-
PDF
- Split View
-
Views
-
Cite
Cite
Ling Min, Qin Hu, Yaoyao Li, Jiao Xu, Yizan Ma, Longfu Zhu, Xiyan Yang, Xianlong Zhang, LEAFY COTYLEDON1-CASEIN KINASE I-TCP15-PHYTOCHROME INTERACTING FACTOR4 Network Regulates Somatic Embryogenesis by Regulating Auxin Homeostasis , Plant Physiology, Volume 169, Issue 4, December 2015, Pages 2805–2821, https://doi.org/10.1104/pp.15.01480
Close - Share Icon Share
Abstract
Somatic embryogenesis (SE) is an efficient tool for the propagation of plant species and also, a useful model for studying the regulatory networks in embryo development. However, the regulatory networks underlying the transition from nonembryogenic callus to somatic embryos during SE remain poorly understood. Here, we describe an upland cotton (Gossypium hirsutum) CASEIN KINASE I gene, GhCKI, which is a unique key regulatory factor that strongly affects SE. Overexpressing GhCKI halted the formation of embryoids and plant regeneration because of a block in the transition from nonembryogenic callus to somatic embryos. In contrast, defective GhCKI in plants facilitated SE. To better understand the mechanism by which GhCKI regulates SE, the regulatory network was analyzed. A direct upstream negative regulator protein, cotton LEAFY COTYLEDON1, was identified to be targeted to a cis-element, CTTTTC, in the promoter of GhCKI. Moreover, GhCKI interacted with and phosphorylated cotton CINCINNATA-like TEOSINTE BRANCHED1-CYCLOIDEA-PCF transcription factor15 by coordinately regulating the expression of cotton PHYTOCHROME INTERACTING FACTOR4, finally disrupting auxin homeostasis, which led to increased cell proliferation and aborted somatic embryo formation in GhCKI-overexpressing somatic cells. Our results show a complex process of SE that is negatively regulated by GhCKI through a complex regulatory network.
Somatic embryogenesis (SE) is a process in which somatic cells are reprogrammed to generate a completely new embryo in response to external stimuli without the fusion of gametes. The process of SE resembles zygotic embryogenesis, and developmental and regulatory mechanisms of SE could provide an accessible reference for studying the earliest developmental events of the zygotic embryo in the lifecycle of higher plants (Mordhorst et al., 1997). In addition, SE is a key step in the realization of genetic transformation, somatic hybridization, and somaclonal variation screening in most plants, especially cash crops (Zeng et al., 2006). Cotton (Gossypium hirsutum), as a key fiber crop, requires a highly successful regeneration procedure from somatic cells to perform genetic manipulation. Although we have conducted research on SE in cotton by expression profile analysis and showed that transcriptional regulation in SE is a complex process with the interaction of multiple molecules (Zeng et al., 2006; Yang et al., 2012), the detailed functions of key transcriptional regulators and the mechanisms underlying the different developmental processes are less well understood, and only a few genes that regulate SE in cotton have been identified (Hu et al., 2011).
SE comprises two styles: direct SE and indirect SE, in which embryos are formed from explant tissues without or with a callus phase, respectively. During SE in cotton, nonembryogenic callus (NEC) and embryogenic callus (EC) are present (Yang et al., 2012), indicating that SE occurs in an indirect style, which consists of four different phases: dedifferentiation from explants, cell division and proliferation, transition from NEC to EC, and transition from EC to somatic embryos (Filonova et al., 2000; Yang et al., 2012).
During the past three decades, numerous transcription factors, kinases, and types of hormones involved in SE in different species have been identified. Arabidopsis (Arabidopsis thaliana) PLANT GROWTH ACTIVATOR6 (PGA6) belonging to the homeodomain transcription factor family and identical to WUSCHEL (WUS) plays a critical role in SE. Gain-of-function and loss-of-function pga6 mutants can promote and compromise the vegetative to embryogenic transition, respectively (Zuo et al., 2002). LEAFY COTYLEDON1 (LEC1), which encodes a heme-activated protein3 (HAP3) subunit of the CCAAT box-binding factor complex, has been shown to be necessary for SE. Mutations in LEC1 cause defective embryo maturation, and thus, LEC1 participates mainly in promoting embryonic cell differentiation during late SE developmental stages (Lotan et al., 1998). Arabidopsis LEC2, FUSCA3 (FUS3), and ABSCISIC ACID INSENSITIVE3 (ABI3)-encoding B3 domain transcription factors are essential for several features of somatic embryo development (Braybrook et al., 2006; Stone et al., 2008). AGAMOUS-LIKE15 (AGL15) is an MADS (for MCM1, agamous, deficiens, and serum response factor)-domain transcription factor that is primarily expressed during embryogenesis. There are no significant differences between agl15 and the wild type in terms of SE, but agl15/agl18 double mutants display a compromised ability to produce somatic embryos (Heck et al., 1995; Zheng et al., 2009).
Other than transcription factors, kinases have been shown to be involved in SE. Kinases play vital roles in signal transduction pathways, regulating downstream signaling proteins by phosphorylation. The most famous type of kinase involved in SE is SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE (SERK). Five putative SERK genes in Arabidopsis have been characterized, and the overexpression of AtSERK1 strengthened the capacity of suspension cells to undergo SE. In addition, expression analysis of three alfalfa (Medicago sativa) SERK genes revealed that these genes are expressed in early-stage embryogenic tissues but not in nonembryogenic tissues or the mature embryo (Hecht et al., 2001; Becraft, 2002; Nolan et al., 2003). These results suggest that SERKs play a conserved role in mediating SE in plants.
In addition to transcription factors and kinases, hormone homeostasis and stress also have been proposed to be key plant growth factors in promoting the dedifferentiation of explants or initiating an embryogenic pathway. Auxin is an important growth regulator for embryo induction (Yang et al., 2012), but cytokinin, abscisic acid (ABA), ethylene, jasmonates, and brassinosteroids also play roles in initiating SE (Sagare et al., 2000; Depuydt and Hardtke, 2011). Interestingly, all of the above-mentioned genes involved in SE are related to auxin, among which LEC1 is known to up-regulate the auxin biosynthesis gene YUCCA10 (YUC10) by binding to its promoter (Junker et al., 2012). Overexpression of LEC1 could substitute for exogenous auxin and induce SE (Lotan et al., 1998). LEC2 was found to bind directly to the regulatory regions of YUC4 and activate the auxin signaling pathway in somatic cells to promote the formation of embryonic cells (Stone et al., 2008). In addition, the expression of SERK1 is also responsive to auxin treatment (Nolan et al., 2003; Gazzarrini et al., 2004).
Casein kinase I (CKI) is a highly conserved Ser/Thr protein kinase that has been implicated in cell proliferation, apoptosis, tumorigenesis, and development in mammals (Gross and Anderson, 1998; Peters et al., 1999; Price, 2006). In yeasts and plants, CKI is involved in regulating sugar signaling (Moriya and Johnston, 2004), root development (Liu et al., 2003), and rice (Oryza sativa) flowering time (Dai and Xue, 2010). In our previous study, we showed that both transgenic overexpression of GhCKI in Arabidopsis and the induction of high expression of GhCKI by high temperature in early-stage anthers in a high temperature-sensitive cotton line caused male sterility. We have speculated that increased GhCKI expression inhibited the activities of starch synthases; disrupted the homeostasis of Glc, indole-3-acetic acid (IAA), and ABA; and caused anther abortion (Min et al., 2013, 2014). Additionally, GhCKI was found to be expressed strongly in NEC and globular embryos, whereas expression was low in embryonic callus, and overexpressing GhCKI led to a failure of SE and very low levels of transgenic plant production (Min et al., 2013); we, therefore, considered GhCKI as a potential regulator of SE. To understand how GhCKI alters cell fate during the transition from NEC to somatic embryos, molecular and genetic experiments were performed. Based on the analysis of upstream and downstream regulatory networks involving GhCKI, we found that a unique gene network (LEC1-CKI-CINCINNATA-like TEOSINTE BRANCHED1-CYCLOIDEA-PCF transcription factor15 [TCP15]-PHYTOCHROME INTERACTING FACTOR4 [PIF4]) regulates auxin homeostasis to affect the fate of callus cells during SE.
RESULTS
Kinetics of Cotton SE in Response to GhCKI Transcription
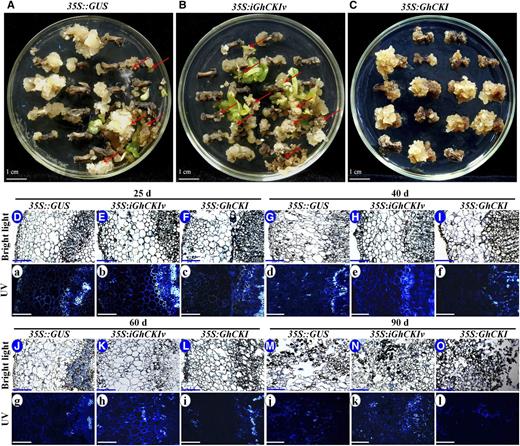
Our previous study showed that overexpressing GhCKI led to a failure of SE and a very low level of transgenic plant regeneration (Min et al., 2013). To further investigate the role of GhCKI in SE, we transformed cotton with gene constructs for both the overexpression (35S:GhCKI) and a variable region RNA interference (RNAi; 35S:iGhCKIv), with 35S::GUS serving as a negative control (Supplemental Fig. S1). Explants transformed with 35S::GUS and 35S:iGhCKIv underwent SE, producing embryonic callus and somatic embryos, and most explants of 35S:iGhCKIv showed more evidence of SE than 35S::GUS explants (Fig. 1, A and B). However, 35S:GhCKI explants failed to undergo SE after induction for 90 d (Fig. 1C). We obtained only two transgenic cotton lines carrying the 35S:GhCKI after more than 20 transformation experiments using more than 4,000 explants. The two lines showed little increase in GhCKI expression and exhibited male sterility (Min et al., 2013). However, 45 and 83 lines carrying the 35S:iGhCKIv and 35S::GUS were isolated from 400 explants, respectively. To confirm the SE phenotypes caused by 35S:iGhCKIv, hypocotyl sections from 5-d-old seedlings of T3 plants of 35S:iGhCKIv-34 showing the lowest expression (Supplemental Fig. S2A) and 35S::GUS transformants (with GUS staining) were cultured for callus induction. As shown in Supplemental Figure S2, B–D, more than 95% (94 of 100, 97 of 100, and 95 of 100) of the explants from 35S:iGhCKIv-34 transgenic plants produced embryonic callus and somatic embryos at 90 d (Supplemental Fig. S2, C and D), whereas approximately 50% (45 of 100, 49 of 100, and 54 of 100) of the explants from 35S::GUS produced somatic embryos (Supplemental Fig. S2, B and D). These results suggest that GhCKI plays a role in inhibiting SE.
