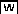-
PDF
- Split View
-
Views
-
Cite
Cite
Huachun Wang, Njabulo Ngwenyama, Yidong Liu, John C. Walker, Shuqun Zhang, Stomatal Development and Patterning Are Regulated by Environmentally Responsive Mitogen-Activated Protein Kinases in Arabidopsis, The Plant Cell, Volume 19, Issue 1, January 2007, Pages 63–73, https://doi.org/10.1105/tpc.106.048298
Close - Share Icon Share
Abstract
Stomata are specialized epidermal structures that regulate gas (CO2 and O2) and water vapor exchange between plants and their environment. In Arabidopsis thaliana, stomatal development is preceded by asymmetric cell divisions, and stomatal distribution follows the one-cell spacing rule, reflecting the coordination of cell fate specification. Stomatal development and patterning are regulated by both genetic and environmental signals. Here, we report that Arabidopsis MITOGEN-ACTIVATED PROTEIN KINASE3 (MPK3) and MPK6, two environmentally responsive mitogen-activated protein kinases (MAPKs), and their upstream MAPK kinases, MKK4 and MKK5, are key regulators of stomatal development and patterning. Loss of function of MKK4/MKK5 or MPK3/MPK6 disrupts the coordinated cell fate specification of stomata versus pavement cells, resulting in the formation of clustered stomata. Conversely, activation of MKK4/MKK5-MPK3/MPK6 causes the suppression of asymmetric cell divisions and stomatal cell fate specification, resulting in a lack of stomatal differentiation. We further establish that the MKK4/MKK5-MPK3/MPK6 module is downstream of YODA, a MAPKKK. The establishment of a complete MAPK signaling cascade as a key regulator of stomatal development and patterning advances our understanding of the regulatory mechanisms of intercellular signaling events that coordinate cell fate specification during stomatal development.
This study identifies MPK3/MPK6 and MKK4/MKK5 as key regulators of stomatal development and patterning that function downstream of MAPKKK YODA to coordinate cell fate specification.
INTRODUCTION
Understanding the mechanisms of the coordination of cell fate differentiation is a long-sought goal of developmental biologists. The generation of diverse cell types in multicellular organisms is often associated with asymmetric cell division (Jan and Jan, 1998; Scheres and Benfey, 1999). Asymmetric cell divisions refer to any cell divisions that give rise to two daughter cells with different developmental potentials (Horvitz and Herskowitz, 1992; Jan and Jan, 1998; Scheres and Benfey, 1999). Many examples of asymmetric cell divisions have been described that are associated with plant development, including the first division of the zygote, pollen mitosis I (male microspore division), asymmetric cell divisions of the cortex/endodermis initial during the radial patterning of the root meristem, and asymmetric cell divisions during stomatal formation (Di Laurenzio et al., 1996; Scheres and Benfey, 1999; Nadeau and Sack, 2002b; Bergmann et al., 2004; Lukowitz et al., 2004). However, the regulatory mechanism of how cell fate specification is coordinated through asymmetric cell division in plants remains to be determined.
Stomata are specialized epidermal structures formed by two guard cells surrounding a pore, through which plants absorb CO2 from and release O2 to their environment. The rest of the epidermal surface of land plants is covered with an impermeable layer of wax that prevents water loss. Thus, the development of stomata is critical for plant survival and productivity and is one of the crucial events in land plant evolution and acclimatization (Nadeau and Sack, 2002b; Woodward et al., 2002; Hetherington and Woodward, 2003).
The differentiation of stomata is preceded by an asymmetric cell division of meristemoid mother cells (MMCs; a subset of protodermal cells), which give rise to two morphologically distinct daughter cells: a small triangular meristemoid and a larger neighboring cell (Nadeau and Sack, 2002b). In Arabidopsis thaliana, the meristemoid maintains stem cell–like activity and can undergo additional rounds of asymmetric cell division (Nadeau and Sack, 2002b). After each round of asymmetric cell division, a smaller meristemoid and a larger neighbor cell are generated. The meristemoid eventually differentiates into a small rounded guard mother cell (GMC). The GMC undergoes a single symmetrical cell division to generate a pair of guard cells. Some of the larger neighbor cells generated through asymmetric cell division of MMCs or meristemoids can adopt a MMC cell fate. These neighbor cell–derived MMCs in turn undergo asymmetric cell divisions, giving rise to satellite meristemoids that eventually differentiate into satellite stomata (Nadeau and Sack, 2002b).
In Arabidopsis, stomatal distribution follows a consistent pattern known as the one-cell spacing rule: there is at least one pavement cell between two adjacent stomata (Geisler et al., 2000; Nadeau and Sack, 2002b). The observed frequency of stomata adjoining each other is much lower than predicted by a random distribution (Geisler et al., 2000). This one-cell spacing patterning ensures the optimal balance between photosynthetic capacity (CO2 uptake) and water loss. The presence of epidermal pavement cells surrounding the stomata is also important for ion exchange between guard cells and pavement cells, which regulates stomata opening and closing. It has been suggested that the patterning of stomatal distribution is regulated by position-dependent local intercellular signaling and long-distance systemic cues (Nadeau and Sack, 2002b; Hetherington and Woodward, 2003; Bergmann, 2004). Mutations in genes encoding TOO MANY MOUTHS (TMM; a Leu-rich repeat receptor–like protein), ERECTA (ER), ERECTA LIKE1 (ERL1), and ERL2 (Leu-rich repeat receptor–like protein kinases), STOMATA DENSITY AND DISTRIBUTION1 (SDD1; a subtilisin-like Ser protease), and YODA (YDA; a mitogen-activated protein kinase kinase kinase [MAPKKK]) disrupt stomatal patterning and result in clustered stomata (Berger and Altmann, 2000; Nadeau and Sack, 2002a; Von Groll et al., 2002; Bergmann et al., 2004; Shpak et al., 2005).
