-
PDF
- Split View
-
Views
-
Cite
Cite
Mario Bronzati, Oliver W M Rauhut, Braincase redescription of Efraasia minor Huene, 1908 (Dinosauria: Sauropodomorpha) from the Late Triassic of Germany, with comments on the evolution of the sauropodomorph braincase, Zoological Journal of the Linnean Society, Volume 182, Issue 1, January 2018, Pages 173–224, https://doi.org/10.1093/zoolinnean/zlx029
Close - Share Icon Share
Abstract
The braincase anatomy of the sauropodomorph dinosaur Efraasia minor (Late Triassic, Norian, Löwenstein Formation of Germany) is redescribed in detail, adding new information based on CT scan data. We discuss the evolution of sauropodomorph braincases from a phylogenetic perspective, focusing on non-neosauropodan representatives. For this, we revised braincase characters used in data matrices focused on this assemblage of taxa. This led to the recognition of problems with some of the phylogenetic characters, especially regarding the basal tubera complex, which did not accurately reflect the morphological variation observed among taxa within the group. We also discuss previous misidentifications of the soft tissues associated with the presence of a divided metotic foramen among sauropodomorphs. This has implications for the recognition of the structures associated with braincase foramina in non-sauropodan sauropodomorphs, and we propose that the path for the jugular vein was either through the posterior foramen resulting from this division or through the foramen magnum. Finally, our study demonstrates a series of differences regarding braincase anatomy between non-sauropodan and sauropod taxa. However, it remains unclear if these differences might be due to a drastic morphological change or if they simply reflect the small number of braincase materials of non-neosauropodan sauropods, which might indicate a more stepwise evolution.
INTRODUCTION
Sauropoda includes the largest land animals in earth history (Upchurch, Barrett & Dodson, 2004; Wilson, 2005), which exhibit a peculiar morphology that largely deviates from the body plan of the earliest representatives of Sauropodomorpha (see e.g. Rauhut et al., 2011). The earliest sauropodomorphs are well known from the Late Triassic of South America, and were small, ‘gracile’, and probably bipedal and omnivorous/carnivorous animals (e.g. Langer et al., 1999; Martinez & Alcober, 2009; Ezcurra, 2010; Martinez, Apaldetti & Pol, 2012a; Cabreira et al., 2016). Anatomical transformations related to quadrupedalism, a herbivorous diet, and increase in body size is observed in non-sauropodan sauropodomorph lineages (Upchurch, Barrett & Galton, 2007; Rauhut et al., 2011; McPhee et al., 2015). This shows that the peculiar and conspicuous morphology of sauropods is a product of morphological transformations including those that happened earlier in the evolutionary history of sauropodomorphs (e.g. Upchurch & Barrett, 2000; Parrish, 2005; Barrett & Upchurch, 2007; Bonnan & Senter, 2007; Upchurch et al., 2007; Martinez, 2009; Yates et al., 2010; Pol, Garrido & Cerda, 2011; McPhee et al., 2014, 2015), among lineages once thought to be conservative (Sereno, 2007a). These lineages are classically referred to as ‘prosauropods’ (= non-sauropodan sauropodomorphs), but this grouping in its traditional sense (see e.g. Galton & Upchurch, 2004) is now generally regarded as a paraphyletic array of taxa that are consecutively closer to Sauropoda (e.g. Upchurch et al., 2007; Yates, 2007b; Pol et al., 2011; McPhee et al., 2015; Otero et al., 2015). In this scenario of major evolutionary changes happening among the ‘prosauropodan’ lineages, the conspicuous braincase morphology of sauropods, which may be related to the lowest encephalization quotients amongst amniotes (Hopson, 1979, 1980), might stem from anatomical modifications within non-sauropodan sauropodomorphs. However, this particular structure has received rather limited attention in evolutionary studies of prosauropods.
Braincase anatomy in general has received greater attention in the last few years. The development of computed tomography and virtual reconstruction techniques (e.g. Witmer & Ridgely, 2009; Balanoff, Bever & Ikejiri, 2010; Knoll et al., 2012) together with the recognition of this structure as an important character complex in phylogenetic analyses (Gower, 2002; Gower & Nesbitt, 2006; Brusatte et al., 2010; Nesbitt, 2011; Carrano, Benson & Sampson, 2012; Pol et al., 2013) contributed to placing the braincase at the forefront of archosaur studies. In dinosaur literature, the anatomy of these complex structures has been mainly investigated in detail in theropods (e.g. Sampson & Witmer, 2007; Lautenschlager et al., 2012; Bever et al., 2013), ornithischian dinosaurs (e.g. Evans, Reisz & Dupuls, 2007; Evans, Bavington & Campione, 2009; Sobral, Hipsley & Müller, 2012) and sauropods (e.g. Balanoff et al., 2010; Knoll et al., 2012). For non-sauropodan sauropodomorph dinosaurs, however, the significance of this structure has not yet been explored in its totality. Apart from a few more detailed studies on the anatomy of the braincase (e.g. Galton, 1985; Galton & Kermack, 2010; Martinez, Haro & Apaldetti, 2012b; Apaldetti et al., 2014), the morphology of this structure still plays a small role in morphological descriptions.
We herein redescribe the braincase of Efraasia minor Huene, 1908, a Late Triassic (Norian) sauropodomorph from Germany. The material used as a basis for this study, the skull of the specimen SMNS 12667, was previously described in a paper by Galton & Bakker (1985). However, a more detailed description of the braincase morphology, including data obtained from CT scans, is provided here. The comparative nature of this study led to the recognition of problematic issues in the literature on sauropodomorph braincases. These include the misidentification of structures and problems in the definition of characters used in phylogenetic studies of the group, which are also discussed here.
MATERIAL AND METHODS
Institutional abbreviations
Abbreviations: AMNH, American Museum of Natural History, New York, USA; BMNH, British Museum of Natural History, London, UK; BP, Bernard Price Institute for Palaeontological Research, University of the Witwaaterstrand, Johannesburg, South Africa; MB, Museum für Naturkunde, Berlin, Germany; MCP-PV, Museu de Ciência e Tecnologia da PUC-RS, Porto Alegre, Brazil; PVL, Paleontologia de Vertebrados Lillo, Tucumán, Argentina; PVSJ, Museo de Ciencias Naturales, San Juan, Argentina; SAM, Iziko South African Museum, Cape Town, South Africa; UFSM, Universidade Federal de Santa Maria, Santa Maria, Brazil; YPM, Yale Peabody Museum, New Have, USA; ZPAL, Institute of Paleobiology of the Polish Academy of Sciences, Warsaw, Poland.
Taxonomic history of SMNS 12667
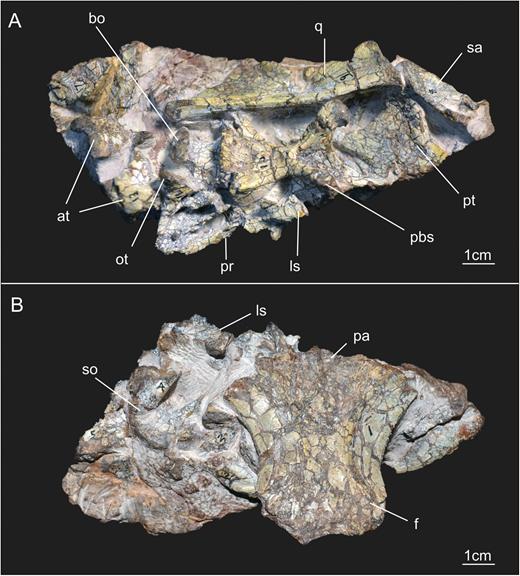
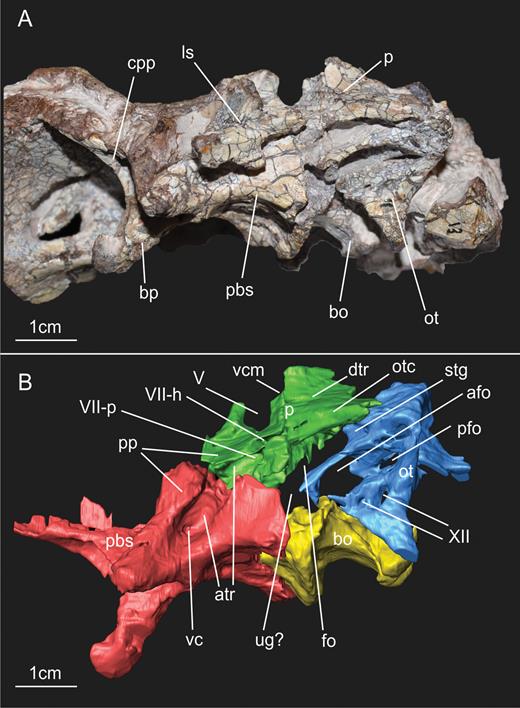
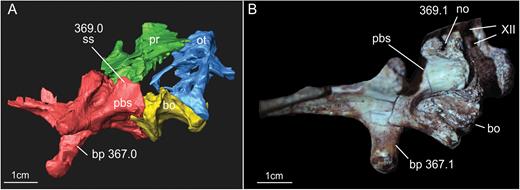
The braincase described here belongs to specimen SMNS 12667 of E. minor, and is housed in the Staatliches Museum für Naturkunde (Stuttgart, Germany). SMNS 12667 consists of a fairly complete skeleton preserved in four blocks (Galton, 1973; Galton & Bakker, 1985), the smallest of which contains the skull elements (Fig. 1). Besides the incomplete skull, preserved bones in the other three blocks include cervical, dorsal, sacral and caudal vertebrae, ribs, gastralia, left and right scapulae, right coracoid, left humerus, metacarpals, left ilium, left and right pubis, left and right femora, right tibia, right fibula, right astragalus, right calcaneum and the proximal end of the right pes (see Galton, 1973). The block in which braincase elements of SMNS 12667 are preserved contains not only these remains but also other cranial bones, such as the quadrate, pterygoid, squamosal, articular and surangular, and also the atlas.
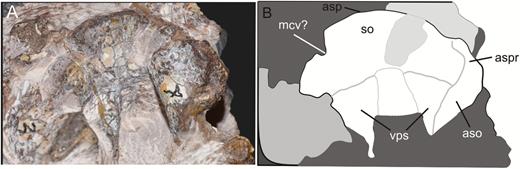
General view of the block containing the braincase of the specimen SMNS 12667 of Efraasia minor. Abbreviations: at, atlas; bo, basioccipital; f, frontals; ls, laterosphenoid; ot, otoccipital; pa, parietal; pbs, parabasisphenoid; pr, prootic; pt, pterygoid; q, quadrate; sa, surangular; so, supraoccipital.
The bones were excavated by the German palaeontologist Eberhard Fraas, who named this specimen in his 1913 paper as a new species of the genus Thecodontosaurus Riley & Stutchbury, 1836, T. diagnosticus Fraas, 1913. At that time, Fraas referred two specimens to T. diagnosticus, SMNS 12667 and another skeleton discovered in the same excavation, SMNS 12668. However, Fraas (1913) did not provide a description or diagnosis, so that Thecodontosaurus diagnosticus Fraas, 1913, has to be regarded as a nomen nudum. Later, Huene (1932) validated the species name and described the material under the name Palaeosaurus(?) diagnosticus. It was not until Galton (1973) that the genus Efraasia Galton, 1973 was firstly proposed, with specimens SMNS 12667 and SMNS 12668 assigned to the species E. diagnosticus. Initial works on SMNS 12667 mainly dealt with the postcranial elements preserved (Fraas, 1913; Huene, 1932; Galton, 1973). Huene (1932) described the braincase in a very preliminary way. Following further preparation of the block containing the cranial elements, Galton & Bakker (1985) presented the first detailed description of the skull remains, including the braincase. In their paper, the authors also proposed a new taxonomic change, suggesting that the specimen SMNS 12667 should be considered a junior synonym of Sellosaurus gracilis von Huene, 1908 (see also Galton, 1985).
In a more recent study, Yates (2003) conducted a taxonomic analysis of the sauropodomorph materials of the Löwenstein Formation, Late Triassic, Germany. Yates came to the conclusion that sauropodomorph fossils coming from this formation belong to two different genera, Plateosaurus von Meyer, 1837, including P. gracilis Huene, 1908 and P. engelhardti von Meyer, 1837, and Efraasia. Together with other materials, SMNS 12667 was assigned to the latter genus, but under the species name E. minor, which was first proposed by Huene (1908) as a new species of Teratosaurus Huene, 1908 (see Galton, 1973). This taxonomic assignment to E. minor proposed by Yates (2003) has been adopted widely in the literature (e.g. Yates et al., 2010; Apaldetti et al., 2011; Pol et al., 2011; McPhee et al., 2014), and is also the one followed in this study.
Systematic terminology
Here we follow the definitions proposed by Galton & Upchurch (2004) and Yates (2007a) for Sauropodomorpha and Sauropoda, respectively.
CT scan procedure
The block containing the braincase of the specimen SMNS 12667 was scanned in a Nanotom Scan (GE Sensing & Inspection Technologies GmbH, Wunstorf, Germany), located at the Zoologische Staatssammlung München (Bavaria State Collection of Zoology, Munich, Germany). In a 55-min scanning procedure (voltage: 80 kV; current: 240 μA; 0.1 mm, diamond filter), 1651 x-ray slices were generated, which yielded a volume data set with the following dimensions: 2063 × 1553 × 2398 with 3.1 μm voxel size. Because of the poor contrast between matrix and bones, an automatic volume rendering did not bring any result. The slices obtained in the scanning procedure were therefore downsampled by half and then segmented in the software Amira (version 5.3.3; Visage Imaging, Berlin, Germany) by hand.
RESULTS
In the following description, we employed traditional anatomical and directional terms such as ‘anterior’ and ‘posterior’ rather than using the veterinary terms ‘cranial’ and ‘caudal’, respectively. Taxa used for comparisons are detailed in Table 1.
List of comparative taxa used in the present study
| Taxon . | Source of information . |
|---|---|
| Adeopapposaurus mognai | PVSJ 568; PVSJ 610; Martinez, 2009 |
| Coloradisaurus brevis | PVL 3967; Apaldetti et al., 2014 |
| Dicraeosaurus hansemanni | MB.R.2379.1-3 |
| Eoraptor lunensis | PVSJ 512; Sereno et al., 2012 |
| Giraffatitan brancai | MB.R.2180.22.1-4 |
| Herrerasaurus ischigualastensis | PVSJ 407; Sereno & Novas, 1993 |
| Massospondylus carinatus | SAM-PK-K1314 |
| Massospondylus kaalae | SAM-PK-K1325; Barrett, 2009 |
| Melanorosaurus readi | NMQR 3314; Yates, 2007b |
| Melanorosaurus sp. | NMQR 1551; Nair et al., 2015 |
| Panphagia protos | PVSJ 874; Martinez et al., 2012b |
| Pantydraco caducus | BMNH - P.24; P.141/1; Galton & Kermack, 2010 |
| Plateosaurus | MB.R.5586-1; SMNS 13200; Prieto-Márquez & Norell, 2011 |
| Riojasaurus incertus | PULR 56 |
| Sarahsaurus aurifontanalis | Rowe, Sues & Reisz, 2011 |
| Saturnalia tupiniquim | MCP 3845-PV |
| Silesaurus opolensis | ZPAL Ab III/361; ZPAL Ab III/362 |
| Thecodontosaurus antiquus | Benton et al., 2000 |
| Tornieria africana | MB.R.2386 |
| Unaysaurus tolentinoi | UFSM 11069 |
| Sauropoda indet. | MB.R.2387.1-3,4; Remes, 2006 |
| Taxon . | Source of information . |
|---|---|
| Adeopapposaurus mognai | PVSJ 568; PVSJ 610; Martinez, 2009 |
| Coloradisaurus brevis | PVL 3967; Apaldetti et al., 2014 |
| Dicraeosaurus hansemanni | MB.R.2379.1-3 |
| Eoraptor lunensis | PVSJ 512; Sereno et al., 2012 |
| Giraffatitan brancai | MB.R.2180.22.1-4 |
| Herrerasaurus ischigualastensis | PVSJ 407; Sereno & Novas, 1993 |
| Massospondylus carinatus | SAM-PK-K1314 |
| Massospondylus kaalae | SAM-PK-K1325; Barrett, 2009 |
| Melanorosaurus readi | NMQR 3314; Yates, 2007b |
| Melanorosaurus sp. | NMQR 1551; Nair et al., 2015 |
| Panphagia protos | PVSJ 874; Martinez et al., 2012b |
| Pantydraco caducus | BMNH - P.24; P.141/1; Galton & Kermack, 2010 |
| Plateosaurus | MB.R.5586-1; SMNS 13200; Prieto-Márquez & Norell, 2011 |
| Riojasaurus incertus | PULR 56 |
| Sarahsaurus aurifontanalis | Rowe, Sues & Reisz, 2011 |
| Saturnalia tupiniquim | MCP 3845-PV |
| Silesaurus opolensis | ZPAL Ab III/361; ZPAL Ab III/362 |
| Thecodontosaurus antiquus | Benton et al., 2000 |
| Tornieria africana | MB.R.2386 |
| Unaysaurus tolentinoi | UFSM 11069 |
| Sauropoda indet. | MB.R.2387.1-3,4; Remes, 2006 |
Specific collection numbers represent specimens analysed first-hand by the authors, whereas other comparative data were obtained from the literature listed within the table.
List of comparative taxa used in the present study
| Taxon . | Source of information . |
|---|---|
| Adeopapposaurus mognai | PVSJ 568; PVSJ 610; Martinez, 2009 |
| Coloradisaurus brevis | PVL 3967; Apaldetti et al., 2014 |
| Dicraeosaurus hansemanni | MB.R.2379.1-3 |
| Eoraptor lunensis | PVSJ 512; Sereno et al., 2012 |
| Giraffatitan brancai | MB.R.2180.22.1-4 |
| Herrerasaurus ischigualastensis | PVSJ 407; Sereno & Novas, 1993 |
| Massospondylus carinatus | SAM-PK-K1314 |
| Massospondylus kaalae | SAM-PK-K1325; Barrett, 2009 |
| Melanorosaurus readi | NMQR 3314; Yates, 2007b |
| Melanorosaurus sp. | NMQR 1551; Nair et al., 2015 |
| Panphagia protos | PVSJ 874; Martinez et al., 2012b |
| Pantydraco caducus | BMNH - P.24; P.141/1; Galton & Kermack, 2010 |
| Plateosaurus | MB.R.5586-1; SMNS 13200; Prieto-Márquez & Norell, 2011 |
| Riojasaurus incertus | PULR 56 |
| Sarahsaurus aurifontanalis | Rowe, Sues & Reisz, 2011 |
| Saturnalia tupiniquim | MCP 3845-PV |
| Silesaurus opolensis | ZPAL Ab III/361; ZPAL Ab III/362 |
| Thecodontosaurus antiquus | Benton et al., 2000 |
| Tornieria africana | MB.R.2386 |
| Unaysaurus tolentinoi | UFSM 11069 |
| Sauropoda indet. | MB.R.2387.1-3,4; Remes, 2006 |
| Taxon . | Source of information . |
|---|---|
| Adeopapposaurus mognai | PVSJ 568; PVSJ 610; Martinez, 2009 |
| Coloradisaurus brevis | PVL 3967; Apaldetti et al., 2014 |
| Dicraeosaurus hansemanni | MB.R.2379.1-3 |
| Eoraptor lunensis | PVSJ 512; Sereno et al., 2012 |
| Giraffatitan brancai | MB.R.2180.22.1-4 |
| Herrerasaurus ischigualastensis | PVSJ 407; Sereno & Novas, 1993 |
| Massospondylus carinatus | SAM-PK-K1314 |
| Massospondylus kaalae | SAM-PK-K1325; Barrett, 2009 |
| Melanorosaurus readi | NMQR 3314; Yates, 2007b |
| Melanorosaurus sp. | NMQR 1551; Nair et al., 2015 |
| Panphagia protos | PVSJ 874; Martinez et al., 2012b |
| Pantydraco caducus | BMNH - P.24; P.141/1; Galton & Kermack, 2010 |
| Plateosaurus | MB.R.5586-1; SMNS 13200; Prieto-Márquez & Norell, 2011 |
| Riojasaurus incertus | PULR 56 |
| Sarahsaurus aurifontanalis | Rowe, Sues & Reisz, 2011 |
| Saturnalia tupiniquim | MCP 3845-PV |
| Silesaurus opolensis | ZPAL Ab III/361; ZPAL Ab III/362 |
| Thecodontosaurus antiquus | Benton et al., 2000 |
| Tornieria africana | MB.R.2386 |
| Unaysaurus tolentinoi | UFSM 11069 |
| Sauropoda indet. | MB.R.2387.1-3,4; Remes, 2006 |
Specific collection numbers represent specimens analysed first-hand by the authors, whereas other comparative data were obtained from the literature listed within the table.
General aspects of the braincase
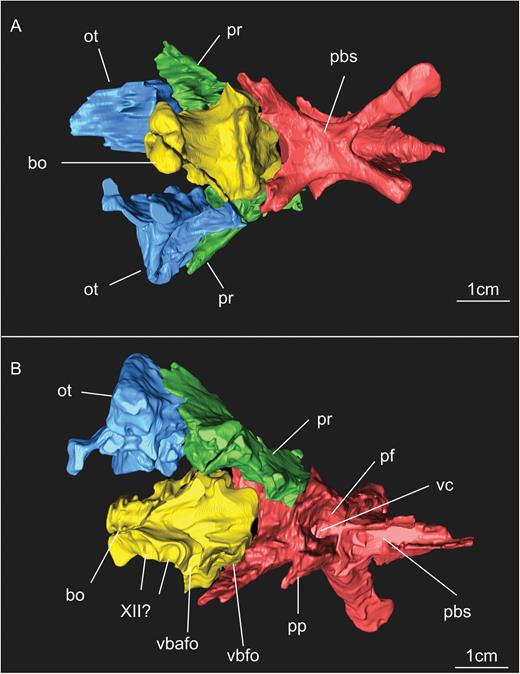
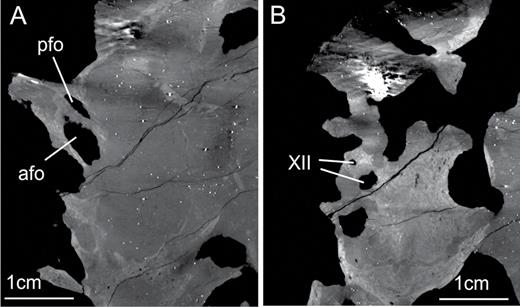
Braincase bones that are preserved include the parabasisphenoid (= parasphenoid + basisphenoid – sensuGower & Weber, 1998), basioccipital, otoccipitals (= exoccipital + opisthotic – sensuSampson & Witmer, 2007), prootics, left laterosphenoid, supraoccipital, frontals and a fragment of the anterior portion of the parietals (Fig. 1). Based on the CT scan data, it is very likely that most of the separation between bones of SMNS 12667 did not happen due to breakage, but through disarticulation, testifying to the skeletally immature status of the specimen at the time of its death (see below). The frontals, the left laterosphenoid and the supraoccipital are displaced from their original position. The assemblage of bones including the basioccipital, parabasisphenoid, prootics and otoccipitals, which represent the ventral and lateral portion of the braincase, are preserved almost in the position these bones would occupy in the animal in life, although only the left prootic and otoccipital are still articulated with each other (Fig. 2). Most of the left side of the braincase is visible, except for the contact of the parabasisphenoid and prootic (hidden by the displaced laterosphenoid), and also the anteriormost region of the parabasisphenoid (Fig. 1). With the data from the CT scan, it was possible to reconstruct the morphology of the entire lateral surface of these bones and also to access details of some of the cranial openings. Moreover, the CT scan showed that the right prootic and otoccipital are partially preserved inside the matrix (Fig. 2). Finally, CT scan data also show that most of the medial surface of the bones of the lateral wall and also the floor of the braincase are preserved in a way that makes an accurate reconstruction of the internal elements (e.g. soft tissues such as the inner ear) impossible (see also Supporting Information S1).
