 | Yll058wp | Yll058wp | Putative protein important in sulfur metabolism |
Average fold change after cross-gel analysis of three independent 2D analyses.
Cytoplastic proteins with fold change > 3, with MEF1 overexpression (bdf1Δ[MEF1 H]) vs. in the bdf1Δ strain, under 0.5 M NaCl for 45 min
| Spot ID | Ratio* | Protein | Gene | function |
| 8 | −6.15 | Ctk2p | YJL006C | Beta-subunit of C-terminal domain kinase I (CTDK-I) affect transcription, pre-mRNA 3′ end processing and translational fidelity |
| 210 | +3.10 | Dst1p | YGL043W | General transcription elongation factor TFIIS enables RNA polymerase II to read through blocks to elongation |
| 486 | −5.26 | Bmh2p | YDR099W | 14-3-3 protein controls proteome at post-transcriptional level |
| 567 | −3.20 | Erg26p | YGL001C | C-3 sterol dehydrogenase involved in ergosterol biosynthesis |
| 599 | −4.80 | Adh1p | YOL086C | Alcohol dehydrogenase required for the reduction of acetaldehyde to ethanol |
| 731 | −3.95 | Cys4p | YGR155W | Cystathionine beta-synthase, synthesis of cystathionine from serine and homocysteine and hydrogen sulfide generation |
| 765 | −4.83 | Yll058wp | Yll058wp | Putative protein important in sulfur metabolism |
| Spot ID | Ratio* | Protein | Gene | function |
| 8 | −6.15 | Ctk2p | YJL006C | Beta-subunit of C-terminal domain kinase I (CTDK-I) affect transcription, pre-mRNA 3′ end processing and translational fidelity |
| 210 | +3.10 | Dst1p | YGL043W | General transcription elongation factor TFIIS enables RNA polymerase II to read through blocks to elongation |
| 486 | −5.26 | Bmh2p | YDR099W | 14-3-3 protein controls proteome at post-transcriptional level |
| 567 | −3.20 | Erg26p | YGL001C | C-3 sterol dehydrogenase involved in ergosterol biosynthesis |
| 599 | −4.80 | Adh1p | YOL086C | Alcohol dehydrogenase required for the reduction of acetaldehyde to ethanol |
| 731 | −3.95 | Cys4p | YGR155W | Cystathionine beta-synthase, synthesis of cystathionine from serine and homocysteine and hydrogen sulfide generation |
| 765 | −4.83 | Yll058wp | Yll058wp | Putative protein important in sulfur metabolism |
Average fold change after cross-gel analysis of three independent 2D analyses.
Two representative 2D gels are shown, with the mitochondrial proteomes after adaptation to 0.5 M NaCl. (a) Virtual 2D gel image of bdf1Δ for analysis. Black solid line circles: The protein spots identified in both two gels. White dotted line circles: The protein spots were not identified in bdf1Δ strain; and (b) virtual 2D gel image of bdf1Δ [MEF1 H] for analysis. The protein spots that were identified by MALDI-TOF/MS-MS are indicated (Tables 2 and 3). Red circles: The proteins with an increasing expression in bdf1Δ [MEF1 H] vs. bdf1Δ. Blue circles: The proteins with a decreasing expression in bdf1Δ [MEF1 H] vs. bdf1Δ. Molecular mass (on the right) and pI (on the bottom) were presented.
The genetic confirmation of the mitochondrial proteomics results. Yeast cells were grown to an exponential phase. An aliquot of 4 μL from a 10-fold serial dilution was spotted onto YPD and YPD plates supplemented with the indicated concentrations of NaCl or onto a YPG plate. Cells were incubated at 30 °C for 3 days. WT, wild type.
We then analyzed the effects of overexpression of these six genes in bdf1Δ. Overexpression of ATP1, ATP2, or COR1 did not cause any obvious change to salt sensitivity of bdf1Δ strain. However, overexpression of PDB1or ILV5 enhanced the salt resistance of bdf1Δ to some degree (Fig. 6, Lines 2, 8, and 9), and none of these modified strains could grow on yeast peptone glycerol (YPG) plates (Fig. 6, Lines 8–12). Double deletion of POR1 and BDF1 is lethal, likely because POR1 is important for cell survival, oxidative stress resistance, and respiratory growth (Martinez-Campa et al., 2003; Outten et al., 2005; Tahara et al., 2007).
Discussion
The relationship among Bdf1p, Hda1p, and Mef1p affected the salt resistance of bdf1Δ
As a transcription factor, Bdf1p preferentially binds to the acetylated histone H4 tail to initiate gene expression via the formation of basal TFIID (Matangkasombut & Buratowski, 2003; Huisinga & Pugh, 2004; Martinez-Campa et al., 2003; Zhang et al.,2005). Hda1p, on the other hand, is a deacetylase that removes acetylated histones and represses genes involved in carbon utilization and stress response (Robyr et al., 2007). Deletion of HDA1 increases histone acetylation (Wu et al., 2001a). We believe that deletion of HDA1 in bdf1Δ may activate the expression of certain salt-resistant genes that were repressed by bdf1Δ mutant.
