-
PDF
- Split View
-
Views
-
Cite
Cite
Sahin Coban, Murat Kekilli, Seyfettin Köklü, Approach and Management of Patients with Chronic Hepatitis B and C During the Course of Inflammatory Bowel Disease, Inflammatory Bowel Diseases, Volume 20, Issue 11, 1 November 2014, Pages 2142–2150, https://doi.org/10.1097/MIB.0000000000000126
Close - Share Icon Share
Abstract
Inflammatory bowel disease and chronic viral hepatitis are 2 distinct but common conditions throughout the world. Mostly, both need life-long follow-up. Since immunosuppressive drugs remain corner stones of inflammatory bowel disease management, one should be aware of the concomitant presence of chronic viral hepatitis in such patients to prevent serious (even fatal) outcomes. Recently, new treatment options have become available in the treatment of both inflammatory bowel disease and chronic viral hepatitis. In this review, we have discussed and summarized current treatment and follow-up strategies for those 2 important public health issues in light of available literature.
Inflammatory Bowel Disease and Hepatitis B
Viral hepatitis B is a common health problem all over the world. It has been estimated that about 3 billion people have been exposed to the hepatitis B virus (HBV), and over 350 million people have chronic HBV infection.1 Chronic HBV infection can be divided into 3 stages according to laboratory and histopathology findings: active, inactive HBV carriers, and resolved HBV.2
Active chronic HBV is defined as HBV DNA levels ≥2000 IU/L with elevated alanine aminotransferase levels. Inactive HBV carriers are defined by HBV DNA levels ≤ 2 IU/L and normal alanine aminotransferase levels, and antiviral therapy is not indicated at this stage.
Treatment is indicated if the patient is in active stage irrespective of the immune status. Antiviral prophylaxis has to be given to patients treated with immunosuppressant drugs because viral reactivation may occur regardless of DNA levels. Antiviral therapy is not needed for patients with resolved HBV but they have to be monitored during the course of immunosuppressant therapy, and prophylactic therapy should be given as needed based on DNA levels.
Inflammatory bowel disease (IBD) is a group of chronic inflammatory conditions of the gastrointestinal tract. The major types of IBD are Crohn's disease (CD) and ulcerative colitis (UC). In recent years, treatment of these two conditions has been markedly improved with the use of immunosuppressants and biological therapies. Over time, these drugs have been used earlier and more extensively for longer duration. Subsequently, concerns associated with immunosuppressive treatment have grown among physicians working in the field of IBD care.3 One of these concerns is the reactivation of HBV infection. Immunosuppresants and biological therapies have a considerable impact on the natural course of HBV infection, and there are many questions that need to be answered to explain the association between IBD and HBV. In this article, we reviewed the management of patients with IBD and viral hepatitis B or C.
Prevalence of HBV Infection in Patients with IBDs
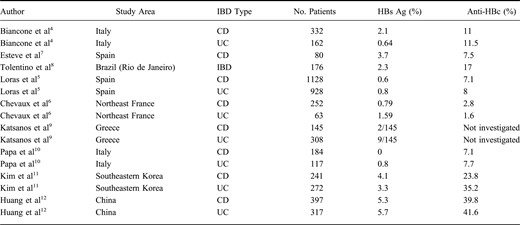
In some prior studies, a significantly higher prevalence of HBV infection has been reported in patients with IBD than in controls; thus, patients with longstanding IBD seem to be at higher risk for viral hepatitis compared with general population because of the need for endoscopic procedures, surgery, and blood transfusions, suggesting nosocomial transmission of the virus. Consistent with this argument, Biancone et al4 reported that antibodies to hepatitis B core protein (anti-HBc) were present in 11% of the patients with IBD, a rate that was statistically significantly higher than in controls. However, in a recent study reported by Loras et al,5 the risk of hepatitis B reactivation has been established in patients with IBD in Spain. In that prospective cross-sectional nationwide study, the prevalence of current and/or past hepatitis B biologic markers was similar to that of general population but lower than reported in studies. Similarly, in a recent study from France, it was observed that HBcAb positivity among IBD patients was comparable with that of the general French population.6 Therefore, decreasing prevalence of viral hepatitis B in patients with IBD reported by studies from both countries suggests that preventative measures such as vaccination, use of disposable materials, implementation of the World Health Organization's blood transfusion safety programs, better aseptic perioperative procedures, and decontamination rules in endoscopy are effective, and may explain the reduced risk of HBV infection. However, in eastern countries the prevalence is still high. Studies evaluating the prevalence of HBV infection among patients with IBD in different countries are summarized in Table 1.