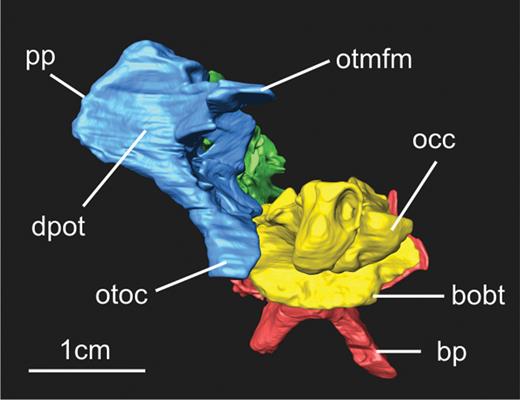
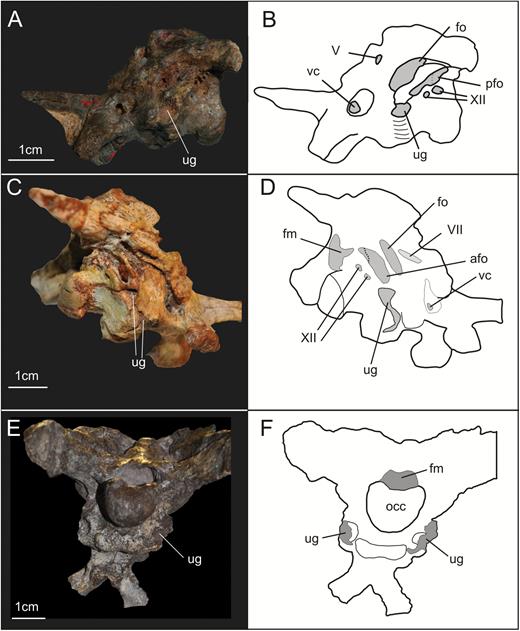
Results of the segmentation of CT scan data showing some of the braincase bones of the specimen SMNS 12667 preserved in the block – the laterosphenoid was omitted because it was strongly displaced from its original position (but see Fig. 11). A, ventral view of the braincase. B, dorsal view of the braincase (right prootic and otoccipital were excluded in order to show details of the dorsal surface of basioccipital and parabasisphenoid). Abbreviations: bo, basioccipital; ot, otoccipital; pbs, parabasisphenoid; pf, pituitary fossa; pp, preotic pendant; pr, prootic; vbafo, ventral border of the anterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis; vbfo, ventra border of the fenestra ovalis; vc, vidian canal; XII, foramen for cranial nerve XII.
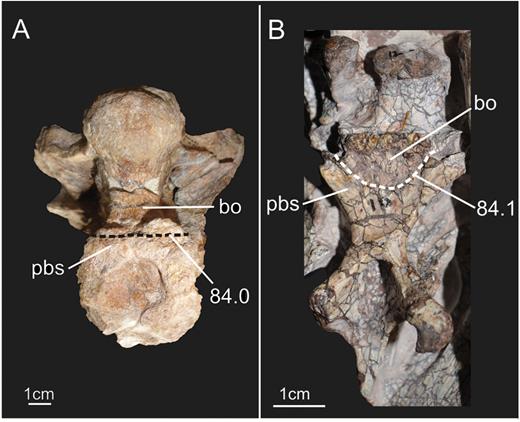
In the original description by Galton & Bakker (1985), the authors point out that the elements of the ventral surface of the braincase (cultriform process of the parasphenoid, proximal part of the basipterygoid processes, basal tubera and occipital condyle) were positioned at the same dorsoventral level, a condition classically regarded as the plesiomorphic condition for Sauropodomorpha (Yates, 2007b). A different interpretation from that of Galton & Bakker (1985) was given by Yates (2003), who stated that the occipital condyle is located slightly dorsally in relation to the ventral elements of the braincase. Determining the exact condition in E. minor is not trivial, because of fractures, displacement and complete disarticulation of some elements. In the basioccipital, a line of fracture is present slightly posterior to the basioccipital component of the basal tubera, indicating a ventral dislocation of the posterior portion of the basioccipital, including the condyle. Furthermore, the parabasisphenoid and basioccipital were almost completely disarticulated from each other, in a way that makes it impossible to determine if the basioccipital was displaced ventrally or if it is in its original position. Thus, to securely establish the position of the occipital condyle in relation to the ventral margin of the braincase would require more complete material with less displacement of its elements. However, if the condyle was displaced dorsally, this displacement was rather small, and certainly considerably less than that seen in sauropodomorphs such as Plateosaurus, Massospondylus carinatus Owen, 1854, or Coloradisaurus brevis Bonaparte, 1978.
Frontal
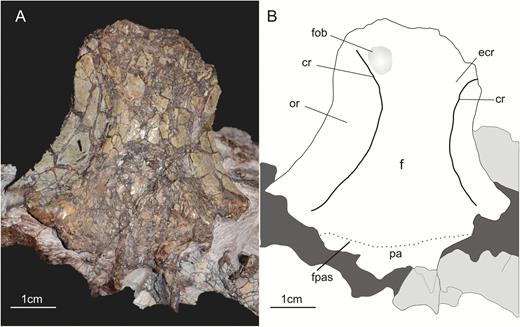
The frontals (Fig. 3) were originally identified as parietals by Huene (1932). Galton & Bakker (1985) correctly re-identified these bones as the frontals, and also mention in their paper that a small portion of the parietals is attached to the posterior margin of the frontals. Both frontals are preserved in SMNS 12667 (Fig. 3) and exposed in ventral view. There is no traceable suture between the left and right elements, nor was it possible to visualize bone limits in the CT scan. However, as sutures are rather untraceable in the entire braincase, and the frontals are affected by numerous fractures, the lack of suture might be related to the preservation rather than representing real fusion of the left and right elements. In Plateosaurus (Prieto-Márquez & Norell, 2011) and Massospondylus kaalae Barrett, 2009, it is possible to observe a suture in the midline between both bones in the ventral surface. Likewise, early sauropodomorphs such as Panphagia protos Martinez & Alcober, 2009 and Eoraptor lunensis Sereno et al., 1993 do not have fused frontals. Thus, it seems unlikely that these bones would have been fused in Efraasia. The frontals are longer than wide. The total length of the frontal is about 45 mm, and its maximum width, in the posterior third of the bone, is 25 mm. This is an intermediate condition in relation to Panphagia (Martinez et al., 2012b), in which the anteroposterior length is twice the width of the frontal, and Plateosaurus (AMNH 6810), in which this ratio is approximately 1.5. Due to preservation, the right frontal shows the distinction between two regions of the ventral surface of the bone, the orbital and endocranial roofs (Fig. 3), better than the left element. The orbital roof corresponds to the region of the frontal that forms the dorsal border of the orbit, whereas the endocranial cavity houses the olfactory tract (Sampson & Witmer, 2007). Both regions are delimited by a single crest (the crista cranii – see e.g. Martinez et al., 2012b), a condition similar to Saturnalia tupiniquim Langer et al. 1999, Plateosaurus (AMNH 6810), M. kaalae, and most other sauropodomorphs. Panphagia exhibits two parallel crests between the distinct regions of the ventral surface of the frontal (Martinez et al., 2012b). The crest in SMNS 12667 is developed as a broad, transversely rounded ridge that is not offset from either the surface of the orbital nor the endocranial facet, but marks a change in orientation of the ventral surfaces. The crest runs parallel to the lateral margin of the frontals, which is concave in ventral view, for most of its length. The anterior portion of the orbital roof is not completely preserved but it is possible to see that the crest converges laterally towards the lateral margin of the frontal in the anterior portion of the frontals. Thus, the width of the orbital roof remains more or less the same along the anteroposterior axis of the ventral surface, but decreases towards its anterior end, at about the level where the fossa for the olfactory bulb is located on the endocranial roof of the bone (see below). The orbital roof of SMNS 12667 is slightly concave transversely and raises dorsally towards the lateral margin. The lateral margin of the frontal is slightly vaulted, being more raised dorsally at its midpoint than its anterior and posterior ends, resulting in an anteroposteriorly concave aspect for the ventral surface of the orbital roof in lateral view. In anterior view, at the level where the frontal reaches its maximum dorsal projection, the angle between the ventral and dorsal surfaces of the bone is approximately 30°, a condition similar to that in Panphagia (Martinez et al., 2012b), M. kaalae and Pantydraco caducus Galton et al., 2007.
At about mid-length of the frontal, the area between the orbital facets is slightly narrower than each of the latter (Fig. 3). However, this area widens gradually both posteriorly towards the roof of the endocranial cavity and anteriorly towards the olfactory bulbs and the antorbital skull roof. The fossa for the olfactory bulb is located at the anterior third of the endocranial roof, being positioned closer to the crest delimitating the orbital roof than to the medial limit of the bone. It is developed as a very shallow, subcircular fossa, deeper at its centre than at its corners, and with lengths varying from approximately 7 mm anteroposteriorly to 5 mm transversely. In the posteriormost region of the bone, the surface of the endocranial roof is slightly wider than the orbital margin. In this respect, the morphology of SMNS 12667 is mostly similar to Panphagia (Martinez et al., 2012b). In Plateosaurus (AMNH 6810), the surface corresponding to the orbital roof is also wider than the one corresponding to the roof of the endocranial cavity at the mid-length of the frontal. However, in this taxon, the difference is much more marked, with the bone surface of the orbital roof being three to four times wider than that of the endocranial roof. The condition in Pantydraco differs from that in Efraasia, Panphagia and Plateosaurus (AMNH 6810) in that even at the mid-length of the bones, the medial surface of the frontal is wider than the orbital roof.
A, ventral view of the frontals of the specimen SMNS 12667 of Efraasia minor. B, schematic drawing of A. Abbreviations: cr, crest; ecr, endocranial roof; f, frontals; fob, fossa for the olfactory bulb; fpas, fronto-parietal suture; or, orbital roof; pa, parietal.
The posterior margin of the frontal extends slightly beyond the posterior limit of the orbital roof (Fig. 3). The posterior region of the articulated frontals of SMNS 12667 is deeply concave transversely. The concavity is deepest posteriorly but decreases gradually anteriorly towards the median portion of the bones. Anteriorly, the ventral surface is flat except for the region of the fossa for the olfactory bulb. The articular facets for the prefrontals and postorbitals are not preserved.
Parietal
Only the anteriormost portion of the parietals is preserved in SMNS 12667, visible in ventral view (Fig. 3). The total anteroposterior length of the preserved portion of the main body of the left parietal is 7 mm, and it is no more than 3 mm in the right parietal. The parietals articulate with the frontals anteriorly. The suture can be more easily recognized on the left side, but its exact course is not entirely clear. From the left lateral limit of the preserved parietal the suture runs posteromedially, giving a concave aspect to the anterior margin of the parietal in the medial portion of the bone, similar to the morphology observed in Adeopapposaurus mognai Martinez, 2009. In contrast, Plateosaurus has a parietal with a straight anteromedial margin, and Panphagia with a concave anterior margin.
The anterolateral ramus of the parietal of SMNS 12667 probably contacted the laterosphenoid ventrally, together forming the anteromedial border of the external and internal supratemporal fenestra. The anterolateral ramus of the parietal is preserved only in the left parietal. It has a triangular shape, with a linear anterior margin with a total length of 9 mm, and a concave posterior margin, which formed the anterior and medial margin of the supratemporal fenestra. The anterolateral ramus extends laterally until the level of the medial limit of the orbital roof of the frontal. As the preserved posterolateral margin of the frontal curves anterolaterally and does not extend posteriorly, this indicates an absence of the frontals from the anterior margin of the supratemporal fenestra, with the anterolateral ramus of the parietal contacting the postorbital in this region. However, given the preservation of the specimen and the variation of the composition of the anterior border of the supratemporal fenestra (i.e. if frontals participate or not) observed in sauropodomorphs (Martinez et al., 2012b), this remains speculative.
In its medial portion, the ventral surface of the parietals is concave, following the concavity in the posterior portion of the frontal described above. The concavity in the ventral surface diminishes progressively laterally until the level of the medial limit of the anterolateral ramus. From this point the surface becomes concave up to the lateral limit of the preserved part of the anterolateral ramus of the parietal.
Basioccipital
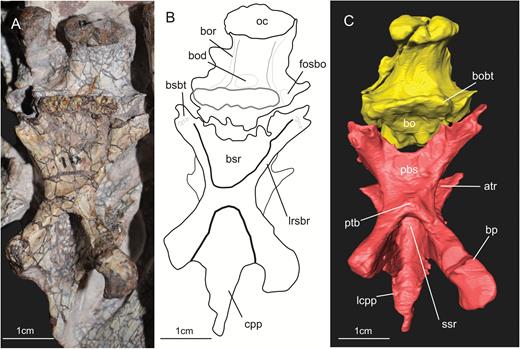
The basioccipital forms the posteroventral portion the braincase (Figs 2, 4). In SMNS 12667, only the ventral and lateral portion of the bone is visible. The CT scan showed that the dorsal part of the basioccipital, which is hidden in the matrix, is partially damaged (Fig. 2), but some inferences about its morphology are still possible. Bones contacting the basioccipital include the parabasisphenoid anteriorly and the otoccipital dorsolaterally. Except for a possible fragment of the otoccipital still being attached to the basioccipital, the latter bone is completely isolated from other elements, as revealed by CT scan data.
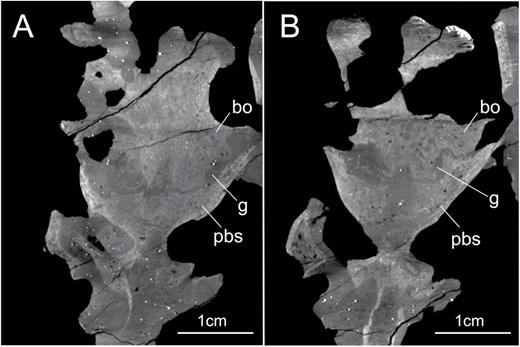
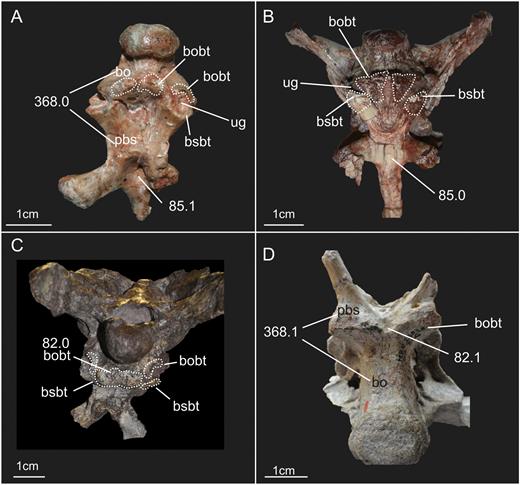
A, ventral view of the basioccipital and parabasisphenoid of the specimen SMNS 12667 of Efraasia minor. B, schematic drawing of (A) C, virtual reconstruction of (A). Abbreviations: atr, anterior tympanic recess; bo, basioccipita; bobt, basioccipital component of the basal tubera; bod, basioccipital depression; bor, basioccipital ridge; bp, basipterygoid process; bsr, basisphenoid recess; bsbt, basisphenoidal component of the basal tubera; cpp, cultriform process of the parabasisphenoid; fosbo, fossa of the basioccipital; lcpp, lamina of the cultriform process of the parabasisphenoid; lrsbr, lateral ridge of the subsellar recess; pbs, parabasisphenoid; ssr, subsellar recess.
In SMNS 12667, the dorsal portion of the basioccipital forms the major part of the floor of the braincase (Fig. 2), with a small contribution of the otoccipital to the lateroposterior portion at the level of the occipital condyle. This condition is similar to other sauropodomorphs, such as Plateosaurus (see Galton, 1985), Leyesaurus tolentinoi Apaldetti et al., 2011, Adeopapposaurus, Coloradisaurus brevis, and Melanorosaurus Haughton, 1924 (see Galton, 1985). Thus, in SMNS 12667 and other sauropodomorphs, the posteriormost surface of the basioccipital forming the floor of the braincase is narrower than the anterior part at the level of the basioccipital component of the basal tubera (Fig. 2).
The posterior portion of the dorsal surface of the basioccipital is transversely concave, resulting in a U shape of the floor of the posterior part of the braincase and the beginning of the neural canal in posterodorsal view, as in Plateosaurus. The anterodorsal portion of the basioccipital forms the ventral border of the anterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis (Fig. 2 – see discussion below for the terms anterior and posterior foramen of the otoccipital). Prieto-Márquez & Norell (2011) stated that the border of the metotic foramen of Plateosaurus (see discussion below) is formed by the parabasisphenoid, but we disagree with their interpretation based on the pictures provided in the manuscript (Prieto-Márquez & Norell, 2011: fig. 27A), and on the analysis of another specimen of Plateosaurus (MB.R.5586-1), which also shows that this border is formed by the basioccipital. Leyesaurus also exhibits the same morphology as Plateosaurus and Efraasia.
For the description of the ventral portion of the basioccipital of SMNS 12667 (Fig. 4), two regions are delimited, an anterior one, which represents the region of the basioccipital anterior to the basioccipital component of the basal tubera, and a posterior one, posterior to this structure. Because this division is based on the basioccipital component of the basal tubera, this structure will be dealt with first.
The basal tubera of sauropodomorphs are usually formed by two ossifications, with contributions from the parabasisphenoid and basioccipital (see Yates, 2004). In the previous description of SMNS 12667, Galton & Bakker (1985) considered the basioccipital/parabasisphenoid suture to traverse the basal tubera. In this case, the latter structure would represent the anterior limit of the basioccipital. We agree with Galton & Bakker (1985) in respect to the structures that they indicated as being part of the basal tubera complex of SMNS 12667 (see Galton & Bakker, 1985: fig. 2C). Thus, this complex consists of a sharp and straight median transverse ridge and a bulbous lateral expansion on either side, which is lower than the ridge and marked by a deep incision extending from lateral into its central part. The latter was considered to be an unossified, cartilaginous part by Galton & Bakker (1985: 3). However, the transverse ridge is only formed by the basioccipital (i.e. it is part of the basioccipital component of the basal tubera), and the median contact between basioccipital and parabasisphenoid lies anterior to this ridge. In SMNS 12667, the basioccipital has thus a broadly triangular anteromedial projection that extends anteriorly between the two posterolateral projections of the parabasisphenoid. This morphology is the same as observed in other non-sauropodan sauropodomorphs, such as Adeopapposaurus, Massospondylus, Pantydraco, Unaysaurus tolentinoi Leal et al., 2004, Coloradisaurus, Anchisaurus polyzelus Hitchcock, 1865, and Plateosaurus. Sauropods, such as Giraffatitan brancai Janensch, 1914 and Dicraeosaurus hansemanni Janensch, 1914, have a more linear and horizontal contact between both bones. The anterior surface of the transverse ridge shows rugose striations, as already mentioned by Galton & Bakker (1985).
Because the basal tubera morphology of the basioccipital of SMNS 12667 may be confused with a structure in theropods named the basituberal web by Bakker, Williams & Currie (1988; = intertuberal lamina, Witmer & Ridgely, 2010), it is worth commenting on the difference between both. As the term used by Witmer & Ridgely (2010) indicates, the lamina in theropods connects the left and right parts of the basisphenoidal component of the basal tubera. In SMNS 12667, the ridge is part of the basioccipital components of the basal tubera, and is not located between basisphenoidal components of the tubera, but situated slightly posterior to these. Furthermore, as in other sauropodomorphs (e.g. Plateosaurus, Massospondylus), the posterior surface of the basioccipital basal tubera in SMNS 12667 is very rugose, related to the muscle attachment in this area (Romer, 1956; Snively & Russell, 2007), and not a smooth lamina as in the theropods exhibiting a similar structure (Bakker et al., 1988). So far, a lamina similar to that seen in some theropods is unknown in sauropodomorphs.
As in other sauropodomorphs (e.g. Coloradisaurus, Plateosaurus, Massospondylus), SMNS 12667 exhibits a distinctive neck in the posterior region of the basioccipital, separating the occipital condyle from the main body of the bone (Figs 2, 4). The condyle of SMNS 12667 is formed by two components, the basioccipital ventrally and mediodorsally, and the otoccipital laterodorsally. The same condition is present in other sauropodomorphs, such as M. carinatus, M. kaalae, Plateosaurus and Melanorosaurus, but it differs from that in Coloradisaurus, in which the otoccipital contribution to the condyle is minimal, with most of the structure being formed solely by the basioccipital. In SMNS 12667, the dorsolateral limit of the basioccipital in the occipital condyle is well marked by the disarticulation of the basioccipital and the otoccipital in this region on the left side of the braincase. However, a small fragment of the otoccipital might still be attached to the basioccipital on the right side. The condyle is not entirely preserved dorsally, and marks of preparation and an unclear limit between bones and sediment in the CT scan data do not allow a secure interpretation of its morphology in posterior view. As preserved, the condyle has a width of 15 mm, 10 mm of which correspond to the basioccipital component of the condyle, and 5 mm to the otoccipital component (2.5 mm on each side).
In SMNS 12667, the portion of the basioccipital delimited by the occipital condyle posteriorly and by the basioccipital component of the basal tubera anteriorly is trapezoidal in shape in ventral view, with the anterior and posterior margins forming parallels sides (Fig. 4). This trapezoidal outline is due to the fact that the lateral wall of the basioccipital just behind the tubera is not strictly vertical but slopes laterodorsally, and is thus visible in ventral view. In Plateosaurus (MB.R.5586-1; Prieto-Márquez & Norell, 2011), the lateral side of the basioccipital is more vertically oriented in the anterolateral region, resulting in a more rectangular shape for this portion of the bone in ventral view.
The ventral side of the basioccipital of SMNS 12667 exhibits a shallow longitudinal groove delimited by two parallel longitudinal ridges (Fig. 4B). The groove extends from the neck of the occipital condyle to the posterior limit of the medial ridge forming the basioccipital component of the basal tubera, where it becomes deeper and wider. The lateral ridges mark the transition from the ventral to the lateral surface of the basioccipital. In other sauropodomorphs (e.g. M. carinatus, Melanorosaurus, Plateosaurus, Giraffatitan), these ridges also extend from the occipital condyle to the basioccipital component of the basal tubera. The ridges, and consequently the fossa between them, are evident to different degrees among sauropodomorpha. In Plateosaurus (MB.R.5586-1) and the sauropod Giraffatitan, the ridges and the groove are easily recognized in ventral view. On the other hand, these structures are much less pronounced in another specimen of Plateosaurus (AMNH 6810), and are absent (or imperceptible) in some taxa, such as Saturnalia and Adeopapposaurus. Regarding the groove in SMNS 12667, it exhibits a deeper, semicircular fossa in its most anterior part, just posterior to the transverse ridge of the basioccipital component of the basal tubera (Fig. 4B). This fossa is also present in other sauropodomorphs, such as Aardonyx celestae Yates et al., 2010; Giraffatitan and Plateosaurus. In Plateosaurus (MB.R.5586-1) and Aardonyx, the fossa is deeper than in SMNS 12667.
Laterally and slightly anterior to the semicircular fossa, the basioccipital surface exhibits another depression, which marks the division of medial and lateral portions of the basioccipital component of the basal tubera (Fig. 4B). The depression is only preserved on the right side and corresponds to a similar structure observed by Prieto-Márquez & Norell (2001: fig. 30B, ‘fos bo’) in Plateosaurus (AMNH 6810). However, it seems that in SMNS 12667, the fossa does not have a well-defined ventral limit, as is the case in Plateosaurus (Prieto-Márquez & Norell, 2011). In the latter, the fossa also marks the division between the two portions of the basioccipital component of the tubera.
CT scan data indicate a complete separation of the basioccipital and parabasisphenoid in SMNS 12667 (Fig. 5). A complete disarticulation between the basioccipital and the parabasisphenoid is usually observed in braincase materials of individuals of sauropodomorphs regarded as juveniles (e.g. Fedak & Galton, 2007; Galton & Kermack, 2010), indicating that SMNS 12667 is a juvenile specimen of E. minor (see also Galton & Bakker, 1985; Yates, 2003). Similar disarticulation between the basioccipital and parabasisphenoid is present in the braincases of Unaysaurus and Anchisaurus (Fedak & Galton, 2007). Regarding Anchisaurus, recent papers have considered this specimen as representing a juvenile (see Fedak & Galton, 2007; Yates, 2010). In Unaysaurus, there is only an incipient contact between the bones by a connection between the basioccipital and basisphenoidal component of the tubera. Although not treated as a juvenile in its original description (Leal et al., 2004), ongoing study of the specimen of Unaysaurus has found characteristics supporting this assessment (J. Bittencourt, pers. comm.). In the holotype of Pantydraco, probably a very immature specimen (Galton & Kermack, 2010), the parabasisphenoid and basioccipital are completely disarticulated from each other. This indicates a very weak junction between these two bones in earlier ontogenetic stages of Sauropodomorpha, before complete maturity of the animals.
X-ray slices obtained from the CT scan procedure showing the complete separation of basioccipital and parabasisphenoid in two distinct regions of the braincase of the specimen SMNS 12667 of Efraasia minor. The region depicted in (A) is more dorsally located in relation to the region depicted in (B). Abbreviations: bo, basioccipital; g, gap; pbs, parabasisphenoid.
Parabasisphenoid
The parabasisphenoid forms the anterior part of the floor of the braincase (Figs 2, 4). In SMNS 12667, the parabasisphenoid would have contacted the basioccipital posteriorly, the otoccipital posterodorsally, the prootic dorsally and potentially the laterosphenoid anterodorsally. The parabasisphenoid possesses a series of associated structures, which include the cultriform process, basipterygoid processes, subsellar and basisphenoid recesses, and a part of the basal tubera (in addition to the basioccipital component, as detailed above).