Schematic representation and histological observation of explants at different induction stages for 35S::GUS, 35S:iGhCKIv, and 35S:GhCKI transformants. A to C, Transformants from 35S::GUS (A), 35S:iGhCKIv (B), and 35S:GhCKI (C) induced for 90 d. No ECs were observed in the GhCKI-overexpressed explants (35S:GhCKI), but most explants of 35S:iGhCKIv showed high-quality EC (red arrows), better than the control explants (35S::GUS). D to O. Cross sections of explants from 35S::GUS (D, G, J, and M), 35S:iGhCKIv (E, H, K, and N), and 35S:GhCKI (F, I, L, and O) induced for 25 (D–F), 40 (G–I), 60 (J–L), and 90 d (M–O) under bright light condition. No significant differences were observed in the explants transformed with three type constructs at 25, 40, 60, and 90 d under bright light. a to l, Cross sections of explants from 35S::GUS, 35S:iGhCKIv, and 35S:GhCKI were stained with aniline blue to detect cell growth and death as shown by bright blue fluorescence under UV light condition. Explants of 35S::GUS (a, d, g, and j), 35S:iGhCKIv (b, e, h, and k), and 35S:GhCKI (c, f, i, and l) were induced for 25 (a–c), 40 (d–f), 60 (g–i), and 90 d (j–l). No significant differences were observed in the density of blue fluorescence in explants at 25 d. However, explants of 35S:iGhCKIv and 35S:GhCKI at 40, 60, and 90 d showed strong and weak blue fluorescence, respectively, compared with 35S::GUS (control). Bars = 1 cm (A–C) and 100 μm (D–O and a–l).
To search for potential reason of GhCKI-inhibited SE, changes in explants transformed with 35S::GUS, 35S:iGhCKIv, and 35S:GhCKI were determined at 25, 40, 60, and 90 d based on histological analysis (Fig. 1, D–O). Little change was observed in the cross sections of explants transformed with three type constructs at 25 (Fig. 1, D–F), 40 (Fig. 1, G–I), 60 (Fig. 1, J–L), and 90 d (Fig. 1, M–O) under bright light. To detect the programmed cell death of explants transformed with three type constructs, cross sections of explants were stained by aniline blue and observed under UV light (Fig. 1, a–l). No significant difference in the strength of fluorescent signal of aniline blue under UV light was observed at 25 d. However, obvious differences were observed in transformants for longer induction (Fig. 1, d–l). Compared with 35S::GUS (Fig. 1, d and g), the fluorescent signals were enhanced and inhibited in 35S:iGhCKIv and 35S:GhCKI explants after induction for 40 (Fig. 1, e and f) and 60 d (Fig. 1, h and i), respectively. In addition, more regularly shaped and content-rich cells with strong aniline blue staining were found in 35S::GUS and 35S:iGhCKIv 90-d induction callus (Fig. 1, M, N, j, and k), whereas 35S:GhCKI callus contained irregular and contentless cells with weak aniline blue staining (Fig. 1, O and l). These results suggest that GhCKI plays a role in inhibiting programmed cell death during SE.
Expression Pattern of GhCKI during SE
To uncover the basis of the differential SE of 35S:GhCKI and 35S::GUS (containing natural GhCKI expression), a more detailed expression pattern analysis was performed by comparing the activities of a cloned fragment of the native cotton casein kinase I promoter (ProGhCKI) and 35S. Hypocotyl sections of 5-d-old seedlings of 35S::GUS and ProGhCKI:GUS T3 transgenic cotton plants were used to induce SE in vitro, and changes in GUS staining during SE were determined for explants that were cultured for 0, 3, 7, 25, 40, 60, and 90 d (Supplemental Fig. S3, A and B). The results revealed a large accumulation of GUS protein throughout the entire SE process in 35S::GUS transgenic explants. However, lower accumulations of GUS protein were detected in ProGhCKI::GUS, especially in 25-, 40-, 60-, and 90-d explants. This GhCKI expression pattern was confirmed by quantitative reverse transcription (qRT)-PCR in wild-type explants during SE (Supplemental Fig. S3C). Somatic embryos usually begin to form when explants are cultured for 40 d in cotton (Yang et al., 2012). Thus, we considered that overexpression of GhCKI might block the possibly necessary decrease in expression of GhCKI from 25 to 90 d to block SE progression. The decreased expression of GhCKI in the 25- to 90-d cultured explants suggests that there may exist a suppressor binding site upstreaming GhCKI, and this was further investigated.
Functional Analysis of Promoter Fragments of GhCKI during SE in Arabidopsis
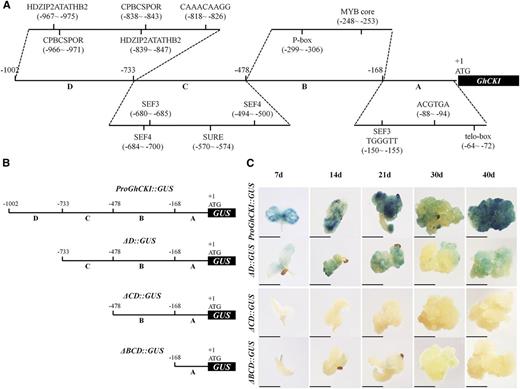
As a first step to understand the potential upstream mechanism regulating GhCKI during SE, four distinct regions based on the location of known cis-elements were cloned, with a view to analyze the functions of the ProGhCKI (Fig. 2A). The regions from −1 to −168 bp, from −169 to −478 bp, from −479 to −733 bp, and from −734 to −1,002 bp of the ProGhCKI were designated as regions A to D, respectively. Region A contains two elements, one from −72 to −64 bp (TTAGGGTTT) and another from −94 to −88 bp (ACGTGA), which were identified as telo boxes required for the activation of gene expression in root primordia and root hairs, respectively. A soybean (Glycine max) embryo factor binding site from −150 to −155 bp (TGGGTT) was also found in region A. Region B contains a CGGTTG motif that serves as a core recognition site for all MYELOBLASTOSIS proteins (MYB; Urao et al., 1993) and a P box (AACCTAAC) for MYB26 protein binding. Region C contains a core sequence of the sulfur-responsive element containing the auxin response factor binding sequence and three soybean embryo factor binding sites. Region D contains two HOMEODOMAIN-LEUCINE ZIPPER2 binding consensus sequence TAATGATTA, which were found to interact with Arabidopsis homeobox gene2 (Ohgishi et al., 2001), a variant of the CC(A/T)6GG motif (CAAACAAGG) for the MADS-domain protein AGL15 (Tang and Perry, 2003), and two cytokinin-dependent elements for cytokinin-enhanced protein binding.
Diagram showing the deletion of the ProGhCKI and expression patterns of GUS driven by full and truncated ProGhCKI sequence in transgenic Arabidopsis plants. A, Structure of the ProGhCKI sequence. Regions A to D ranged from −1 to −168, from −169 to −478, from −479 to −733, and from −734 to −1,002 bp of ProGhCKI, respectively. Partially predicted cis-regulatory elements in different regions of ProGhCKI are labeled. B and C, Deletion of the ProGhCKI fused to GUS protein (B), and comparison of GUS expression in transformed Arabidopsis among different constructs (C). ƊBCD, ƊCD, and ƊD indicate regions A, A + B, and A + B + C, respectively. The 7, 14, 21, and 30 d indicate different culture times. CPBCSPOR, Cytokinin-dependent element; HDZIP2ATATHB2, HOMEODOMAIN-LEUCINE ZIPPER2 binding consensus sequence; SEF, soybean embryo factor; SURE, sulfur-responsive element. Bars = 2 mm.
To identify the core regulatory region responsible for SE regulation in ProGhCKI, three progressive 5′ deletions of ProGhCKI together with the whole sequence were fused with GUS (Fig. 2B) and used to transform Arabidopsis. The expression patterns were determined in cultures induced from T3 seeds germinated on Arabidopsis seed somatic embryogenesis (ASSE) medium (“Materials and Methods”) as shown in Figure 2C. Region A alone (ƊBCD) showed weak GUS staining in the 7-, 14-, 21-, and 30-d cultures (Fig. 2C). Promoters lacking the C and D region (ƊCD) could not drive GUS expression in any of the culture stages. Strong staining for ProGhCKI::GUS and ƊD::GUS in the meristem and vascular tissues was observed in 7- and 14-d cultures. At 21 and 40 d, the cultures from ProGhCKI::GUS and ƊD::GUS showed strong GUS activity. Higher GUS activities were detected at all stages of the cultured explants transformed with ProGhCKI::GUS compared with other deletion constructs.
Based on these results, we speculate that the cis-elements in region B may provide the binding site for repressors of GhCKI during SE and that regions C and D may contain cis-elements for the binding of activators of GhCKI affecting SE (Fig. 2C). However, either ƊD::GUS or ProGhCKI::GUS showed significantly impaired GUS activity in the 30-d cultures (Fig. 2C) when EC and somatic embryos were generated at this stage (Lotan et al., 1998). Considering that the GUS activity of ProGhCKI::GUS transgenic tissue was significantly lower than in 35S::GUS explants at 25, 40, 60, and 90 d postinduction (Supplemental Fig. S3, A and B), we speculate that the repressors of GhCKI may bind to region B and then suppress the transcription of GhCKI at 30-d Arabidopsis cultures. In addition, we hypothesize that potential repressors of GhCKI might be highly expressed in EC and somatic embryos under normal conditions.