Mitogen-activated protein kinase (MAPK) cascades are three-kinase signaling modules that are evolutionarily conserved across eukaryotes (Ichimura et al., 2002). They play important functions in regulating both stress responses and plant growth and development (Tena et al., 2001; Zhang and Klessig, 2001; Nakagami et al., 2005; Pedley and Martin, 2005). MKK4 and MKK5 together with MPK3 and MPK6 were previously shown to be downstream of several MAPKKKs and to function in plant responses to environmental stimuli (Tena et al., 2001; Zhang and Klessig, 2001; Nakagami et al., 2005; Pedley and Martin, 2005). Here, we establish that the MKK4/MKK5-MPK3/MPK6 signaling module functions downstream of YDA as a key regulator of stomatal development and patterning. In loss-of-function mutants of MKK4/MKK5 or MPK3/MPK6, the one-cell spacing rule is disrupted, resulting in stomata being clustered together. In a gain-of-function mutant of MKK4/MKK5, stomatal development is suppressed and the epidermis is composed solely of jigsaw puzzle–like pavement cells. These findings suggest that MAPK cascade activity determines the coordination of stomata versus pavement cell specification on the epidermis.
RESULTS
MPK3 and MPK6 Are Functionally Overlapping Key Regulators of Stomatal Development and Patterning
Stomatal Development in Wild-Type, mpk6−/− MPK3RNAi, and Rescued mpk3−/− mpk6−/− Seedlings.
(A), (C), and (E) Seedlings at 7 d after germination.
(B), (D), and (F) Confocal images of the abaxial epidermis of developing cotyledons.
Cell outlines were visualized with propidium iodide staining. The distribution of stomata on the cotyledons of wild-type seedlings follows the one-cell spacing rule. By contrast, cotyledons of mpk6−/− MPK3RNAi and rescued mpk3−/− mpk6−/− seedlings are covered with excessively clustered stomata. Bars = 1 mm in (A), (C), and (E) and 10 μm in (B), (D), and (F).
To conditionally rescue the embryo lethality of the mpk3−/− mpk6−/− double mutants, MPK6 was put under the regulation of a steroid-inducible system (GVG -MPK6) (Aoyama and Chua, 1997) and stably transformed into mpk3−/− mpk6+/− plants. Homozygous GVG-MPK6 transgenic plants in the mpk3−/− mpk6+/− background were identified in the T3 generation. To induce GVG-MPK6 expression during embryogenesis, dexamethasone (Dex; a steroid) was applied by spraying once every 3 d after bolting until all seeds had matured. In the T4 generation, one-quarter of the seedlings segregated as mpk3−/− mpk6−/−, suggesting that steroid-inducible GVG-MPK6 expression successfully rescued the embryo lethality of mpk3−/− mpk6−/−. However, after germination, all of the rescued mpk3−/− mpk6−/− seedlings were arrested at the cotyledon stage and were unable to initiate true leaves (Figure 1E). Dex treatment was not able to rescue the mpk3−/− mpk6−/− seedlings further. The cotyledon epidermis of these rescued mpk3−/− mpk6−/− seedlings was composed of clustered stomata, and the one-cell spacing rule was disrupted (Figure 1F). Both mpk6−/− MPK3RNAi and the rescued mpk3−/− mpk6−/− seedlings showed the same stomatal development and patterning defects, which convincingly demonstrates that MPK3 and MPK6 are key regulators of stomatal development and patterning.
MKK4 and MKK5 Are Functionally Redundant Regulators of Stomatal Development and Patterning
In the Arabidopsis genome, there are 10 predicted MAPKKs (Ichimura et al., 2002). Previous studies demonstrated that MKK4 and MKK5 are upstream kinases for MPK3 and MPK6 in the stress-responsive signaling cascade (Yang et al., 2001; Asai et al., 2002; Ren et al., 2002). To determine whether MKK4 and MKK5 also function as upstream kinases of MPK3 and MPK6 in the stomatal development and patterning signal pathway, we generated RNAi gene-silencing plants of MKK4 and MKK5.
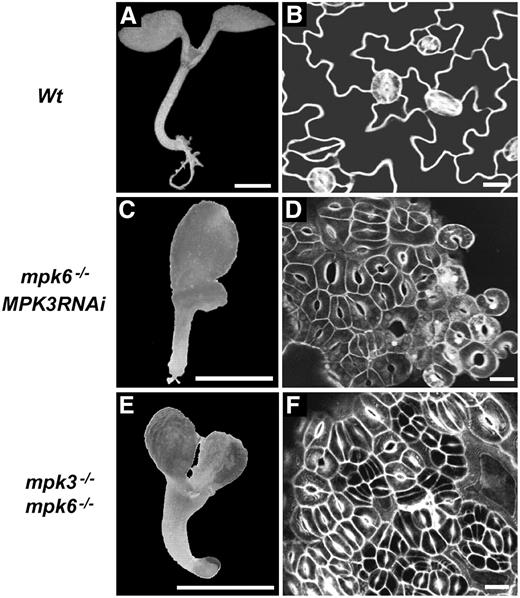
Stomatal Development and Patterning Defects in MKK4 and MKK5 Loss-of-Function Seedlings.
Confocal images of the abaxial epidermis of developing cotyledons at 7 d after germination.
(A) Stomatal development follows the one-cell spacing rule in vector control transgenic plants.
(B) and (C) MKK4RNAi (B) and MKK5RNAi (C) transgenic seedlings have stomatal clusters with two to three stomata.
(D) Cotyledons of tandem MKK4-MKK5RNAi transgenic seedlings are covered by stomata; no jigsaw puzzle–like pavement cells are observed. The inset shows a representative MKK4-MKK5RNAi transgenic seedling.
Bars = 10 μm except for the inset in (D), where the bar = 1 mm.
To test whether MKK4 and MKK5 are overlapping negative regulators of stomatal development and patterning, we generated tandem MKK4 and MKK5 RNAi transgenic plants (MKK4-MKK5RNAi). More than 85% of the MKK4-MKK5RNAi transgenic plants were arrested at the cotyledon stage and showed dramatic stomatal development and patterning defects (Figure 2D). In some cases, the entire epidermal layer of the cotyledon was composed exclusively of stomata; no pavement cells were observed, and the one-cell spacing pattern was completely disrupted (Figure 2; see Supplemental Figure 2 online). Silencing of MKK4 and MKK5 generated the same stomatal development and patterning phenotype as that of the rescued mpk3−/− mpk6−/− and the mpk6−/− MPK3RNAi transgenic plants, suggesting that they may function in the same stomatal development and patterning pathway.