Screening for HBV Before Institution of Treatment
Screening procedures and viral serology testing need to be routinely planned in patients with IBD before initiation of immunosuppressive therapy.13 Today, it is generally accepted that all patients with IBD should be screened if they have been exposed to HBV. Indeed, screening for HBV should be performed at the time of diagnosis of IBD, rather than waiting until initiation of treatment with immunosuppressants or anti-tumor necrosis factor (TNF) agents.14,15 This recommendation is based on the potential fatal outcomes of HBV reactivation and the need to use effective and safe anti-HBV drugs to prevent reactivation.16 Hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (HBsAb), and hepatitis B core antibody (HBcAb) are recommended tests for screening at the time of IBD diagnosis. Also, the American Association for the Study of Liver Diseases (AASLD) guidelines recommend HBV screening for populations with an intermediate or high HBV prevalence (.2%) and patients requiring immunosuppression, including patients with IBD.
Vaccination
The patients with IBD are more susceptible to infections, many of which can be prevented with vaccines.17 In many studies, a low rate of seroprotection and adherence to vaccination was shown in immunized IBD patients.15,17,18 During the course of their disease, many patients will need to receive treatment with immunosuppressants such as corticosteroids, purine antimetabolites such as thiopurines, and biological drugs. Thus, it is important to consider which patients would be at risk for acquiring an infection while they receive medication.16 Vaccination program against HBV usually begins at birth. Successful HBV vaccination schedules have achieved a dramatic reduction in HBV transmission in many countries.19
For patients diagnosed with IBD, immunization should be scheduled after completing clinical and laboratory workup at the time of diagnosis and before starting any treatment with immunomodulating and/or biological agents.20 Healthcare professionals should determine an appropriate vaccine schedule after immunocompetence of the patients has been evaluated on a case-by-case basis. It has been recommended that the best time to evaluate any IBD patient for vaccination requirements is at the time of initial IBD diagnosis because patients with IBD are likely to need immunosuppressive therapy during the course of their disease.13,–15 Additionally, response rates to HBV vaccination are significantly lower in patients who receive immunosuppressive or anti-TNF agents than those who do not.21
The prevalence of chronic HBV infection in IBD patients is comparable with the general population. A HBV vaccine is recommended for all seronegative and immunocompromised patients.22 Invasive procedures such as endoscopies and surgery may predispose patients with IBD to hepatitis B infection.20 In addition, using immunosuppressive agents may lead to reactivation of a latent infection.3,7
Despite aforementioned recommendations and the presence of significant risk factors, vaccines are currently under-prescribed in patients with IBD.23 This suggests that immunization against viral hepatitis is uncommon.