The Transcription of GhCKI Is Putatively Suppressed by GhLEC1 by Binding to the cis-Element CTTTTC in Region B of ProGhCKI
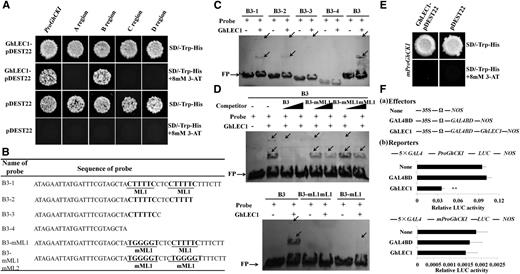
To search for potential upstream repressors of GhCKI during SE, the B region of the ProGhCKI sequence was used to screen an Arabidopsis transcription factor library using the yeast (Saccharomyces cerevisiae) one-hybrid (Y1H) system (Ou et al., 2011). Among the transcription factors found to bind region B of ProGhCKI in yeast, AtLEC1, necessary for embryonic cell differentiation (Lotan et al., 1998), was a candidate. Therefore, the homologous protein of AtLEC1 in cotton, GhLEC1, was identified. The full-length open reading frame (ORF) of GhLEC1 consists of 651 nucleotides encoding a peptide of 216-amino acid residues with a predicted molecular mass of 24 kD (Supplemental Fig. S4A). GhLEC1 was found to bind the full ProGhCKI and the region B of ProGhCKI in 1:1 Y1H experiments (Fig. 3A).
Expression of GhCKI was directly suppressed by GhLEC1 by binding to the cis-element CTTTTC upstream of GhCKI. A, Y1H assays showing the binding of GhLEC1 to region B in the promoter of GhCKI. Regions A to D ranged from −1 to −168, from −169 to −478, from −479 to −733, and from −734 to −1,002 bp of ProGhCKI, respectively. Empty pDEST22 vector was used as a negative control. The growth of transformants on SD-Trp-His + 8 mm 3-AT medium showed that GhLEC1 was able to bind to the ProGhCKI. B, Truncated B3 region of the ProGhCKI sequences and different mutated versions as indicated in the text. The underlined ML1 and mML1 are the CTTTTC core sequence and mutated CTTTTC (TGGGGT) sequence, respectively. C and D, EMSA analysis of GhLEC1 protein interacting with dig-labeled oligonucleotide as shown in B. C, The binding status between GhLEC1 and different B3 regions of ProGhCKI deleted progressively in the 3′ to 5′ direction. D, GhLEC1 binding to the B3 region of ProGhCKI was confirmed by competition experiments by ML1 core sequence mutation. Lower shows no binding between GhLEC1 and B3 with two ML1 mutations. Black arrows in C and D indicate shifted bands. FP, Free probe. E, GhLEC1 failed to bind ProGhCKI when two ML1s were mutated (mProGhCKI) in the Y1H assays. Empty pDEST22 vector served as a negative control. SD-Trp-His + 8 mm 3-AT medium was used to check the binding. F, Transcriptional suppression of GhLEC1 on the promoter of GhCKI in vivo. Fa, Effectors prepared for cotton EC protoplast transient assays. Nos, nopaline synthase; 35S, promoter of cauliflower mosaic virus; Ω, translation enhancer. Fb, Schematic representation of the reporters and GhLEC1 suppression of GhCKI transcription as revealed by the relative LUC activity. The effectors (none, GAL4BD, and GhLEC1) and reporters (ProGhCKI:LUC and mProGhCKI:LUC) were cotransformed. GhLEC1 displayed high and no repression activity for the transcription of wild-type and mutated ProGhCKIs, respectively. None and GAL4BD served as negative controls. Asterisks indicate statistically significant differences between GhLEC1 and two negative controls (**; P < 0.01, Student's t test).
To determine the exact functional binding site for GhLEC1 in region B, six distinct regions (B1–B6) progressing from the 5′ to the 3′ direction in region B were synthesized (Supplemental Fig. S4B). Next, we incubated His-tagged recombinant GhLEC1 (Supplemental Fig. S4C) with the B1 to B6 double-stranded DNA oligonucleotide probes, respectively, to evaluate binding using in vitro electrophoretic mobility shift assays (EMSAs); two shifted bands indicative of GhLEC1 binding to B3 were detected. Next, region B3 was deleted progressively in the 3′ to the 5′ direction to design four probes (B3-1–B3-4) for EMSA (Fig. 3B). In each lane, two mobility shifts, dig-tagged B3-1 and B3-2, were observed in the polyacrylamide gels, and B3-2 showed a slightly reduced intensity of the shifted bands compared with B3-1. In contrast, only one weak band was found in B3-3, and no interaction was detected between GhLEC1 and B3-4 (Fig. 3C). These results indicated that CTTTTC (called an ML motif [ML1]) is a putative binding site for GhLEC1.
To further confirm this possibility, competition experiments were performed. As shown in Figure 3D, the binding of GhLEC1 to B3 could be effectively competed by adding excessive amounts of unlabeled B3 probe (10× and 50×). Furthermore, unlabeled B3 probes containing one and two ML1-mutated (TGGGGT) sequences could be partially and completely competed for binding of GhLEC1 to the B3 fragment of the ProGhCKI (Fig. 3D). Parallel experiments indicated that GhLEC1 was unable to bind labeled B3 probes containing two ML1-mutated regions (Fig. 3D). In addition, ProGhCKI containing two ML1 mutations (mProGhCKI) was also cloned and mated with GhLEC1 in yeast. No visible clones were observed (Fig. 3E). These results indicate that the ML1 core sequence of ProGhCKI is required for GhLEC1 binding.
Moreover, the interaction between the GhLEC1 and ProGhCKI was quantified using a dual-luciferase reporter system in cotton protoplasts. Compared with negative controls, GhLEC1 strongly down-regulated the activity of the luciferase (LUC) reporter in response to ProGhCKI, whereas this activity was not changed in mProGhCKI compared with the controls (Fig. 3F). These results show that GhLEC1 putatively suppresses the transcription of GhCKI by binding to the ML1 sequences of the B region of ProGhCKI in vitro.
GhCKI Interacts with and Phosphorylates GhTCP15 in Vitro
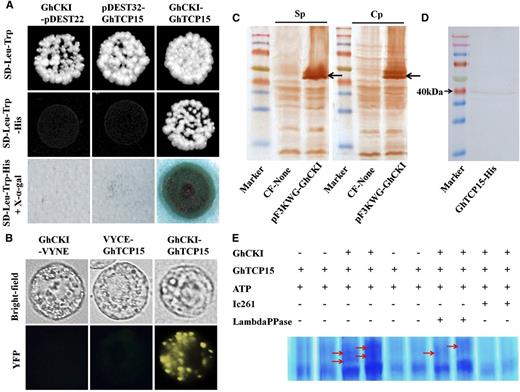
To understand the regulatory network of which GhCKI is a component during SE, full-length GhCKI was used to screen two yeast two-hybrid (Y2H) libraries: one Arabidopsis transcription factor library and one cotton EC library; 64 and 28 candidate GhCKI-interacting proteins were identified in the two libraries, respectively. Among these proteins, we identified a class I TCP transcription factor (AtTCP15) from the Arabidopsis transcription factor library and a protein from an upland cotton EC complementary DNA (cDNA) library that was completely homologous to Gossypium barbadense (Gb)TCP (Supplemental Fig. S5; Hao et al., 2012). GbTCP has the greatest similarity to AtTCP15, and therefore, the protein was also named GhTCP15. Two recent studies have shown that AtTCP15 is involved in the regulation of cell division, proliferation, and differentiation (Kieffer et al., 2011; Li et al., 2012). Thus, the ORF of GhTCP15, encoding 344 amino acids with a calculated M r of 37.6, was cloned and mated to GhCKI. The result showed that GhCKI interacted with GhTCP15 (Fig. 4A), and the result was further verified by β-galactosidase and bimolecular fluorescence complementation (BiFC) assays using cotton protoplasts from embryonic callus (Fig. 4, A and B).
GhCKI interacted with and phosphorylated GhTCP15. A, GhCKI interacts with GhTCP15 in Y2H assays. The empty pDEST22 and pDEST32 were used as negative controls. Blue colonies on SD-Trp-Leu-His + X-α-gal medium indicated positive interactions. B, BiFC assay showing the interaction of GhCKI with GhTCP15 in vivo. The yellow fluorescence indicates a positive interaction. The expression of GhCKI or GhTCP15 alone (GhCKI-VYNE or VYCE-GhTCP15) in cotton protoplasts was assessed as a negative control. YFP, Yellow fluorescence protein. C, GhCKI protein expressed by cell-free protein expression assays were detected by western blot. CF-None indicates a mixture containing no DNA as a negative control reaction. pF3KWG-GhCKI indicates cell-free expression mixture with expressed GhCKI. Sp and Cp indicate antibodies prepared using the specific and conserved domain sequences, respectively, of the GhCKI protein as probes. Black arrows indicate hybridization signals. D, Induced expression of His-tagged GhTCP15 was verified by western blot. The M r of expressed GhTCP15 was consistent with the calculated M r (37.6). E, Phosphorylation of GhTCP15 by GhCKI was verified by in vitro phosphorylation assays using Phos-tag SDS-PAGE. Ic261 is a CKI inhibitor. Red arrows indicate positive phosphorylation. LambdaPPase, λ-PPase.
Because GhCKI interacted with GhTCP15 and because the amino acid sequence of GhTCP15 was found to contain several consensus CKI phosphorylation sites (Supplemental Fig. S6), it was necessary to determine whether GhCKI phosphorylates GhTCP15. Therefore, GhCKI and GhTCP15 proteins were synthesized using high-yield wheat (Triticum aestivum) germ cell-free and prokaryotic protein expression systems, respectively (Fig. 4, C and D). The GhCKI and GhTCP15 proteins were subjected to in vitro phosphorylation assays and visualized by SDS-PAGE containing Phos-tag and MnCl2. As shown in Figure 4E, GhTCP15 was phosphorylated by GhCKI, and the kinase activity of GhCKI was disrupted by an inhibitor of CKI (Ic261). The impaired density of the phosphorylation band of GhTCP15 corresponded to the appearance of λ-phosphatase, which suggested that GhCKI represents a typical CKI and is able to use GhTCP15 as a substrate for phosphorylation in vitro.