In SMNS 12667, the parasphenoid is completely fused to the basisphenoid (Fig. 4), as in all other dinosaurs (Currie, 1997) and archosauriforms (Walker, 1990; Bittencourt et al., 2014). Because of this it is necessary to emphasize that, despite recognizing a portion of the parabasisphenoid as the cultriform process and treating it as a distinct region in the description herein, it is impossible to precisely delimitate the posterior limit of the process and the suture between parasphenoid and basisphenoid. Furthermore, it is very likely that only the most proximal part of the cultriform process is preserved in SMNS 12667, because, by comparison with other non-sauropodan sauropodomorphs that have a more completely preserved cultriform process (e.g. Saturnalia, Plateosaurus, Pantydraco), the anteroposterior length of this structure might be greater than the length of the rest of the braincase. Only the ventral surface of the parabasisphenoid and parts of the lateral sides are exposed in SMNS 12667, but more details of its morphology can be established with the help of the CT scan data.
The parabasisphenoid is slightly longer (25 mm) than wide (22 mm) between the basal tubera and the base of the cultriform process (Figs 2, 4). In ventral view, it is notably X-shaped, being strongly constricted in its central part and expanding rapidly laterally and posteriorly towards the basisphenoidal portions of the basal tubera and anteriorly towards the basipterygoid processes. The lateral expansion of both of these structures is approximately equal, but the minimal width of the bone of c. 8.5 mm is less than half of the width across the basal tubera (c. 22 mm).
In lateral view, the ventral margin of the parabasisphenoid between the proximal limit of the basipterygoid process and the basisphenoidal component of the tubera is curved, with its posterior and anterior ends located ventrally in respect to the surface between them. Dorsally, a deep excavation in the lateral surface of the bone corresponds to the anterior tympanic recess of theropods in respect to its relative position (Witmer, 1997). The surface posteroventral to this recess is flat, and the lateral side of the bone would have contacted the prootic dorsally and the otoccipital posteriorly.
Posterior to the cultriform process, the dorsal surface of the parabasisphenoid has the pituitary fossa preserved anteriorly, which is separated from the posteriormost surface by a vertical wall of bone perforated by the vidian canal (or foramen for the internal carotid artery) medioventrally. This canal is represented by a single opening in Efraasia, similar to the condition observed in Thecodontosaurus, which indicates that the right and left carotids and enter the pituitary fossa through a single foramen. On the other hand, Adeopapposaurus (PVSJ 568), Massospondylus (BP/1/5231) and Plateosaurus (MB.R.5586-1) exhibit two small foramina in this region, indicating that the left and right carotid enter pituitary fossa trough separate openings. However, it is necessary to point out that this region of the braincase is poorly preserved in both Efraasia and Thecodontosaurus. Thus, the presence of a single opening in Efraasia might be an artefact, especially because the septum dividing the canal in Adeopapposaurus and Plateosaurus consists of a thin and delicate structure.
The basisphenoidal component of the basal tubera is a bulbous structure located at the tip of the posterolateral projections of the parabasisphenoid (Fig. 4). From the anterior limit of the parabasisphenoid/basioccipital contact, the length of the projections is c. 8 mm. The surface of the tubera shows a series of small and shallow circular pits that represent the scars of the muscle attachment in this region. The pits are present in the posterior end of the ventral surface of the projection, and also in the posteroventral corner of the lateral side of parabasisphenoid.
Here we adopt the term basisphenoid recess (sensuWitmer, 1997) to refer to the depression on the ventral surface of the parabasisphenoid (Fig. 4), located anterior to the posterolateral projections of the tubera and posteriorly to the subsellar recess (see below). In SMNS 12667, the depression is very shallow and anteriorly defined by a protuberance on the ventral surface of the parabasisphenoid located between the proximal bases of the basipterygoid processes. A protuberance is also observed in other taxa, such as Plateosaurus (Prieto-Márquez & Norell, 2011), Unaysaurus and Adeopapposaurus (PVSJ 568), whereas other taxa, such as Massospondylus, Coloradisaurus and Pantydraco do not possess a protuberance in this region of the parabasisphenoid. The lateral limit of the basisphenoid recess of SMNS 12667 is more distinguishable on the right side of the braincase. On this side, a low-rounded ridge extends along the lateral margin of the ventral surface of the parabasisphenoid. Posteriorly, this ridge becomes confluent with the basisphenoidal component of the basal tubera.
The presence of a basisphenoid recess is a widespread characteristic among non-sauropodan sauropodomorphs, probably being present in all the members of the group (pers. obs.). As mentioned above, the usage of terms shallow and deep is subjective, but obvious differences are also notable in relation to the depth of the basisphenoid recess among different taxa. The basisphenoid recess of SMNS 12667 is shallow, resembling more the morphology observed in taxa such as Adeopapposaurus and Pantydraco, than the one of Coloradisaurus, which exhibits a deeper basisphenoid recess. However, a basisphenoid recess as deep as that observed in most theropod taxa (e.g. Rauhut, 2003, 2004) is not observed in non-sauropodan sauropodomorphs. In SMNS 12667, as in other non-sauropodan sauropodomorphs we analysed, the recess does not have a clearly defined posterior limit in the parabasisphenoid, but fades towards the ventral surface of the basioccipital projected between the posterolateral margins of the parabasisphenoid. This differs from theropods (Witmer, 1997; Rauhut, 2004; Sampson & Witmer, 2007) and sauropods such as Tornieria africana Fraas, 1908, in which the recess is also clearly defined posteriorly, configuring a rounded/circular outline to this structure in ventral view. In SMNS 12667, the shape of the recess in the parabasisphenoid is triangular/trapezoidal, similar to the condition in other non-sauropodan sauropodomorphs analysed for this study. The ridges delimiting the basisphenoid recess laterally correspond to the transitional surface between the ventral and lateral side of the parabasisphenoid (Fig. 6). This transitional surface, from the ventral side of the bone to the level of the anterior tympanic recess laterally, was named the lateral lamina of the basisphenoid (= crista ventrolateralis in Theropoda; Sampson & Witmer, 2007, following Kurzanov, 1976) by Apaldetti et al. (2014) in their redescription of the skull of Coloradisaurus. In SMNS 12667, this transitional surface is not developed as a lamina, but rather as a rounded lateral edge.
A, ventrolateral view of the braincase of the specimen SMNS 12667 of Efraasia minor. B, virtual reconstruction of (A) (excluding the laterosphenoid) detailing the cranial openings. Abbreviations: afo, anterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis; atr, anterior tympanic recess; bo, basioccipital; bp, basipterygoid process; cpp, cultriform process of the parabasisphenoid; dtr, dorsal tympanic recess; fo, fenestra ovalis; ls, laterosphenoid; mpp, attachment region of the m. protractor pterygoideus; ot, otoccipital; otc, otosphenoidal crest; p, prootic; pbs, parabasisphenoid; pfo, posterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis; pp, preotic pendant; stg, stapedial groove; ug, unossified gap; vc, vidian canal; vcm, path of the mid-cerebral vein; V, notch of the fifth cranial nerve (trigeminal); VII-h, foramen for hyomandinbular ramus of the seventh cranial nerve (facial); VII-p, foramen for palatine ramus of the seventh cranial nerve (facial); XII, foramina for the 12th cranial nerve (hypoglossal).
The lateral surface of the parabasisphenoid of SMNS 12667 is better exposed on the right side of the braincase, with the left side being partially covered by matrix and hidden by the dislocated right laterosphenoid (Fig. 6A). Nevertheless, with CT scan data, it is possible to access the whole morphology of the lateral portion of the bone, and the region of the parabasisphenoid that would have made contact with the prootic in the left side of the braincase (Fig. 6B). Although the precise limits of the bones are still uncertain, it is possible that the separation of parabasisphenoid and both prootic and otoccipital on the left side of the braincase happened in the original region of articulation between these bones, based on the morphological similarity of what is preserved of the parabasisphenoid on both sides (Fig. 2 and Supporting Information S1).
The excavation on the lateral side of the parabasisphenoid (Fig. 6) is topologically correlated to the structure usually named the anterior tympanic recess in theropods (Witmer, 1997; Rauhut, 2004), which is also present in representatives of the Avemetatarsalia lineage outside Dinosauria (Nesbitt, 2011; Bittencourt et al., 2014; pers. obs.), in the non-archosaurian archosauriform Euparkeria capensis Broom, 1913 (Sobral et al., 2016), and in all the non-sauropodan sauropodomorphs analysed for this study. In SMNS 12667, the anterior tympanic recess is mainly located in the parabasisphenoid, but its posterodorsal limit is within the anteroventral limit of the lateral surface of the prootics (see below). The anteroventral limit of the recess in the parabasisphenoid is situated close to the base of the basipterygoid process. From this point, the recess extends posterodorsally, occupying approximately one-third of the lateral surface of the bone. From the anteroventral to the posterodorsal limit, the length of the recess is approximately 15 mm. The lateral surface of the parabasisphenoid roofing the dorsal limit of the anterior tympanic recess is the region of the preotic pendant. This structure is formed by the parabasisphenoid and the prootics (see description below).
The aperture of the vidian canal lies in the anteroventral portion of the anterior tympanic recess of SMNS 12667 (Fig. 6). The vidian canal represents the opening through which the internal carotid artery and the palatine branch of the facial nerve (VII) enter the internal cavity of the braincase (Galton, 1985; Sampson & Witmer, 2007). In their description of the skull of Coloradisaurus, Apaldetti et al. (2014) treated the vidian canal and the foramen for the internal carotid artery as two distinct structures. This would make Coloradisaurus distinct from SMNS 12667 and other sauropodomorphs; however, it rather reflects a confusion in the usage of different terms related to the same structure. As explained in Müller, Sterly & Anquetin (2011), the path of the internal carotid artery varies among amniotes. However, in those groups where a vidian canal is present, by definition, it represents the aperture through which the internal carotid artery enters the braincase. The dorsal opening in Coloradisaurus might be related to the palatine branch of the facial nerve (see below).
The aperture of the vidian canal in the lateral surface of the parabasisphenoid connects to an aperture located at the ventromedial portion of the posterior limit of the pituitary fossa (Fig. 2). The pituitary fossa (or sella turcica), which houses the pituitary gland (Galton, 1985), is a structure present in the anterior portion of the dorsal surface of the parabasisphenoid, posterodorsal to the cultriform process. The posterior limit of the pituitary fossa is a wall of bone (c. 2 mm thick and 7 mm tall), which would have contacted the prootics dorsally at the region of dorsum sellae, which is not preserved in SMNS 12667. The lateral borders of the pituitary fossa correspond to the medial surface of the portion of the parabasisphenoid that laterally forms the preotic pendant. From its ventral limit, the lateral margins of the fossa diverge posterolaterally. Thus, the pituitary fossa is triangular in outline in anterior view, but with a rounded ventral apex (Fig. 2), similar to the morphology observed in Plateosaurus. Posterior to the wall of bone defining the fossa, the shape of the dorsal region of the parabasisphenoid follows the general morphology of the corresponding ventral surface described above.
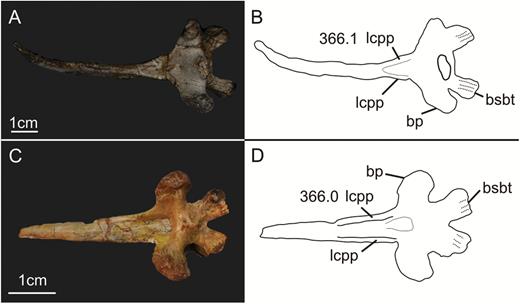
Because of its basically identical morphology and position to the condition seen in many theropods, we interpret the deep ventral concavity at the base of the cultriform process (Fig. 4) as the subsellar recess (sensuWitmer, 1997). The term subsellar recess is widely used in the literature on theropod braincases (e.g. Rauhut, 2004; Sampson & Witmer, 2007; Paulina-Carabajal, 2011; Bever et al., 2013), but has not yet been applied to sauropodomorphs. In fact, not only is the use of the term subsellar recess uncommon in studies on sauropodomorphs, but even the respective structure is rarely described. One of the few exceptions is the paper by Gow (1990), in which the author used the term ‘blind pocket’ to refer to the subsellar recess of M. carinatus; however, no detailed description or comparisons with other taxa were provided. Nevertheless, notable differences regarding the depth of the subsellar recess among sauropodomorphs are obvious (although the usage of deep and shallow may be subjective). The recess of SMNS 12667 is very deep, similar to the condition in Coloradisaurus and Plateosaurus (MB.R.2285-1). Other taxa, such as Pantydraco, M. carinatus (only SAM-PK-K1314) and Giraffatitan, exhibit a shallower recess (see discussion below). Although not mentioned in the description by Galton & Bakker (1985), subsequent phylogenetic studies (e.g. Yates, 2007b; Yates et al., 2010; Apaldetti et al., 2011; Pol et al., 2014; McPhee et al., 2014, 2015) treated the braincase of Efraasia as possessing a deep transverse septum between the basipterygoid processes (character 83 of Yates, 2007b). These authors probably interpreted the posterior border of the deep subsellar recess (sensuWitmer, 1997) as such a septum, but the bony connection between the processes is actually low when compared to other taxa (see discussion below).
The right basipterygoid process of SMNS 12667 is entirely preserved, lacking only a small fragment of the anteromedial surface distally, whereas only the proximal part of the left process is preserved (Figs 2, 4, 6). The relatively robust proximal portion of the basipterygoid process is formed by a complex array of bony struts that results in a roughly T- to H-shaped cross section of this part. Thus, the ventral part of the base of the process is formed by a stout vertical strut between the anterior tympanic recess and the subsellar recess; this strut has a slightly transversely expanded ventral surface in its proximal part. More dorsally, the base of the basipterygoid process is formed by a thin anterodorsally and medially directed lamina that extends from the basipterygoid process towards the cultriform process and thus forms the dorsolateral wall of the subsellar recess, and a more robust, almost horizontal lamina that arises from the dorsal roof of the anterior tympanic recess. The distal part of the basipterygoid process is lateromedially compressed, as preserved on the right side. In its distalmost portion, where the process would have contacted the pterygoid, the tip of the basipterygoid process curves laterally. The process projects ventrolaterally, as in Unaysaurus, Giraffatitan, Massospondylus and Thecodontosaurus. Establishing the anteroposterior orientation of the basipterygoid processes can be problematic because it is sometimes difficult to determine the exact orientation of the parabasisphenoid in the braincase. Using Plateosaurus as an example, Prieto-Márquez & Norell (2011) stated in their description of AMNH 6810 that the basipterygoid process projects anteriorly in this specimen. However, according to our interpretation of the illustrations (Prieto-Márquez & Norell, 2011: fig. 27), the processes are clearly posteroventrally oriented in AMNH 6810, as in other specimens of Plateosaurus (e.g. MB.R.5581.6; MB.R.1937; SMNS 13200). In SMNS 12667, the basipterygoid processes are notably anteriorly oriented. A vertical or even posterior orientation of these processes would imply an inclination of the ventral surface of the parabasisphenoid of more than 45° in relation to the anteroposterior axis, and consequently a strong verticalization of the internal cavity of the braincase. This is very unlikely, and not supported by the relative position of the basioccipital (and thus the occipital condyle) towards the parabasisphenoid. At about the level of the proximal limit of the cultriform process (Figs 2, 4), the distance between the medial margins of the basipterygoid processes is c. 10 mm, but the bases of the processes converge posteromedially to a minimal distance of 2 mm at the posterior end of the subsellar recess. This approximation of the proximal portions of the basipterygoid processes in SMNS 12667 resembles the morphology observed in Thecodontosaurus, rather than that of Adeopapposaurus, Coloradisaurus, Massospondylus and Plateosaurus, which have a greater separation of the basipterygoid processes proximally.
The cultriform process of the parabasisphenoid is only partially preserved (Figs 2, 4). The exact proximal limit of the process is difficult to establish because the process arises gradually from the laminae of the basipterygoid processes mentioned above and the subsellar recess. The preserved portion is no longer than 15 mm anteroposteriorly and 9 mm high. In dorsal view, the lateral margins of the proximal portion of the cultriform process contact each other dorsally anterior to the pituitary fossa in sauropodomorphs such as Plateosaurus and Saturnalia, forming a short, closed canal. In SMNS 12667, the lateral margins do not converge dorsally (Fig. 2B), but it is not possible to affirm if this represents the original morphology or results from the poor preservation of the fossil in this region. In anterior view, the cultriform process is U-shaped, with its lateral margins diverging laterodorsally from each other.
The ventral surface of the proximal part of the cultriform process of SMNS 12667 is concave transversely (Fig. 4), as in other sauropodomorphs, such as Coloradisaurus, Plateosaurus, Massospondylus and the sauropod Giraffatitan. The lateral margins of the concave surface are delimited by sharp-rimmed laminae, which extend from the bases of the basipterygoid processes to the lateral edges of the cultriform process. In contrast, Plateosaurus, Massospondylus, Giraffatitan and Coloradisaurus do not exhibit such a sharp lamina (triangular lateral lamina of the parasphenoid rostrum in Apaldetti et al., 2014), but rather have a more rounded crest, which extends from the basipterygoid process to the cultriform process. In lateral view, the anteroventral border of the laminae is notably concave. In SMNS 12667 and some other taxa, such as Massospondylus and Coloradisaurus, these laminae/crests are inclined ventrolaterally, so that their bases are parallel to each other along their entire length, whereas the laminae diverge posteroventrally in anterior view. They fade into the ventral surface of the cultriform process slightly anterior to the level of the anterior end of the basipterygoid processes, from where the ventral surface becomes slightly convex transversely (Fig. 4). A different condition is present in Plateosaurus, Unaysaurus, Sarahsaurus aurifontanalis Rowe et al., 2011, and in the sauropod Giraffatitan. In these taxa, the two ridges converge medially in the portion of the cultriform process where the surface becomes flat/convex, resulting in a triangular shape of the concavity on the ventral surface of the proximal portion of the process, providing a well-defined anterior limit for the subsellar recess (see below).
Prootic
Both prootics of SMNS 12667 are preserved (Fig. 2) but the description provided here is mainly based on the left element (Figs 6, 7), as the right prootic is preserved inside the matrix and its surface is greatly damaged. The left prootic has its lateral surface exposed, except for the anteroventral surface that contacted the parabasisphenoid ventrally (Figs 6A, 7). This area is covered by the right laterosphenoid in the block, but can be visualized with CT scan data (Fig. 6B). The CT scan also shows that the medial surface of this bone, including the inner ear cavity, is greatly damaged. The only recognizable feature in the medial surface is a large depression in the region that corresponds to the position of the flocculus of the cerebellum. However, any detail of this structure, or of the semicircular canals of the inner ear within the prootic, is impossible based on the CT scan data.
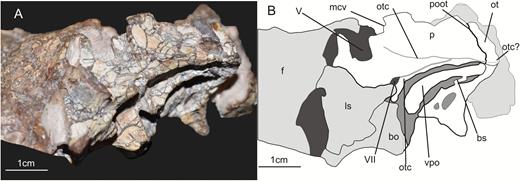
Lateral view of the left prootic of the specimen SMNS 12667 of Efraasia minor. Abbreviations: bs, bony strut; f, frontal; ls, laterosphenoid; ot, otoccipital; otc, otosphenoidal crest; p, prootic; pbs, parabasisphenoid; poot, posterior limit of of the prootic overlapping the otoccipital; vcm, notch of the mid-cerebral vein; vpo, ventral process of the otoccipital; V, notch of the trigeminal nerve; VII, foramen for the cranial nerve VII.
The prootic forms most of the laterodorsal wall of the braincase. In SMNS 12667, the prootic is still articulated with the paroccipital process of the otoccipital posteriorly (Fig. 7). The posterodorsal part of the prootic probably contacted the parietal dorsally, and potentially also the supraoccipital, as in some other sauropodomorphs, such as Thecodontosaurus (Benton et al., 2000), Adeopapposaurus (Martinez, 2009) and Plateosaurus (Galton, 1984). Other contacts of the prootic include the parabasisphenoid anteroventrally and the laterosphenoid anterodorsally. Several foramina either pierce the prootic or are bordered by this element in conjunction with other bones. These include the foramina for cranial nerves V and VII, the opening for the mid-cerebral vein, and the fenestra ovalis (Figs 6, 7).
The surface of the prootic overlapping the otoccipital represents the posterior third of a subrectangular bone surface that extends from the base of the paroccipital process posterior to the level of the notch for the mid-cerebral vein anteriorly (Fig. 7). The rectangle is flexed in its anterior third, so that the anterior part is directed slightly anterodorsally, whereas the posterior part is oriented considerably posterodorsally; this flexure results in a notched anterior half of the dorsal margin. The maximal length of this subrectangular surface is 19 mm and its height is 11 mm posteriorly and 9 mm anteriorly, it being delimited by the otosphenoidal crest (= crista prootica; see Sampson & Witmer, 2007) ventrally. The anterodorsal margin of this surface would have contacted the laterosphenoid, enclosing the aperture of the mid-cerebral vein (see below). Posteriorly, the dorsal part of the prootic overlaps the otoccipital, thus forming the anterior part of the base of the paroccipital process, as in other archosaurs. The surface of this dorsal portion of the prootic is anteroposteriorly concave towards the anterior rim, but flat in its posterior half. In theropods, this is the position where the dorsal tympanic recess is located (Witmer, 1997; Rauhut, 2004). Our observations indicate that a very shallow concavity is present among many non-sauropodan sauropodomorphs (e.g. Plateosaurus, Adeopapposaurus, Massospondylus), including Efraasia, but it is not developed as a pneumatic recess as in some theropod taxa (Witmer, 1997).
As noted above, the dorsal portion of the prootic is separated from the ventral part by a bony crest. We here adopt the term otosphenoidal crest instead of crista prootica, because, as discussed by Sampson & Witmer (2007), the crest extends beyond the limits of the prootics in some taxa. In SMNS 12667, the posterior end of the crest is unclear due to preservation, but it seems that the crest extended posteriorly onto the proximal part of the paroccipital process (Fig. 7). At the ventral margin of the subrectangular surface described above, the crest is developed as a low rounded ridge, and it defines the dorsal margin of the fenestra ovalis and the laterodorsal margin of the stapedial groove on the bases of the paroccipital process. The crest bifurcates anteriorly at the anteroposterior level where the dorsal surface of the prootic curves anterodorsally (Figs 6, 7). The dorsal component of this bifurcation extends anteriorly towards the notch for the mid-cerebral vein, where it meets the anterior border of the bone at about the mid-height of this notch. In the anterior portion, the crest becomes more prominent and overhangs the more ventral part of the lateral side towards the foramen. It thus might have formed the dorsal border of a posterior course of the mid-cerebral vein on the outside of the braincase. This anterodorsal component of the otosphenoidal crest is not present in all taxa, and in those taxa where it is present, it is often not regarded as part of the structure (see e.g. Martinez et al., 2012b). Usually, the crest extends only ventrally or anteroventrally, bordering the foramen for the facial nerve (Sampson & Witmer, 2007). However, as we see no discontinuity between the ridge extending anteriorly and the posterior component of the otosphenoidal crest, we consider this anterodorsal component as part of the otosphenoidal crest in SMNS 12667. This anterodorsal component is also observed in Panphagia (see Martinez et al., 2012b: fig. 8C), Massospondylus (BP/1/5231) and Plateosaurus (MB.R.5581.6), but not in Adeopapposaurus.
The portion of the otosphenoidal crest that extends anteroventrally borders the foramen for the facial nerve (Fig. 7 – see below). As is the case in other non-sauropodan sauropodomorphs, such as Plateosaurus, Melanorosaurus, Adeopapposaurus and Massospondylus, the portion of the crest bordering the facial foramen is low and do not expand laterally in SMNS 12667. In contrast, many sauropods, such as Dicraeosaurus, Tornieria and Giraffatitan, exhibit a well-developed, high and laterally expanded otosphenoidal crest. In these taxa, the crest is developed as a sheet of bone projecting lateroposteriorly, hiding the fenestra ovalis in lateral view. Anteroventral to the foramen for the nerve VII, the otosphenoidal crest becomes confluent with the posterior rim of the preotic pendant.
The preotic pendant (sensuMadsen & Welles, 2000; = ala basisphenoidalis, see Sampson & Witmer, 2007) is a structure usually formed by a bone expansion in the anteroventral portion of the prootics and the dorsal margin of the parabasisphenoid (Figs 2, 6, 7), dorsal to the vidian canal (Sampson & Witmer, 2007). This structure is present in dinosauriforms (e.g. Silesaurus opolensis Dzik, 2003), and also in all dinosaur clades (pers. obs.). However, as for the subsellar and basisphenoid recesses, it is usually more developed in the braincase of some theropod taxa, where it is expanded as a posteroventrally directed lamina (e.g. Chure & Madsen, 1996, 1998; Rauhut, 2004; Sampson & Witmer, 2007). Sauropodomorph taxa, such as Plateosaurus, Melanorosaurus, Thecodontosaurus and Massospondylus, exhibit relatively well-developed preotic pendants, but they do not overlap a significant portion of the anterior tympanic recess in lateral view, as in some theropods. In SMNS 12667, there is no such well-developed structure, with the preotic pendant projecting no more than 2 mm ventrally in the area of the anterior tympanic recess. The length of the preotic pendant surface of SMNS 12667 in the prootic, measured from the ventral border of the foramen for the facial nerve up to the anteroventral limit of the bone, is c. 5 mm. The preotic pendant marks the anterodorsal limit of the anterior tympanic recess. The posterodorsal limit of the preotic pendant is more difficult to establish, as the dorsal surface becomes confluent with the surface of the prootic anteroventral to the notch of the trigeminal nerve, which does not participate in the preotic pendant. The portion of the pendant formed by the parabasisphenoid is similar in size to the one formed by the prootic, and it overhangs the dorsal margin of the anterior tympanic recess in this bone. The preotic pendant represents the attachment surface for the m. protractor pterygoideus (Figs 6, 7), as in other sauropodomorphs (Prieto-Márquez & Norell, 2011; Martinez et al., 2012b) and theropods (Sampson & Witmer, 2007).