Constitutively Active MKK4/MKK5 Can Switch off Stomatal Cell Fate Initiation
The activation loop of plant MAPKKs has a consensus sequence S/TXXXXXS/T that is different from the S/TXXXS/T motif in mammalian MAPKKs (Ichimura et al., 2002). Constitutively active mutants of plant MAPKKs can be generated by mutating the conserved Ser/Thr in the activation loop to Asp (D) (Yang et al., 2001; Ren et al., 2002).
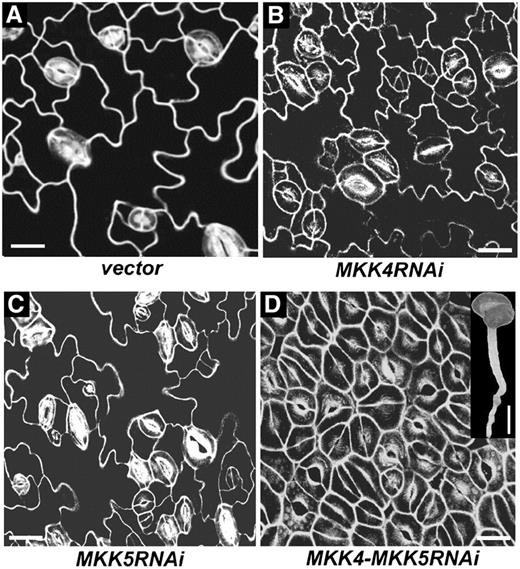
Gain-of-Function MAPKK Suppresses Stomatal Development through Endogenous MPK3 and MPK6.
(A) Without Dex induction, stomatal development in Dex-inducible GVG-Nt-MEK2DD transgenic seedlings is the same as that in the wild type (cf. Figure 2A).
(B) With Dex (0.02 μM) induction, epidermal cells of GVG-Nt-MEK2DD transgenic seedlings rarely undergo asymmetric cell divisions, and no stomatal differentiation is observed.
(C) and (D) Loss of function of either MPK3 (C) or MPK6 (D) reverses the no-stomate phenotype of GVG-Nt-MEK2DD transgenic seedlings.
Bars = 10 μm.
It has been shown that the induction of MKK4DD, MKK5DD, and Nt-MEK2DD transgene expression can activate endogenous MPK3 and MPK6 (Yang et al., 2001; Ren et al., 2002; Liu and Zhang, 2004). To further demonstrate that MKK4, MKK5, and Nt-MEK2 are genetically upstream to MPK3 and MPK6 in the stomatal development pathway, we crossed GVG-Nt-MEK2DD into the mpk3−/− or mpk6−/− mutant background. In contrast with GVG-Nt-MEK2DD plants, GVG-Nt-MEK2DD mpk3−/− and GVG-Nt-MEK2DD mpk6−/− plants have normal stomatal development: the stomatal patterning follows the one-cell spacing rule (Figure 3). Both mpk3−/− and mpk6−/− can suppress the Nt-MEK2DD gain-of-function phenotype, suggesting that this is a dosage-dependent phenotype. When either downstream MAPK is missing, the strength of signaling output will be reduced, which will lead to a reversal of the no-stomate phenotype. This result indicates that Nt-MEK2DD functions through both MPK3 and MPK6, supporting the hypothesis that MKK4 and MKK5 are upstream of MPK3 and MPK6 in regulating stomatal development and patterning. To better understand the role of the MKK4/MKK5-MPK3/MPK6 module in stomatal development and patterning, we set out to identify the upstream MAPKK kinase (MAPKKK).
YDA Is the Potential Upstream MAPKKK of MKK4/ MKK5-MPK3/MPK6 in Regulating Stomatal Development and Patterning
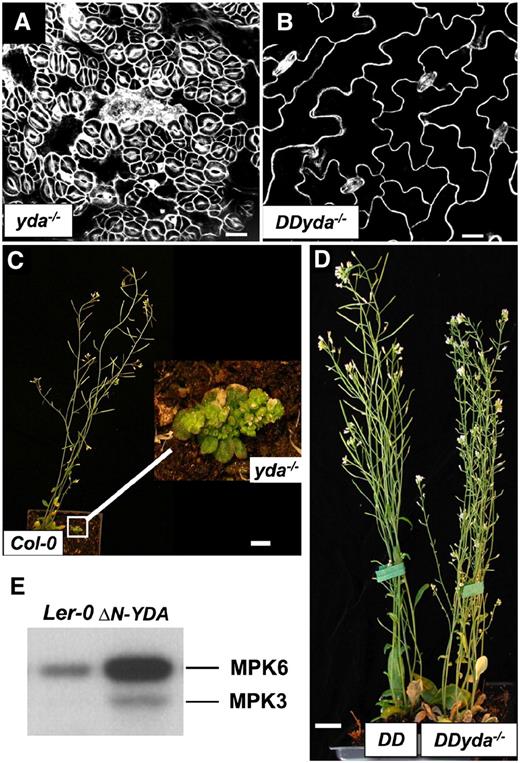
Loss-of-function mutants of YDA have clustered stomata, whereas gain-of-function mutants of YDA result in no stomatal differentiation (Bergmann et al., 2004). Gain and loss of function of MKK4/MKK5-MPK3/MPK6 show similar phenotypes as the corresponding mutants of YDA, indicating that YDA could be the upstream protein kinase of MKK4/MKK5-MPK3/MPK6 in regulating stomatal development and patterning.
Epistatic Interaction of YDA, Nt-MEK2DD, MPK3, and MPK6.
(A) Loss of function of YDA results in a clustered-stomata phenotype.
(B) Gain-of-function GVG-Nt-MEK2DD suppresses the clustered stomata phenotype in yda−/−. The GVG-Nt-MEK2DD yda−/− double mutant is labeled as DDyda−/− for simplicity.
(C) yda−/− plants have an extremely dwarfed stature, compact rosette leaves, and an abnormal clustered inflorescence.
(D) DDyda−/− plants are approximately the same size as the control DD (GVG-Nt-MEK2DD) plant and have normal expanded rosette and extended inflorescence development.
(E) MPK3 and MPK6 are activated in the ΔN-YDA mutant, as shown by in-gel kinase assay of MAPK activity. Ler-0, Landsberg erecta.