However, effective vaccination is provided to 12% of IBD patients both with and without immunosuppressive therapy. It was reported that the unique factors related to higher efficacy of the vaccine was younger age, and the number of immunomodulatory drugs that patients take. The use of higher doses of antigen or administration of the fourth dose is sometimes required to achieve increased response to the vaccine. The cutoff rate for response in the general population is associated with reaching surface antibody levels (HBsAb) that are equal to or greater than 10 mU/mL.20,22 Patients who do not respond to the standard dosage of vaccine should be exposed to a more extensive vaccination schedule such as administration of a second, higher dose (40 mg) of HBV surface antigen and/or repeated administration of the whole schedule. The traditional HBV vaccination schedule covers 3 doses at 0, 1, and 6 months. If faster vaccination is needed, the doses can be administered at monthly intervals (at 1 and 2 mo).3,20
Verification of HBsAb seroconversion is recommended 1 or 2 months after the last dose of the vaccine to check whether the vaccine has been effective.23 The efficacy of the vaccine depends on the intensity of immunomodulators and biologic agents. Some authors suggest that a higher HBsAb concentration is needed in these patients, with titers greater than 100 mU/mL considered as protective. Annual verification of the level of HBsAb titers and administration of a booster dose are recommended particularly in patients with IBD who receive immunosuppressive treatment.16
Clinical Practice
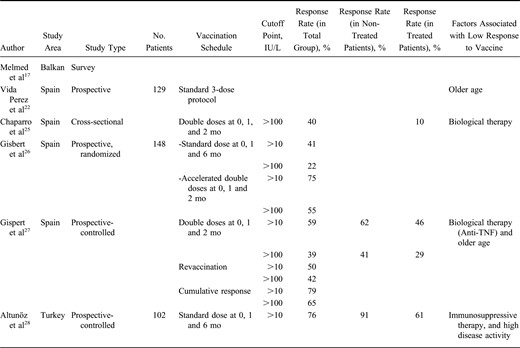
Vaccination rates show variability in different studies. In a study by Melmed et al,17 the risk of exposure and immunization status were assessed among patients receiving therapy. The study was performed based on the questionnaires completed by the patients; their responses showed that although 44% of the individuals had at least 1 risk factor for transmission of hepatitis B such as having blood transfusions or tattoos, only 28% of the patients had been vaccinated against HBV infection. In another study conducted by Gupta et al,24 it was indicated that 49% of gastroenterologists had never recommended vaccinations for patients with IBD. In a Spanish multicenter cross-sectional study,5 the authors found that rate of vaccination against HBV was only 12% in patients with IBD (Table 2). Therefore, it is essential to increase awareness of importance of immunization among physicians who manage IBD in their patients.21 Consequently, low rates of vaccination may lead to HBV infection in patients with IBD, although it may easily be avoided using a standard vaccination schedule.15 With this approach, we can provide patients protection against the infectious complications associated with immunosuppressants and biological therapies.15 Universal HBV vaccination programs have been put into practice in a successful manner in many countries to diminish the occurrence of HBV infection.
Schedule for HBV Vaccination
The regular immunization program usually includes administration of 3 vaccine doses, at 0, 1, and 6 months.13,19,21 To increase final anti-HBs titers, longer intervals might be used, but it does not affect seroconversion rates.19 Although lower anti-HBs titers are detected when intervals between injections are shortened, hastened immunization schedules (i.e., at 0, 1, and 2 months) can also be effective.29
Three scheduled doses are given at 0, 1, and 6 months if a combined hepatitis A and hepatitis B vaccine is administered. Modified dosing regimens, consisting of doubling the standard antigen dose, or adding the fourth dose (at 0, 1, 2, and 6 months), may increase response rates.16,30,–33 Such a schedule is usually needed in IBD patients with or without immunosuppression, as the duration of immune response to vaccine is changed in these patients.
Postvaccination test for anti-HBs titers is recommended for high-risk subjects such as patients with IBD who would require immunosuppressant therapy, although it is not necessary after vaccination of healthy adults.24,34,35 Because the response rate to the HBV vaccine is lower in patients with IBD than normal individuals, testing for serological immunity should be done 1 to 2 months after completing the vaccination schedule to establish whether there is a need for revaccination.19,35
An anti-HBs level of ≥10 mIU/mL obtained 1 to 3 months after completion of the primary vaccination schedule is considered to be a reliable marker for protection against HBV infection according to the World Health Organization.6,19,29,35,–39 Patients found to have anti-HBs titers below 10 mIU/mL after the primary vaccine schedule should be revaccinated with the second 3-dose schedule. Most of those patients who have not responded to the primary vaccine schedule would probably respond to the second 3-dose schedule.3,–24,29,–35 Even if patients initially achieve sufficient antibody titers after vaccination, over time anti-HBs titers frequently become undetectable.40,41 However, almost all healthy persons continue to be protected against HBV infection, even when anti-HBs concentrations fall below 10 mIU/mL.35 That is why vaccine-induced immunologic memory is sustained for at least 12 years in nonimmunocompromised persons despite reduced anti-HBs titers.38 However, there are limited data on the duration of immune memory in immunocompromised patients after vaccination against HBV. Thus, annual testing to evaluate anti-HBs levels and administration of booster doses when anti-HBs levels fall below 10 mIU/mL are recommended for immunocompromised persons such as IBD patients, particularly those who receive immunosuppressant therapy.35
Factors affecting the efficacy of vaccination
The primary 3-dose vaccine schedule confers effective protection against HBV in >95% of healthy individuals.19,35 However, immunosuppressive conditions are usually associated with reduced immunological status after vaccination.19 The response rate to HBV vaccine in patients with IBD seems to be lower, especially in those receiving immunosuppressants or biological therapy.21 The response rates to HBV vaccine vary between 33% and 60% in different studies (Table 2). When the cutoff level for sufficient protection against HBV was set at >100 mIU/mL, the response rate was lower.