In SMNS 12667, the notch for the trigeminal nerve (V) is located dorsally and slightly posteriorly to the attachment surface for the m. protractor pterygoideus (Fig. 6), as in all other sauropodomorphs (e.g. Plateosaurus, Panphagia, Coloradisaurus, Melanorosaurus). As is typical for members of the group, the foramen for cranial nerve V in SMNS 12667 is bordered by the prootic ventrally and posteriorly, and would be enclosed by the laterosphenoid anterodorsally (Yates, 2007b; Prieto-Márquez & Norell, 2011; Martinez et al., 2012b; Apaldetti et al., 2014). In Thecodontosaurus, the foramen for cranial nerve V is completely enclosed by the prootic (Benton et al., 2000), which is also observed in the ornithischian Dysalotosaurus lettowvorbecki Virchow, 1919 (Sobral et al., 2012), but this represents an unusual configuration for non-sauropod sauropodomorphs, which was not observed in any other taxa we examined for this study (pers. obs.). In SMNS 12667, the anteroventral margin of the trigeminal notch formed by the prootic is broken and slightly dislocated. As preserved, this margin has a length of 5 mm, which is also the width of the foramen. However, because of the breakage, part of the anteroventral margin is ventrally dislocated, and its total length could be some millimetres greater than the width of the notch. The ventral border of the notch is concave. The posterodorsal margin of the notch in the prootic is shorter than the anteroventral margin (3 mm). The small triangular projection of the prootic forming the posterodorsal notch of the trigeminal nerve also forms the anteroventral margin of the notch for the mid-cerebral vein. In contrast to the concave ventral margin of the notch for the trigeminal nerve, the notch for the mid-cerebral vein has a more straight ventral margin. As with the trigeminal foramen, the laterosphenoid probably enclosed the notch for the mid-cerebral vein anterodorsally. A complete separation of the foramen for the trigeminal nerve and mid-cerebral vein is also observed in other taxa, such as Plateosaurus and Adeopapposaurus, but not in Coloradisaurus. In this taxon, there is a single notch for the trigeminal nerve and the mid-cerebral vein, with the latter probably passing through the dorsal portion of the opening (Apaldetti et al., 2014).
Posteroventral to the trigeminal notch, other openings in the prootic correspond to the foramen for the facial nerve (VII). Galton & Bakker (1985) did not mention the passage for this nerve, probably because the region was obscured by matrix, but with the CT scan data, it was possible to identify the internal and external apertures in the prootic related to this nerve (Fig. 6). As is typical for dinosaurs, an opening is found posteroventral to the trigeminal foramen, associated with the otosphenoidal crest. Regarding the relationship between the otosphenoidal crest and the foramen for cranial nerve VII in non-sauropodan sauropodomorphs, several statements can be found in the literature. Gow (1990) stated that the crest borders the opening of the foramen for the facial nerve in Massospondylus (BP/1/5231) anteriorly, but noted a second ridge ventral to it, which he called the crista subfacialis. On the other hand, Galton (1985) mentioned that it borders the posterior margin in Plateosaurus, and Martinez et al. (2012b) considered the foramen to be enclosed by the crista in Panphagia. However, it seems that the difference stated by the authors is not the result of different morphologies among taxa, but different interpretations of what was regarded as the ventral ramus of the otosphenoidal crest bordering the facial nerve foramen. In SMNS 12667, there is no indication that the crest runs only along the anterior or posterior margin of the foramen. The borders of the foramen are continuous with the otosphenoidal crest both anteroventrally and posterodorsally (although the crest is slightly damaged in this region). It thus seems that the crest bifurcates to enclose the foramen for the facial nerve, as described for Panphagia (Martinez et al., 2012b). This morphology is also observed in Plateosaurus (MB.R.5586-1, AMNH 6810), Melanorosaurus (NMQR 1551), Adeopapposaurus, Thecodontosaurus and in the sauropod Spinophorosaurus nigerensis Remes et al., 2009 (Knoll et al., 2012).
In SMNS 12667, the aperture of the facial nerve on the medial side of the prootic is circular, with a diameter of approximately 5 mm, but its shape and size may be slightly distorted, given the poor preservation of the medial surface of the bone (see Supporting Information S1). After leaving the brain, the facial nerve of dinosaurs becomes subdivided and exhibits two distinct rami, the hyomandibular and palatine rami (Galton, 1985; Sampson & Witmer, 2007). In SMNS 12667, there are two foramina on the lateral side of the prootic, which probably represents separate exits for the two distinct rami of the facial nerve. One of these is visible on the lateral side of the prootic at the otosphenoidal crest posteroventral to the trigeminal foramen, and probably represents the passage of the hyomandibular ramus of the facial nerve (Galton, 1985). This foramen has an elliptical shape, 6 mm long from the posterodorsal to the anteroventral limit and it is 3 mm wide at its mid-length, although these values have quite certainly been exaggerated by breakage. According to Galton (1985), this ramus in Plateosaurus turns posterodorsally after passing through the external aperture of the foramen. Based on the CT scan data, it is possible to trace the initial way of the hyomandibular ramus in SMNS 12667. As in Plateosaurus, the nerve turns posteriorly and then runs posterodorsally along the otosphenoidal crest. The second branch of the facial nerve, the palatine ramus, runs anteroventrally and joins the internal carotid artery below the dorsal lamina of the parabasisphenoid, with both entering the vidian canal together (Galton, 1985). In SMNS 12667, the second opening for the facial nerve is a circular aperture (3–4 mm diameter) located at the posterodorsal corner of the anterior tympanic recess, separated from the foramen of the hyomandibular ramus by a thin crest that encloses the latter posteroventrally. This opening probably represents the passage for the palatine ramus, as the CT data show that both foramina converge on the same medial aperture within the prootic. An aperture in this area, at the posterodorsal corner of the anterior tympanic recess, is not present in Plateosaurus and Panphagia. In these taxa, there is a single lateral opening for cranial nerve VII. A similar condition as in SMNS 12667 is probably present in Coloradisaurus. The region of the anterior tympanic recess is not very well preserved in the braincase of Coloradisaurus, but a foramen was probably present at the dorsoventral corner of this structure (pers. obs.). The presence of this foramen was also mentioned by Apaldetti et al. (2014: fig. 6B), but these authors interpreted it as the vidian canal. However, this opening is probably not related to the vidian canal in Coloradisaurus, as the foramen for the passage of the carotid is usually located anteroventrally in the anterior tympanic recess. Likewise, Gow (1990) noted a small foramen in a juvenile braincase of Massospondylus posteroventral to the foramen for the facial nerve, which he interpreted as the passage of a blood vessel. However, the position of this foramen closely corresponds to that for the palatine ramus of the facial nerve in SMNS 12667, as Gow (1990: 60) notes that this foramen is separated from the facial foramen by a small crest underneath the latter, which he termed ‘crista subfacialis’. Thus, we regard this foramen as a probable separate exit for the palatine branch of the nerve in Massospondylus. Interestingly, Gow noted that such a foramen is not present in adult braincases of Massospondylus, indicating the possibility of ontogenetic variation in this character.
Otoccipital
According to Sampson & Witmer (2007), the exoccipital and opisthotic are completely fused in dinosaurs, forming a single element for which they used the name otoccipital, which is the term adopted here. In SMNS 12667, both otoccipitals are preserved, although the right element, which is only visible with CT scan, is considerably damaged (Fig. 2). The left element is visible in the matrix, but additional details of its morphology are shown by CT scan data. The description herein is solely based on the left otoccipital (Figs 6–8).
The otoccipital of dinosaurs usually contacts most of the bones of the braincase, including the supraoccipital medially, the parabasisphenoid anteroventrally, the basioccipital posteroventrally and the prootic anterodorsally (Galton, 1985; Sampson & Witmer, 2007). In SMNS 12667, the otoccipital is completely disarticulated from other bones, except for the contact with the prootic, with the latter overlapping the former anterolaterally at the base of the paroccipital process (Figs 2, 7). Structures that are associated with the otoccipital, being formed exclusively by it or in conjunction with other bones, are the paroccipital process, the occipital condyle, the foramen magnum, the anterior and posterior foramina between the exoccipital pillar and the fenestra ovalis.
As mentioned above, the otoccipital forms the laterodorsal portion of the occipital condyle (Fig. 8). This part of the otoccipital is developed as a pyramidal posterior projection at the posteromedioventral edge of the bone (Figs 6, 8). Laterally, the projection has a total extension of 8 mm from its posterior to its anterior tip, the latter here delimited as the ventral margin for the posterior foramen for the hypoglossal nerve. The ventromedial portion of this pyramidal projection contacts the basioccipital at the occipital condyle, and also forms part of the posterolateral surface of the floor of the braincase (Fig. 2). This contribution of the otoccipital to the floor of the braincase is similar to the condition observed in other sauropodomorphs (e.g. Plateosaurus, Coloradisaurus and Massospondylus). Dorsal to the pyramidal projection, the medial side of the otoccipital forms the lateral and part of the dorsal margins of the foramen magnum (Fig. 8). In occipital view, the surface of the otoccipital bordering the foramen extends dorsolaterally from the ventral limit of the foramen magnum to a point where it curves medially and assumes a dorsomedial orientation. This change in the orientation occurs approximately at the mid-height of the foramen magnum, at the same dorsoventral level where the ventral limit of the proximal base of the paroccipital is located laterally. Furthermore, the portion of the otoccipital bordering the dorsal half of the foramen magnum projects slightly further posteriorly than the portion of the bone in the ventral half of the border. The exact contribution of the otoccipital to the dorsal margin of the foramen magnum, and consequently the supraoccipital contribution to it, cannot be determined precisely. In Coloradisaurus, Thecodontosaurus, Plateosaurus and Adeopapposaurus, the contribution of the supraoccipital in the dorsal margin of the foramen magnum is greater than that by the otoccipital. A different morphology is observed in Melanorosaurus, which has a reduced contribution of the supraoccipital to the border of the foramen magnum (less than one-third of the dorsal border). In SMNS 12667, the condition probably differed from Melanorosaurus, being more similar to that of the other sauropodomorphs mentioned above, given the extent of the medial projection of the otoccipital in the region corresponding to the dorsal border of the foramen magnum (Fig. 8). The total height of the foramen magnum is difficult to establish because of the dislocation of the elements and incompleteness of the dorsal margin. Based on the morphology of the otoccipital and basioccipital, the foramen had a total height of c. 15 mm, which is about three times greater than the height of the occipital condyle (Fig. 8).
Posterior view of the braincase of the SMNS 12667 specimen of Efraasia minor (right otoccipital is excluded due to the poor preservation and higher level of displacement of the element). Abbreviations: bobt, basioccipital component of the basal tubera; bp, basipterygoid process; dpot, depression in the posterior portion of the otoccipital; occ, occipital condyle; otoc, otoccipital contribution to the occipital condyle; otmfm, otoccipital contribution to the margin of the foramen magnum; pp, proximal portion of the left paroccipital process.
In occipital view, the otoccipital forms a rectangular to subquadrangular surface that projects dorsolaterally and slightly posteriorly lateral to the dorsal half of the foramen magnum (Fig. 8). This surface is slightly depressed at its centre, so that it is medioventrally-dorsolaterally concave. This morphology was also observed in this region in all other sauropodomorph taxa studied. The ventromedial limit of the rectangular surface is confluent with the pyramidal posterior projection described above. Laterally, the contact between these two parts is formed by a 2 to 3 mm thick, rounded ridge that anteriorly forms the posterior border for the posterior opening of the hypoglossal nerve (XII) and the metotic foramen. The dorsolateral limit of the rectangular concave surface represents the base of the paroccipital process. However, only the proximal-most part of the paroccipital process is preserved in SMNS 12667 (Fig. 8), which does not allow the recognition of any further details of this structure.
In sauropodomorphs, as in dinosaurs generally, several cranial foramina are partially or completely enclosed by the otoccipital (Galton, 1985; Sampson & Witmer, 2007). On the lateral side of the otoccipital of SMNS 12667, two smaller posteroventrally placed foramina and two larger (Fig. 9) dorsally and more anteriorly placed openings are placed in a lateral depression on the bone below the paroccipital process, which corresponds to the paracondylar fossa in theropods (e.g. Bever et al., 2013). The two most ventral and posterior foramina represent openings for the hypoglossal nerve (XII), and are completely enclosed by the otocipital (Figs 6, 7, 9). All non-sauropodan sauropodomorph taxa we observed (e.g. Adeopapposaurus, Coloradisaurus, Massospondylus, Melanorosaurus, Plateosaurus, Thecodontosaurus) also have two openings for the hypoglossal nerve. In Sauropoda, this condition is not present in all members, and some taxa show a single opening for cranial nerve XII (e.g. Paulina-Carabajal, Carballido & Currie, 2014). In SMNS 12667, the external aperture of the posterior foramen for the hypoglossal nerve is elliptical, 5 mm tall and 3 mm wide at its mid-length. The anterior foramen is circular and considerably smaller than the posterior one, with a diameter of approximately 1.5 mm. The same pattern in the relative size of these openings, with the posterior being larger than the anterior one, is observed in other non-sauropodan sauropodomorphs. One aspect that varies is the relative position of the ventral margin of these openings. In SMNS 12667 and Thecodontosaurus, the ventral margin of both foramina are aligned horizontally, whereas in taxa such as Adeopapposaurus, Plateosaurus and Melanorosaurus, the ventral margin of the anterior foramen is located ventral to the ventral margin of the posterior foramen.
X-ray slices obtained from the CT scan procedure showing cranial opening in the braincase of the specimen SMNS 12667 of Efraasia minor. A, anterior and posterior foramina in the left otoccipital between the exoccipital pillar and the fenestra ovalis. B, the two foramina for the cranial nerve XII (hypoglossal). Abbreviations: afo, anterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis; pfo, posterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis; XII, foramina for the hypoglossal nerve.
Dorsal and anterodorsal to the foramina for the hypoglossal nerve, there are two other foramina completely enclosed by the otoccipital (Figs 6, 7, 9). In their description of the braincase, Galton & Bakker (1985) considered the posterior of these foramina as the foramen lacerum, and the anterior one as the foramen jugularis. According to these authors, the former is the opening related to cranial nerves IX–XI, whereas the latter represents the opening for the jugular vein. Based on previous studies on archosaur braincases (e.g. Bellairs & Kamal, 1981; Walker, 1990; Gower & Weber, 1998; Sampson & Witmer, 2007; Sobral et al., 2012), we come to a different interpretation (see discussion below). In this study, we name the posterior foramen as the ‘posterior foramen between the exoccipital pillar and the fenestra ovalis’, whereas the anterior foramen is treated here as the ‘anterior foramen between the exoccipital pillar and the fenestra ovalis’ (see discussion below).
We here consider the name foramen lacerum posterior (= foramen jugularis; Orliac, 2009) as inappropriate because this opening might not represent the path of the jugular vein (see discussion below). The aperture of this ‘posterior foramen’ in the medial portion of the otoccipital is about two to three times the size of the aperture of the posterior foramen for the hypoglossal nerve (Figs 6, 7). The aperture is located in the posterodorsal corner of the lateral margin of the otoccipital, ventral to the proximal portion of the paroccipital process. Laterally, the aperture has a more elliptical shape, with a length of 6 mm from the posterodorsal to the anteroventral margin, and a width of 2 mm at its widest portion. Anteriorly, a bony strut (we prefer not to use any specific name for this strut, as for example, prevagal strut or metotic strut – see more in the discussion below) separates the posterior and anterior foramina. In lateral view, the strut is about 3 mm and 2 mm wide in its dorsal and ventral portion, respectively. Although the lateral surface of the bony strut is depressed even below the level of the paracondylar fossa around the openings for the hypoglossal nerve, it extends laterally dorsally to become confluent with the dorsolateral surface of the prootic, ventral to the portion of the otoccipital overlapped by the prootic and the proximal portion of the parocipital process. Its ventral limit is located 3 mm more medially in relation to the external openings for the hypoglossal nerve.
We here consider the term ‘anterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis’ preferable to foramen jugularis (Galton, 1985), since this opening does not represent the foramen for the passage of the jugular vein (see discussion below). This anterior foramen (Fig. 6) is posterodorsally defined by the bony strut separating it from the posterior foramen described above. Anteriorly, it is defined by a ventral process of the otoccipital. The total length of this foramen from its posterodorsal margin to the anteroventral limit cannot be determined because the ventral margin is not preserved. However, this foramen is considerably larger than the other three foramina within the otoccipital. As in other dinosaurs, a ventral process (Fig. 7) of the otoccipital extends from the bases of the paroccipital process posterodorsally to the contact with the parabasisphenoid anteroventrally (Sampson & Witmer, 2007). It marks the separation between the anterior foramen and the fenestra ovalis anteriorly. This process has been also named the crista interfenestralis (see Sampson & Witmer, 2007), but we prefer to avoid this term because it has been used to name the process separating the fenestra ovalis from the fenestra pseudorotunda, which might not be the case in SMNS 12667 (see discussion below). In SMNS 12667, this crest is developed as a lateromedially expanded sheet of bone. The lateral margin of this sheet is 1 mm wide, whereas medially its width is c. 5 mm ventrally and c. 3 mm dorsally.
Anterior to the ventral process of the otoccipital is located the fenestra ovalis (= fenestra vestibuli; Fig. 7). In contrast to the other cranial openings described above, the fenestra ovalis is not completely enclosed by the otoccipital, but is anteriorly bordered by the prootic (Fig. 6). The anterodorsal margin of this foramen is defined by the otosphenoidal crest of the prootic, as described above. Ventrally, the opening of the fenestra ovalis reaches the posterior portion of the parabasisphenoid, but this part is slightly dislocated from its original position in SMNS 12667. The fenestra ovalis is an elongated foramen with an elliptical shape. The total length of this foramen from the anteroventral to the posterodorsal margin is approximately 14 mm, with a maximum width of 5 mm.
Supraoccipital
The supraoccipital is partially preserved and visible only in anteroventral view (Fig. 10). CT scan shows that the posterodorsal margin of the bone is damaged so that it is impossible to observe any detail of its morphology. As in most non-sauropodan sauropodomorphs, the supraoccipital was strongly inclined anterodorsally, so that the dorsal part of the occiput faced posterodorsally.
In previous phylogenetic data matrices (Yates, 2007b; Apaldetti et al., 2014; McPhee et al., 2014, 2015), the supraoccipital of Efraasia has been treated as being wider than high as in other sauropodomorphs, such as Thecodontosaurus, Panphagia and Pantydraco, but in contrast to the condition of Coloradisaurus and Plateosaurus, in which the supraoccipital is as high as wide. Based on the preserved and visible part of the supraoccipital in SMNS 12667, this bone has a maximum width of 25 mm. The height of the preserved supraoccipital is 18 mm. However, a part of the posteroventral portion of the supraoccipital, including the surface that probably formed part of the border of the foramen magnum, is missing (Fig. 10). It is thus not possible to establish the condition in SMNS 12667 with certainty.
A, anteroventral view of the supraoccipital of the specimen SMNS 12667 of Efraasia minor. B, schematic drawing of (A). Abbreviations: aso, articulation surface with the otoccipital; asp, articulation surface with the parietal; aspr, articulation surface with the prootic; mcv, notch for the mid-cerebral vein; so, supraocipital; vps, ventral projection of the ventral surface of the supraoccipital.
The supraoccipital forms a narrow, tunnel-like roof of the endocranial cavity posteriorly between robust ventral projections laterally (Fig. 10). Anterior to the projections, the supraoccipital expands both laterally and dorsally, up to a dorsally widely arched and laterally slightly concave margin that would have contacted the parietal wings. On the right side of the anterodorsal margin, there is a notch that most probably represents the path of the mid-cerebral vein towards its posterior exit, as in Panphagia (Martinez et al., 2012b). This area is poorly preserved on the left side of the supraoccipital, but at least a small, matrix-filled incision is present here in the same position. Anteroventral to the incision in the margin, a large recess is found on the medial side of the supraoccipital on either side, which probably housed the posterior venous sinus associated with the mid-cerebral vein. Sauropodomorphs show some variation regarding the path of this vein in the supraoccipital. In some taxa, such as Coloradisaurus and Plateosaurus, the foramen for the vein is completely enclosed by the supraoccipital. In Efraasia, the anterior margin of the supraoccipital would have contacted the parietals, so the mid-cerebral vein would have exited the braincase between the supraoccipital and the ventral margin of the parietal wings.
It is worth mentioning here the variation in the nomenclature adopted in different studies on sauropodomorph braincases regarding the vein associated with the foramen or notch in the supraoccipital (in case the vein passes between the supraoccipital and parietal). In the original description of Panphagia, Martinez & Alcober (2009) identified the notch in the supraoccipital as the path for vena capitis dorsalis, which is the same term adopoted in Apaldetti et al. (2014) to refer to the vein associated with the foramen in the supraoccipital of Coloradisaurus. Posteriorly, Martinez et al. (2012b) refers to the notch of Panphagia as the path for the external occipital vein. Still, Yates (2007b) label the foramen in the supraoccipital as the path for the mid-cerebral vein (vena cerebralis media).
According to Sampson & Witmer (2007), a foramen in the anteromedial portion of the supraoccipital of the theropod Majungasaurus Depéret, 1896 is related to the posterior exit of the mid-cerebral vein, which becomes the external occipital vein after exiting the skull. Still according to the authors, the exit of the vena capitis dorsalis is located at the juncture between parietal, laterosphenoids and possibly the prootic, lateral to the exit of the mid-cerebral vein. Thus, as the foramen in the supraoccipital of sauropodomorphs such as Plateosaurus and Coloradisaurus is located in the same position of the foramen described for Majungasaurus in Sampson & Witmer (2007), we here consider the foramen or notch in the supraoccipital of sauropodomorphs as associated with the mid-cerebral vein (or external occipital vein), as previously identified by Yates (2007b) and Martinez et al. (2012b).
Posteroventrally, the ventral margin of the supraoccipital would have contacted the otoccipital posteriorly, and the prootics anteriorly (Fig. 10). The ventral projections are 11 mm long dorsoventrally, 9 mm thick lateromedially and have a preserved anteroposterior length of 9 mm. The anteroventral surface of the supraoccipital between the ventral projections formed the posterodorsal portion of the internal cavity of the braincase, including the dorsal margin of the foramen magnum in the posterior limit of the bone. The surface between these two projections is concave. Anteriorly, the surface between the projections is 5 mm wide, but as the projections diverge laterally at the posteroventral end of the bone, the surface widens to 8 mm at the preserved posterior end.
Laterosphenoid
The left laterosphenoid is preserved (Fig. 11), but has been displaced from its original connections to other bones of the braincase (Figs 2, 6A). As in other dinosaurs, the laterosphenoid would have contacted the prootic posteroventrally, the parietal and, possibly the frontal dorsally, and the postorbital dorsolaterally. The laterosphenoid might furthermore have contacted an orbitosphenoid anteriorly, as is the case in Plateosaurus (Galton, 1985), and the parabasisphenoid ventrally.
Left laterosphenoid of the specimen SMNS 12667 of Efraasia minor in lateral (A), anterolateral (B) and medial (C) views. Abbreviations: alpl, anterolateral process of the laterosphenoid; ca, crista antotica; dmpl, dorsomedial process of the laterosphenoid; ppl, posterior process of the laterosphenoid; vpl, ventral process of the laterosphenoid; III, path of the cranial nerve III (oculomotor); IV, path of the cranial nerve IV (trochlear); V, path of the cranial nerve V (trigeminal).