Bars = 10 μm in (A) and (B) and 1 cm in (C) and (D).
GVG-Nt-MEK2DD also rescued the pleiotropic growth and developmental defects in yda−/− (Figures 4C and 4D). Only a very small percentage of yda−/− mutants can survive in the soil. They have pleiotropic growth and developmental defects, including extremely dwarfed stature, compact rosette leaves, and abnormal flowers (with petal, carpel, ovule, and anther developmental defects) (Figure 4C) (Lukowitz et al., 2004). However, GVG-Nt-MEK2DD yda−/− plants were comparable in size to wild-type plants and have normal-looking expanded rosette leaves and long inflorescences (Figure 4D). These results indicate that Nt-MEK2 or MKK4/MKK5 could be a downstream kinase of YDA in multiple growth and developmental pathways.
MPK3 and MPK6 Are Activated in Constitutively Active ΔN-YDA Plants
YDA belongs to the MEKK1/Ste11/Bck1 class of MAPKKKs. Removing the N-terminal negative regulatory domain of YDA (ΔN-YDA) was proposed to allow YDA to become constitutively active (Lukowitz et al., 2004). To determine whether YDA is an upstream MAPKKK of MPK3 and MPK6, we tested the kinase activity of MPK3 and MPK6 in ΔN-YDA seedlings. As shown in Figure 4E, both MPK3 and MPK6 were activated in ΔN-YDA plants. This result provides biochemical evidence that MPK3 and MPK6 are downstream MAPKs of YDA.
A truncated gain-of-function MAPKKK may create nonspecific activation of MKKs and MPKs that are not normally activated by that MAPKKK. As a control experiment, we generated transgenic plants overexpressing ΔANP1 and ΔMEKK1 that are known to activate MPK3 and MPK6 in stress response pathways (Kovtun et al., 2000; Asai et al., 2002). We did not observe any effects on stomatal development in these gain-of-function transgenic plants (data not shown). Loss-of-function mutants of ANP1 and MEKK1 also did not show any stomatal development defects (H. Jin, personal communication; data not shown). These results suggest that YDA functions specifically upstream of MPK3 and MPK6 in regulating stomatal development.
MKK4/MKK5 Regulate the Frequency of Asymmetric Cell Divisions and Stomatal Cell Fate Differentiation
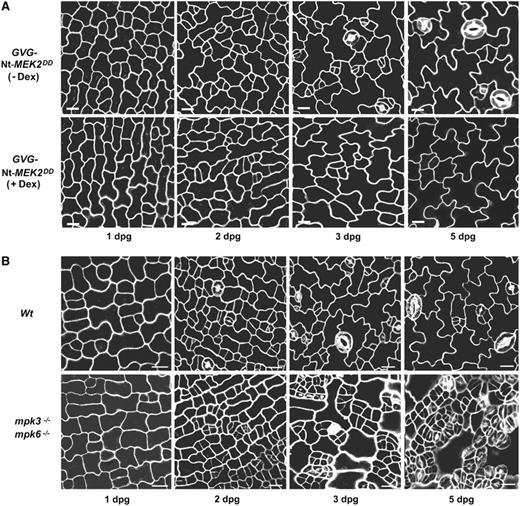
Time-Course Analysis of Epidermal Development in GVG-Nt-MEK2DD Seedlings and the Rescued mpk3−/− mpk6−/− Seedlings.
(A) Without Dex induction, stomatal development in GVG-Nt-MEK2DD seedlings is the same as in the wild type. With Dex (0.02 μM) induction, epidermal cells rarely undergo asymmetric cell divisions, and no stomatal differentiation is observed.
(B) In wild-type seedlings, stomatal development follows the one-cell spacing rule. However, in the rescued mpk3−/− mpk6−/− seedlings, meristematic precursor cells undergo ectopic cell divisions that produce near-isodiametric progeny, which eventually all differentiate into stomata.
The abaxial epidermis of developing cotyledons was imaged by confocal microscopy after staining with propidium iodide. dpg, days after germination. Bars = 10 μm.
However, asymmetric cell division is only a precondition for coordinated stomatal differentiation, and asymmetric cell division does not always lead to stomatal cell fate specification. As shown in Figure 5A, a few asymmetric cell divisions, as indicated by the formation of meristemoids, did occur at an earlier stage in the GVG-Nt-MEK2DD gain-of-function mutant. The meristemoids that were produced by these few asymmetric cell divisions frequently arrested at the GMC stage and were unable to proceed to differentiate into stomata. This is similar to what has been observed in 35S:SDD1 transgenic plants (Von Groll et al., 2002) and tmm/er mutant plants (Shpak et al., 2005). This result suggests that the ultimate cell fate specification of stomata can be uncoupled from the asymmetric cell divisions at later stages during stomatal development. This finding agrees with previous observations that, when two meristemoids arise adjacent to each other, one will be arrested (Geisler et al., 2000). This suggests the existence of additional signaling events that can modify stomatal cell fate specification decisions at later stages. In the case of the GVG-Nt-MEK2DD gain-of-function mutant, the increased MAPK cascade activity may suppress the commitment of the meristemoid to a stomatal cell fate after asymmetric cell division events.
Time-Course Analysis of Stomatal Development in the Rescued mpk3−/− mpk6−/−
To further investigate the cause of clustered stomata in the rescued mpk3−/− mpk6−/− seedlings, we examined epidermal development at different times after germination until 5 d after germination. Epidermal development in the rescued mpk3−/− mpk6−/− seedlings showed no differences from that in wild-type seedlings at 1 d after germination (Figure 5B). This finding suggests that the stomatal development and patterning defects in the rescued mpk3−/− mpk6−/− seedlings are likely to be postembryonic. By 2 d after germination, asymmetric cell divisions had occurred in both the wild type and the rescued mpk3−/− mpk6−/−. By 3 d after germination, more ectopic cell divisions had occurred in the rescued mpk3−/− mpk6−/− compared with the wild type. The division planes of these cell divisions were randomly oriented, and they were frequently anticlinal to existing stomata. The progeny of these ectopic cell divisions were approximately equal in size. By 5 d after germination, the one-cell spacing rule was strictly followed in the wild type, whereas clustered stomata were produced in the rescued mpk3−/− mpk6−/−. This result suggests that the ectopically overproduced small cells in mpk3−/− mpk6−/− all eventually differentiated into stomata.