Several risk factors for unresponsiveness have been demonstrated in healthy population including chronic diseases associated with immunosuppression, male gender, older age, smoking, and obesity.35 For IBD, there are limited data on the factors affecting the response to HBV vaccination. The use of immunosuppressive or anti-TNF agents, older age, and disease activity have been identified as risk factors in these studies (Table 2). Overall, the response rate to HBV vaccination seems to be quite low in patients with IBD, even in those not receiving immunosuppressant or anti-TNF agents.22,25 However, in a study conducted by Gisbert et al,26 it was demonstrated that the response rate may be increased by the administration of double dose schedule.
Effect of Immunosuppressive Therapy on HBV Vaccination in Patients with IBD
Immunosuppressive therapy including corticosteroids, methotrexate, or azothiopurine and anti-TNF agents including infliximab, adalimumab, or certolizumab pegol might cause to reactivation of HBV infection in patients with IBD who are HBV carriers. The risk of HBV reactivation seems to be associated with the intensity of immunosuppressive therapy.20
Prophylaxis and Treatment
To prevent reactivation of HBV, prophylaxis has to be considered in patients who are HBs antigen positive without active viral replication before undergoing chemotherapy or immunosuppressant therapy.42,–44 Prophylaxis has been found to be useful in patients with IBD, despite availability of limited data. In some randomized controlled trials, it has been reported that prophylaxis with lamivudine is useful to prevent reactivation of HBV in patients with IBD.42,45,46 A meta-analysis involving 21 studies to evaluate the effect of lamivudine for prophylaxis of HBV reactivation showed that lamivudine decreased mortality in patients taking immunosuppressant drugs (odds ratio: 0.36; 95% confidence interval, 0.23–0.56).47
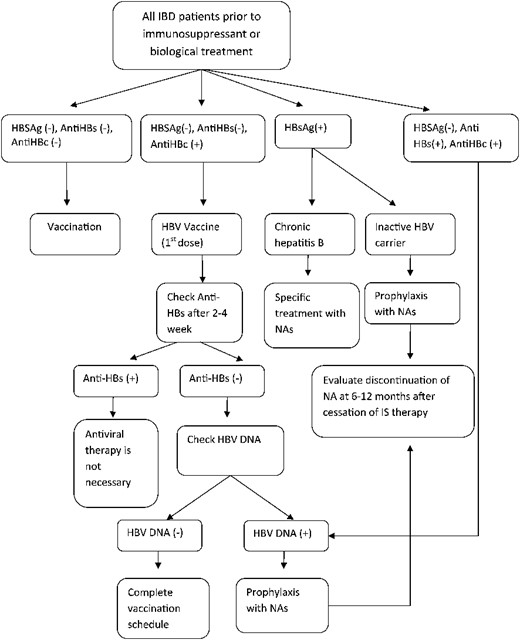
The European Association for the Study of the Liver,47 The American Association for the Study of Liver Disease,2 and the European Crohn's and Colitis Organization16 recommend that early introduction of nucleoside/nucleotide analogues (NAs) be considered in all HBsAg positive patients requiring immunosuppressive or biological therapy. An antiviral therapy should be administered to such patients who HBsAg-positive with or without active viral replication, regardless of the number and type of immunosuppressant drugs used (Fig. 1).2,48
An algorithm for the management of patients with IBD according to HBV infection status.