The laterosphenoid seems to be somewhat incomplete and is mainly exposed in anterior and lateral view (Fig. 11). The bone formed the anterior and anterolateral wall of the braincase and can be subdivided into an anterior and a lateral surface, which meet at an angle of approximately 90°. An anterolateral process (= the postorbital process of Prieto-Márquez & Norell, 2011) extends from the dorsal part of the junction of these two surfaces and would have possibly contacted the postorbital laterally. The extremity of the process with the articular facet for the postorbital seems to be missing, however. As preserved, this process is relatively short in SMNS 12667, being about one-fourth of the total anteroposterior length of the laterosphenoid. This is also the condition in Massospondylus, whereas Plateosaurus (Prieto-Márquez & Norell, 2011) has an anterolateral process that is slender and with a length corresponding to half of the length of the laterosphenoid in lateral view. In how far the condition in Efraasia might be owed to the missing portion of the process cannot be established. As is the case in Massospondylus (Gow, 1990), the laterosphenoid of SMNS 12667 is longer anteroposteriorly than wide transversely. In lateral view, the laterosphenoid is triangular to trapezoidal in outline, becoming higher anteriorly. The dorsal part of the laterosphenoid is considerably concave anteroposteriorly especially dorsally where it turns into the anterolateral process. In this area, the lateral side curves gradually into the posterior side of the anterolateral process. Anteriorly, it is separated from the anterior side by a low, but well-defined crista antotica (Madsen, McIntosh & Berman, 1995), which separates the orbital cavity anteriorly from the adductor chamber posteriorly (Sampson & Witmer, 2007). Towards the anterior end of the laterosphenoid as seen in lateral view, there is a slender, roughly triangular ventral process (= the parabasisphenoid process of Prieto-Márquéz & Norell, 2011) with a slightly concave posterior margin; this margin most probably represents the anterior border of the trigeminal foramen. This process widens slightly ventrally, where it would have contacted the anterior portion of the prootic and eventually the parabasisphenoid, at the region of the preotic pendant. It is dorsally overhung by the lateroventral extension of the dorsal part of the laterosphenoid, resulting in a short, longitudinal, ventrally open channel anterior to the trigeminal foramen. Galton & Bakker (1985: fig. 2) interpreted this channel as conducting a branch of the mid-cerebral vein. However, in other dinosaurs that show such a channel or a groove anterior to the trigeminal foramen, this is usually interpreted as the passage of the ophthalmic branch of the trigeminal nerve (V1; e.g. Sampson & Witmer, 2007; Sobral et al., 2012), and we consider this the more likely interpretation here as well.
In anterior view (Fig. 11), the laterosphenoid is narrow ventrally, but gradually expands dorsally towards the anterolateral process. In articulation with the rest of the braincase, the anterior surface of the laterosphenoid would have faced slightly anteroventrally. A large indentation is present in its medial rim just dorsal to the level of the overhanging lateral shelf. This indentation is higher than wide and becomes slightly wider dorsally. Galton & Bakker (1985) identified this opening as the foramen for the optic nerve, II (see also Prieto-Márquez & Norell, 2011, for the same interpretation in Plateosaurus). However, in dinosaurs in which the orbitosphenoid is preserved, this nerve usually exits the braincase through this bone (e.g. Gow, 1990; Currie & Zhao, 1993; Sampson & Witmer, 2007), and thus this opening is probably for the passage of the oculomotor nerve (III), as interpreted by Gow (1990) in Massospondylus. At the dorsal margin of the anterior surface of the laterosphenoid, a wider, but lower second indentation is present medially. As noted by Galton & Bakker (1985), this opening represents the passage of the fourth (trochlear) cranial nerve.
Based on CT scan data, it is possible to examine the medial surface of the laterosphenoid (Fig. 11). Although the medial surface of the bone is not well preserved, some details can be observed. In SMNS 12667, the medial surface of the main body of the laterosphenoid (excluding the processes) is concave, being deeper around the centre of this region. This is similar to the condition in other non-sauropodan sauropodomorphs with laterosphenoids visible in medial view, such as Plateosaurus (AMNH 6810) and Massospondylus. Based on comparisons with these two taxa, a small protuberance located at the level of the proximal portion of the anterolateral process of SMNS 12667 corresponds to the proximal portion of a dorsomedial process (= the frontal process of Prieto-Márquez & Norell, 2011). Prieto-Márquez & Norell (2011: fig. 26C) indicated the path of cranial nerve IV as being immediately ventral to this dorsomedial process. However, in SMNS 12667, there is a notch at the anterior margin of the laterosphenoid, followed by a concave surface on the medial side, which might correspond to the path of cranial nerve IV. If this indeed represents the path for the trochlear nerve, this nerve would be more ventrally located in relation to the dorsomedial process in SMNS 12667 than it is in Plateosaurus (as indicated by Prieto-Márquez & Norell, 2011).
DISCUSSION
The discussion is divided into two main sections. First we discuss morphological aspects of the braincase of sauropodomorphs. This part of the discussion deals with the interpretation of soft tissues associated with two foramina located in the otoccipital of sauropodomorphs, here treated as the ‘anterior and posterior foramina between the exoccipital pillar and the fenestra ovalis’. Another morphological trait discussed here is the presence of an unossified gap between the parabasisphenoid, basioccipital and the otoccipital in the braincase of sauropodomorphs. In the second section of the discussion, we provide an analysis of the evolution of non-neosauropodan sauropodomorph braincases from a comparative perspective. For this, a phylogenetic framework in which to trace the evolutionary history of each particular trait is necessary. The topology used here is based on a numerical analysis using a data set that is modified from the recent study of McPhee et al. (2015), focusing on non-neosauropodan sauropodomorph relationships. In the course of our study, we identified problematic aspects in phylogenetic characters representing braincase anatomy that have been used in phylogenetic studies of sauropodomorphs. The problematic aspects are mainly related to character definition, which does not accurately represent the morphology observed in the specimens, and the scoring of taxa in the matrix. Accordingly, we discuss some of the phylogenetic characters related to braincase anatomy that are present in the matrix, and also new characters proposed in this study, the phylogenetic history of which is also discussed here (see Appendix for more details).
Considerations on the morphology of the braincase of sauropodomorphs
The division of the metotic fissure and the course of the jugular vein
Our survey of the literature indicates that previous studies of braincases of sauropodomorphs (e.g. Galton, 1985; Galton & Bakker, 1985; Benton et al., 2000; Yates, 2007b) probably misidentified the soft tissues associated with the two foramina in the otoccipital located between the exoccipital pillar and the fenestra ovalis. Very detailed explanations regarding the development of the metotic fissure in archosaurs and the presence of two foramina between the exoccipital pillar and the fenestra ovalis have been provided in the literature (Gower & Weber, 1998; Gower, 2002, Sampson & Witmer, 2007; Sobral et al., 2012). These have implications for the exit route of cranial nerve X (vagus nerve) and the posterior cephalic vein (= internal jugular vein of some authors; Gower, 2002; Sobral et al., 2012), and also for the development of a secondary tympanic membrane (Gower & Weber, 1998; Sampson & Witmer, 2007), the latter being related to a refinement of the auditory system (Müller & Tsuji, 2007). A brief overview is presented here in order to clarify our point regarding the nature of the two foramina in the otoccipital of sauropodomorph dinosaurs.
According to Gower & Weber (1998), the metotic fissure is a gap present during embryonic stages, which is positioned between the otic capsule and the basicranium of the chondocranium (see also Bellairs & Kamal, 1981; Rieppel, 1985). During the embryonic stage, cranial nerves X and XI and usually the posterior cephalic vein pass through the metotic fissure. During ontogeny, this structure can then persist as a single opening, or can become subdivided by a prevagal strut sensuGower & Weber (1998). In the first case, the single opening of adults should be referred to as the metotic foramen, and represents the opening for cranial nerves IX to XI and possibly the posterior cephalic vein. If the metotic fissure becomes subdivided, the anterior opening should be referred to as the fenestra pseudorotunda (= fenestra cochleae in Sampson & Witmer, 2007), whereas Gower & Weber (1998) suggest the term vagal foramen be used for the posterior opening, which has variously also been called the jugular foramen. Cranial nerve X and XI and, possibly, the posterior cephalic vein pass through this posterior foramen in adults. The path of cranial nerve IX is more plastic among archosaurs, and this nerve can exit the internal cavity through different paths (Sobral et al., 2012). Likewise, whereas the vagal nerve (X) always seems to pass through this posterior foramen (when present), the course of the posterior cephalic vein is more variable, and this vessel might exit the braincase through this foramen or the foramen magnum (see Gower & Weber, 1998; Gower, 2002; Sampson & Witmer, 2007). In those taxa in which the fissura metotica becomes divided, the anterior opening, the fenestra pseudorotunda, represents the lateral opening of the recessus scala tympani and is covered by a secondary tympanic membrane (see also Rieppel, 1985; Sampson & Witmer, 2007). Although the presence of a divided metotic fissure (i.e. the presence of a fenestra pseudorotunda and a vagal foramen in adults – see Sampson & Witmer, 2007) is observed in both major clades of extant archosaurs (Crocodylia and Aves), it might be better explained as independent acquisitions in the pseudosuchian and avemetatarsalian lineages (Gower & Weber, 1998).
Gower (2002) furthermore noted the presence of an additional foramen posterodorsal to the metotic foramen in the non-crocodylomorph pseudosuchians Batrachotomus Gower, 1999 and Postosuchus Chatterjee, 1985. This foramen in Batrachotomus is associated with a venal sinus on the interior of the braincase, and was thus interpreted as a separate opening for the posterior cephalic vein only (Gower, 2002). Thus, according to Gower (2002), Batrachotomus and Postosuchus do not have a divided metotic foramen in a fenestra pseudorotunda and a vagal foramen (i.e. there is no formation of a secondary tympanic membrane).
In respect to sauropodomorphs, earlier interpretations (see e.g. Galton, 1985; Galton & Bakker, 1985) of the soft tissues associated with the foramina here treated as ‘anterior and posterior foramina of the otoccipital between the exoccipital pillar and the fenestra ovalis’ differ from that suggested by Gower & Weber (1998) and Gower (2002) for archosaurs in general. In the original description of the braincase of SMNS 12667, Galton & Bakker (1985) interpreted the anterior foramen as the foramen jugularis, through which the internal jugular vein would pass. According to the authors of that study, cranial nerves IX–XI would exit through the posterior foramen, which they called foramen lacerum posterior. This nomenclature was followed in subsequent studies on the braincase of sauropodomorphs (e.g. Benton et al., 2000; Yates, 2007b). A first problem of this nomenclature adopted by Galton & Bakker (1985) is that the term foramen lacerum posterior is equivalent to the term foramen jugularis (Orliac, 2009), which is the name the authors used to refer to the anterior foramen.
Furthermore, the interpretation of Galton & Bakker (1985), identifying the anterior foramen (their foramen jugularis) as the path of the jugular vein, is problematic. In cases where a divided metotic foramen is present, the posterior cephalic vein (internal jugular vein in Galton & Bakker, 1985) may either pass through the foramen magnum, as reported for lepidosaurs and crocodiles (see Gower, 2002; Sobral et al., 2012), or this vein passes through the posterior of the two foramina, the vagal foramen. In the latter case, if the posterior foramen is relatively small, this might be an indication that the vein drained mainly into the occipital sinus, as proposed for abelisaurids in Sampson & Witmer (2007). As explained by Bellairs & Kamal (1981), a vein passes through the metotic fissure in embryonic stages of reptiles. However, this vein disappears during ontogeny and then the posterior cephalic vein of adults leaves the skull through the foramen magnum in lepidosaurs and at least some crocodiles. On the other hand, in cases where the metotic foramen is undivided (i.e. there is no formation of a fenestra pseudorotunda, which is covered by a secondary tympanic membrane, and a vagal foramen), but there is an additional foramen at the level of the exoccipital pillar, this foramen might represent the path for the posterior cephalic vein (Gower, 2002).
Given the scenario explained above, it would thus be highly unusual that the posterior cephalic vein passes through the anterior opening, as proposed by Janensch (1935), followed by Galton (1985), Galton & Bakker (1985) and subsequent studies on the braincase of sauropodomorphs (e.g. Yates, 2007b; Martinez, 2009; Apaldetti et al., 2014). According to our survey of the literature, there are two possible scenarios regarding the path of the posterior cephalic vein and the nature of the two foramina of SMNS 12667, and thus for other non-sauropodan sauropodomorphs. One scenario is that our anterior foramen corresponds to the fenestra pseudorotunda (sensuGower & Weber, 1998) and that it was covered by a secondary tympanic membrane (Gower & Weber, 1998; Sampson & Witmer, 2007). In this case, our posterior foramen would be equivalent to the vagal foramen (sensuGower & Weber, 1998). Given the reduced size of this foramen, it probably represented the path for the vagus nerve, but not for the posterior cephalic vein, which would pass through the foramen magnum (Sampson & Witmer, 2007). The second scenario is that SMNS 12667 has an undivided metotic foramen (sensuGower & Weber, 1998), and our posterior foramen actually corresponds to the path of the posterior cephalic vein, as argued for some other archosaurs, whereas the anterior foramen would likely correspond to the path for cranial nerves IX–XI (Gower, 2002). In the latter case, one possibility is that the undivided metotic foramen seen in sauropods is related to a configuration in which the posterior cephalic vein leaves the braincase through the foramen magnum, as in many lepidosaurs and crocodiles.
The presence of an unossified gap in sauropodomorph braincases
The term unossified gap has been used in braincase studies to refer to unossified areas of the braincase that remain cartilaginous throughout life, whereas the braincase usually ossifies extensively in reptiles (Gower & Sennikov, 1996; Gower & Weber, 1998). Unossified gaps in different regions of the chondocranium and presenting different morphologies were recognized in the braincase of diapsids (Gower & Weber, 1998). In the context of non-archosaur archosauriform and non-crocodylomorph pseudosuchian braincases, the presence of such structures has received great attention in previous studies (Gower & Sennikov, 1996; Gower, 2002; Gower & Nesbitt, 2006; Nesbitt, 2011; Sookias et al., 2014). In these forms, an unossified gap occurs between the components of the basioccipital and parabasisphenoid that form the basal tubera and at the ventral end of the ventral ramus of the otoccipital, which separates the fenestra ovalis from the posterior foramen in the otoccipital (Gower & Sennikov, 1996; Gower & Weber, 1998).
Some of the sauropodomorph braincases we analysed also exhibit an unossified area (Fig. 12) that is topologically equivalent to the unossified gap (sensu Gower & Webber, 1998; Gower, 2002) of some non-archosaurian archosauriforms and non-crocodylomorph pseudosuchians. This structure is also present in Adeopapposaurus, Unaysaurus, Massospondylus, Melanorosaurus, Plateosaurus, Leyesaurus and Thecodontosaurus (already pointed out by Benton et al., 2000). Above we discussed the significance of the junction between the parabasisphenoid and basioccipital as a possible indicator of the level of the maturity of the individual. Nevertheless, even in braincases of presumed adult individuals (Plateosaurus, Melanorosaurus, Massospondylus, Thecodontosaurus, Leyesaurus) in which the basioccipital and parabasisphenoid are firmly attached to each other, an unossified gap is still present between basioccipital, parabasisphenoid and otoccipital. Finally, we do not think that the existence of an unossified gap in the braincase of non-sauropodan sauropodomorphs can be attributed to preservation or sampling bias, given that we analysed most of the non-sauropodan sauropodomorph taxa that have braincase elements preserved first-hand (Table 1).
Posterolateral view of the braincases of the specimens YPM 2192 of Thecodontosaurus antiquus (A, B) and PVSJ 568 of Adeopapposaurus mognai (C, D), and posteroventral view of the braincase of the specimen SMNS 13200 of Plateosaurus (E, F) illustrating the presence of an unossified gap in sauropodomorph braincases. Abbreviations: afo, anterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis; fm, foramen magnum; fo, fenestra ovalis; occ, occipital condyle; pfo, posterior foramen of the otoccipital between the exoccipital pillar and the fenestra ovalis; ug, unossified gap; vc, vidian canal; V, foramen for the trigeminal nerve; VII, foramen for the facial nerve; XII, foramina for the hypoglossal nerve.
Phylogenetic analysis
Recent phylogenetic analyses (e.g. Martinez, 2009; Apaldetti et al., 2012, 2014; McPhee et al., 2014, 2015; Otero et al., 2015) focused on non-neosauropodan sauropodomorph relationships mostly represent extensions of two data matrices, those of Yates (2007b) and Upchurch et al. (2007). These matrices differ slightly in taxon composition and characters, and the results of both show some disagreements regarding the arrangement of the classic ‘prosauropods’ (see Galton & Upchurch, 2004; Sereno, 2007a). Results of Yates (2007b), and of the analyses derived from his data matrix (e.g. Otero & Pol, 2013; Apaldetti et al., 2014; McPhee et al., 2014, 2015; Otero et al., 2015), exhibit the majority of these taxa as a series of consecutive sister groups of less inclusive clades containing Sauropoda. On the other hand, the results of Upchurch et al. (2007) and subsequent analyses extending this data set (e.g. Martinez, 2009; one of the analyses presented in Apaldetti et al., 2011) have found taxa such as Plateosauridae and Massospondylidae (‘the core prosauropods’ – Sereno, 2007a) forming a monophyletic Prosauropoda (but see Yates et al., 2010).
Nevertheless, the data matrices presented by Upchurch et al. (2007) and Yates (2007b) include almost the same set of phylogenetic characters related to braincase anatomy. The only difference between the set of characters is character 64 used by Upchurch et al. (2007) – ‘Ossification of the extremity of the basal tubera: complete, so that the basioccipital and parabasisphenoid form a single rugose tuber (0); unossified, with the basioccipital forming a ventrally facing platform of unfinished bone that abuts a similarly unfinished caudally facing wall of the parabasisphenoid (1)’. This character was originally proposed by Yates & Kitching (2003), but was later excluded from Yates (2007b) and subsequent analyses based on it. This character obviously describes the presence of an unossified gap (Gower & Sennikov, 1996; Gower & Weber, 1998; Gower, 2002) between the basioccipital, parabasisphenoid and otoccipital in the braincase of sauropodomorphs discussed above.
We revised the phylogenetic characters related to braincase anatomy used in McPhee et al. (2015), which, in turn, represent one of the most recent versions of the data matrix based on Yates (2007b). It is worth mentioning that characters of McPhee et al. (2015) that are related to braincase anatomy modified in relation to the way they are presented in Yates (2007b), as is also the case for previous studies using an extended version of this data set (e.g. Yates et al., 2010; Pol et al., 2011; Apaldetti et al., 2014; Otero et al., 2015). The revision resulted in the recognition of problematic issues regarding character definition and character scoring. Accordingly, we here propose modifications of character definitions and/or in the character states attributed to some taxa. Following revision and the addition of new characters, a phylogenetic analysis was carried out using TNT (Goloboff, Farris & Nixon, 2008) under the following parameters: random seed 0; 10 000 replicates; hold 10; TBR (tree bi-section reconnection) for branch swapping. The analysis recovered a total of 144 MPTs (most parsimonious trees), 1248 steps long. We used the prunnelsen command of TNT (Goloboff & Szumik, 2015) in order to identify unstable taxa in the analysis. This procedure identifies Blikanasaurus as an unstable OTU (Operational Taxonomic Unit) in this analysis. A reduced strict consensus tree (Fig. 13) excluding Blikanasaurus was used as the framework to analyse aspects of the evolution of the braincase in the sauropodomorph lineage (see Supporting Information 2 for a Nexus file containing the matrix used in the analysis).
Strict consensus tree of the 144 MPTs recovered in the phylogenetic analysis. Taxon names written in black indicate that at least one character related to the braincase anatomy could be scored in the data matrix for that taxon. Boxes indicate transformations associated to respective branches.
Despite the substantial modifications in the data matrix (see Appendix), the results of our analysis agree with that of McPhee et al. (2015). The reduced consensus tree is well resolved (more than 90% of the nodes). The classical ‘prosauropods’ are found paraphyletic in relation to Sauropoda. Nevertheless, some less inclusive groups are found among this assemblage of taxa. Our results confirm the affinity of Unaysaurus to Plateosaurus, with both taxa forming Plateosauridae. Our reduced consensus tree also depicts a monophyletic Massospondylidae (Fig. 13), which is found more closely related to sauropods than to plateosaurids. Finally, within Sauropoda, the non-neosauropodan taxa are majorly found as consecutive sister groups of less inclusive clades containing Neosauropoda.
In the following discussion of braincase characters, numbers follow those in the data set of our analysis; however, these are the same as those in the data matrices of McPhee et al. (2015) and previous analyses (e.g. Pol et al., 2011; Apaldetti et al., 2014, McPhee et al., 2014; Otero et al., 2015), using an expanded version of the Yates (2007b) matrix, except for the new characters proposed here.
Revison of previous characters and new characters related to braincase anatomy
Deep septum in the interbasipterygoid space vs. deep subsellar and basisphenoid recesses – character 85 of this study and of Yates (2007b)
This character was previously defined as: ‘Deep septum spanning the interbasipterygoid space: absent (0) or present (1)’. The evolution of this trait was recently discussed by Apaldetti et al. (2014). In their study, these authors considered five OTUs to have the state ‘1’: Anchisaurus, Coloradisaurus, Efraasia, Plateosaurus and Riojasaurus incertus Bonaparte, 1967. In the course of our study, we found anatomical variation regarding the morphology of the ‘septum’ among taxa scored with state ‘1’. Furthermore, we were unable to identify anatomical congruence to clearly distinguish these taxa coded with state ‘1’ from those coded with state ‘0’, as some of the taxa coded ‘0’ have a morphology that matches the morphology of taxa coded with state ‘1’. This problem stems from the lack of a clear statement about the nature of the septum and what really represents the interbasipterygoid space.
The basipterygoid processes arise from the ventral surface of the main body of the parabasisphenoid anteriorly, and their bases are connected to the ventrolateral edge of the cultriform process by a curved lamina. The median space between the anterior and posterior limits of left and right basipterygoid processes is the region of the subsellar recess (sensuWitmer, 1997). In taxa that have a deep subsellar and also a deep basisphenoid recess, there is a transverse wall or septum of bone spanning between the bases of the basipterygoid process that separates these two recesses. This is the case, for example, in Coloradisaurus (see Apaldetti et al., 2014: fig. 6c), Riojasaurus and Anchisaurus (see Fedak & Galton, 2007: fig. 6a). This morphology is more obvious in theropods, in which both recesses are typically better developed than in sauropodomorphs [the septum is treated as the interbasipterygoideal lamina of the basisphenoid in Witmer & Ridgely (2010); or basisphenoid web in Bakker et al. (1988) and Rauhut (2004)]. However, in contrast to Coloradisaurus, Riojasaurus and Anchisaurus, the other two taxa scored with state ‘1’, Efraasia and Plateosaurus, have a much more shallow basisphenoid recess. In this case, there is no septum in these taxa, as the bone spanning between the basipterygoid processes only forms the posterior wall of the subsellar recess. What has probably been treated as a septum in these two taxa is thus the posterior margin of the deep subsellar recess. Therefore, it is clear that there is a difference in the morphology of taxa coded with state ‘1’. Whereas Riojasaurus, Coloradisaurus and Anchisaurus have a vertical sheet of bone between the subsellar and basisphenoid recesses, Efraasia and Plateosaurus do not possess such a structure because they do not possess a deep basisphenoid recess. Thus, it seems that what has been coded as the presence of a deep septum between the interbasipterygoid process is actually related to the depth of the subsellar recess.
Furthermore, we also found problematic aspects for taxa that were coded as ‘0’ that should be considered. Leyesaurus exhibits a morphology that strongly resembles that of Efraasia, in having a deep subsellar recess but a shallow basisphenoid recess. However, whereas Efraasia is coded as ‘1’, Leyesaurus is coded as ‘0’. In addition, a structure similar to that described by Fedak & Galton (2007: fig. 6a) as a ridge (i.e. septum) between the basipterygoid processes in Anchisaurus is clearly present in Eoraptor (see Sereno, Martinez & Alcober, 2012: fig. 29), but the latter was coded with state ‘0’. Finally, neotheropods usually have well-defined subsellar and basisphenoid recesses (Witmer, 1997; Rauhut, 2004), and, as a consequence, they also have a deep septum in the interbasipterygoid space. However, the OTU Neotheropoda was also coded with state ‘0’ in all previous analyses using an updated version of Yates (2007b) matrix.
The differences between our interpretations and those of previous studies when coding this character could be attributed to the implicit subjectivity of the word ‘deep’, used in the character definition. However, as shown by the example of Efraasia and Leyesaurus described above, this problem may also arise from different interpretations of what constitutes a septum spanning the interbasipterygoid space. Although neither Efraasia nor Leyesaurus do possess a septum, the deep subsellar recess was misinterpreted as a septum in the former, but not in the latter.
Here we propose a modification of the definition of character 85 (Fig. 14) in order to avoid subjectivity and try to minimize conflicts in coding in future studies. Thus, the character is here defined as: subsellar recess: maximum width equal or greater than the dorsoventral height (0); maximum width smaller than the dorsoventral height (1).
The results of our analysis show that the presence of a subsellar recess state ‘1’ occurs in all taxa belonging to the clade containing Efraasia and Neosauropoda (Fig. 14). The only sauropodomorpha scored with state ‘0’ in the data matrix was Pantydraco, whereas Massospondylus was scored with states 0 and 1. For this taxon, state ‘0’ was observed in the specimen SAM-PK-K1314 (Fig. 14A), whereas the specimen BP/1/5231 exhibits a deep subsellar recess, conforming to the morphology of state ‘1’. Thus, although the recess is not as developed as the subsellar recess of some theropod taxa (Witmer, 1997), it is clear that a similar structure is also present and considerably developed in Sauropodomorpha, but was not mentioned in previous studies.