There are three possible scenarios for Bdf1p and Hda1p to insert their opposite regulatory effects. (1) One protein is dominant and it affects more of the phenotypes; the dominant protein may even regulate the second protein directly. (2) Neither is dominant and these two proteins compete for their shared common target genes. (3) These two proteins have different target genes that lead to opposite physiological responses.
Our results showed that Bdf1p regulated HDA1 expression directly and positively (Fig. 1) both under normal and salt stress conditions. Hda1p regulated MEF1 expression directly and negatively (Fig. 3). Moreover, Mef1p, a mitochondrial translation elongation factor, related to the salt resistance capacity of bdf1Δ by regulating mitochondrial proteins. This indicated that Bdf1p might regulate MEF1 via Hda1p activity. These results suggest the contribution of Bdf1p and Hda1p to salt stress response follows the first possibility mentioned above.
When HDA1 was deleted in bdf1Δ, MEF1 expression was higher than in bdf1Δ (Fig. 3a), and the salt sensitivity was reduced (Fig. 1a). MEF1 transcriptional levels increased in response to a decline in HDA1 expression in bdf1Δ. We supposed that BDF1 deletion induced the transcription of MEF1 at a basal level. But the increase was not sufficiently high, especially under NaCl stress (Figs 3a and 1b). Meanwhile, the deletion of HDA1 and BDF1 both increased the expression of MEF1, however, their deletion showed different phenotypes in response to salt stress. Our previous transcriptome analysis of bdf1 mutant under salt stress showed that among the mitochondrial genes, the expression of mitochondrial translation elongation factor MEF1 decreased obviously (Liu et al., 2007). This indicated that the expression change of MEF1 was not the sole reason but higher transcription of MEF1 was required to compensate the salt stress sensitivity and mitochondrial dysfunction in bdf1Δ (Figs 1 and 4).
The inhibition by high copies of MEF1 to salt sensitivity, high level of ROS, nuclear instability, or abnormal respiration of bdf1Δ was more effective than by low copies of MEF1, suggesting that this compensation was dose dependent.
Intact mitochondrial function is a key element in salt resistance of bdf1Δ
Mitochondrial function is related to nuclear genome instability (Towpik, 2005), and the presence of mitochondrial DNA can protect yeast against salt stress-induced apoptosis (Allen & Balin, 1989; Eisenberg et al., 2007; Gao et al., 2011). The BDF1 gene deletion strain showed significant mitochondrial dysfunction under salt stress conditions (Fig. 4). Mef1p regulates protein translation and synthesis in the mitochondria (Vambutas et al., 1991) and is integral to protein distribution, respiration, mitochondrial genome maintenance, and chronological life span (Vambutas et al., 1991; Proszynski et al., 2005; Burtner et al., 2011). In this study, we found that MEF1 overexpression in bdf1Δ could suppress the mitochondrial dysfunction of bdf1Δ, for instance, the growth returning to normal on a nonfermentation carbon source, decreased levels of ROS or improved stability of nucleus, and finally the phenotype of bdf1Δ was completely restored. Therefore, intact mitochondrial functions maintained by MEF1 played a key role in enhancing salt resistance of bdf1Δ (Fig. 4). Further introduction of HDA1 resulted in poorer growth on salt stress, more accumulation of ROS, and more scattered nuclear fragments (Fig. 4a–c, Line 5; Fig. 2, Line 6) than bdf1Δ [MEF1L]. At the meanwhile, in the view of transcriptional level control among MEF1, HDA1 and BDF1, HDA1 might repress mitochondria function by the transcriptional regulation of MEF1. The relation between MEF1 and HDA1 played important roles in these progresses, and they were likely the important targets of the BDF1-specific salt stress response.
Mitochondrial proteins affected by Mef1p played a role in the compensation of salt sensitivity
The mitochondrial proteomics analysis revealed that expression of six mitochondrial proteins was significantly changed when MEF1 was overexpressed. These proteins were mainly related to energy metabolism and mitochondrial stability: ATP synthase subunits (Atp1p, Atp2p), respiration (Cor1p), isoleucine branched-chain biosynthesis (Ilv5p), pyruvate conversion into acetyl-CoA (Pdb1p), and mitochondrial porin (Por1p, voltage-dependent anion channel). Atp1p, Ilv5p, and Pdb1p were also reported to participate in mitochondrial nucleoid composition and mtDNA integrity maintenance (Chen et al., 2005). Our analysis showed that only PDB1, ILV5, or ATP2 overexpression in bdf1Δ enhanced the salt resistance of strains to a certain degree. However, among MEF1-affected proteins, no one could replace the role of MEF1 in the complete recovery of salt sensitivity mitochondrial respiration and nuclei stability.