Currently, there is a consensus that HBV prophylaxis should be introduced at least 7 days before institution of therapy, and maintained for 6 months to 1 year after finishing chemotherapy, because reactivation of HBV may occur after completion of treatment.2,3,34 For patients with IBD, the European Crohn's and Colitis Organization recommends that antiviral prophylaxis should be initiated between 1 and 3 weeks before administration of immunosuppressant or biological therapy and maintained for 6 months after completion of the therapy.7,16
The most common drug used for HBV prophylaxis is lamivudine, but resistance is the major concern for this drug, which usually develops after prolonged use and has been identified in up to 70% of cases after 5 years of treatment.49,50 Resistance associated with lamivudine has also been detected in patients on long-term anti-TNF therapy.7 Therefore, this drug may be suitable for short-term prophylaxis during chemotherapy; however, because immunosuppressive therapy may be needed indefinitely in patients with IBD, more potent NAs should be preferred. Alternative NAs including tenofovir, adefovir, telbivudine, and entecavir have not been assessed for prophylaxis in patients with IBD; however, tenofovir and entecavir have been associated with lowest rates of resistance in the long-term, thus one of them should be preferred for prophylaxis in patients with IBD. If lamivudine, telbivudine, or adefovir is used, physicians should be vigilant for drug resistance, and they must closely follow serum aminotransferases and HBV DNA levels.
Interferon-α must not be used for HBV prophylaxis, as it may exacerbate CD, and it may lead to additional bone marrow suppression.16 Regular use of antiviral prophylaxis is not recommended in HBsAg negative and HBcAb positive patients and in those who have resolved hepatitis B1,51 (Fig. 1). This approach is not applicable for patients undergoing chemotherapy and particularly when rituximab is used. In such cases, antiviral prophylaxis should be performed.51,–54 However, virological markers as well as HBV DNA should be monitored carefully and on an continual basis. Early detection of viral reactivation and treatment with NAs at an early phase is needed in these patients in the course of chemotherapy or biological therapy55,56 (Fig. 1).
In conclusion, novel therapies including immunosuppressants, immunological and biological drugs that are used in patients with IBD are associated with an increased risk for HBV reactivation. For this reason, all patients with IBD should be screened for HBV markers before start of treatment and the best time is at the time of initial diagnosis. Screening for HBsAg, anti-HBs, and anti-HBc levels is recommended by the current guidelines not only to detect HBV infection but also to detect occult HBV. HBsAg-positive patients who will take immunosuppressive or biological therapy such as anti-TNF agents should receive NAs irrespective of HBV DNA level. Prophylaxis with NAs has to be introduced at least 1 week prior to initiation of treatment. Because immunosuppressive drugs may be needed indefinitely, NAs with a high genetic barrier such as tenofovir or entecavir should be preferred to avoid drug resistance. In addition, patients with positive anti-HBc without HBsAg and undetectable viral DNA should be followed carefully and closely for reactivation.
IBD and Hepatitis C
Patients with IBD are considered to be at increased risk for hepatitis C virus (HCV) infection because of higher frequency of endoscopic, surgical, and transfusion procedures needed to effectively control the disease. However, recent epidemiologic surveys have shown that the prevalence of HCV infection in patients with IBD is similar to or even lower than in the general population.5,57 IBD management has been marked by the increased use of different types of immunosuppressant medications and by the advances in biological therapies in the last few years. Increasing evidence in favor of immunosuppressive drugs suggests that they are now being used more often and earlier in the course of the IBD.20 Many questions concerning the relationship between IBD and HCV infection remain unanswered. Such questions are particularly relevant as treatment with immunosuppressant medications has a clear impact on the natural history and clinical consequences of HCV infection, but it is not clear which factors increase the risk of HCV reactivation in patients treated with biologics and other immunosuppressants. Progression to liver cirrhosis seems to be similar to that previously observed in nonimmunosuppressed infected patients.58 Thus, administration of immunosuppressive drugs, as is common in IBD, does not seem to increase progression to end-stage liver disease.59,60 Finally, the efficacy and safety of antiviral therapy have not been established in patients with IBD, and more information is needed on the risk of exacerbation of IBD in patients treated with antiviral therapy.