GhLEC1, GhCKI, and GhTCP15 Affect Callus Proliferation
Through Y1H and Y2H experiments, GhLEC1 and GhTCP15 were identified as regulatory genes upstream and downstream of GhCKI. To further confirm the effects of GhCKI on SE, we used Arabidopsis-overexpressing GhCKI (OE25-6, with moderate GhCKI expression, showed normal male fertility similar to the wild type and OE15-7, with high GhCKI expression, showed male sterility) that was previously generated (Min et al., 2013). In addition, because a multiple sequence alignment of the conserved domain of CKI, Arabidopsis CASEIN KINASE1 (AtCKL1), and AtCKL2 showed a high degree of nucleotide sequences conservation with GhCKI (Supplemental Fig. S7A), we also transformed Arabidopsis plants using an RNAi vector containing the conserved region of GhCKI (35S:iGhCKIc; Supplemental Fig. S1B). Among 16 independent transformants, two 35S:iGhCKIc lines (conserved region/domain of GhCKI RNAi [CDi-1] and CDi-6) containing only one copy and showing significantly down-regulated expression of AtCKL1 and AtCKL2 were used for further study (Supplemental Fig. S7, B and C). In addition, Arabidopsis were transformed with a GhLEC1 overexpression (Lov6-29) construct to produce lines Lov6-29 and Lov7-11 (Supplemental Fig. S8, A and B). The lec1-1 mutant (CS8101) was obtained from the Arabidopsis Biological Resource Center. It was found that the expressions of AtCKL1 and AtCKL2 were down-regulated by overexpressing GhLEC1 but up-regulated in lec1-1 mutants (Supplemental Fig. S8C), suggesting that LEC1 acts as a repressor of the transcription of GhCKI.
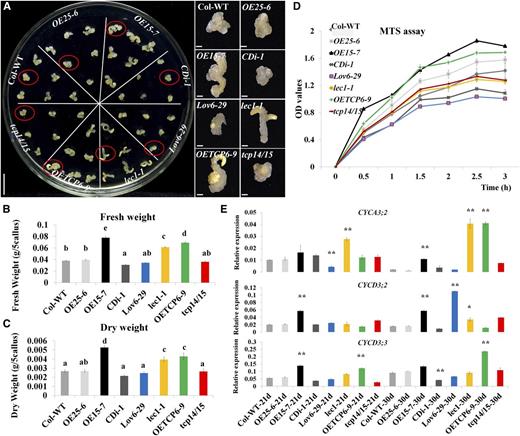
To analyze the role of GhTCP15 further, a homozygous T3 line with a high expression level of GhTCP15 (OETCP6-9; Hao et al., 2012) and the tcp14-4/tcp15-3 double mutant (tcp14/tcp15; AtTCP14 and AtTCP15 are two closely related genes; Kieffer et al., 2011) were collected. Then, Columbia-0 (Col-0) wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/tcp15 (Table I) seeds of Arabidopsis were cultured on ASSE medium to conduct a holistic analysis of the effect on callus formation and growth. After induction for 21 d, OE15-7, OETCP6-9, and lec1-1 explants showed a greater callus fresh and dry weights than controls (Fig. 5, A–C). Lov6-29, CDi-1, and tcp14/tcp15 produced smaller fresh and dry weights of callus compared with the Col-0 wild type and OE25-6 (Fig. 5, A–C). Moreover, for longer induction up to 30 d, the trends for callus fresh and dry weights for the eight genotypes were similar with those of 21 d (Supplemental Fig. S9). These results indicated that GhLEC1, GhCKI, and GhTCP15 influence callus mass during SE.
The correspondent construct and description for Arabidopsis transgenic line
| Code . | Construct . | Description . |
|---|---|---|
| Col-0 wild type | The wild type in a Col-0 background | |
| OE25-6 | 35S:GhCKI | Moderate GhCKI expression in Arabidopsis (Min et al., 2013) |
| OE15-7 | 35S:GhCKI | High GhCKI expression in Arabidopsis (Min et al., 2013) |
| CDi-1 | 35S:iGhCKIc | Down-regulated expression of AtCKL1 and AtCKL2 in Arabidopsis |
| CDi-6 | 35S:iGhCKIc | Down-regulated expression of AtCKL1 and AtCKL2 in Arabidopsis |
| Lov6-29 | 35S:GhLEC1 | High GhLEC1 expression in Arabidopsis |
| Lov7-11 | 35S:GhLEC1 | High GhLEC1 expression in Arabidopsis |
| lec1-1 | 3850:1003 | AtLEC1 transfer DNA insertion mutant (CS8101) |
| OETCP6-9 | 35S:GbTCP/35S:GhTCP15 | High GbTCP/GhTCP15 (GhTCP15 protein completely homologous to GbTCP) expression in Arabidopsis (Hao et al., 2012) |
| tcp14/tcp15 | tcp14-4/tcp15-3 | AtTCP14 transfer DNA insertion mutant crosses with tcp15-3 mutant (Salk_011491; Kieffer et al., 2011) |
| Code . | Construct . | Description . |
|---|---|---|
| Col-0 wild type | The wild type in a Col-0 background | |
| OE25-6 | 35S:GhCKI | Moderate GhCKI expression in Arabidopsis (Min et al., 2013) |
| OE15-7 | 35S:GhCKI | High GhCKI expression in Arabidopsis (Min et al., 2013) |
| CDi-1 | 35S:iGhCKIc | Down-regulated expression of AtCKL1 and AtCKL2 in Arabidopsis |
| CDi-6 | 35S:iGhCKIc | Down-regulated expression of AtCKL1 and AtCKL2 in Arabidopsis |
| Lov6-29 | 35S:GhLEC1 | High GhLEC1 expression in Arabidopsis |
| Lov7-11 | 35S:GhLEC1 | High GhLEC1 expression in Arabidopsis |
| lec1-1 | 3850:1003 | AtLEC1 transfer DNA insertion mutant (CS8101) |
| OETCP6-9 | 35S:GbTCP/35S:GhTCP15 | High GbTCP/GhTCP15 (GhTCP15 protein completely homologous to GbTCP) expression in Arabidopsis (Hao et al., 2012) |
| tcp14/tcp15 | tcp14-4/tcp15-3 | AtTCP14 transfer DNA insertion mutant crosses with tcp15-3 mutant (Salk_011491; Kieffer et al., 2011) |
| Code . | Construct . | Description . |
|---|---|---|
| Col-0 wild type | The wild type in a Col-0 background | |
| OE25-6 | 35S:GhCKI | Moderate GhCKI expression in Arabidopsis (Min et al., 2013) |
| OE15-7 | 35S:GhCKI | High GhCKI expression in Arabidopsis (Min et al., 2013) |
| CDi-1 | 35S:iGhCKIc | Down-regulated expression of AtCKL1 and AtCKL2 in Arabidopsis |
| CDi-6 | 35S:iGhCKIc | Down-regulated expression of AtCKL1 and AtCKL2 in Arabidopsis |
| Lov6-29 | 35S:GhLEC1 | High GhLEC1 expression in Arabidopsis |
| Lov7-11 | 35S:GhLEC1 | High GhLEC1 expression in Arabidopsis |
| lec1-1 | 3850:1003 | AtLEC1 transfer DNA insertion mutant (CS8101) |
| OETCP6-9 | 35S:GbTCP/35S:GhTCP15 | High GbTCP/GhTCP15 (GhTCP15 protein completely homologous to GbTCP) expression in Arabidopsis (Hao et al., 2012) |
| tcp14/tcp15 | tcp14-4/tcp15-3 | AtTCP14 transfer DNA insertion mutant crosses with tcp15-3 mutant (Salk_011491; Kieffer et al., 2011) |
| Code . | Construct . | Description . |
|---|---|---|
| Col-0 wild type | The wild type in a Col-0 background | |
| OE25-6 | 35S:GhCKI | Moderate GhCKI expression in Arabidopsis (Min et al., 2013) |
| OE15-7 | 35S:GhCKI | High GhCKI expression in Arabidopsis (Min et al., 2013) |
| CDi-1 | 35S:iGhCKIc | Down-regulated expression of AtCKL1 and AtCKL2 in Arabidopsis |
| CDi-6 | 35S:iGhCKIc | Down-regulated expression of AtCKL1 and AtCKL2 in Arabidopsis |
| Lov6-29 | 35S:GhLEC1 | High GhLEC1 expression in Arabidopsis |
| Lov7-11 | 35S:GhLEC1 | High GhLEC1 expression in Arabidopsis |
| lec1-1 | 3850:1003 | AtLEC1 transfer DNA insertion mutant (CS8101) |
| OETCP6-9 | 35S:GbTCP/35S:GhTCP15 | High GbTCP/GhTCP15 (GhTCP15 protein completely homologous to GbTCP) expression in Arabidopsis (Hao et al., 2012) |
| tcp14/tcp15 | tcp14-4/tcp15-3 | AtTCP14 transfer DNA insertion mutant crosses with tcp15-3 mutant (Salk_011491; Kieffer et al., 2011) |
Ectopic expression of GhLEC1, GhCKI, and GhTCP15 in Arabidopsis affects the callus proliferation. A, Callus growth of the Col-0 wild type (WT), OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/15 at 21 d. Calluses in red circles are highlighted on the right. Bars = 1 cm (culture plate) and 1 mm (highlighted pictures). B and C, Determination of the fresh weight (B) and dry weight (C) of the Col-0 wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/15 cultures cultured for 21 d. The fresh and dry weights were calculated as grams of callus from five explants. D, MTS assays. Protoplasts (2 × 105) were prepared from cultures of the Col-0 wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/15 after 21 d of culturing and then cultured in 100 μL of W5 with MTS for 0.5 to 3 h for detecting OD values. (E) Expression levels for cell proliferation-related genes (CYCA3;2, CYCD3;2, and CYCD3;3) were validated by qRT-PCR in cultures of the Col-0 wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/15 after induction for 21 and 30 d. The data in B to D represent the means ± sem of biologically independent experiments (n ≥ 3). Values in B and C not sharing a common letter were considered statistically significant (SSR; P < 0.05). Asterisks in D indicate statistically significant differences between different lines and the wild type (Student’s t test). *, P < 0.05; **, P < 0.01.