Stomatal Cell Fate Marker Genes Are Upregulated in MKK4-MKK5RNAi Plants and Downregulated in GVG-Nt-MEK2DD Plants
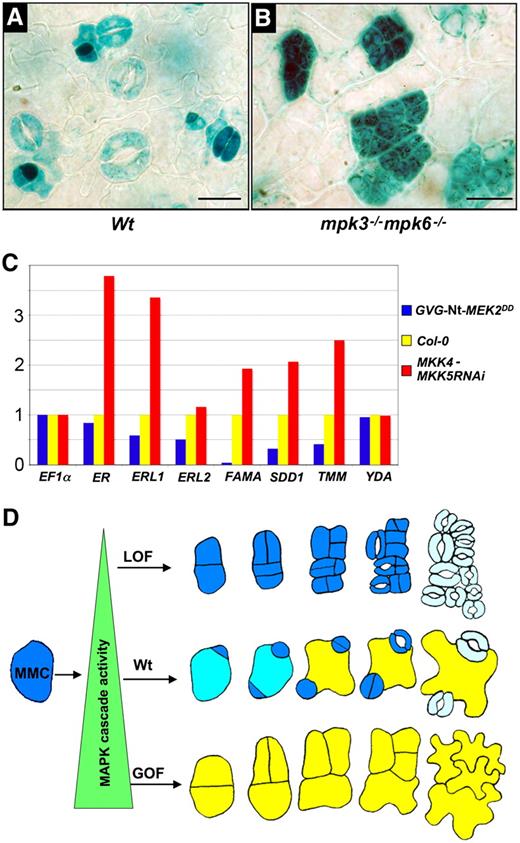
Expression of Stomatal Cell Fate Marker Genes in Loss- and Gain-of-Function MAPK Cascade Mutants.
(A) In the wild type, expression of the stomatal cell fate marker gene ERL1:GUS is restricted to the progeny of cells that divide asymmetrically (meristemoids, GMCs, young guard cells, neighbor cells/stomata lineage ground cells, and mature guard cells).
(B) In the rescued mpk3−/− mpk6−/−, ERL1:GUS expression is detected in all of the small near-isodiametric cells.
(C) Stomatal cell fate genes are upregulated in the MKK4-MKK5RNAi loss-of-function mutant and downregulated in the GVG-Nt-MEK2DD gain-of-function mutant (induced by 0.02 μM Dex). The levels of mRNA in pooled seedlings (>30) were determined by quantitative RT-PCR. After being normalized to EF1α, the relative levels to those in control seedlings are shown. Three biological repeats were performed, and similar results were obtained. Col-0, Columbia.
(D) A model depicts the function of the YDA-MKK4/MKK5-MPK3/MPK6 cascade in regulating asymmetric cell division and coordinating cell fate specification. This MAPK cascade functions as a rheostat-like molecular switch in coordinating stomatal cell fate specification. In the gain-of-function (GOF) MAPK cascade, high MAPK activity suppresses the asymmetric cell division of the MMC and the differentiation of stomata. In wild-type plants, the MMC undergoes normal asymmetric cell division; the coordinated cell fate specification between meristemoids and neighbor cells helps maintain the one-cell spacing rule. The loss-of-function (LOF) MAPK cascade disrupts the orientation, the frequency, and the polarity of asymmetric cell divisions, resulting in cell fate coordination defects and clustered stomata.
To determine whether other stomatal cell fate genes have a similar expression pattern as ERL1, we tested the expression level of other known stomatal cell fate genes in MKK4-MKK5RNAi and Dex-induced GVG-Nt-MEK2DD plants. Real-time RT-PCR results demonstrated that the stomatal cell fate genes ERL1, ERL2, TMM, and SDD1 were upregulated in MKK4-MKK5RNAi seedlings and downregulated in GVG-Nt-MEK2DD plants (Figure 6C).
DISCUSSION
The MAPK Signaling Cascade Functions as a Key Regulator of Cell Fate Coordination during Stomatal Development
Coordinated cell fate specification is critical to plant growth and development. We report that MPK3 and MPK6, two Arabidopsis MAPKs, and their upstream MAPKKs, MKK4 and MKK5, are key regulators of stomatal development. We further establish that YDA is likely to be the upstream MAPKKK in this MAPK cascade. Loss of function of this MAPK cascade leads to a stomate-only phenotype (Figure 2D). Gain-of-function activation of this MAPK cascade produces a pavement cell–only phenotype (Figure 3B). Together, these results suggest that this MAPK cascade is an important regulator of cell fate coordination during stomata versus pavement cell specification (Figure 6D).
In MKK4/MKK5-MPK3/MPK6 cascade loss-of-function mutants, stomata in a cluster were often arranged in different orientations and differed in size, suggesting that they were produced sequentially and iteratively (Figure 5B; see Supplemental Figure 4 online). This is similar to what has been observed in tmm (Geisler et al., 2000; Nadeau and Sack, 2002b). In the most severe case, the MKK4/MKK5 tandem RNAi transgenic plants showed a stomate-only phenotype (Figure 2; see Supplemental Figure 2 online). As shown in Supplemental Figure 2 online, the ectopic guard cells frequently were aligned in parallel, reminiscent of what has been observed in four-lips (flp) (Yang and Sack, 1995; Lai et al., 2005). FLP, along with its close homolog MYB88, belongs to the R2R3-type MYBs. Both FLP and MYB88 are expressed in GMCs and function in restricting the cell cycling of GMCs and promoting the terminal differentiation of guard cells (Yang and Sack, 1995; Lai et al., 2005). However, as shown in Figure 5, the stomatal patterning defects in the MKK4/MKK5-MPK3/MPK6 cascade loss-of-function mutants start before GMC formation. Several lines of evidence support the notion that the stomatal patterning defects in the yda−/− mutant occur before GMCs are specified as well (Bergmann et al., 2004). Together, these results suggest that the stomatal development and patterning defects in the YDA-MKK4/MKK5-MPK3/MPK6 MAPK cascade mutants most likely start before GMC specification.