Immunosuppressive Agents and Hepatitis C
The risk of HCV reactivation under biologics and other immunosuppressants seems to be very low. Currently, there is no consensus for HCV screening before initiation of immunosuppressive therapy in IBD patients infected with HCV.16
A recent multicenter study61 reported that IBD patients with HBV infection had a greater frequency of elevations in liver function tests compared with patients with IBD with HCV infection when both groups of patients were treated with immunosuppressive medications. Eight out of 51 patients with positive HCV-RNA (15.7%) developed liver dysfunction. All of them had been treated with corticosteroids except for 1 patient who received azathioprine. Interestingly, none of them were given anti-TNF agents. Seven patients had mild elevation in hepatic enzyme levels, and 1 patient developed fatal liver failure, which was related to treatment with corticosteroids.
Steroids
Corticosteroids are commonly used to treat acute exacerbations of IBD, but they may negatively affect natural history of HCV infection by increasing viral replication, a phenomenon observed in the postliver transplant population. To date, researchers have not directly addressed the effect of corticosteroids on HCV in patients with IBD.62 Traditionally, it seems reasonable to assume that corticosteroids used to treat patients with IBD have no harmful effect on the natural history of HCV.16 However, one patient had an acute flare of HCV infection after discontinuing corticosteroids.63 In addition, available data on HCV recurrence after liver transplantation suggest that corticosteroids should be tapered slowly to limit the risk of early recurrent HCV infection.64,65 With the availability of other medications for IBD, it may be in the best interest for HCV patients with IBD to avoid the use of corticosteroids when possible.66
Azathioprine and Others
There are no data on the safety of immunosuppressive drugs in HCV patients with IBD. Azathioprine, mycophenolate mofetil, and cyclosporine have been used in patients with hepatitis C undergoing liver transplantation and have shown potential anti-HCV activity.67,68 Azathioprine has antiviral activity against HCV in vitro.69 Data from patients with HCV in the posttransplant setting indicate that azathioprine can be safely used in patients with IBD.16 A separate study on patients with HCV with arthropathy showed no detrimental effect of treatment with methotrexate.70 The use of immunomodulators in IBD patients infected with HCV seems to be associated with low risk, although long-term studies are needed to establish their safety in this setting.71
Anti-TNF Agents
Anti-TNF agents have been shown to be highly effective for the treatment of IBD.66 TNF-α may play a role in the pathogenesis of HCV. Elevated TNF-α levels are a negative predictor for response to HCV treatment, so inhibition of TNF-α may be potentially beneficial for HCV clearance.72 Infliximab, certolizumab pegol, and adalimumab reduce inflammation through TNF-α inhibition. Except for some case reports, currently, there are no data to suggest that TNF-α antagonists are dangerous in IBD patients infected with HCV. However, TNF-α antagonists are used rather extensively in patients with rheumatoid arthritis. A case series of 24 rheumatoid arthritis patients infected with HCV who were treated with TNF-α antagonists (21 patients with etanercept, 3 patients with infliximab) showed no adverse effect on hepatic biochemical tests or HCV viral load.73 Additionally, anti-TNF antagonists have been successfully used as adjuvant therapy in patients undergoing specific antiviral treatment for HCV infection with ribavirin (RBV) and interferon.74 Lin et al66 also demonstrated the safety of using interferon-based therapy in patients with IBD.
Treatment of HCV in Patients with IBD
Current first-line treatment regimen for HCV includes pegylated interferon-α (PEG-IFNα) with RBV.20 HCV therapy has been revolutionized recently by the development and approval of direct-acting antiviral agents (DAAs). These drugs target specific enzymes involved in viral replication. During 2012, at least 30 additional DAAs were in various stages of clinical development. DAAs such as HCV protease inhibitors, polymerase inhibitors, and NS5A inhibitors among others can achieve high cure rates when combined with PEG-IFNα and RBV, and promising results may be obtained when they are used in combination.75 Triple therapy with PEG-IFNα, RBV, and a protease inhibitor has replaced PEG-IFNα and RBV alone as the new standard of care for the treatment of chronic HCV infection.