Changes in organ size and mass rely on alterations in cell size, number, or both (Mizukami and Fischer, 2000). In general, the cell numbers in the developing organ depend essentially on cell proliferation (Mizukami and Fischer, 2000). To analyze the mechanism by which GhCKI affected callus mass, we compared the callus mass ratio in the Col-0 wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/tcp15 at two time points (21/0 d and 30/21 d; Supplemental Fig. S9). The callus proliferation rates of OE15-7 and OETCP6-9 were significantly higher and those of Lov6-29 and CDi-1 were significantly lower than those of the Col-0 wild type and OE25-6. However, no significant differences in proliferation rate were seen between tcp14/tcp15 and lec1-1 and the Col-0 wild type or OE25-6 (Supplemental Fig. S9). We also used the tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt [MTS]) and an electronic coupling agent (phenazine ethosulfate) to test proliferation at the biochemical level. Protoplasts (2 × 105) from 21-d-old callus from each line were prepared for MTS assays; then, the optical density (OD) value at 490 nm was determined every 0.5 h for 3 h using a microplate reader. After 0.5 h, OE15-7 showed significantly higher OD values than the other lines. From 1 to 3 h, the OD values of eight genotype lines in MTS assays (Fig. 5D) showed the same trends as callus proliferation rate analysis (Supplemental Fig. S9). In addition, the expressions of some cyclin-dependent protein kinase genes (Cyclin-dependent protein kinase3;2 [CYCA3;2], CYCLIN D3;2 [CYCD3;2], and CYCD3;3) associated with cell proliferation (Vandepoele et al., 2002; Dewitte et al., 2007) were markedly increased in OE15-7 and OETCP6-9 and significantly decreased in Lov6-29 and CDi-1 in 21- and 30-d cultures compared with those of OE25-6 and the Col-0 wild type. However, the expressions of three cyclins were slightly changed in lec1-1, except CYCA3;2 and CYCD3;2, which were significantly increased in lec1-1 30-d cultures. In tcp14/tcp15, the expression of three cyclins was slightly increased, except the expression of CYCD3;3 was significantly decreased in 21 d (Fig. 5E). These results were consistent with the MTS assays (Fig. 5D). Taken together, GhLEC1, GhCKI, and GhTCP15 may affect callus mass by regulating cell proliferation.
GhLEC1-GhCKI-GhTCP15 Regulates Cell Proliferation by Regulating Auxin Biosynthesis
It is well established that different cellular auxin signaling cascades are translated into different cellular responses, including cell expansion, cell proliferation, and cell differentiation (Benjamins and Scheres, 2008; Vanneste and Friml, 2009; Ishida et al., 2010). A high concentration of auxin has often been shown to function as an efficient initiator of callus formation and proliferation during SE, when the explants are exposed at an early stage (Leyser, 2002). In our previous study, GhCKI was found to be involved in the auxin signaling pathway (Min et al., 2014). To investigate the effects of GhCKI on auxin metabolism, the IAA contents were determined for the 21-d-induced Arabidopsis explants (Fig. 6A). The OE15-7, OETCP6-9, and lec1-1 explants showed the highest IAA contents; the Col-0 wild type, OE25-6, and tcp14/tcp15 showed medium IAA contents, and CDi-1 and Lov6-29 showed the lowest IAA contents. The IAA contents were reflected in the expression of auxin biosynthesis-related genes (AtYUC2, AtYUC5, and AtYUC8; Fig. 6B). IAA content and the expression of IAA biosynthesis genes were consistent with the trends of callus mass and callus proliferation rate of the eight genotypes lines. These results indicated that GhLEC1, GhCKI, and GhTCP15 may affect cell proliferation by influencing the homeostasis of endogenous IAA through regulating the expression level of auxin biosynthesis genes.
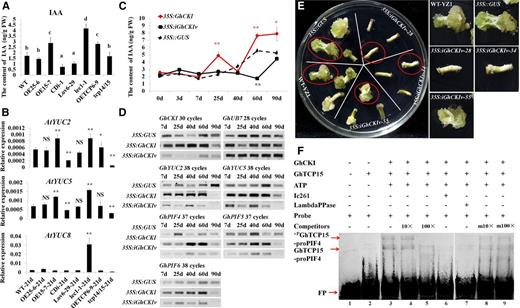
GhCKI is involved in auxin biosynthesis by regulating the transcription of GhPIF4 through phosphorylating GhTCP15. A, IAA contents of the Col-0 wild type (WT), OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/15 in 21-d cultures. The data represent the means ± sem of biologically independent experiments (n ≥ 3). Values not sharing a common letter were considered statistically significant (SSR; P < 0.05). B, Expression levels of auxin biosynthesis genes were validated by qRT-PCR in the Col-0 wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/15 21-d cultures. The data represent the means ± sem of biologically independent experiments (n ≥ 3). Asterisks indicate statistically significant differences between different lines and the wild type (Student’s t test). NS, Not significant; *, P < 0.05; **, P < 0.01. C, Determination of IAA content in 35S::GUS, 35S:GhCKI, and 35S:iGhCKIv cultures at different culture time points of transgenic cotton explants. The data represent the means ± sem of three biologically independent experiments. Red asterisks indicate statistically significant differences between 35S:GhCKI and 35S::GUS. Black asterisks indicate statistically significant differences between 35S:iGhCKIv and 35S::GUS. FW, Fresh weight; *, P < 0.05; **, P < 0.01. D, RT-PCR showing the expression of PIFs and auxin biosynthesis genes in 35S::GUS, 35S:GhCKI, and 35S:iGhCKIv transgenic cotton explants. GhUB7 was used as a control; 7 to 90 d indicate that explants transformed with different constructs were cultured on callus induction medium without antibiotics for 2 d (coculture) followed by transfer to induction medium containing antibiotics and cultured for 7 to 90 d. E, Homozygous 35S:iGhCKIv transgenic lines were cultured on medium without applying exogenous auxin. Wild-type YZ1 and 35S::GUS act as controls and showed significantly increases in callus mass accompanied by the appearance of somatic embryos. However, the inhibition of callus induction in 35S:iGhCKIv-28 and -34 cotton explants, leading to SE abortion, was observed. Calluses in red circles are highlighted on the right. Bars = 1 cm. F, EMSA showing the effects of GhCKI on the binding of GhTCP15 to labeled GhTP4 probe carrying a GTGGGACC core element in the promoter of GhPIF4. Lanes 2 to 6, Untreated GhTCP15 (lane 2) or GhTCP15 preincubated with GhCKI and ATP (lane 3), 10× or 100× unlabeled GhTP4 added to lane 3 reaction solution (lanes 4 and 5), and the CKI inhibitor Ic261 added to lane 3 (lane 6). To examine the effect of dephosphorylation, the mixture in lane 3 was incubated with λ-PPase (lane 7). Additionally, 10× or 100× unlabeled mproPIF4 was added to lane 3 (lanes 8 and 9, respectively). Ic261 is the CKI inhibitor; 10× or 100× indicate 10× or 100× unlabeled ProPIF4, and m10× or m100× indicate m10× or m100× unlabeled mproPIF4. +PGhTCP15-ProPIF4 and GhTCP15-ProPIF4 indicate phosphorylated GhTCP15 and GhTCP15 binding to labeled ProPIF4, respectively. FP, Free probe; FW, fresh weight; LambdaPPase, λ-PPase.
GhCKI Might Regulate Auxin Biosynthesis by Regulating the Transcription of GhPIF4 via Phosphorylating GhTCP15
GhCKI and auxin were implicated in the SE process as suggested by the increased levels of IAA detected in 21-d callus of the Arabidopsis GhCKI overexpression line (OE15-7; Fig. 6A) and cotton explants derived from 35S::GUS, 35S:GhCKI, and 35S:iGhCKIv (Fig. 6C). Compared with 35S::GUS, the 35S:GhCKI explants showed significantly higher levels of IAA at 25, 60, and 90 d, and 35S:iGhCKIv showed significantly lower levels of IAA at 60 d. The IAA contents also were reflected in the expression of auxin biosynthesis-related genes (GhYUC2 and GhYUC5) in cotton explants (Fig. 6D). Then, the hypocotyls of the homozygous 35S:iGhCKIv lines were directly induced on medium lacking exogenous auxin, with wild-type Yuzhao NO.1 (YZ1) and 35S::GUS serving as controls. As shown in Figure 6E, wild-type YZ1, 35S::GUS, and 35S:iGhCKIv-55 (the expression of GhCKI was slightly decreased; Supplemental Fig. S2A) showed significant increases in callus mass accompanied by the appearance of somatic embryos. However, the inhibition of callus induction in 35S:iGhCKIv-28 and 35S:iGhCKIv-34 cotton explants leading to SE abortion was observed. These results further support a link between GhCKI and IAA during SE.
It is well established that PIFs directly regulate multiple auxin biosynthesis genes by binding to G-box sequences in Arabidopsis genes promoters (Franklin et al., 2011). To further characterize a possible link between GhCKI and IAA, the expressions of three cotton PIFs were measured by reverse transcription (RT)-PCR (Fig. 6D), and the results showed that GhPIF4, GhPIF5, and GhPIF6 were expressed to higher levels in 35S:GhCKI cultures but to a reduced extent in 35S:iGhCKIv cultures compared with 35S::GUS, and this is consistent with the changes in the expression of GhYUC2 and GhYUC5 (Fig. 6D). We, therefore, speculate that GhCKI may regulate auxin signaling during SE by GhPIFs regulation of YUC genes, although no direct interaction between GhCKI and GhPIFs was found.