Braincases of four different sauropodomorphs illustrating the distinct morphologies associated with character states of characters 85, 82 and 368 (number after point indicates the respective character state). A, specimen UFSM 11069 of Unaysaurus (ventral view); B, specimen SAM-PK-K1314 of Massospondylus (ventral view); C, specimen SMNS 13200 of Plateosaurus (posteroventral view); D, specimen MB.R.2387.3, an indeterminate Sauropoda (posteroventral view – anteroposterior axis is inverted in relation to other braincases in the figures). Abbreviations: bo, basioccipital; bobt, basioccipital component of the basal tubera; bsbt, basisphenoidal component of the basal tubera; pbs, parabasisphenoid; ug, unossified gap.
The anterior limit of the subsellar recess – new character (366 of this study)
Here we propose a new character, related to the lamina (e.g. in the case of Efraasia and Coloradisaurus) or ridge (e.g. in the case of Plateosaurus and Massospondylus) that extends from the basipterygoid process onto the ventral surface of the cultriform process of the parasphenoid. As detailed above in the description of the parabasisphenoid, the extension of these ridges on the cultriform process of the parasphenoid converge medially in some taxa, whereas in others, as in Efraasia, the extension of the ridges extend parallel to each other until they fade away into the cultriform process distally. When these ridges converge medially, the subsellar recess has a marked anterior end, which is triangular in shape in ventral view. When the ridges do not converge medially, the anterior limit of the subsellar recess is not well marked, with its ventral margin being confluent with the ventral margin of the cultriform process (Fig. 15).
Ventral view of the parabasisphenoid of the specimens AMNH 6810 of Plateosaurus engelhardti (A, B) and PVSJ 568 of Adeopapposaurus mognai showing the two different morphologies associated with character states of character 366 (number after point indicates the respective character state) in sauropodomorphs. Abbreviations: bp, basipterygoid process; bsbt, basisphenoidal component of the basal tubera; lcpp, lamina on the cultriform process of the parabasisphenoid.
The new character (number 366 in the character list) is proposed as follows: laminae/ridges extending from the basipterygoid process onto the parasphenoid rostrum: extend parallel until they fade into the ventral margin of the cultriform process (0); converge anteromedially on the ventral surface of the cultriform process (1). Character state ‘0’ is scored for, for example, Efraasia, and Massospondylus. Character state ‘1’ is scored for, for example, Plateosaurus, and Melanorosaurus (undescribed specimen NM QR 1551 – Nair et al., 2015).
This character was only scored for nine of the OTUs within Sauropodomorpha in this study. Our results indicate two independent events of a modification from state ‘0’ to ‘1’, one in the branch leading to the clade including Plateosaurus and Unaysaurus (Plateosauridae) and the other at the branch leading to Melanorosaurus.
The relative position of the components of the basal tubera – character 82 of this study and of Yates (2007b)
Romer (1956) defined the basal tubera as structures present in the region of the basisphenoid and basioccipital contact related to the attachment of hypaxial musculature, with the contribution of each bone varying among groups. More recently, Snively & Russell (2007) have shown that in the case of living archosaurs, the majority of the surface consisting of the occipital plate and the posterior region of the basisphenoid represent areas for the attachment of neck musculature (e.g. m. rectus capitis ventralis, m. longissimus capitis). It has long been recognized that the basal tubera of sauropodomorphs have a basioccipital and a parabasisphenoid component (see e.g. Yates, 2010), and the relative position of the basal tubera components of each one of the two bones was translated into a phylogenetic character for the group. Character 82 of Yates (2007b) was thus defined as: ‘shape of basal tuberae: knob-like, with basisphenoidal component rostral to basioccipital component (0), or forming a transverse ridge, with the basisphenoidal component lateral to the basioccipital component (1)’.
Problematic aspects of this character were identified in the course of our analyses of sauropodomorph braincase materials. The first is related to the morphology described by each of the character states. Efraasia (SMNS 12667) is scored in previous data matrices as having state ‘1’, and matches the morphology described by the character state as possessing a transverse ridge (see Figs 2, 4). However, in Efraasia, the transverse ridge is actually formed by the basioccipital component of the tubera only. In other taxa, such as Coloradisaurus, Massospondylus and Melanorosaurus, part of the basioccipital component is also medial to the basisphenoid components, but in these taxa the basioccipital components are not entirely connected to form a ridge (see e.g. Apaldetti et al., 2014; Fig. 14), but are separated by a shallow recess, which results in a knob-like aspect for the structure. It thus does not correspond to the ridge-like shape as described in character state ‘1’. On the other hand, in contrast to this discontinuous and knob-like appearance of the basal tubera in these forms, some neosauropods exhibit a laminar basioccipital component of the basal tubera. In these taxa, the basioccipital forms has a laminar aspect that connects to the posterolateral projections of the parabasisphenoid, where the tubera are located (Fig. 14 – see also Tschopp, Mateus & Benson, 2015). This laminar morphology of the basioccipital component of the basal tubera is also observed in other archosaurs (Gower, 2002; Nesbitt, 2011). Thus, even if the basioccipital component of the basal tubera is located posterior to the basisphenoidal one, we could not recognize a knob-like morphology as predicted by character state ‘0’. Removing the statement regarding the shape of the basioccipital component does not suffice, as this character has a second problematic aspect.
In the way the character is defined, it is implied that the basioccipital components of the basal tubera are either medially or posteriorly located in relation to the basisphenoid component, excluding a morphology in which both possibilities are present (Fig. 14). Yates (2010) already discussed that the basioccipital component is not entirely posteriorly or medially located in relation to the basisphenoid component, and recommended that the scoring for the character should be based on where the major portion of the former structure is positioned. However, Yates (2010) did not take into account the entire set of structures that together correspond to the basioccipital component of the basal tubera in some sauropodomorphs. Taxa such as Adeopapposaurus, Melanorosaurus, Pantydraco, Plateosaurus and Unaysaurus exhibit multiple protuberances in the basioccipital that can be considered as different basioccipital components of the tubera when previous definitions of the term are applied (Romer, 1956; Snively & Russell, 2007). These taxa possess protuberances on the medial surface of the basioccipital, which are medially (or posteriorly) located in relation to the posterolateral projections of the parabasisphenoid that form the component of the tubera of this bone, and also protuberances located on the lateral surface of the basioccipital, posterior to the basisphenoid component of the tubera (Fig. 14). Therefore, it is not possible to establish which portion of the structure forms the majority of the basioccipital tubera, as suggested by Yates (2010).
After our survey of the literature (see e.g. Tschopp et al., 2015), together with first-hand analysis of sauropod specimens, we could not recognize any sauropod taxon (if Melanorosaurus is not included in the group) that has part of the basioccipital component of the basal tubera (either a knob or a ridge-like structure) medially located in relation to the basisphenoidal component, as observed in non-sauropodan taxa, such as Efraasia. It is very likely that this was the variation intended to be captured in character state ‘2’ of character 84 of Yates (2007b): ‘… with the basal tuberae being separated by a deep caudally opening U-shaped fossa’ – see below. The left and right portion of the basal tuberae of sauropods correspond to two well-defined anchorage surfaces for muscle attachments, either globular or box-like, as defined in Tschopp et al. (2015 – see character 82 of that study), that are separated by a U/V-shaped fossa.
To incorporate this variation in the morphology of the basal tubera into information for the phylogenetic analysis focusing on non-neosauropodan sauropodomorphs, a modified version of character 82 is proposed as follows: ‘Basioccipital component of the basal tubera, medial component in relation to the parabasisphenoidal components: present (0); absent (1)’.
Our results indicate a single event within Sauropodomorpha in which a transformation from state ‘0’ to state ‘1’ occurred, at the branch leading to Sauropoda. It is important to mention here that the medial component of the basioccipital basal tubera is located posterior to the anterior projection of the bone treated in character 84 (see below). Thus, we consider both characters as independent.
The variation regarding the morphology of the basioccipital component of the basal tubera is probably related to differences in the neck musculature among taxa. Snively & Russell (2007) demonstrated differences in muscle numbers and their corresponding points of insertion in the braincases of birds and crocodiles. For the former, the authors indicate a single muscle inserting on the tuberosities of the basioccipital, the m. rectus capitis ventralis, also present in crocodiles. However, crocodiles have a second muscle that also inserts on basioccipital tuberosities, the m. longissimus capitis. It is beyond the scope of this work to provide any statement regarding the homology of the insertion areas of muscles in sauropodomorphs with those of birds or crocodiles, but future studies might show that the variation seen in the development of the basal tubera in different groups of archosaurs might reflect differences in neck musculature.
Junction of the parabasisphenoid and basioccipital – new character (84 of this study)
Character 84 of Yates (2007b) was defined as: ‘ridge formed along the junction of the parabasisphenoid and the basioccipital, between the basal tuberae: present with a smooth rostral face (0), present with a median fossa on the rostral face (1), or absent with the basal tuberae being separated by a deep caudally opening U-shaped fossa (2)’.
A first problem to be considered here is related to the character locator (sensuSereno, 2007b) – along the junction of the parabasisphenoid and basioccipital, between the basal tuberae. The ventral surface of the parabasisphenoid of sauropodomorphs, such as Efraasia, Pantydraco and Plateosaurus, all coded as having a ridge along the junction between basioccipital and parabasisphenoid, has two posterolateral projections that form the basal tubera component of this bone at their distal ends (see Figs 4, 14). In these taxa, the posterior margin of the parabasisphenoid has a ‘V/U’ shape in ventral view (Fig. 16) and the basioccipital exhibits a median anterior projection, which forms the contact with the parabasisphenoid in this region (see basioccipital description). For these taxa, previously coded with ‘0’ or ‘1’ (see e.g. Yates, 2007b), no ridge was observed at the junction of the parabasisphenoid and the basioccipital, either in this region or laterally. Based on an examination of the scoring for this character in the data matrix of Yates (2007b), we suppose that what was possibly coded as a ridge is in fact part of the basioccipital portion of the tubera that is medially located in relation to the basisphenoid component (see description of the basioccipital and Figs 4, 16). All taxa scored with state ‘0’ or ‘1’ (ridge present) in character 84 are also scored with state ‘1’ for character 82 (basioccipital component medially located; see discussion above). On the other hand, taxa scored as ‘2’ (ridge absent) have state ‘0’ for character 82 (basioccipital component posteriorly located). The only exception is Pantydraco, which was coded with state 0 for both characters. However, first-hand analysis of the material showed that Pantydraco clearly has a part of the basioccipital component of the basal tubera medially located in relation to the basisphenoid tubera, and this component was probably regarded as a ridge. Further evidence that the basioccipital component of the basal tubera was treated as a ridge in the junction between the basioccipital and the parabasisphenoid is that early interpretations of the contact between these two bones assumed a linear contact at the level of the basal tubera (see e.g. Galton & Bakker, 1985: fig. 4).
Ventral view of braincases of two specimens of sauropodomorphs illustrating the two different morphologies associated with character states of character 84 (number after point indicates the respective character state). A, specimen MB.R.2386 of the neosauropod Tornieria; B, specimen SMNS 12667 of Efraasia. The dashed lines mark the suture between the parabasisphenoid and basioccipital. Abbreviations: bo, basioccipital; pbs, parabasisphenoid.
Given the problems detailed here, we propose the exclusion of character 84 of (Yates, 2007b) and a new character 84 that accounts for the details of the junction between the parabasisphenoid and basioccipital (see Fig. 16). The character is thus proposed as follows: basioccipital–parabasisphenoid junction on the ventral surface of the bones: straight line (0); U/V-shaped (1). We considered the morphology in which the anterior portion of the basioccipital projects anteriorly between the two posterolateral projections of the parabasisphenoid as U/V-shaped – in this case, the posterolateral projections of the parabasisphenoid are separated from each other by a significant portion of the basioccipital. Character state ‘0’ is scored for OTUs such as Neosauropoda. Character state ‘1’ is scored for OTUs such as Efraasia and Pantydraco.
The results of our analysis show that, in Sauropodomorpha, all non-sauropodan sauropodomorphs exhibit state ‘1’, which is symplesiomorphic for these taxa (state ‘1’ is found among other early dinosaurs and non-dinosaurian dinosauriforms, but the non-archosaurian archosauriform Euparkeria was treated as having state ‘0’). There was a single modification from state ‘1’ to ‘0’ within the sauropodomorph clade, which happened at the branch leading to Neosauropoda. Nevertheless, it is important to emphasize that we did not score the taxa Spinophorosaurus, Mamenchisaurus Young, 1954, and Shunosaurus lii Dong et al. 1983 in our analysis because we did not analyse the braincase materials first-hand, and the contact between the basioccipital and parabasisphenoid has not been described in detail for these taxa. Thus, it is possible that the modifications in the contact between the parabasisphenoid and basioccipital happened earlier in the evolution of Sauropodomorpha.
The exit of the mid-cerebral vein in the lateral surface of the braincase – character 80 of this study and of Yates (2007b)
The mid-cerebral vein pierces the endocranial cavity on the lateral surface of the braincase and exits it through a foramen in the occiput (Galton, 1985; Sampson & Witmer, 2007). In sauropodomorphs, the foramen for the posterior exit of the mid-cerebral vein (sometimes called the external occipital vein; Sampson & Witmer, 2007) leaves the cavity on the occipital surface of the skull and can be enclosed solely by the supraoccipital or have its borders formed by the supraoccipital and parietal as discussed above. This difference in the exit route is used as a phylogenetic character (character 73 of Yates, 2007b). Likewise, the exit of the vein on the lateral surface of the braincase is also variable and has also been used as a phylogenetic character. Character 80 of Yates (2007b) has been stated as: ‘Exit of the mid-cerebral vein: through trigeminal foramen (0) or through a separate foramen anterodorsal to trigeminal foramen (1)’.
This character was proposed by Rauhut (2003) in an analysis of the phylogenetic relationships of theropod dinosaurs. However, it is necessary to make a small modification to this character in the context of a phylogenetic analysis focusing on sauropodomorph dinosaurs. The problem with this character is related to the location of the foramen for the mid-cerebral vein as stated in character state ‘1’. In contrast to the description of this character state, in taxa such as Efraasia and Plateosaurus the separate (or partially separate) foramen for the mid-cerebral vein is located posterodorsal to the trigeminal foramen. However, the morphology of Efraasia (Fig. 6) and Plateosaurus is not the only condition present among sauropodomorphs. Shunosaurus presents a separate foramen for the mid-cerebral vein that is located anterodorsal to the trigeminal foramen (Chatterjee & Zheng, 2002), as described by the character state originally.
Thus, we propose a subtle modification in the statement of character 80, with the modified version as follows: Exit of the mid-cerebral vein: through trigeminal foramen (0) or through a separate foramen (1). With this modification, we intend to avoid misinterpretation in future studies while coding this character as the position of the separate for the mid-cerebral vein varies among taxa. Character state ‘0’ is scored in, for example, Coloradisaurus, whereas character state ‘1’ is scored for OTUs such as Plateosaurus and Shunosaurus.
In the context of our analysis, the only sauropodomorph to exhibit state ‘0’ is Coloradisaurus.
Orientation of the basipterygoid process – new character (367 of this study)
The different orientations of the basipterygoid processes were used as character states of phylogenetic characters in previous studies of archosaurs and dinosaurs (e.g. Wilson, 2002; Butler, Upchurch & Norman, 2008; Nesbitt, 2011; Bittencourt et al., 2014). Here we also propose to use this variation as a phylogenetic character in the context of non-neosauropodan sauropodomorphs. As detailed in the comparative description of the basipterygoid processes, there is some variation regarding their orientation in distinct sauropodomorph taxa. However, some considerations should be made in order to avoid confusion when coding this character.
Orienting the braincase can be difficult because it is not always the case that the braincase is found entirely preserved and associated with the rest of the skull. For sauropodomorphs outside Sauropoda, one possibility might be to use the orientation of the foramen magnum as a landmark to determine the orientation of the braincase. In non-neosauropodan sauropodomorph taxa, the foramen magnum typically faces posteriorly when the braincase is seen in lateral view. However, among sauropodomorphs, a different condition is observed in taxa within the Neosauropoda clade, such as diplodocoids (Salgado, 1999), in which the foramen magnum faces posteroventrally. Thus, in order to make this character more adequate in the context of analysis of sauropodomorph taxa, we use the angle between the basipterygoid processes and the cultriform process of the parasphenoid as a proxy for this character. It is worth mentioning that Wilson (2002) used the angle between the basipterygoid processes in order to capture the variation in the orientation of these structures. Despite agreeing with the approach adopted in Wilson (2002), we decided to analyse the angle formed between the basipterygoid process and the cultriform process of the parabasisphenoid, because, as part of the parabasisphenoid, these structures are more commonly found in articulation than are the basipterygoid process and the skull roof (i.e. frontals and parietals). For example, SMNS 12667 has anteroventrally projected basipterygoid processes, exhibiting an acute angle between these structures and the parasphenoid rostrum. On the other hand, Plateosaurus has ventrally/posteroventrally projected basipterygoid processes, and the angle between these structures and the cultriform process is obtuse.
New character (number 367 in the characters list) is thus proposed as follows: angle between basipterygoid process and cultriform process of the parabasisphenoid: < 90° (0); 90° (1); > 90° (2). Character state ‘0’ is scored, for example, for Efraasia, state ‘1’ is scored for, for example, Thecodontosaurus and state ‘2’ is scored for, for example, Plateosaurus.
Our analysis shows a transformation from state ‘0’ to ‘1’ in the branch leading to the clade including Leyesaurus, Massospondylus and Adeopapposaurus. This represents further evidence that these taxa form a clade within Massospondylidae (e.g. Apaldetti et al., 2014; Otero et al., 2015). Tracing this character in the phylogeny shows that there are multiple transformations within Sauropodomorpha, but, as shown for the clade including Leyesaurus, Massospondylus and Adeopapposaurus, this character may be important in establishing smaller subclades within Sauropodomorpha in future works.
Relative length of the parabasisphenoid – new character (368 of this study)
Sauropods exhibit an anteroposteriorly short parabasisphenoid in comparison to ‘prosauropod’ taxa. The relative length of the parabasisphenoid was used as a phylogenetic character in other studies of archosaurs (char. 56 in Rauhut, 2003; char. 53 in Nesbitt, 2011; and char. 51 in Bittencourt et al., 2014). We took a different approach than that adopted in previous studies in order to quantify and translate this variation into a phylogenetic character. The new character (number 368 in the character list) is proposed as follows: length of the parabasisphenoid (from the proximal limit of the basipterygoid process to the basisphenoidal component of the basal tubera) in relation to the length of the basioccipital (from the basioccipital component of the basal tubera to the posterior limit of the condyle): longer or equal (0); shorter (1). Character state ‘0’ is scored for many non-sauropodan sauropodomorphs, such as Efraasia, Plateosaurus and Massospondylus, whereas state ‘1’ is scored for Neosauropoda (e.g. Giraffatitan, Dicraeosaurus, Tornieria).
As noted above, the parasphenoid and basisphenoid are fused in dinosaurs into a single element, the parabasisphenoid (Sampson & Witmer, 2007). Thus, we used the basipterygoid processes as the markers of the anterior limit of the parabasisphenoid. Regarding the basioccipital, we decided to use the medial component of the basioccipital basal tubera in order to mark an anterior limit, as some taxa do not have the anterior triangular projection of the basioccipital described above. In taxa that do exhibit such a projection, the basioccipital component of the basal tubera is located in the posterior limit of it. Thus, the area posterior to the basal tubera is topologically congruent for taxa with or without the anterior projection.
Characters that are based on two structures that can vary might be problematic (see e.g. Simões et al., 2016), but we believe that this ratio represents the notable reduction of the parabasisphenoid observed in Eusauropoda rather well. Indeed, state ‘1’ is found only in members of Eusauropoda and its sister group, Spinophorosaurus.
Notch in the posterodorsal margin in the lateral surface of the parabasisphenoid – new character (369 of this study)
After first-hand analysis of specimens of Massospondylus and Adeopapposaurus, we found that both taxa have a notch in the posterodorsal margin of the lateral portion of the parabasisphenoid, right below the fenestra ovalis in lateral view (Fig. 17; see also Martinez, 2009: fig. 10C). Here we propose a new character related to the presence/absence of this notch in the parabasisphenoid (obs. despite recognizing this notch, we did not find any indication of a soft-tissue structure that might be associated with it in the literature).
Lateroventral view of the braincases of the specimens SMNS 12667 of Efraasia minor (A, virtual reconstruction) and SAM-PK-K1314 of Massospondylus carinatus illustrating different morphologies associated with character states of characters 367 and 369 (number after point indicates the respective character state). Abbreviations: bo, basioccipital; bp, basipterygoid process; no, notch; ot, otoccipital; pbs, parabasisphenoid; pr, prootic; ss, smooth surface; XII, foramina for the hypoglossal nerve.
New character (number 369 in the list of characters) is proposed as follows: Notch in the posterodorsal margin of the lateral portion of the parabasisphenoid: absent (0); present (1). State ‘0’ is scored for, for example, Efraasia and Plateosaurus, whereas state ‘1’ is scored for Adeopapposaurus and Massospondylus.
The presence of the notch in the parabasisphenoid has only been observed in Adeopapposaurus and Massospondylus, and thus represents further evidence that these taxa form a clade within Massospondylidae. It is worth mentioning that the parabasisphenoid of Leyesaurus (a member of the clade containing Massospondylus and Adeopapposaurus) is incomplete, and this OTU was coded with ‘?’ in our analysis.
Divided/undivided metotic fissure – new character (370 of this study)
Following from the discussion of the subdivision of the metotic fissure presented above, we here propose a new character to account for this variation in sauropodomorphs. The new character (number 370 in the list of characters) is proposed as: ‘Number of foramina in the otoccipital between the exoccipital pillar (excluding the foramina for the hypoglossal nerve) posteriorly and fenestra ovalis anteriorly: one (0), two (1)’. Character state ‘0’ is scored for e.g. Plateosaurus. Character state ‘1’ is scored for Efraasia, among others.
The presence of two foramina in this region of the otoccipital is observed in all non-sauropodan sauropodomorph OTUs, except for Plateosaurus. Regarding Thecodontosaurus, Benton et al. (2000) report a morphology that would be similar to that in Massospondylus in Gow (1990) and Plateosaurus in Galton (1985). However, these taxa differ in morphology. The specimen of Massospondylus (BP/1/5231) analysed by Gow (1990) has two foramina that correspond to the two foramina seen in Efraasia. Regarding Plateosaurus, there is only a subdivision in the metotic foramen of the specimens, with a smaller opening forming a notch at the posterodorsal edge of the metotic foramen (Galton, 1985; Prieto-Márquez & Norell, 2011). Thus, Plateosaurus is the only OTU belonging to Sauropodomorpha but not to Sauropoda that was scored with state ‘0’ in our analysis.
Regarding sauropods, no evidence of the presence of two foramina is observed in the taxa for which information is available, including Shunosaurus (Chatterjee & Zheng, 2002), Giraffatitan, Dicraeosaurus, Spinophorosaurus (Knoll et al., 2012), Apatosaurus Marsh, 1877 (Balanoff et al., 2010) and an indeterminate titanosaurian (Sues et al., 2015). In the context of our analyses, a change to a condition of a single foramen in Sauropodomorpha happens at the branch leading to the clade containing Shunosaurus and Neosauropoda. However, stating the presence of a single foramen for the whole Neosauropoda clade may be misleading because, as neosauropods are not the focus of our study, we did not conduct an extensive review of all the braincase materials preserved in this clade (see Table 1).
Presence/absence of an unossified gap – new character (371 of this study)
New character (number 371 in the list of characters) is proposed as follows: ‘Unossified gap between the basioccipital and basisphenoidal component of the basal tubera and ventral ramus of the opisthotic: absent (0); present (1)’. Character state ‘0’ is scored for OTUs such as Neosauropoda and Saturnalia. Character state ‘1’ is scored for OTUs such as Thecodontosaurus and Plateosaurus (Fig. 12). Gower (2002) and Nesbitt (2011) already discussed the relation of the unossified gap and the cochlear recess. However, the character we proposed here does not deal with the morphology of this recess, but only with the presence and absence of the gap.
In our analysis, the only taxon within Sauropodomorpha but outside Sauropoda that exhibits state ‘0’ is Saturnalia. All other sauropodomorph OTUs outside Sauropoda that we analysed first-hand and that were scored for this character have an unossified gap between the basioccipital, parabasisphenoid and otoccipital. In E. minor, it seems likely that such a gap was present (as pointed out by Galton & Bakker, 1985), although this cannot be said with certainty due to displacement of elements. In the left side of the braincase, for example, the gap between the basal tubera of the basisphenoid and the basioccipital is much bigger than that between the basioccipital and parabasisphenoid medially. Nevertheless, a secure statement requires material in which this region is better preserved. On the other hand, we did not find any evidence for the presence of such a gap in the sauropods analysed for this study.