The effect of interferon on IBD is unclear. Several case reports suggest a potential benefit of interferon in patients with UC.76,77 A Cochrane review including 3 prospective studies of interferon-α and interferon-β in patients with UC concluded that interferon was not a successful therapy for UC.78
Some studies have demonstrated that patients with HCV with IBD who were treated with nonpegylated interferon-α monotherapy have a sustained virological response that is similar to that of non-IBD.79 No difference in adverse events were observed between patients with IBD and non-IBD controls nor were exacerbation in gastrointestinal symptoms observed.62 Other studies have analyzed the efficacy of PEG-IFNα combined with RBV regimen in CD patients with HCV, and concluded that their efficacy is comparable with that reported in patients without CD.3,79 In a study by Scherzer et al,60 which investigated the use of CD medications for HCV treatment, 3 patients received azathioprine, 4 received mesalamine, and 1 received mycophenolate mofetil, and 3 were not given any medication. During HCV treatment, 6 of the 11 patients had increased frequency of symptoms related to CD and required a more intensive medical therapy with steroids, mesalamine, or antibiotics.
No differences were detected in the incidence of adverse events between HCV patients with or without IBD treated with interferon-α.79,80 However, Peyrin-Biroulet et al39 reported 8 cases of severe pancytopenia in patients receiving azathioprine for IBD and RBV plus PEG-IFNα for chronic hepatitis C. Similarly, Chaparro et al81 reported a case of pancytopenia in a patient with CD who was treated with azathioprine and PEG-IFN with RBV. These results suggest that ribavirin may interact with the metabolism of azathioprine, thus increasing the risk of developing myelotoxicity. Therefore, treatment with ribavirin and azathioprine should be tailored individually and close monitoring of patients is necessary throughout the treatment.
Interferon-α is known to stimulate a Th1-type immune response, and CD is usually characterized by a Th1-type immune response.82 Scherzer et al60 analyzed the safety of interferon-α plus ribavirin therapy in CD patients with HCV infection and reported that gastrointestinal symptoms may be temporarily exacerbated. However, increased CD activity is easily managed with short-term glucocorticoid therapy. Thus, antiviral therapy and interferon-α with ribavirin should be given to CD patients with HCV infection to prevent long-term sequelae of chronic hepatitis.60
No published clinical trial data were identified through literature search on the safety and efficacy of DAAs for the treatment of HCV in patients with IBD. Boceprevir and telaprevir are commonly well tolerated. The most common side effects of boceprevir- and telaprevir-based triple therapy are those typically associated with pegIFNα and RBV treatment. Drug interactions are a particularly important consideration when using HCV protease inhibitors (e.g., simeprevir, telaprevir, or boceprevir) and should be assessed thoroughly before use of these agents. Patients treated with boceprevir or telaprevir who receive concomitant corticosteroid therapy may have decreased antiviral efficacy with a potential increase in drug-resistant variants.75,83 The effect of sofosbuvir on IBD is unclear. Sofosbuvir with or without ribavirin is generally well tolerated, although it has known interactions with certain agents which need to be established before use. At present, there are no data to suggest that DAAs are unsafe in patients with IBD with HCV.84
Hepatitis C therapy does not seem to worsen the course of IBD when the disease is adequately controlled and maintenance treatment established. The natural history and clinical consequences of HCV infection have been shown not to be affected by drugs used to treat IBD, although few data exist in patients with IBD. HCV treatment with PEG-IFN and RBV may exacerbate gastrointestinal symptoms; and therefore, the decision to treat HCV needs to be individualized.62 If possible, only stable patients with IBD should be considered for HCV treatment, with close monitoring for evidence of exacerbation of IBD symptoms while on treatment. The risk–benefit ratio for HCV treatment should be determined on an individual basis in patients with IBD.
References
Author notes
Reprints: Seyfettin Köklü, MD, Baglarbasi Mahallesi, Duman Sokak, 55/11, Keçiören, Ankara, Turkey 06300 (e-mail: gskoklu@yahoo.com).
The authors have no conflicts of interest to disclose.