Interestingly, there is an interaction between GhCKI and GhTCP15 (Fig. 4); to examine whether the GhTCP15 phosphorylated by GhCKI affected DNA binding to GhPIF promoters, we searched the class I TCP binding sequence GTGGGNCC and the classes I and II TCP common binding element site II (TGGGCC/T; Trémousaygue et al., 2003) in the region 1.8-kb upstream of GhPIF4, GhPIF5, and GhPIF6. The typical class I TCP binding sequence GTGGGNCC (Trémousaygue et al., 2003) appeared in the GhPIF4 promoter (ProPIF4) from −935 to −942 bp. To investigate the nature of the protein complex binding the promoter, a purified His-tagged GhTCP15 was used to perform EMSA analysis after incubation of the proteins with GhCKI and ATP. In the absence of GhCKI and ATP, GhTCP15 only weakly bound to ProPIF4 to form a complex (Fig. 6F, lane 2). Incubation of GhTCP15 with GhCKI and ATP before EMSA resulted in a strong increase in the DNA-binding activity of GhTCP15 (Fig. 6F, lane 3). However, after the addition of 10× and 100× unlabeled ProPIF4 to compete the binding or the addition of the CKI inhibitor Ic261, the binding activities were impaired (Fig. 6F, lanes 4–6). The DNA-binding activity was also impaired when the GhTCP15 protein was pretreated with GhCKI and ATP and then incubated with λ-phosphatase before EMSA (Fig. 6F, lane 7). In contrast, incubation of GhTCP15 with both GhCKI and ATP using the GhPIF4 promoter mutant (mproPIF4) as the competitor resulted in no detectable differences in binding activity (Fig. 6F, lanes 8 and 9). The results implied that GhCKI phosphorylates GhTCP15 to regulate the transcription of GhPIF4 and thereby, may regulate auxin biosynthesis to affect cell proliferation.
GhLEC1, GhCKI, and GhTCP15 Affect Cell Differentiation
GhLEC1, GhCKI, and GhTCP15 may affect cell proliferation by regulating auxin biosynthesis, but whether GhLEC1, GhCKI, and GhTCP15 affect the production of competent embryogenic cells and subsequent formation of EC and somatic embryos requires further investigation. Somatic embryos can develop from EC derived from competent embryogenic cells. Genes known to induce SE when overexpressed include PGA6/WUS and BABY BOOM (BBM), which play predominant roles in maintaining embryonic cell identity (Boutilier et al., 2002; Zuo et al., 2002; Hecht et al., 2001). Therefore, the expression levels of PGA6/WUS and BBM were examined at 21 d in callus derived from the Col-0 wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/tcp15.
No significant differences in the expression of PGA6/WUS and BBM were observed in the Col-0 wild type, OE25-6, OE15-7, lec1-1, OETCP6-9, and tcp14/tcp15 callus. However, in CDi-1 and Lov6-29 tissues, the expression of PGA6 and BBM genes was slightly increased compared with that of the Col-0 wild type (Supplemental Fig. S10A). The results indicate that AtLEC1 mutant (lec1-1) and overexpression of GhCKI and GhTCP15 did not affect the production of competent embryogenic cells from NEC. To check whether GhLEC1, GhCKI, and GhTCP15 expressions were associated with the formation of EC and somatic embryos, the expression levels of AtLEC1, AtFUS3, and AtABI3 in 30-d callus of eight genotypes lines were analyzed by qRT-PCR (Supplemental Fig. S10B). AtLEC1 was expressed at low levels in OE15-7, OETCP6-9, and tcp14/tcp15 but undetectable in lec1-1. AtFUS3 and AtABI3 genes were highly expressed in 30-d-old callus of CDi-1 and Lov6-29 and expressed at intermediate levels in the Col-0 wild type, OE25-6, lec1-1, and tcp14/tcp15 but expressed at low levels in OE15-7 and OETCP6-9 (Supplemental Fig. S10B).
The 30-d-old callus represents the stage in which somatic embryos are formed (Lotan et al., 1998), which is illustrated by transmission electron microscopy (TEM). The cultures of OE15-7, OETCP6-9, and lec1-1 consisted of abnormal cells with a large central vacuole, thin cytoplasm, and few organelles; the other cultures consisted of cells with identical diameters and dense cytoplasms, small vacuoles, rich starchy grains, and large nuclei (Supplemental Fig. S11). In plants, nonembryogenic cells are characterized by a large central vacuole and thin cytoplasm, whereas EC cells typically have dense cytoplasm, small vacuoles, and large nuclei. In addition, the mitochondria in OE15-7, OETCP6-9, and lec1-1 cultured cells showed a complete outer membrane and no internal vacuolization, whereas cells of the Col-0 wild type, OE25-6, CDi-1, Lov6-29, and tcp14/15 displayed internal vacuolization in the mitochondrion. These observations suggest that overexpression of GhLEC1 may promote somatic embryo formation, whereas overexpression of GhCKI and GhTCP15 suppresses somatic embryo formation by promoting cell proliferation and decreasing the competence for embryogenic cell differentiation.
DISCUSSION
GhCKI Is a Negative Regulator of Somatic Embryo Formation
The Y1H system revealed that GhLEC1 binds to the promoter of GhCKI. LEC1 is required for the normal development of both early and late embryo morphogenetic events and efficiently promotes embryonic cell differentiation from vegetative cells; the lec1-1 mutation causes defective embryo maturation (Lotan et al., 1998). However, a credible target binding site for LEC1 has not been identified previously. LEC1, which encodes an HAP3 subunit of the CCAAT, is presumed to interact with the other HAP subunits to form a complex that binds a CCAAT box (Lotan et al., 1998). However, no consensus CCAAT DNA sequence was found in the promoter of GhCKI. In addition, LEC1 belongs to the NUCLEAR FACTOR Y B subunit (NF-YB) family, and many studies have shown that some NF-YB members (such as LEC2, ABI3, and FUS3) can bind to the RY/Sph (for pyrimidines and purine repeat element/SphI restriction site element) motif ([A/C]TGCATG; Braybrook et al., 2006), whereas no typical RY/Sph motif was found within ProGhCKI. Based on the expression profile analysis of the 5′ deleted ProGhCKI, we found that region B was sufficient for GhLEC1 binding. EMSAs were performed between GhLEC1 and the 5′ deletions of region B in ProGhCKI, and the results suggested that B3 is critical for the GhLEC1 binding. Additional EMSAs showed that ML1 (CTTTTC) is the specific recognition site for GhLEC1, which is the first LEC1 binding site to be identified.
Previous studies have found genes that are regulated by LEC2 through microarray analysis (Braybrook et al., 2006), and AGL15 was determined to be a direct target gene based on the Chromatin Immunoprecipitation-chip approach and Affymetrix tiling arrays (Zheng et al., 2009). However, no reports have described direct target genes of LEC1. Confirmation of the ML1 motif provided evidence for the identification of potential targets that are directly regulated by LEC1. We and others have shown that ectopic overexpression of LEC1 promoted cellular processes by enhancing embryo induction in vegetative cells, suggesting that the LEC1 gene is necessary and sufficient for the induction of somatic embryo formation. As a direct target of GhLEC1, GhCKI showed an opposite expression pattern compared with GhLEC1, and it was confirmed by EMSAs and LUC assays that GhLEC1 directly suppressed the expression of GhCKI. Because CDi-1 showed phenotypes similar to GhLEC1 overexpression lines (Fig. 5) and because overexpression of GhCKI in cotton and Arabidopsis inhibited somatic embryo formation, it is possible that GhCKI plays a role distinct from that of GhLEC1 in SE and acts as a negative regulator of somatic embryo formation.
Both GhCKI and GhTCP15 Are Key Genes in Cellular Proliferation
We have described the interaction of GhCKI with GhTCP15 and their similar expression patterns during SE. Overexpression of GhTCP15 resulted in a phenotype similar to that observed for the overexpression of GhCKI (Fig. 5). TCP proteins are plant-specific transcription factors with important roles in multiple plant developmental events and associated with cell proliferation (Li et al., 2005; Kieffer et al., 2011). Li et al. (2005) reported that classes I and II TCP factors function in an opposite manner in plant cell growth and proliferation, with class II TCP factors inhibiting but class I factors promoting cell proliferation. GhTCP15, a homolog of AtTCP15, is a class I TCP protein. Therefore, we propose that GhTCP15 and its interactive protein GhCKI are both critical for cell proliferation, because they may regulate the expression of cell cycle-associated genes. Class I TCP proteins regulate the expressions of the CYCB1;1 gene in the G2/M phase of the cell cycle and proliferation cellular nuclear antigen 2 during the G1/S transition to control the balance between cell proliferation and endoreduplication (Boudolf et al., 2004). Class II TCP proteins reduce the expression of histones and cyclins (cell cycle marker genes) to negatively regulate cell growth (Gaudin et al., 2000). Interestingly, CYCP3;1, CYCP3;2, and one cyclin A gene were all up-regulated in Arabidopsis root or cotton fibers by overexpressing GbTCP (GhTCP15; Hao et al., 2012), and the binding site for TCP protein was often found together with a telo box in the promoters of cell cycle-related genes (Trémousaygue et al., 2003). In our Y1H assays, AtTCP4 potentially regulated GhCKI (data not shown), and the telo box was also found in the promoter of GhCKI (Fig. 3). GhCKI is a member of the CKI family, and studies in mammals have shown that CKI is involved in circadian rhythms, DNA repair pathways, and cell cycle progression, among others (Dhillon and Hoekstra, 1994; Peters et al., 1999; Behrend et al., 2000). Considering these findings together, we propose that GhCKI might participate in or regulate cell cycle progression. Thus, it is possible that the overexpression of GhCKI or GhTCP15 accelerates the cell cycle and ultimately, causes excessive callus proliferation.
In addition to the excessive callus proliferation observed in both GhCKI- and GhTCP15-overexpressing Arabidopsis, more branches, more flowers, and shorter siliques with few seeds were also observed in the GhTCP15 highly overexpressing lines (Hao et al., 2012), which were similar to those observed in OE15-7 with high expression of GhCKI (Min et al., 2013). These data suggest that both GhCKI and GhTCP15 cooperatively regulate multiple developmental processes.
GhCKI Coordinates Cell Proliferation and Differentiation by Altering Auxin Homeostasis
We found that GhCKI positively regulates the callus mass, with a smaller mass in the RNAi line CDi-1 and a larger mass in the overexpresser OE15-7. Increase in callus mass is determined primarily by cell proliferation rather than water uptake, which was shown by the increasing dry weight (Fig. 5C). This finding suggests that GhCKI positively regulates cell proliferation. Somatic embryo formation is regulated by the coordination of cellular proliferation and differentiation, and plant hormones are involved in regulating these processes. Auxin, ethylene, ABA, GA, brassinosteroids, and cytokinin have been reported to participate in callus formation, development, and SE processes (Goren et al., 1979; Depuydt and Hardtke, 2011). Of these, auxin and cytokinin are the most studied hormones in SE; the balance between these two types of hormones determines cell proliferation and differentiation (Skoog and Miller, 1957).