Asymmetric cell division has been shown to associate with stomatal cell fate specification in Arabidopsis (Geisler et al., 2000). It is believed that perturbation of the frequency of asymmetric cell divisions and the orientation of the asymmetric division plane will ultimately disrupt stomatal development and patterning (Berger and Altmann, 2000; Nadeau and Sack, 2002b; Sack, 2004; Serna, 2004; Shpak et al., 2005). Multiple aspects associated with asymmetric cell division could be regulated by the YDA-MKK4/MKK5-MPK3/MPK6 that may contribute to the stomatal patterning defect in the MAPK cascade mutant. This MAPK cascade could function to (1) restrict the asymmetric cell division frequency of the meristemoid, (2) maintain the polarity of the asymmetric cell division, and (3) coordinate cell fate specification of the progeny of asymmetric cell divisions. We have demonstrated that activation of MPK3/MPK6 by gain of function of the MAPKK reduces the frequency of asymmetric cell divisions and suppresses stomatal cell fate specification, producing a pavement cell–only phenotype (Figure 5A; see Supplemental Table 1 online). The pavement cell–only phenotype was observed in the gain-of-function ΔN-YDA plants as well (Bergmann et al., 2004). In the rescued mpk3−/− mpk6−/− seedlings, we observed ectopically overproduced small cells that express the stomatal lineage cell marker ERL1:GUS, suggesting that they might be the products of ectopic asymmetric cell divisions with indistinguishable polarity. If this holds true, it is plausible to propose that loss of the polarity of asymmetric cell divisions in the rescued mpk3−/− mpk6−/− causes disrupted cell fate coordination of the progeny cells, producing clustered stomata. However, as noted previously, ERL1 is also expressed in the proliferating cells in the leaf primordia and functions in promoting cell proliferation (Shpak et al., 2004, 2005). We cannot exclude the possibility that ERL1:GUS expression in the clustered ectopically produced small cells might simply mean that they are proliferating. Future research with asymmetric cell division–specific markers will help us to further address this question.
A Model of MAPK Cascade Function in Position-Dependent Intercellular Communication That Coordinates Stomatal Cell Fate Specification and Regulates Stomatal Patterning
It was proposed that the oriented asymmetric cell division, in which the satellite meristemoid is placed away from existing stomata, is the major mechanism to maintain the one-cell spacing patterning of stomatal development (Geisler et al., 2000; Nadeau and Sack, 2002b). As shown in Figure 5B and Supplemental Figure 4 online, disoriented asymmetric cell divisions often associate with clustered stomatal formation. However, the disoriented asymmetric cell division is unable to fully explain the all-stomate phenotype in the loss-of-function MAPK cascade mutants (Figure 2; see Supplemental Figure 2 online). As discussed above, the ectopic cell divisions observed in the rescued mpk3−/− mpk6−/− seedlings may represent asymmetric cell divisions with indistinguishable polarities. Thus, the loss of asymmetric cell division polarity may lead to the disruption of cell fate coordination between the daughter cells, which eventually all differentiate into stomata (Figure 5). If this is the case, it is reasonable to propose that within the stomatal cell lineage, the maintenance of polarity in the progeny of asymmetric cell divisions is another key mechanism in establishing the one-cell spacing pattern. The function of this MAPK cascade may be to maintain the polarity of asymmetric cell divisions and to coordinate cell fate specification between the daughter cells (Figure 6D).
In the epidermal layer, two meristemoids from two neighboring MMCs either signal each other to divide away from one another, or one becomes arrested so that two stomata will not develop next to each other (Geisler et al., 2000). It is unknown how cells from different stomatal cell lineages coordinate their fates. It was suggested that position-dependent intercellular signaling is used to maintain the one-cell spacing pattern (Geisler et al., 2000; Nadeau and Sack, 2002b). The stomate-only phenotype in the MKK4/MKK5 double RNAi transgenic plants (Figure 2; see Supplemental Figure 2 online) could also indicate that all of the protodermal cells are competent to assume MMC cell fate and enter the stomatal development pathway. In the MKK4/MKK5 double mutant, the cell fate coordination between progeny cells from different MMCs is disrupted, resulting in stomata derived from different MMCs being adjacent to each other. This finding suggests that the YDA-MKK4/MKK5-MPK3/MP6 cascade could also be a major component of position-dependent intercellular signaling between cells from different stomatal cell lineages.
Besides the proposed function of regulating the polarity of asymmetric cell divisions during stomatal development, MPK3/MPK6 also regulates the polarity of zygote asymmetric cell division. In mpk3−/− mpk6−/−, the unequal cell division of the zygote is disrupted. The resulting apical and basal cells are approximately equal in size (see Supplemental Figure 5 online). This is similar to what has been observed in the yda−/− mutant (Lukowitz et al., 2004). The abnormal zygote asymmetric cell division in mpk3−/− mpk6−/− results in the disruption of cell fate coordination between the apical and basal cells and leads to improper development of the embryo (see Supplemental Figure 1 online). As asymmetric cell divisions are associated with various cell fate specifications in plants, future research to understand the molecular mechanism of how this MAPK cascade regulates the polarity of asymmetric cell division and cell fate coordination during stomata and embryo development will have a significant impact on our understanding of cell fate specification in plants in general.
The MAPK Cascade Is the Potential Integrating Point of Environmental Signals and Developmental Signals That Regulate Stomatal Development
In stress-responsive signaling pathways, ANP1 and MEKK1 can serve as upstream kinases for MKK4/MKK5 and MPK3/MPK6 based on gain-of-function analysis using a protoplast transient transformation system (Kovtun et al., 2000; Asai et al., 2002). However, no stomatal development and patterning defects were observed in loss-of-function ANP1 or MEKK1 plants (H. Jin, personal communication; data not shown), suggesting that the same MAPKK-MAPK module can assemble with different MAPKKKs and transduce different input signals.
In Arabidopsis, there are ∼60 putative MAPKKKs, 10 MAPKKs, and 20 MAPKs (Ichimura et al., 2002). The limited number of MAPKKs compared with a relatively large number of MAPKKKs suggests that MAPKKs and MAPKs are the converging points of MAPK cascade signaling. Together with previous studies, our results suggest that the MKK4/MKK5-MPK3/MPK6 module has dual functions in both the stomatal development and patterning pathway and the stress-responsive pathways. In stomatal development, the function of this module is to transduce the endogenous and exogenous signals in the target cells and to specify the optimal differentiation ratio of stomata versus pavement cells in leaf epidermis, thus ensuring maximum performance of the plant. In stress responses, activation of this module confers resistance to both biotic and abiotic stresses (Tena et al., 2001; Zhang and Klessig, 2001; Nakagami et al., 2005; Pedley and Martin, 2005).