Braincase evolution in Sauropodomorpha
For some of the phylogenetic characters related to the anatomy of the braincase, our results demonstrate that non-sauropodan sauropodomorphs exhibit a condition that is distinct from that observed in sauropod taxa present in our analysis, namely a basioccipital component of the basal tubera composed of multiple protuberances, including a protuberance medially located in relation to the basisphenoidal component of the tubera, main body of the parabasisphenoid that is relatively longer than the basioccipital, the presence of two foramina in the otoccipital between the exoccipital pillar and the fenestra ovalis, the presence of an unossified gap (but not in Saturnalia), the U/V shape contact between parabasisphenoid and basioccipital, the presence of an unossified gap. Some of these character states found in non-sauropodan taxa (e.g. a parabasisphenoid proportionally longer than the basioccipital; the U/V contact between the basioccipital and the parabasisphenoid) represent the plesiomorphic condition within Sauropodomorpha and thus highlight that the anatomy of the Sauropoda is unique among Sauropodomorpha, and strongly deviates from the anatomy of taxa outside this clade. Others (e.g. a basal tuberae with a medial basisphenoidal component, the presence of an unossified gap) are apomorphic when compared to sauropodomorph ‘outgroups’, which exhibits the same character states found among sauropods, and might thus be seen as supporting the monophyly of part of the non-sauropodan sauropodomorphs. However, the paraphyletic array of these taxa is strongly supported by postcranial characters, which override the cranial similarities in the current analysis (see also Apaldetti, Pol & Yates, 2012).
We conducted exploratory phylogenetic analyses in order to test the hypothesis of a monophyletic ‘Prosauropoda’ using our data set. Character 82 could potentially indicate the monophyly of non-sauropodan sauropodomorphs within the minimal clade containing Pantydraco and Melanorosaurus, whereas character 371 could potentially indicate the monophyly of the non-sauropodan within the minimal clade containing Thecodontosaurus and Melanorosaurus. Constrained analyses indicate that 66 additional steps are necessary in order to recover a monophyletic ‘Prosauropoda’ including the non-sauropodan sauropodomorphs within the clades above mentioned (including or not including Pantydraco). However, these hypotheses are still much less unlikely than a scenario of a less inclusive ‘Prosauropoda’ formed by the ‘core prosauropods’ (including plateosaurids and massospondylids), for which we found no additional evidence based on the braincase anatomy. Only 20 additional steps are necessary to obtain a monophyletic ‘Prosauropoda’ equivalent to the clade obtained in Upchurch et al. (2007), which do not include taxa such as Thecodontosaurus, Pantydraco and Efraasia.
It is worth discussing here the results of our analysis in the context of the different definitions already proposed for Sauropoda. The first phylogenetic definition for Sauropoda based on results of a numerical analysis of a data matrix was presented by Salgado, Coria & Calvo (1997), which defined the group as the least inclusive clade including Vulcanodon Raath, 1972 and Eusauropoda. An additional definition was proposed by Yates (2007b – the definition adopted here; but see Peyre de Fabrègues, Allain & Varriel, 2015), which defined Sauropoda as the least inclusive clade that includes Saltasaurus loricatus (Bonaparte & Powell, 1980) but not Melanorosaurus readi. In this context, our statement that most of the character states observed in sauropods differ from the character states of the non-sauropodan sauropodomorphs holds true when either the definition of Salgado et al. (1997) or the one of Yates (2007a) is applied (see Fig. 13). However, Wilson & Sereno (1998) also proposed a phylogenetic definition for Sauropoda, which consider the group as including all sauropodomorphs closer to Saltasaurus than to Plateosaurus. In the context of this definition, a series of transformations related to the characters discussed above would still happen within Sauropoda. However, the presence of taxa such as massospondylids and Anchisaurus in Sauropoda would indicate that some members of Sauropoda have braincase morphology mostly similar to the one of the non-sauropodan representatives. Nevertheless, one aspect of the definition of Wilson & Sereno (1998) should be taken into account. It was proposed at a time when some studies (e.g. Galton, 1990; Sereno, 1999) were finding support for a monophyletic ‘Prosauropoda’ including plateosaurids and massospondylids. However, in the context of the most recent analysis of sauropodomorphs, which shows that even the ‘core prosauropods’ (massospondylids and plateosaurids) do not form a monophyletic group that excludes sauropods, the definition of Sauropoda proposed by Wilson & Sereno (1998) fell into disuse (see also McPhee et al., 2014; Peyre de Fabrègues et al., 2015).
Finally, there is a lack of braincase materials and/or of detailed descriptions (the only exception being Spinophorosaurus) of this structure in taxa more closely related to Neosauropoda than to Melanorosaurus (here considered as the sister group of Sauropoda). This hampers the reconstruction of the character transformations at the base of Sauropoda at the moment. Thus, only with the description and/or discovery of additional braincase materials will it be possible to achieve a detailed scenario of the anatomical transformations in the braincase anatomy of taxa within Sauropoda (i.e. if the anatomical modifications happened at the branch leading to Sauropoda or in less inclusive clades of Sauropoda).
CONCLUSIONS
Braincase anatomy still plays a minor role in descriptive works of sauropodomorphs, with this structure usually being much less detailed than other parts of the skull or postcranium. Here we supplement the original work of Galton & Bakker (1985) on the braincase of E. minor, and carry out a comparative description in order to illustrate morphological variation in sauropodomorphs.
The usually short descriptions of the braincase probably also had an impact on the use of characters related to this complex structure formed by multiple elements in phylogenetic analyses, and may have led to the problematic aspects of the characters used in previous analysis discussed above. For example, the braincase (including frontals and parietals, elements that are also part of the skull roof) was only represented by 13 characters out of 365 in the matrix of McPhee et al. (2015). In this study, we propose seven new characters related to the anatomy of the braincase. We further highlight some issues that we consider as problematic when coding phylogenetic characters related to braincase anatomy in a data matrix for Sauropodomorpha. Setting aside the problems with the term in general and the distinction between characters and character states (see Forey & Kitching, 2000), phylogenetic characters represent our translation of the observable morphological variation into the basic units of a cladistic analysis (Freudenstein, 2005). As our explanation of the evolutionary history of a group or even only single morphological traits is based on a phylogenetic hypothesis, proper definition of the phylogenetic characters is crucial, as our hypothesis ultimately relies on these characters (Rieppel & Kearney, 2007; Simões et al., 2016). Trying to clarify the criteria used when formulating these fundamental units is a way of avoiding problems of interpretations that can cause, for example, conflicts in coding or inability to recognize the transformation series proposed for such a character. With this contribution, we therefore intend to clarify aspects of phylogenetic characters related to the anatomy of braincase in sauropodomorphs as an attempt to minimize differences in interpretations in future studies. Nevertheless, our points and suggestions are not definitive, and will certainly be subject to changes in future studies that provide more data on this complex structure.
Finally, our study indicates that the braincase anatomy of sauropods is majorly a result of modifications that happened within this clade. This contrasts with the evolution of other parts of the organisms. As mentioned above, it has been demonstrated that the peculiar anatomy of sauropods is a result of modifications that took place earlier in the evolutionary history of Sauropodomorpha. In this case, one factor that needs to be taken into account is a potential inability to translate existent morphologies in the braincase anatomy of ‘prosauropod’ lineages into phylogenetic characters that could indicate a stepwise acquisition of the features observed in sauropods. Still, it is unclear if the braincase anatomy of sauropods is the product of a rapid and drastic morphological change that took place within this clade or if it reflects the small number of braincases of non-neosauropodan sauropods preserved, which might exhibit a mixture of plesiomorphic and apomorphic traits in the context of Sauropodomorpha.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Supporting Information S1. Additional files containing the results of the digital segmentation of the specimen SMNS 12667.
Supporting Information S2. Nexus file for the phylogenetic analysis.
ACKNOWLEDGEMENTS
We are especially thankful to Rainer Schoch for loan of SMNS 12667 and to Gertrud Rößner and Bernhard Ruthensteiner for the CT scan of the material. Atila A. S. da Rosa, Daniela Schwarz, Diego Abelin, Gabriela Cisterna, Hilary Ketchum, Jaime Powell, Jonah Choniere, Paul Barrett, Rainer Schoch, Ricardo Martinez, Sandra Chapman and Zaituna Erasmus helped and/or provided the access to materials in collections, which we are thankful for. We are also thankful to those that contributed by sharing data used in this study: Blair McPhee, Diego Pol, Jay Nair, Jonathas Bittencourt, Kimberley Chapelle, Emanuel Tschopp. Felipe Montefeltro and Max C. Langer greatly contributed to aspects regarding the discussion on phylogenetic characters. We are also thankful to Gabriela Sobral and one other anonymous reviewer that greatly contributed to the quality of this study. TNT is a free program made available by the Willi Hennig Society. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) – Science without Borders (grant 246610/2012-3 to M.B.) and by the Deutsche Forschunsgsgemeinschaft (grant RA 1012/12-1 to O.W.M.R.).
REFERENCES
APPENDIX
The characters used in the phylogenetic analyses represent a modified version of the data set of McPhee et al. (2015), which in turn corresponds to an updated version of many previous works on Sauropodomorpha phylogeny (e.g. Yates, 2007b; Otero & Pol, 2013; Apaldetti et al., 2014; McPhee et al., 2014).
Characters 80, 82, 84 and 85 were modified (see main text) and rescored in the matrix. Characters 366–371 represent new characters proposed in this study – modified and new characters are highlighted in bold in the character list below. Character 365 was excluded from the analysis because we do not agree with the logical basis of the character (i.e. using only the total length of the femur instead of a relative measurement can be problematic, for example, because of ontogenetic variation). Character 86 was also excluded from the analysis because it lacks a more explicit definition (i.e. the depth of the parasphenoid rostrum varies anteriorly, but there is no specification in the character statement regarding where the measurements should be taken); and also because of problematic character scoring in previous analyses (i.e. many taxa that have only the ventral surface of the parasphenoid rostrum visible were coded for this character – e.g. Coloradisaurus, Efraasia). Additional modifications from the data matrix of McPhee et al. (2015) consist of rescoring character 58 for all taxa, and rescoring character 74 for the taxa Panphagia and Eoraptor with ‘?’.
Following McPhee et al. (2015), the following characters were set as additive (also followed by ORDERED in the characters list below): 8, 13, 19, 23, 40, 57, 69, 92, 102, 117, 121, 131, 144, 147, 149, 150, 157, 162, 167, 169, 170, 177, 207, 210, 225, 230, 237, 245, 255, 257, 270, 283, 304, 310, 318, 338, 351, 354, 356, 361, 365. A Nexus file with the data matrix is provided in the Supporting Information S2.
Characters list
1. Skull to femur ratio: greater than 0.6 (0); less than 0.6 (1).
2. Lateral plates appressed to the labial side of the premaxillary, maxillary and dentary teeth: absent (0); present (1).
3. Relative height of the rostrum at the posterior margin of the naris: more than 0.6 the height of the skull at the middle of the orbit (0); less than 0.6 the height of the skull at the middle of the orbit (1).
4. Foramen on the lateral surface of the premaxillary body: absent (0); present (1).
5. Distal end of the dorsal premaxillary process: tapered (0); transversely expanded (1).
6. Profile of premaxilla: convex (0); with an inflection at the base of the dorsal process (1).
7. Size and position of the posterolateral process of premaxilla: large and lateral to the anterior process of the maxilla (0); small and medial to the anterior process of the maxilla (1).
8. Relationship between posterolateral process of the premaxilla and the anteroventral process of the nasal: broad sutured contact (0); point contact (1); separated by maxilla (2). Ordered.
9. Posteromedial process of the premaxilla: absent (0); present (1).
10. Shape of the anteromedial process of the maxilla: narrow, elongated and projecting anterior to lateral premaxilla–maxilla suture (0); short, broad and level with lateral premaxilla–maxilla suture (1).
11. Development of external narial fossa: absent to weak (0); well developed with sharp posterior and anteroventral rims (1).
12. Development of narial fossa on the anterior ramus of the maxilla: weak and orientated laterally to dorsolaterally (0); well developed and forming a horizontal shelf (1).
13. Size and position of subnarial foramen: absent (0); small (no larger than adjacent maxillary neurovascular foramina) and positioned outside of narial fossa (1); large and on the rim of, or inside, the narial fossa (2). Ordered.
14. Shape of subnarial foramen: rounded (0); slot-shaped (1).
15. Maxillary contribution to the margin of the narial fossa: absent (0); present (1).
16. Diameter of external naris: less than 0.5 of the orbital diameter (0); greater than 0.5 of the orbital diameter.
17. Shape of the external naris (in adults): rounded (0); subtriangular with an acute posteroventral corner (1).
18. Level of the anterior margin of the external naris: anterior to the mid-length of the premaxillary body (0); posterior to the mid-length of the premaxillary body (1).
19. Level of the posterior margin of external naris: anterior to, or level with the premaxilla–maxilla suture (0); posterior to the first maxillary alveolus (1); posterior to the mid-length of the maxillary tooth row and the anterior margin of the antorbital fenestra (2). Ordered.
20. Dorsal profile of the snout: straight to gently convex (0); with a depression behind the naris (2).
21. Elongate median nasal depression: absent (0); present (1).
22. Width of anteroventral process of nasal at its base: less than the width of the anterodorsal process at its base (0); greater than the width of the anterodorsal process at its base (1).
23. Nasal relationship with dorsal margin of antorbital fossa: not contributing to the margin of the antorbital fossa (0); lateral margin overhangs the antorbital fossa and forms its dorsal margin (1); overhang extensive, obscuring the dorsal lachrymal–maxilla contact in lateral view (2). Ordered.
24. Pointed caudolateral process of the nasal overlapping the lachrymal: absent (0); present (1).
25. Anterior profile of the maxilla: slopes continuously towards the rostral tip (0); with a strong inflection at the base of the ascending ramus, creating a rostral ramus with parallel dorsal and ventral margins (1).
26. Length of rostral ramus of the maxilla: less than its dorsoventral depth (0); greater than its dorsoventral depth (1).
27. Shape of the main body of the maxilla: tapering posteriorly (0); dorsal and ventral margins parallel for most of their length (1).
28. Shape of the ascending ramus of the maxilla in lateral view: tapering dorsally (0); with an anteroposterior expansion at the dorsal end (1).
29. Rostrocaudal length of the antorbital fossa: greater than that of the orbit (0); less than that of the orbit (1).
30. Posteroventral extent of medial wall of antorbital fossa: reaching the anterior tip of the jugal (0); terminating anterior to the anterior tip of the jugal (1).
31. Development of the antorbital fossa on the ascending ramus of the maxilla: deeply impressed and delimited by a sharp, scarp-like rim (0); weakly impressed and delimited by a rounded rim or a change in slope (1).
32. Shape of the antorbital fossa: crescentic with a strongly concave posterior margin that is roughly parallel to the anterior margin of the antorbital fossa (0); subtriangular with a straight to gently concave posterior margin (1); antorbital fossa absent (2).
33. Size of the neurovascular foramen at the posterior end of the lateral maxillary row: not larger than the others (0); distinctly larger than the others in the row (1).
34. Direction that the neurovascular foramen at the posterior end of the lateral maxillary row opens: posteriorly (0); anteriorly, ventrally or laterally (1).
35. Arrangement of lateral maxillary neurovascular foramina: linear (0); irregular (1).
36. Longitudinal ridge on the posterior lateral surface of the maxilla: absent (0); present (1).
37. Dorsal exposure of the lachrymal: present (0); absent (1).
38. Shape of the lachrymal: dorsoventrally short and block-shaped (0); dorsoventrally elongate and shaped like an inverted L (1).
39. Orientation of the lachrymal orbital margin: strongly sloping anterodorsally (0); erect and close to vertical (1).
40. Length of the anterior ramus of the lachrymal: greater than half the length of the ventral ramus (0); less than half the length of the ventral ramus (1); absent altogether (2). Ordered.
41. Web of bone spanning junction between anterior and ventral rami of lachrymal: absent and antorbital fossa laterally exposed (0); present, obscuring posterodorsal corner of antorbital fossa (1).
42. Extension of the antorbital fossa onto the ventral end of the lachrymal: present (0); absent (1).
43. Length of the posterior process of the prefrontal: short (0); elongated, so that total prefrontal length is equal to the anteroposterior diameter of the orbit (1).
44. Ventral process of prefrontal extending down the posteromedial side of the lachrymal: present (0); absent (1).
45. Maximum transverse width of the prefrontal: less than 0.25 of the skull width at that level (0); more than 0.25 of the skull width at that level (1).
46. Shape of the orbit: subcircular (0); ventrally constricted making the orbit subtriangular (1).
47. Slender anterior process of the frontal intruding between the prefrontal and the nasal: absent (0); present (1).
48. Jugal–lachrymal relationship: lachrymal overlapping lateral surface of jugal or abutting it dorsally (0); jugal overlapping lachrymal laterally (1).
49. Shape of the suborbital region of the jugal: an anteroposteriorly elongate bar (0); an anteroposteriorly shortened plate (1).
50. Jugal contribution to the antorbital fenestra: absent (0); present (1).
51. Dorsal process of the anterior jugal: present (0); absent (1).
52. Ratio of the minimum depth of the jugal below the orbit to the distance between the anterior end of the jugal and the anteroventral corner of the infratemporal fenestra: less than 0.2 (0); greater than 0.2 (1).
53. Transverse width of the ventral ramus of the postorbital: less than its anteroposterior width at mid-shaft (0); greater than its anteroposterior width at mid-shaft (1).
54. Shape of the dorsal margin of postorbital in lateral view: straight to gently curved (0); with a distinct embayment between the anterior and posterior dorsal processes (1).
55. Height of the postorbital rim of the orbit: flush with the posterior lateral process of the postorbital (0); raised so that it projects laterally to the posterior dorsal process (1).
56. Postfrontal bone: present (0); absent (1).
57. Position of the anterior margin of the infratemporal fenestra: behind the orbit (0); extends under the rear half of the orbit (1); extends as far forward as the mid-length of the orbit (2). Ordered.
58. Frontal contribution to the supratemporal fenestra: present (0); absent (1).
59. Orientation of the long axis of the supratemporal fenestra: longitudinal (0); transverse (1).
60. Medial margin of supratemporal fossa: simple smooth curve (0); with a projection at the frontal/postorbital–parietal suture producing a scalloped margin (1).
61. Length of the quadratojugal ramus of the squamosal relative to the width at its base: less than four times its width (0); greater than four times its width (1).
62. Proportion of infratemporal fenestra bordered by squamosal: more than 0.5 of the depth of the infratemporal fenestra (0); less than 0.5 of the depth of the infratemporal fenestra (1).
63. Squamosal–quadratojugal contact: present (0); absent (1).
64. Angle of divergence between jugal and squamosal rami of quadratojugal: close to 90° (0); close to parallel (1).
65. Length of jugal ramus of quadratojugal: no longer than the squamosal ramus (0); longer than the squamosal ramus (1).
66. Shape of the rostral end of the jugal ramus of the quadratojugal: tapered (0); dorsoventrally expanded (1).
67. Relationship of quadratojugal to jugal: jugal overlaps the lateral surface of the quadratojugal (0); quadratojugal overlaps the lateral surface of the jugal (1); quadratojugal sutures along the ventrolateral margin of the jugal (2).
68. Position of the quadrate foramen: on the quadrate–quadratojugal suture (0); deeply incised into, and partly encircled by, the quadrate (1); on the quadrate–squamosal suture, just below the quadrate head (2).
69. Shape of posterolateral margin of quadrate: sloping anterolaterally from posteromedial ridge (0); everted posteriorly creating a posteriorly facing fossa (1); posterior fossa deeply excavated, invading quadrate body (2). Ordered.
70. Exposure of the lateral surface of the quadrate head: absent, covered by lateral sheet of the squamosal (0); present (1).
71. Proportion of the length of the quadrate that is occupied by the pterygoid wing: at least 70% (0); greater than 70% (1).
72. Depth of the occipital wing of the parietal: less than 1.5 times the depth of the foramen magnum (0); more than 1.5 times the depth of the foramen magnum (1).
73. Position of foramina for mid-cerebral vein on occiput: between supraoccipital and parietal (0); on the supraoccipital (1).
74. Postparietal fenestra between supraoccipital and parietals: absent (0); present (1).
75. Shape of the supraoccipital: diamond-shaped, at least as high as wide (0); semilunate and wider than high (1).
76. Orientation of the supraoccipital plate: erect to gently sloping (0); strongly sloping forward so that the dorsal tip lies level with the basipterygoid processes (1).
77. Orientation of the paroccipital processes in occipital view: slightly dorsolaterally directed to horizontal (0); ventrolaterally directed (1).
78. Orientation of the paroccipital processes in dorsal view: posterolateral forming a V-shaped occiput (0); lateral forming a flat occiput (1).
79. Size of the post-temporal fenestra: large fenestra (0); a small hole that is much less than half the depth of the paroccipital process (1).
80. Exit of the mid-cerebral vein: through trigeminal foramen (0) or through a separate foramen (1) (modified fromRauhut, 2003).
81. Shape of the floor of the braincase in lateral view: relatively straight with the basal tuberae, basipterygoid processes and parasphenoid rostrum roughly aligned (0); bent with the basipterygoid processes and the parasphenoid rostrum below the level of the basioccipital condyle and the basal tuberae (1); bent with the basal tuberae lowered below the level of the basioccipital and the parasphenoid rostrum raised above it (2).
82. Basioccipital component of the basal tubera, medial component in relation to the parabasisphenoidal component: present (0); absent (1) (modified from Yates, 2007).
83. Length of the basipterygoid processes (from the top of the parasphenoid to the tip of the process): less than the height of the braincase (from the top of the parasphenoid to the top of the supraoccipital) (0); greater than the height of the braincase (from the top of the parasphenoid to the top of the supraoccipital) (1).
84. Basioccipital–basisphenoid junction on the ventral surface of the bones: straight line (0); U/V-shaped (1) (this study).
85. Subsellar recess: maximum width equal or greater than the dorsoventral height (0); maximum width smaller than the dorsoventral height (1) (this study).
86. Dorsoventral depth of the parasphenoid rostrum: much less than (0), or about equal to (1), the transverse width (Yates, 2003). EXCLUDED.
87. Shape of jugal process of ectopterygoid: gently curved (0); strongly recurved and hook-like (1).
88. Pneumatic fossa on the ventral surface of the ectopterygoid: present (0); absent (1).
89. Relationship of the ectopterygoid to the pterygoid: ectopterygoid overlapping the ventral surface of the pterygoid (0); ectopterygoid overlapping the dorsal surface of the pterygoid (1).
90. Position of the maxillary articular surface of the palatine: along the lateral margin of the bone (0); at the end of a narrow anterolateral process due to the absence of the posterolateral process (1).
91. Centrally located tubercle on the ventral surface of palatine: absent (0); present (1).
92. Medial process of the pterygoid forming a hook around the basipterygoid process: absent (0); flat and blunt-ended (1); bent upward and pointed (2). Ordered.
93. Length of the vomers: less than 0.25 of the total skull length (0); more than 0.25 of the total skull length (1).
94. Position of jaw joint: no lower than the level of the dorsal margin of the dentary (0); depressed, well below this level (1).
95. Shape of upper jaws in ventral view: narrow with an acute rostral apex (0); broad and U-shaped (1).
96. Length of the external mandibular fenestra: more than 0.1 of the length of the mandible (0); less than 0.1 of the length of the mandible (1).
97. Caudal end of dentary tooth row medially inset with a thick lateral ridge on the dentary forming a buccal emargination: absent (0); present (1).
98. Height:length ratio of the dentary: less than 0.2; greater than 0.2 (1).
99. Orientation of the symphyseal end of the dentary: in line with the long axis of the dentary (0); strongly curved ventrally (1).
100. Position of first dentary tooth: adjacent to symphysis (0); inset one tooth’s width from the symphysis (1).
101. Dorsoventral expansion at the symphyseal end of the dentary: absent (0); present (1).
102. Splenial foramen: absent (0); present and enclosed (1); present and open anteriorly (2). Ordered.
103. Splenial–angular joint: flattened sutured contact (0); synovial joint surface between tongue-like process of angular fitting in groove of the splenial (1).
104. A stout, triangular, medial process of the articular, behind the glenoid: present (0); absent (1).
105. Length of the retroarticular process: less than the depth of the mandible below the glenoid (0); greater than the depth of the mandible below the glenoid (2).
106. Strong medial embayment behind glenoid of the articular in dorsal view: absent (0); present (1).
107. Number of premaxillary teeth: four (0); more than four (1).
108. Number of dentary teeth (in adults): less than 18 (0); 18 or more (1).
109. Arrangement of teeth within the jaws: linearly placed, crowns not overlapping (0); imbricated with distal side of tooth overlapping mesial side of the succeeding tooth (1).
110. Orientation of the maxillary tooth crowns: erect (0); procumbent (1).
111. Orientation of the dentary tooth crowns: erect (0); procumbent (1).
112. Teeth with basally constricted crowns: absent (0); present (1).
113. Tooth–tooth occlusal wear facets: absent (0); present (1).
114. Mesial and distal serrations of the teeth: fine and set at right angles to the margin of the tooth (0); coarse and angled upwards at an angle of 45° to the margin of the tooth (1).
115. Distribution of serrations on the maxillary and dentary teeth: present on both the mesial and distal carinae (0); absent on the posterior carinae (1); absent on both carinae (2).
116. Long axis of the tooth crowns distally recurved: present (0); absent (1).
117. Texture of the enamel surface: entirely smooth (0); finely wrinkled in some patches (1); extensively and coarsely wrinkled (2). Ordered.