Interestingly, LEC1 up-regulated the auxin biosynthesis gene YUC10 (Junker et al., 2012). In our study, GhLEC1 directly but negatively regulated GhCKI, which is involved in the auxin (Min et al., 2014) biosynthetic pathways, indicating that GhLEC1 and GhCKI have essential functions in auxin homeostasis during SE. AtTCP15 was involved in the alteration in auxin homeostasis (Kieffer et al., 2011). Furthermore, a high concentration of auxin has often been shown to function as an efficient initiator of callus formation and proliferation during SE when the explants are exposed at an early stage (Leyser, 2002). However, a high concentration of auxin inhibited the callus differentiation and somatic embryo formation (Filonova et al., 2000). Thus, we propose that auxin homeostasis plays roles in the balance of cell proliferation and cell differentiation. Overexpression of GhCKI or GhTCP15 disrupted auxin homeostasis (Fig. 6, A and C), which altered the transition from cell proliferation to cell differentiation, resulting in the arrest of somatic embryo formation.
CONCLUSION
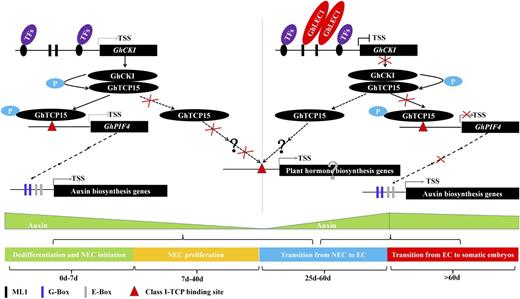
Based on an integration of the relationships between different morphologies and biochemical changes during SE in the Arabidopsis and cotton lines, we speculate that GhCKI plays a positive role in explant dedifferentiation, NEC formation, and cell proliferation by phosphorylating GhTCP15. Phosphorylated GhTCP15 has an enhanced binding to the promoter of GhPIF4 to regulate the transcription of GhPIF4, thereby regulating auxin biosynthesis at an early stage of SE. During later stages of SE, GhLEC1 expression was initiated, repressing the transcription of GhCKI by binding to the cis-element CTTTTC in the promoter of GhCKI. This might reduce GhTCP15 phosphorylation activity and decrease the transcription of GhPIF4 and auxin biosynthesis genes to promote the transition from cell proliferation to cell differentiation and somatic embryo formation (Fig. 7).
Schematic diagram illustrating how GhCKI cooperates with the upstream GhLEC1 and the downstream GhTCP15 to regulate SE by modulating the auxin signaling pathway by GhPIF4. Without GhLEC1 expression, GhCKI expression is enhanced, GhCKI kinases phosphorylate GhTCP15 at an early stage of SE (explant dedifferentiation, NEC formation, and cell proliferation), and there is increased binding of phosphorylated GhTCP15 to the promoter of GhPIF4, which promotes the expression of auxin biosynthesis genes. When the expression of GhLEC1 is initiated in the late stage of SE, the expression of GhCKI is inhibited, leading to reduced phosphorylation of GhTCP15. Thus, the transcriptions of GhPIF4 and auxin biosynthesis genes are impaired, leading to a decrease in the accumulation of auxin, which promotes the transition from NEC to EC, thereby facilitating somatic embryo formation. +P, Phosphorylation; TF, transcription factor; TSS, transcriptional start site.
MATERIALS AND METHODS
Plant Materials and Culture Medium
Seeds of YZ1 (cotton [Gossypium hirsutum]), Arabidopsis (Arabidopsis thaliana) transgenic plants (Col-0), and the mutant lines (lec1-1 [Arabidopsis; ecotype Wassilewskija] and tcp14/15 [Arabidopsis; Col-0]) were sterilized and washed as previously described (Zhang et al., 2006; Hu et al., 2011). Cotton seeds were germinated on one-half-strength Murashige and Skoog medium supplemented with 1.5% (w/v) Glc and solidified with 0.25% (w/v) phytagel (Sigma) at 28°C in the dark for 7 d. The hypocotyls of the seedling were cut into 0.5- to 0.8-cm sections and used as explants for callus induction or transformation. Callus induction was carried out on basic Murashige and Skoog medium and B5 vitamins (MSB medium) containing 3% (w/v) Glc, 0.25% (w/v) phytagel, 0.1 mg L−1 2,4-dichlorophenoxyacetic acid, and 0.1 mg L−1 kinetin for 25 d. Then, explants were subcultured on MSB medium supplemented with 3% (w/v) Glc, 0.25% (w/v) phytagel, 0.5 mg L−1 indolebutyric acid, and 0.1 mg L−1 kinetin for induction of EC or sampling. ECs were used for protoplast isolation and cultured on MSB medium containing 3% (w/v) Glc, 0.25% (w/v) phytagel, 0.5 mg L−1 indolebutyric acid, and 0.15 mg L−1 kinetin. The sterilized Arabidopsis seeds were cultured on ASSE medium (basic MSB medium containing 2.0 mg L−1 2,4-dichlorophenoxyacetic acid, 1.0 mg L−1 6-BA, 0.3 g L−1 casein acid hydrolysate, 0.5 g L−1 Gln, 0.5 g L−1 Pro, 3% (w/v) Suc, and 0.25% (w/v) phytagel, pH 5.8) at 28°C in the dark.
Vector Construction and Transformation
The 1,002-bp upstream sequence of the GhCKI start codon (ProGhCKI) was cloned (Min et al., 2013), and the putative cis-elements were predicted using the PLACE Database (Higo et al., 1999). ProGhCKI and its three progressive 5′ deletions were identified and inserted into Gateway vector pGWB433 (Nakagawa et al., 2007) and fused to GUS by attB with attP sites (BP) recombination and attL with attR sites (LR) recombination (Invitrogen) to obtain vectors ƊBCD::GUS, ƊCD::GUS, ƊD::GUS, and ProGhCKI::GUS. The isolation of GhCKI and GhTCP15 cDNAs and the construction of their overexpression vectors were performed as previously described (Hao et al., 2012; Min et al., 2013). An empty pCAMBIA2300S vector (35S::GUS) served as a control. The full-length GhLEC1 ORF was obtained from RNA extracted from cotton EC as a template, and the encoded protein was found to be 53% homologous to the AtLEC1 protein using Clustalx 1.83. The GhLEC1 overexpression vector was constructed with pK2GW7 by BP and LR reactions using primer pair Lov-Forward/Reverse (F/R). Two RNAi constructs carrying GhCKI conserved and variable regions (35S:iGhCKIc and 35S:iGhCKIv) were constructed using pHellsgate 4 through BP recombination reactions. The GhCKI, GhTCP15, and GhLEC1 overexpression constructs; ƊBCD::GUS, ƊCD::GUS, and ƊD::GUS constructs; and ProGhCKI::GUS and 35S:iGhCKIc constructs were used to transform Arabidopsis (Col-0) by the floral dip method (Zhang et al., 2006). GhCKI overexpression and 35S:iGhCKIv, ProGhCKI::GUS, and 35S::GUS constructs were introduced into cotton (YZ1) by transforming hypocotyl sections by Agrobacterium tumefaciens (Jin et al., 2005). The primers used in this study are listed in Supplemental Table S1.
GUS Activity and Histochemical and Microscopic Analyses
The histochemical localization of GUS activity in ProGhCKI::GUS, 35S::GUS, ƊBCD::GUS, ƊCD::GUS, and ƊD::GUS cotton and Arabidopsis transformants was performed as described by Min et al. (2013). To evaluate the potential production of EC, cotton explants that were transformed with 35S::GUS, 35S:GhCKI, and 35S:iGhCKIv, callus of transgenic Arabidopsis plants, and mutant lines from different culture times were imaged using a Nikon D40 Camera. The cotton explants were sectioned into 8-μm-thick slices with a microtome, stained with 0.1% aniline blue, and observed according to the work by Min et al. (2014). Electronic microscopic analyses (TEM) of the callus were conducted as previously described (Min et al., 2013).
Y1H Assay
To search for potential upstream regulators of GhCKI during SE, the ProGhCKI sequence was amplified using the primer pair ProGhCKI Y1H-F/R with adapters containing EcoRI-XbaI digestion sites and cloned into the pHisi-1 vector to generate pHisi-1-ProGhCKI. The vectors were linearized with XhoI and then transferred into YM4271 yeast (Saccharomyces cerevisiae) strains to screen an Arabidopsis transcription factor library (Ou et al., 2011). Positive clones were selected on synthetic drop-out media (SD)-Trp-His medium with different concentrations of 3-amino-1,2,4-triazole (3-AT) for 3 to 5 d at 30°C. To characterize the interaction between GhLEC1 and the promoter regions of GhCKI in yeast, the full-length GhLEC1 cDNA was cloned into a pDEST22 vector using the Gateway LR Reaction (Invitrogen) and then transferred into the Y187 yeast strain. The different regions of ProGhCKI and mProGhCKI were amplified using specific primer pairs (Supplemental Table S1) to generate the pHisi-1-ProGhCKIA, -B, -C, and -D, and pHisi-1-mProGhCKI vectors as described above. A mating-based Y1H assay was conducted according to the protocol described by Ou et al. (2011). The primers used in the Y1H assay are listed in Supplemental Table S1.
EMSAs
GhLEC1 and GhTCP15 were cloned into a pET-28a vector for expression induction in Escherichia coli BL21 strain with 1 mm isopropyl-1-thio-b-d-galactopyranoside for 4 h at 18°C. His-tagged GhLEC1 and GhTCP15 were extracted and purified using the MagneHis Protein Purification Kit (Promega). The promoter regions of GhCKI and GhPIF4 were synthesized and used as probes. Probe labeling, DNA-protein binding reactions, and probe shift detection were performed according to the user manual of a 2nd Generation DIG Gel Shift Kit (Roche) as described by Li et al. (2014). The primers and oligonucleotides used in the EMSA are listed in Supplemental Table S1.