Not only may the MKK4/MKK5-MPK3/MPK6 cascade have dual functions, it may also be an integrating point of multiple signals. It is essential that plants are able to sense environmental cues and adjust stomatal development (Gray et al., 2000; Lake et al., 2002; Hetherington and Woodward, 2003; Gitz et al., 2005). In response to UV-B irradiation, plants have decreased stomata density (Gitz et al., 2005). However, how environmental responses are integrated with stomatal developmental programming remains unknown. The MKK4/MKK5-MPK3/MPK6 module is an essential regulator of both biotic and abiotic stress responses and is activated upon UV-B irradiation (Holley et al., 2003). Here, we have shown that the same MAPK module is a central regulator of stomatal development and patterning. It is tempting to propose that this MKK-MPK module is the long-sought molecular hub at which environmental signals impinge on the stomatal development pathway and influence stomatal development (see Supplemental Figure 6 online).
One intriguing question is how the signaling specificity of different MAPK cascades sharing the same MKK-MPK module is maintained. Tissue- or cell-specific expression of input signaling molecules may play a critical role in maintaining the signaling specificity of different MAPK cascades. The stomatal cell fate genes SDD1, TMM, ER, ERL1, and ERL2 are expressed in the MMCs, meristemoids, GMCs, and new guard cells but not in the pavement cells in the epidermal cell layer (Berger and Altmann, 2000; Nadeau and Sack, 2002a; Bergmann et al., 2004; Sack, 2004; Serna, 2004; Shpak et al., 2005). Signaling specificity can be maintained by substrate specificity as well; the same MAPK can activate different substrates that are differentially expressed. The identification of MAPK substrates is urgently needed to address this question.
We have established the YDA-MKK4/MKK5-MPK3/MPK6 cascade as a key component of intercellular interactions to coordinate cell fate specification during stomatal development. Understanding the downstream signaling events of this MAPK cascade will ultimately help elucidate the regulatory mechanisms of intercellular signaling during stomatal development.
METHODS
Plant Growth Conditions
After surface sterilization and imbibition at 4°C for 3 to 5 d, Arabidopsis thaliana seeds were plated on half-strength Murashige and Skoog medium with 0.7% phytagar and appropriate antibiotic for selection. Dex was added to the medium at a final concentration of 0.02 μM. Plates were incubated in a tissue culture chamber at 22°C under continuous light (70 μE·m−2·s−1).
T-DNA Insertional Mutants and Crosses
All three T-DNA insertion mutant alleles of MPK6 were genotyped as described previously (Liu and Zhang, 2004). MPK3 and YDA T-DNA insertional mutants were obtained from the ABRC (mpk3, SALK_151594; yda, SALK_ 105078) (Alonso et al., 2003). Homozygous GVG-Nt-MEK2DD mpk3−/− F3 plants were generated as described previously for GVG-Nt-MEK2DD mpk6−/− plants (Liu and Zhang, 2004). The steroid-inducible GVG-Nt-MEK2DD transgene was followed by hygromycin resistance, and T-DNA insertions were followed by PCR of genomic DNA. Similar results were obtained using crosses of three different mpk6−/− alleles and GVG-Nt-MEK2DD. Data from GVG-Nt-MEK2DD mpk6-2 are shown. Heterozygous yda T-DNA insertion mutants were crossed to homozygous GVG-Nt-MEK2DD, and F1 plants were screened for the presence of yda by PCR genotyping. Seeds from GVG-Nt-MEK2DD yda+/− plants were selected on hygromycin plates to identify GVG-Nt-MEK2DD yda−/− double mutants. Dex (0.02 μM) was added to induce Nt-MEK2DD expression. The yda−/− genotype was confirmed by PCR genotyping.
Generation of Dex-Inducible and RNAi Transgenic Plants
To make Dex-inducible GVG-MPK6, the open reading frame of MPK6 with a Flag epitope at its N terminus was inserted into the XhoI/SpeI sites of pTA7002 vector (Aoyama and Chua, 1997). Steroid-inducible GVG-Nt-MEK2DD, GVG-MKK4DD, and GVG-MKK5DD were described previously (Yang et al., 2001; Ren et al., 2002). Two different MPK3 RNAi constructs were used to silence MPK3 in the mpk6 T-DNA mutant background. They targeted the regions corresponding to 1 to 693 bp and 312 to 1178 bp of MPK3, which were used as the inverted repeats in the pHANNIBAL vector (Wesley et al., 2001). For silencing of MKK4 and MKK5, regions corresponding to 1 to 674 bp of MKK4 and 1 to 647 bp of MKK5 were used. All inverted repeats with the Pdk intron from the pHANNIBAL vector were mobilized into the pBI121 binary vector for transformation. A tandem RNAi construct was used to silence both MKK4 and MKK5. Detailed procedures for making the RNAi constructs are shown in Supplemental Figure 7 online.
Conditional Rescue of the mpk3−/− mpk6−/− Double Mutant
Embryo lethality of the mpk3−/− mpk6−/− double mutant was rescued by transforming the steroid-inducible MPK6 construct into mpk3−/− mpk6+/− plants. T3 homozygous GVG-MPK6 transgenic plants in the mpk3−/− mpk6+/− background were sprayed with Dex (30 μM) once every 3 d starting from 1 week before bolting until all seeds matured. In the T4 generation, one-quarter of the seedlings were mpk3−/− mpk6−/−. Two independent transgenic lines were followed, and the same results were obtained. Data from one of them are shown.
Confocal Microscopy
For each time point, 10 seedlings were observed and imaged. To visualize epidermal cell outlines, the seedlings were immersed in 0.2 mg/mL propidium iodide for 30 min, dissected using a stereoscope, and mounted on slides with the cotyledon abaxial side facing up. Images were taken with a Bio-Rad Radiance 2000 (Carl Zeiss Microimaging) confocal system coupled to an Olympus IX70 inverted microscope.