The present study provides for the first time a possible mechanism for the inhibition of the salt stress sensitivity of bdf1Δ, which involves a recovery of MEF1 overexpression regulated by the relationship among BDF1, HDA1, and MEF1. The mitochondrial proteins, Atp2, Pdb1p, and Ilv5p, played a part in the salt stress response, which was affected by MEF1 overexpression, indicating that energy metabolism and nucleoid maintenance are involved in the salt resistance recovery.
Authors' contributions
L.C. and M.W. contributed equally to this work.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30170021, 30671143 and 30570031).
References
Supporting Information
Table S1. Gene-specific primers used in this study.
Author notes
Editor: Ian Dawes



![MEF1 overexpression recovered the respiration deficiency and nuclear stability of bdf1Δ. (a) MEF1 overexpression recovered the growth of bdf1Δ on a nonfermentable carbon source, glycerol. Yeast cells were grown to an exponential phase. An aliquot of 4 μL from a 10-fold serial dilution was spotted onto a YPG plate. Cells were incubated at 30 °C for 3 days. (b) MEF1 overexpression in bdf1Δ significantly decreased ROS levels. Yeast cells were treated with and without 0.5 M NaCl for 45 min after being grown to an exponential phase. DHR was used to monitor the production of ROS (top lane). (c) MEF1 overexpression improved nucleic genomic stability. Yeast cells were treated with and without 0.5 M NaCl for 45 min after growth to an exponential phase. DAPI staining was used to visualize the position of the chromatin within the nuclei. The numbers representing the strains are as follows: 1, WT; 2, bdf1Δ; 3, bdf1Δ [MEF1 L]; 4, bdf1Δ [MEF1 H]; 5, bdf1Δ [MEF1 L] [HDA1 H]; 6, bdf1Δ [MEF1 H] [HDA1 H] (Table 1). WT, wild type.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsyr/14/4/10.1111_1567-1364.12144/2/m_fyr12144-fig-0004.jpeg?Expires=1716424491&Signature=hnboRmrm7sDl9vtHoSVkI1laR-ARuHEccEgelfHHS-w1SmoxbtWfh6RR8PHumunhmQ7AJNfYRy2u3I-ssT7BX~gavPuaQiG4HP2Pxd06ywu7Ce3Be-0QgarZ62W1bqT0vyvnVvb9n-DkYHysEI1yR0fC9u7KP7vPw1~1nTNB7Qdl35q52OheAO7Xo6XMuV~aDJk2tRx3Z5pDmtWRDbjPDLFnJR9fzGR31NEmg2lYwNjLC~dxh9KtAyAeWEQolydwWv~ODOuv3YyowG5yur0SYeoFuPTh6ntahIeQbnkwEF4abn4jvUT9CcmBC58gr828WFspdv8FIRZkTftM0xCang__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Two representative 2D gels are shown, with the mitochondrial proteomes after adaptation to 0.5 M NaCl. (a) Virtual 2D gel image of bdf1Δ for analysis. Black solid line circles: The protein spots identified in both two gels. White dotted line circles: The protein spots were not identified in bdf1Δ strain; and (b) virtual 2D gel image of bdf1Δ [MEF1 H] for analysis. The protein spots that were identified by MALDI-TOF/MS-MS are indicated (Tables 2 and 3). Red circles: The proteins with an increasing expression in bdf1Δ [MEF1 H] vs. bdf1Δ. Blue circles: The proteins with a decreasing expression in bdf1Δ [MEF1 H] vs. bdf1Δ. Molecular mass (on the right) and pI (on the bottom) were presented.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsyr/14/4/10.1111_1567-1364.12144/2/m_fyr12144-fig-0005.jpeg?Expires=1716424491&Signature=qnoQ5MP1j9tvuCeX~osnRHzP-gO-2dojVWUr1dyiXOYEWqWwxlO1sUZirA-lj7ljGFsf93hTrGgC-l83956jmkSTWBH8kc8vANKlOSR2Ejbh57XQel1HuGFgXUZ8-Zm~ngNd~UvtXZl~Jj-KMhmG-d5ulOK3paKGXrqfFcOT8Be0umBqu7O-6VIGGUttHIYlBoWdsKkMqQA04ySQpJOpfN6YWDVXTSgRazLIw~W2tlTTFbhHVzcqgb65FvialRluwsiFIXa~Y0KWd~iLHLtDBfMV06lig7P~aUjar2MJ9pW5A4SIlfXo-MhBaf7mW0p5kd04-zPC~ctMfRTbqA-BbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)