Dual-Luciferase Reporter Assays
The dual-luciferase reporter assays were performed as described previously (Hao et al., 2010). The fragment of GhLEC1 was obtained by PCR using the primer pair GhLEC1-galactose-responsive transcription factor4 (GAL4) DNA-binding domain (GAL4BD)-F/R (Supplemental Table S1). The PCR product was then ligated into the 35S-pBDGAL4 vector using the In-Fusion HD Cloning Kit (Clontech) to obtain plasmid 35S-GhLEC1 as an effector; the None and 35S-pBDGAL4 vectors served as negative controls. The ProGhCKI sequence containing two CTTTTC core sequences was amplified using the primer pair ProGhCKI-LUC-F/R with adapters containing HindIII-SalI digestion sites using ProGhCKI::GUS vector as a template, and the relevant mProGhCKI sequence containing two CTTTTC-mutated versions was obtained by overlap extension PCR. The two fragments were inserted into GAL4-LUC to generate ProGhCKI-GAL4-LUC and mProGhCKI-GAL4-LUC as reporter constructs, with AtUbiquitin3 (AtUb3)-Renilla-LUC as an internal control. To analyze the interaction between GhLEC1 and ProGhCKI, protoplasts were isolated from cotton ECs according to the work by Yang et al. (2008), and effector, reporter, and internal controls (6, 6, and 0.5 μg, respectively) were cotransformed into protoplasts using polyethylene glycol 4000. The transformed protoplasts were cultured at 28°C in the dark for 16 h, and firefly and Renilla luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega) and the Multimode Plate Reader (PerkinElmer).
Y2H Assay
The bait construct (pDEST32-GhCKI) was prepared as previously described (Min et al., 2013). An Arabidopsis transcription factor library was provided by L. J. Qu, and a cotton SE cDNA library was prepared from ECs using Matchmaker Library Construction and Screening Kits (Clontech). The two libraries were screened using pDEST32-GhCKI by yeast mating. To confirm the interaction between GhCKI and GhTCP15, the full-length coding sequence of GhTCP15 was amplified using the primer pair GhTCP15-pDEST22-F/R and cloned into pDEST22 by the Gateway LR Reaction (Invitrogen) to generate pDEST22-GhTCP15. Next, pDEST22-GhTCP15 was introduced into yeast strain Y187, which was mated with the AH109 yeast strain containing pDEST32-GhCKI and selected on SD-Leu-Trp, SD-Leu-Trp-His, or SD-Leu-Trp-His + X-α-gal.
BiFC Assays
BiFC assays were performed as described (Waadt et al., 2008). For generation of the BiFC constructs, the VenusC:GhCKI was constructed (Min et al., 2013), and the ORF of GhTCP15 was amplified with primer pair GhTCP15-VN-F/R (Supplemental Table S1) and cloned at the SalI site of pVYNE(R) by in-fusion enzyme to obtain the VenusN:GhTCP15. Protoplasts were prepared from cotton ECs, and the following procedures were performed as per our previous description (Min et al.,2013).
Cell-Free Protein Expression
Cell-free protein expression was performed using the TNT SP6 High-Yield Wheat Germ Protein Expression System (Promega). To generate the protein expression vector, the ORF of GhCKI was amplified using the primer pair GhCKI-CF-F/R (Supplemental Table S1) and cloned into the SgfI-PmeI sites in pF3KWG (BYDV) Flexi Vector, resulting in pF3KWG-GhCKI. The mixture of pF3KWG-GhCKI and TNT SP6 High-Yield Wheat Germ Master was incubated at 25°C for 2 h for translation. Mixtures containing no DNA and Luciferase SP6 Control DNA served as negative and positive control reactions, respectively. The translation products were detected by SDS-PAGE followed by blotting with conserved or specific GhCKI antibodies.
In Vitro Phosphorylation and Dephosphorylation Assays
In vitro kinase assays for GhCKI were performed according to the manufacturer’s instructions using the Casein Kinase I Assay Kit (Sigma) with minor modifications. Cell-free-expressed GhCKI as the kinase and E. coli-induced and -expressed GhTCP15 as the substrate protein were mixed with 1× kinase assay buffer (40 mm HEPES, pH 7.5, 130 mm KCl, 10 mm MgCl2, 0.01 mm ATP, 5 mm dithiothreitol, 5 mm β-glycerophosphate, and 0.2 mm sodium orthovanadate; Sigma) and 0.1 mm ATP and distilled, deionized water or inhibitor Ic261 in a total volume of 24 μL. The reactions were incubated at 37°C for 15 min and then stopped by boiling for 5 min. For the dephosphorylation assays, the products of GhTCP15 phosphorylation by GhCKI were incubated with 1× dephosphorylation assay buffer (50 mm HEPES, pH 7.5, 100 mm NaCl, 2 mm dithiothreitol, 0.01% Brij35, and 1 mm MnCl2) and 40 units λ-protein phosphatase (λ-PPase) at 30°C for 1 h. The phosphorylation and dephosphorylation assay products were separated by electrophoresis using Phos-tag SDS-PAGE (8% acrylamide gels, 0.375 m Tris, 0.1% SDS, 0.1 mm MnCl2, and 0.05 mm Phos-tag AAL-107), and the gels were stained, destained, and visualized.
RT-PCR and qRT-PCR
To confirm if the transformants are obtained, RT-PCR and qRT-PCR analyses were performed as described in a previous study (Min et al., 2014). GhUB7 and AtACTIN7 were used as internal controls for cotton and Arabidopsis, respectively. The primers used in the study are listed in Supplemental Table S1.
IAA Determination
For determination of endogenous IAA concentrations, samples were homogenized in 1 mL of 80% cold methanol and shaken at 4°C overnight in the dark. Further extraction was performed as described previously (Liu et al., 2012), and the extract dissolution, filtration, storage, and quantifications of endogenous IAA were according to our previous report (Min et al., 2014).
Callus Proliferation and MTS Assays
Seeds from eight Arabidopsis lines, including the Col-0 wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/15, were cultured on ASSE medium; for each line, more than six replicates were assessed. After culturing for 7, 14, 21, and 30 d, the cultures were harvested and weighed. The average weight of each line from five cultures was used to calculate the proliferation rate.
The MTS assay was also used to measure cell proliferation using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega). In the MTS assays, the protoplast preparation and culture conditions were performed as previously described (Yoo et al., 2007), and the number of protoplasts was counted with a hemacytometer under light microscopy. Protoplasts (2 × 105) were cultured in 100 μL of W5 solution (2 mm 4-morpholineethanesulfonic acid [pH 5.7] containing 154 mm NaCl, 125 mm CaCl2, and 5 mm KCl), and 20 μL of CellTiter 96 Aqueous One Solution reagent was added to quantify MTS at 23°C based on four replicates. The OD values at 490 nm at different time points were determined using a Multimode Plate Reader (PerkinElmer).
Statistics
Each graphical data point represents the results of multiple independent experiments (n ≥ 3), and the means ± sem are shown. The P values for physiological parameters (fresh weight, dry weight, cell proliferation rate, and LUC activity) were evaluated based on the shortest significant ranges (SSRs; P values < 0.05) post hoc analysis, and values that did not share a common letter were considered statistically significant. Statistically significant results for the content of IAA and qRT-PCR were determined using the two-tailed unpaired Student’s t test, and P values < 0.05 were considered statistically significant.
The accession numbers in this article are shown in Supplemental Table S1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Constructs used for cotton and Arabidopsis transformation.
Supplemental Figure S2. Comparison of EC formation in the control and GhCKI variable region RNAi transformants.
Supplemental Figure S3. Expression pattern of GhCKI during SE.
Supplemental Figure S4. Isolation and expression of GhLEC1.
Supplemental Figure S5. Sequence alignment between GbTCP and GhTCP15.
Supplemental Figure S6. Predicted phosphorylation sites by CKI in GhTCP15.
Supplemental Figure S7. Down-regulation expression of GhCKI homologous genes in Arabidopsis by RNAi.
Supplemental Figure S8. Isolation of ectopically overexpressed GhLEC1 Arabidopsis plants.
Supplemental Figure S9. Effects of GhLEC1, GhCKI, and GhTCP15 on callus mass during SE.
Supplemental Figure S10. GhLEC1-GhCKI-GhTCP15 governs the switch from cell proliferation to cell differentiation by regulating the homeostasis of auxin.
Supplemental Figure S11. TEM analyses of cultures after induction for 30 d in the Col-0 wild type, OE25-6, OE15-7, CDi-1, Lov6-29, lec1-1, OETCP6-9, and tcp14/15.
Supplemental Table S1. Oligonucleotides used in this study.
ACKNOWLEDGMENTS
We thank Lijia Qu (Peking University, China) for providing Arabidopsis transcription factors yeast library, Nakagawa Tsuyoshi (Shimane University, Japan) for providing the plasmid pGWB433, Liting Hu and Limin He (Huazhong Agricultural University) for assistance with TEM, and Hongbo Liu and Dongqin Li for assistance with liquid chromatography/mass spectrometry (Huazhong Agricultural University).
Glossary
- ABA
abscisic acid
- ASSE
Arabidopsis seed somatic embryogenesis
- BiFC
bimolecular fluorescence complementation
- cDNA
complementary DNA
- Col-0
Columbia-0
- EC
embryogenic callus
- EMSA
electrophoretic mobility shift assay
- IAA
indole-3-acetic acid
- λ-PPase
λ-protein phosphatase
- MSB medium
Murashige and Skoog medium and B5 vitamins
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- NEC
nonembryogenic callus
- OD
optical density
- ORF
open reading frame
- qRT
quantitative reverse transcription
- RT
reverse transcription
- SE
somatic embryogenesis
- SSR
shortest significant range
- TEM
transmission electron microscopy
- Y1H
yeast one hybrid
- Y2H
yeast two hybrid
LITERATURE CITED
Author notes
This work was supported by the National High-Tech Programme of China 863 (grant no. 2013AA102601) and the National Natural Science Foundation of China (grant no. 31401423).
Address correspondence to xlzhang@mail.hzau.edu.cn.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Xianlong Zhang (xlzhang@mail.hzau.edu.cn).
L.M. and X.Z. designed the experiment; L.M., Q.H., Y.L., J.X., and Y.M. performed the experiments; L.M., L.Z., X.Y., and X.Z. analyzed the data; L.M. and X.Z. wrote the article.
Articles can be viewed without a subscription.