Quantitative RT-PCR Analysis
Total RNA was extracted using RNAqueous (Ambion) according to the manufacturer's instructions. After DNase treatment, 1 μg of total RNA was used for reverse transcription. Quantitative PCR analysis was performed using an Optican 2 real-time PCR machine (MJ Research). Relative levels of each transcript were calculated after being normalized to the EF1α control.
GUS Staining
Seedlings were incubated in GUS staining buffer (10 mM EDTA, 0.1% Triton X-100, 2 mM potassium ferricyanide, 2 mM potassium ferrocyanide, 100 μg/mL chloramphenicol, and 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-glucuronic acid in 50 mM sodium phosphate buffer, pH 7.0) for 6 h at 37°C. The seedlings were then cleared in 20% lactic acid and 20% glycerol and observed on an Olympus IX-70 microscope under Nomarski optics.
Protein Extraction and in-Gel Kinase Assay
Protein was extracted from seedlings and stored at −80°C as described previously (Zhang and Klessig, 1997; Liu and Zhang, 2004). The concentration of protein extracts was determined using the Bio-Rad protein assay kit with BSA as the standard. Myelin basic protein was used as the substrate in the in-gel kinase assay (Liu and Zhang, 2004).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At3g45640 (MPK3), At2g43790 (MPK6), At1g51660 (MKK4), and At3g21220 (MKK5).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The mpk3−/− mpk6−/− Double Mutant Is Embryo-Lethal.
Supplemental Figure 2. Whole Cotyledon Epidermis of a Tandem MKK4-MKK5RNAi Transgenic Plant.
Supplemental Figure 3. Dex Treatment Has No Effect on Stomatal Development and Patterning.
Supplemental Figure 4. Stomata in Clusters Are Formed Sequentially and Iteratively.
Supplemental Figure 5. The MAPK Cascade Regulates Asymmetric Cell Division in the Zygote.
Supplemental Figure 6. Model of the Function of YDA-MKK4/MKK5-MPK3/MPK6 in Stomatal Development.
Supplemental Figure 7. Maps of RNAi- and Dex-Inducible Constructs.
Supplemental Table 1. Percentages of Epidermal Cells with Given Identities in Abaxial Cotyledon Epidermis.
ACKNOWLEDGMENTS
We thank Nam-Hai Chua for the pTA7002 vector; Commonwealth Scientific and Industrial Research Organization Plant Industry for the pHANNIBAL vector; Hailing Jin for sharing unpublished information; Richard Hammond and Jason Doke for genomic DNA preparation and seed collection; the ABRC for the seed stocks; Dominique Bergmann, Keiko Torii, Fred Sack, and Jessica Lucas for sharing seeds and plasmids with us; and Clayton Larue, Kevin Lease, David Chevalier, and Jiangqi Wen for critical reading of the manuscript. This research was supported by grants from the National Science Foundation (to J.C.W. and S.Z.), the University of Missouri Research Board (to S.Z.), and the University of Missouri Food for the 21st Century Program (to J.C.W.). H.W. was supported by a Life Science Predoctoral Fellowship from the University of Missouri-Columbia. N.N. was supported by the Life Science Undergraduate Research Express Program from the University of Missouri-Columbia.
REFERENCES
Alonso, J.M., et al. (
Aoyama, T., and Chua, N.-H. (
Asai, T., Tena, G., Plotnikova, J., Willmann, M.R., Chiu, W.L., Gomez-Gomez, L., Boller, T., Ausubel, F.M., and Sheen, J. (
Berger, D., and Altmann, T. (
Bergmann, D.C. (
Bergmann, D.C., Lukowitz, W., and Somerville, C.R. (
Di Laurenzio, L., Wysocka-Diller, J., Malamy, J.E., Pysh, L., Helariutta, Y., Freshour, G., Hahn, M.G., Feldmann, K.A., and Benfey, P.N. (
Geisler, M., Nadeau, J., and Sack, F.D. (
Gitz, I., Dennis, C., Liu-Gitz, L., Britz, S.J., and Sullivan, J.H. (
Gray, J.E., Holroyd, G.H., van der Lee, F.M., Bahrami, A.R., Sijmons, P.C., Woodward, F.I., Schuch, W., and Hetherington, A.M. (
Hamel, L.P., et al. (
Hetherington, A.M., and Woodward, F.I. (
Holley, S.R., Yalamanchili, R.D., Moura, D.S., Ryan, C.A., and Stratmann, J.W. (
Horvitz, H.R., and Herskowitz, I. (
Jan, Y.N., and Jan, L.Y. (
Kovtun, Y., Chiu, W.L., Tena, G., and Sheen, J. (
Lai, L.B., Nadeau, J.A., Lucas, J., Lee, E.-K., Nakagawa, T., Zhao, L., Geisler, M., and Sack, F.D. (
Lake, J.A., Woodward, F.I., and Quick, W.P. (
Liu, Y., and Zhang, S. (
Lukowitz, W., Roeder, A., Parmenter, D., and Somerville, C. (
Ichimura, K., et al. (
Nadeau, J.A., and Sack, F.D. (
Nadeau, J.A., and Sack, F.D. (September 30,
Nakagami, H., Pitzschke, A., and Hirt, H. (
Pedley, K.F., and Martin, G.B. (
Ren, D., Yang, H., and Zhang, S. (
Sack, F.D. (
Scheres, B., and Benfey, P.N. (
Shpak, E.D., Berthiaume, C.T., Hill, E.J., and Torii, K.U. (
Shpak, E.D., McAbee, J.M., Pillitteri, L.J., and Torii, K.U. (
Tena, G., Asai, T., Chiu, W.L., and Sheen, J. (
Von Groll, U., Berger, D., and Altmann, T. (
Wesley, S.V., et al. (
Woodward, F.I., Lake, J.A., and Quick, W.P. (
Yang, K.Y., Liu, Y., and Zhang, S. (
Yang, M., and Sack, F.D. (
Zhang, S., and Klessig, D.F. (
Zhang, S., and Klessig, D.F. (
Author notes
To whom correspondence should be addressed. Email zhangsh@missouri.edu; fax 573-884-9676.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Shuqun Zhang (zhangsh@missouri.edu).
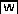
Online version contains Web-only data.