118. Lingual concavities of the teeth: absent (0); present (1).
119. Longitudinal labial grooves on the teeth: absent (0); present (1).
120. Distribution of the serrations along the mesial and distal carinae of the tooth: extend along most of the length of the crown (0); restricted to the upper half of the crown (1).
121. Number of cervical vertebrae: eight or fewer (0); 9–10 (1); 12–13 (2); more than 13 (3). Ordered.
122. Shallow, dorsally facing fossa on the atlantal neurapophysis bordered by a dorsally everted lateral margin: absent (0); present (1).
123. Width of axial intercentrum: less than width of axial centrum (0); greater than width of axial centrum (1).
124. Position of axial prezygapophyses: on the anterolateral surface of the neural arch (0); mounted on anteriorly projecting pedicels (1).
125. Posterior margin of the axial postzygapophyses: overhang the axial centrum (0); flush with the caudal face of the axial centrum (1).
126. Length of the axial centrum: less than three times the height of the centrum (0); at least three times the height of the centrum (1).
127. Length of the anterior cervical centra (cervicals 3–5): no more than the length of the axial centrum (0); greater than the length of the axial centrum (1).
128. Length of middle to posterior cervical centra (cervicals 6–8): no more than the length of the axial centrum (0); greater than the length of the axial centrum (1).
129. Dorsal excavation of the cervical parapophyses: absent (0); present (1).
130. Lateral compression of the anterior cervical vertebrae: centra are no higher than they are wide (0); are approximately 1.25 times higher than wide (1).
131. Relative elongation of the anterior cervical centra (cervicals 3–5): lengths of the centra are less than 2.5 times the height of their anterior faces (0); lengths are 2.5–4 times the height of their anterior faces (1); the length of at least cervical 4 or 5 exceeds four times the anterior centrum height (2). Ordered.
132. Ventral keels on cranial cervical centra: present (0); absent (1).
133. Height of the mid-cervical neural arches: no more than the height of the posterior centrum face (0); greater than the height of the posterior centrum face (1).
134. Cervical epipophyses on the dorsal surface of the postzygapophyses: absent (0); present on at least some cervical vertebrae (1).
135. Posterior ends of the anterior, postaxial epipophyses: with a free pointed tip (0); joined to the postzygapophysis along their entire length (1).
136. Shape of the epipophyses: tall ridges (0); flattened, horizontal plates (1).
137. Epipophyses overhanging the rear margin of the postzygapophyses: absent (0); present in at least some postaxial cervical vertebrae (1).
138. Anterior spur-like projections on mid-cervical neural spines: absent (0); present (1).
139. Shape of mid-cervical neural spines: less than twice as long as high (0); at least twice as long as high (1).
140. Shape of cervical rib shafts: short and posteroventrally directed (0); longer than the length of their centra and extending parallel to cervical column (1).
141. Position of the base of the cervical rib shaft: level with, or higher than the ventral margin of the cervical centrum (0); located below the ventral margin due to a ventrally extended parapophysis (1).
142. Postzygodiapophyseal lamina in cervical neural arches 4–8: present (0); absent (1).
143. Laminae of the cervical neural arches 4–8: well-developed tall laminae (0); weakly developed low ridges (1).
144. Shape of anterior centrum face in cervical centra: concave (0); flat (1); convex (2). Ordered.
145. Ventral surface of the centra in the cervicodorsal transition: transversely rounded (0); with longitudinal keels (1).
146. Number of vertebrae between cervicodorsal transition and primordial sacral vertebrae: 15–16 (0); no more than 14 (1).
147. Lateral surfaces of the dorsal centra: with at most vague, shallow depressions (0); with deep fossae that approach the midline (1); with invasive, sharp-rimmed pleurocoels (2). Ordered.
148. Oblique ridge dividing pleural fossa of cervical vertebrae: absent (0); present (1).
149. Laterally expanded tables at the mid-length of the dorsal surface of the neural spines: absent in all vertebrae (0); present on the pectoral vertebrae (1); present on the pectoral and cervical vertebrae (2). Ordered.
150. Dorsal centra: entirely amphicoelous to amphiplatyan (1); first two dorsals are opisthocoelous (1); cranial half of dorsal column is opisthocoelous (2). Ordered.
151. Shape of the posterior dorsal centra: relatively elongated for their size (0); strongly axially compressed for their size (1).
152. Laminae bounding triangular infradiapophyseal fossae (chonae) on dorsal neural arches: absent (0); present (1).
153. Location of parapophysis in first two dorsals: at the anterior end of the centrum (0); located at the mid-length of the centrum, within the middle chonos (1).
154. Parapophyses of the dorsal column completely shift from the centrum to the neural arch: anterior to the 13th presacral vertebra (0); posterior to the 13th presacral vertebra (1).
155. Orientation of the transverse processes of the dorsal vertebrae: most horizontally directed (0); all upwardly directed (1).
156. Contribution of the paradiapophyseal lamina to the margin of the anterior chonos in mid-dorsal vertebrae: present (0); prevented by high placement of parapophysis (1).
157. Hyposphenes in the dorsal vertebrae: absent (0); present but less than the height of the neural canal (1); present and equal to the height of the neural canal (2). Ordered.
158. Prezygodiapophyseal lamina and associated anterior triangular fossa (anterior infradiapophyseal fossa): present on all dorsals (0); absent in mid-dorsals (1).
159. Anterior centroparapophyseal lamina in dorsal vertebrae: absent (0); present (1).
160. Prezygoparapophyseal lamina in dorsal vertebrae: absent (0); present (1).
161. Accessory lamina dividing posterior chonos from postzygapophysis: absent (0); present (1).
162. Pneumatic excavation of the dorsal neural arches: absent (0); equivocal (e.g. no more than depressions within the infradiapophyseal chambers) (1); sharp-rimmed subfossae or foramina clearly invading bone surface (2). Ordered.
163. Separation of lateral surfaces of anterior dorsal neural arches under transverse processes: widely spaced (0); only separated by a thin midline septum (1).
164. Height of dorsal neural arches, from neurocentral suture to level of zygapophyseal facets: much less than height of centrum (0); subequal to or greater than height of centrum (1).
165. Form of anterior surface of neural arch: simple centroprezygopophyseal ridge (0); broad anteriorly facing surface bounded laterally by centroprezygopophyseal lamina (1).
166. Shape of posterior dorsal neural canal: subcircular (0); slit-shaped (1).
167. Height of middle dorsal neural spines: less than the length of the base (0); higher than the length of the base but less than 1.5 times the length of the base (1); greater than 1.5 times the length of the base (2). Ordered.
168. Shape of anterior dorsal neural spines: lateral margins parallel in anterior view (0); transversely expanding towards dorsal end (2).
169. Cross-sectional shape of dorsal neural spines: transversely compressed (0); broad and triangular (1); square-shaped in posterior vertebrae (2). Ordered.
170. Spinodiapophyseal lamina on dorsal vertebrae: absent (0); present and separated from spinopostzygapophyseal lamina (1); present and joining spinopostzygapophyseal lamina to create a composite posterolateral spinal lamina (2). Ordered.
171. Well-developed, sheet-like suprapostzygapophyseal laminae: absent (0); present on at least the caudal dorsal vertebrae (2).
172. Shape of the spinopostzygapophyseal lamina in middle and posterior dorsal vertebrae: singular (0); bifurcated at its distal end.
173. Shape of posterior margin of middle dorsal neural spines in lateral view: approximately straight (0); concave with a projecting posterodorsal corner (1).
174. Transversely expanded plate-like summits of posterior dorsal neural spines: absent (0); present (1).
175. Last presacral rib: free (0); fused to vertebra (1).
176. Sacral rib much narrower than the transverse process of the first primordial sacral vertebra (and dorsosacral if present) in dorsal view: absent (0); present (1).
177. Number of dorsosacral vertebrae: none (0); one (1); two (2). Ordered.
178. Caudosacral vertebra: absent (0); present (1).
179. Shape of the iliac articular facets of the first primordial sacral rib: singular (0); divided into dorsal and ventral facets separated by a non-articulating gap (1).
180. Deep, medially directed pit excavating the surface of the non-articulating gap of the first primordial sacral rib: absent (0); present (1).
181. Depth of the iliac articular surface of the primordial sacrals: less than 0.75 of the depth of the ilium (0); greater than 0.75 of the depth of the ilium (1).
182. Sacral ribs contributing to the rim of the acetabulum: absent (0); present (1).
183. Posterior and anterior expansion of the transverse processes of the first and second primordial sacral vertebrae, respectively, partly roofing the intercostal space: absent (0); present (1).
184. Length of first caudal centrum: greater than its height (0); less than its height (1).
185. Position of postzygapophyses in proximal caudal vertebrae: protruding with an interpostzygapophyseal notch visible in dorsal view (0); placed on either side of the caudal end of the base of the neural spine without any interpostzygapophyseal notch (1).
186. A hyposphenal ridge on caudal vertebrae: absent (0); present (1).
187. Prezygadiapophyseal laminae on anterior caudals: absent (0); present (1).
188. Depth of the bases of the proximal caudal transverse processes: shallow, restricted to the neural arches (0); deep, extending from the centrum to the neural arch (1).
189. Position of last caudal vertebra with a protruding transverse process: distal to caudal 16 (0); proximal to caudal 16 (1).
190. Orientation of posterior margin of proximal caudal neural spines: sloping posterodorsally (0); vertical (1).
191. Longitudinal ventral sulcus on proximal and middle caudal vertebrae: present (0); absent (1).
192. Length of mid-caudal centra: greater than twice the height of their anterior faces (0); less than twice the height of their anterior faces (1).
193. Cross-sectional shape of the distal caudal centra: oval with rounded lateral and ventral sides (0); square-shaped with flattened lateral and ventral sides (1).
194. Length of distal caudal prezygapophyses: short, not overlapping the preceding centrum by more than a quarter (0); long and overlapping the preceding the centrum by more than a quarter (1).
195. Shape of the terminal caudal vertebrae: unfused, size decreasing towards tip (0); expanded and fused to form a club-shaped tail (1).
196. ‘Weaponized’ dermal spikes on tail: absent (0); present (1).
197. Length of the longest chevron: less than twice the length of the preceding centrum (0); greater than twice the length of the preceding centrum (1).
198. Anteroventral process on distal chevrons: absent (0); present (1).
199. Mid-caudal chevrons with a ventral slit: absent (0); present (1).
200. Longitudinal ridge on the dorsal surface of the sterna plate: absent (0); present (1).
201. Craniocaudal length of the acromion process of the scapula: less than 1.5 times the minimum width of the scapula blade (0); greater than 1.5 times the minimum width of the scapula blade (1).
202. Minimum width of the scapula: greater than 20% of its length (0); less than 20% of its length (1).
203. Caudal margin of the acromion process of the scapula: rises from the blade at angle that is less than 65° from the long axis of the scapula, at its steepest point (0); rises from the blade at angle that is greater than 65° from the long axis of the scapula, at its steepest point (1).
204. Width of dorsal expansion of the scapula: less than the width of the ventral end of the scapula (0); equal to the width of the ventral end of the scapula (1).
205. Flat caudoventrally facing surface on the coracoids between glenoid and coracoid tubercle: absent (0); present (1).
206. Coracoid tubercle: present (0); absent (1).
207. Length of the humerus: less than 55% of the length of the femur (0); 55–65% of the length of the femur (1); 65–70% of the length of the femur (2); more than 70% of the length of the femur (3). Ordered.
208. Shape of the humeral head: weakly developed, rounded in anterior–posterior view but minimally expanded perpendicular to the latter axis (0); flat in anterior–posterior view with only a slightly expanded lateral component (1); domed, being convex/hemispherical in anterior–posterior view with a strong lateral incursion onto the humeral shaft (2) (Unordered).
209. Shape of the deltopectoral crest: subtriangular (0); subrectangular (1).
210. Length of the deltopectoral crest of the humerus: less than 30% of the length of the humerus (0); 30–50% of the length of the humerus (1); greater than 50% of the length of the humerus (2). Ordered.
211. Shape of the anterolateral margin of the deltopectoral crest of the humerus: straight (0); strongly sinuous (1).
212. Rugose pit centrally located on the lateral surface of the deltopectoral crest: absent (0); present (1).
213. Well-defined fossa on the distal flexor surface of the humerus: present (0); absent (1).
214. Transverse width of the distal humerus: less than 33% of the length of the humerus (0); greater than 33% of the length of the humerus (1).
215. Shape of the entepicondyle of the distal humerus: rounded process (0): with a flat distomedially facing surface bounded by a sharp proximal margin (1).
216. Length of the radius: greater than 80% of the humerus (0); less than 80% of the humerus (1).
217. Deep radial fossa, bounded by an anterolateral process, on proximal ulna: absent (0); present (1).
218. Olecranon process on proximal ulna: present (0); absent (1); greatly enlarged olecranon (2).
219. Maximum linear dimensions of the ulnare and radiale: exceed that of at least one of the first three distal carpals (0); less than any of the distal carpals (1).
220. Transverse width of the first distal carpal: less than 120% of the transverse width of the second distal carpal (0); greater than 120% of the transverse width of the second distal carpal (1).
221. Sulcus across the medial end of the first distal carpal: absent (0); present (1).
222. Lateral end of first distal carpal: abuts second distal carpal (0); overlaps second distal carpal (1).
223. Second distal carpal: completely covers the proximal end of the second metacarpal (0); does not completely cover the proximal end of the second metacarpal (1).
224. Ossification of the fifth distal carpal: present (0); absent (1).
225. Length of the manus: less than 38% of the humerus + radius (0); 38–45% of the humerus + radius (1); greater than 45% of the humerus + radius (2). Ordered.
226. Shape of metacarpus: flattened to gently curved and spreading (0); a colonnade of subparallel metacarpals tightly curved into a U shape (1).
227. Proximal width of first metacarpal: less than the proximal width of the second metacarpal (0); greater than the proximal width of the second metacarpal (1).
228. Minimum transverse shaft width of first metacarpal: less than twice the minimum transverse shaft width of second metacarpal (0); greater than twice the minimum transverse shaft width of second metacarpal (1).
229. Proximal end of first metacarpal: flush with other metacarpals (0); inset into the carpus (1).
230. Shape of the first metacarpal: proximal width less than 65% of its length (0); proximal width 65–80% of its length (1); proximal width 80–100% of its length (2); greater than 100% of its length (3). Ordered.
231. Strong asymmetry in the lateral and medial distal condyles of the first metacarpal: absent (0); present (1).
232. Deep distal extensor pits on the second and third metacarpals: absent (0); present (1).
233. Shape of the distal ends of second and third metacarpals: subrectangular in distal view (0); trapezoidal with flexor rims of distal collateral ligament pits flaring beyond extensor rims (1).
234. Shape of the fifth metacarpal: longer than wide at the proximal end with a flat proximal surface (0); almost as wide as it is long with a strongly convex proximal articulation surface (1).
235. Length of the fifth metacarpal: less than 75% of the length of the third metacarpal (0); greater than 75% of the length of the third metacarpal (1).
236. Length of manual digit one: less than the length of manual digit two (0); greater than the length of manual digit two (1).
237. Ventrolateral twisting of the transverse axis of the distal end of the first phalanx of manual digit one relative to its proximal end: absent (0); present but much less than 60° (1); 60° (2). Ordered.
238. Length of the first phalanx of manual digit one: less than the length of the first metacarpal (0); greater than the length of the first metacarpal (1).
239. Shape of the proximal articular surface of the first phalanx of manual digit one: rounded (0); with an embayment on the medial side (1).
240. Shape of the first phalanx of manual digit one: elongate and subcylindrical (0); strongly proximodistally compressed and wedge-shaped (1).
241. Length of the penultimate phalanx of manual digit two: less than the length of the second metacarpal (0); greater than the length of the second metacarpal (1).
242. Length of the penultimate phalanx of manual digit three: less than the length of the third metacarpal (0); greater than the length of the third metacarpal (1).
243. Shape of non-terminal phalanges of manual digits two and three: longer than wide (0); as long as wide (1).
244. Shape of the unguals of manual digits two and three: straight (0); strongly curved with tips projecting well below flexor margin of proximal articular surface (1).
245. Length of the ungual of manual digit two: greater than the length of the ungual of manual digit one (0); 75–100% of the ungual of manual digit one (1); less than 75% of the ungual of manual digit one (2); the ungual of manual digit two is absent (3). Ordered.
246. Phalangeal formula of manual digits two and three: three and four, respectively (0); with at least one phalanx missing from each digit (1).
247. Phalangeal formula of manual digits four and five: greater than 2-0, respectively (0); less than 2-0, respectively (1).
248. Strongly convex dorsal margin of the ilium: absent (0); present (1).
249. Cranial extent of preacetabular process of ilium: does not project further anterior than the anterior margin of the pubic peduncle (0); projects anterior to the cranial margin of the pubic peduncle (1).
250. Shape of the preacetabular process: blunt and rectangular (0); with a pointed, projecting anteroventral corner and a rounded dorsum (1).
251. Depth of the preacetabular process of the ilium: much less than the depth of the ilium above the acetabulum (0); subequal to the depth of the ilium above the acetabulum (1).
252. Length of preacetabular process of the ilium: less than twice its depth (0); greater than twice its depth (1).
253. Buttress between preacetabular process and the supraacetabular crest of the ilium: present (0); absent (1).
254. Medial wall of acetabulum: fully closing acetabulum with a triangular ventral process between the pubic and ischial peduncles (0); partially open acetabulum with a straight ventral margin between the peduncles (1); partially open acetabulum with a concave ventral margin between the peduncles (2); fully open acetabulum with medial ventral margin closely approximating lateral rim of acetabulum (3). Ordered.
255. Length of the pubic peduncle of the ilium: less than twice the anteroposterior width of its distal end (0); greater than twice the anteroposterior width of its distal end.
256. Caudally projecting ‘heel’ at the distal end of the ischial peduncle: absent (0); present (1).
257. Length of the ischial peduncle of the ilium: similar to pubic peduncle (0); much shorter than pubic peduncle (1); virtually absent so that the chord connecting the distal end of the pubic peduncle with the ischial articular surface contacts the postacetabular process (2). Ordered.
258. Length of the postacetabular process of the ilium: between 40 and 100% of the distance between the pubic and ischial peduncles (0); less than 40% of the distance between the pubic and ischial peduncles (1); more than 100% of the distance between the pubic and ischial peduncles (2).
259. Well-developed brevis fossa with sharp margins on the ventral surface of the postacetabular process of the ilium: absent (0); present, ventrally facing (1); present, lateroventrally facing (2). 256; third state (2).
260. Anterior end of ventrolateral ridge bounding brevis fossa: not connected to supracetabular crest (0); joining supracetabular crest (1).
261. Shape of the caudal margin of the postacetabular process of the ilium: rounded to bluntly pointed (0); square ended (1); with a pointed ventral corner and a rounded caudodorsal margin (2).
262. Width of the conjoined pubes: less than 75% of their length (0); greater than 75% of their length (1).
263. Pubic tubercle on the lateral surface of the proximal pubis: present (0); absent (1).
264. Proximal anterior profile of pubis: anterior margin of pubic apron smoothly confluent with anterior margin of iliac pedicel (0); iliac pedicel set anterior to the pubic apron creating a prominent inflection in the proximal anterior profile of the pubis (1).
265. Minimum transverse width of the pubic apron: much more than 40% of the width across the iliac peduncles of the ilium (0); less than 40% of the width across the iliac peduncles of the ilium (1).
266. Position of the obturator foramen of the pubis: at least partially occluded by the iliac pedicel in anterior view (0); completely visible in anterior view (1).
267. Lateral margins of the pubic apron in anterior view: straight (0); concave (1).
268. Orientation of distal third of the blades of the pubic apron: confluent with the proximal part of the pubic apron (0); twisted posterolaterally relative to proximal section so that the anterior surface turns to face laterally (1).
269. Orientation of the entire blades of the pubic apron: transverse (0); twisted posteromedially (1).
270. Craniocaudal expansion of the distal pubis: absent (0); less than 15% of the length of the pubis (1); greater than 15% of the length of the pubis (2). Ordered.
271. Notch separating posteroventral end of the ischial obturator plate from the ischial shaft: present (0); absent (1).
272. Elongate interischial fenestra: absent (0); present (1).
273. Longitudinal dorsolateral sulcus on proximal ischium: absent (0); present (1).
274. Shape of distal ischium: broad and plate-like, not distinct from obturator region (0); with a discrete rod-like distal shaft (1).
275. Length of ischium: less than that of the pubis (0); greater than that of the pubis (1).
276. Ischial component of acetabular rim: larger than the pubic component (0); equal to the pubic component (1).
277. Shape of the transverse section of the ischial shaft: ovoid to subrectangular (0); triangular (1).
278. Orientation of the long axes of the transverse section of the distal ischia: meet at an angle (0); are coplanar (1).
279. Depth of the transverse section of the ischial shaft: much less than the transverse width of the section (0); at least as great as the transverse width of the section (1).
280. Distal ischial expansion: absent (0); present (1).
281. Transverse width of the conjoined distal ischial expansions: greater than their sagittal depth (0); less than their sagittal depth (1).
282. Length of the hindlimb: greater than the length of the trunk (0); less than the length of the trunk (1).
283. Longitudinal axis of the femur in lateral view: strongly bent with an offset between the proximal and distal axes greater than 15° (0); weakly bent with an offset of less than 10° (1); straight (2). Ordered.
284. Shape of the cross section of the mid-shaft of the femur: subcircular (0); strongly elliptical with the long axis orientated mediolaterally (1).
285. Angle between the long axis of the femoral head and the transverse axis of the distal femur: about 30° (0); close to 0° (1).
286. Shape of femoral head: roughly rectangular in profile with a sharp medial distal corner (0); roughly hemispherical with no sharp medial distal corner (1). This character only applies to taxa with a medially, or anteromedially protruding femoral head. It does not apply to outgroup taxa (Euparkeria or Crurotarsi) with proximally directed femoral heads and is coded as unknown in these taxa.
287. Posterior proximal tubercle on femur: well developed (0); indistinct to absent (1).
288. Shape of the lesser trochanter: small rounded tubercle (0); proximodistally orientated, elongate ridge (1); absent (2).
289. Position of proximal tip of lesser trochanter: level with the femoral head (0); distal to the femoral head (1).
290. Projection of the lesser trochanter: just a scar upon the femoral surface (0); a raised process (1).
291. Transverse ridge extending laterally from the lesser trochanter: absent (0); present (1).
292. Height of the lesser trochanter in cross section: less than its basal width (0); at least as high as its basal width (1).
293. Position of the lesser trochanter in anterior view: near the centre of the anterior face of the femoral shaft (0); close to the lateral margin of the femoral shaft (1).
294. Visibility of the lesser trochanter in posterior view: not visible (0); visible (1).
295. Height of the fourth trochanter: a low rugose ridge (0); a tall crest (1).
296. Position of the fourth trochanter along the length of the femur: in the proximal half (0); straddling the midpoint (1).
297. Symmetry of the profile of the fourth trochanter of the femur: subsymmetrical without a sharp distal corner (0); asymmetrical with a steeper distal slope than the proximal slope and a distinct distal corner (1).
298. Shape of the profile of the fourth trochanter of the femur: rounded (0); subrectangular (1).
299. Position of fourth trochanter along the mediolateral axis of the femur: centrally located (0); on the medial margin (1).
300. Extensor depression on anterior surface of the distal end of the femur: absent (0); present (1).
301. Size of the medial condyle of the distal femur: subequal to the fibular + lateral condyles (0); larger than the fibular + lateral condyles (1).
302. Well-developed tibiofibular crest on distal femur: absent (0); present (1).
303. Distal surface of tibiofibular crest: as deep anteroposteriorly as wide mediolaterally or deeper (0); wider mediolaterally than deep anteroposteriorly (1).
304. Tibia:femur length ratio: greater than 1.0 (0); between 0.6 and 1.0 (1); less than 0.6 (2). Ordered.
305. Orientation of cnemial crest: projects anteriorly to anterolaterally (0); projecting laterally (1).
306. Paramarginal ridge on lateral surface of cnemial crest: absent (0); present (1).
307. Position of the tallest point of the cnemial crest: close to the proximal end of the crest (0); about half-way along the length of the crest, creating an anterodorsally sloping proximal margin of the crest (1).
308. Proximal end of tibia with a flange of bone that contacts the fibula: absent (0): present (1).
309. Position of the posterior end of the fibular condyle on the proximal articular surface tibia: anterior to the posterior margin of the proximal articular surface (0); level with the posterior margin of the proximal articular surface (1).
310. Shape of the proximal articular surface of the tibia: transverse width subequal to anteroposterior length (0); transverse width between 0.6 and 0.9 times anteroposterior length (1); anteroposterior length twice the transverse width or higher (2). Ordered.
311. Transverse width of the distal tibia: subequal to its craniocaudal length (0); greater than its craniocaudal length (1).
312. Anteroposterior width of the lateral side of the distal articular surface of the tibia: as wide as the anteroposterior width of the medial side (0); narrower than the anteroposterior width of the medial side (1).
313. Relationship of the posterolateral process of the distal end of the tibia with the fibula: not flaring laterally and not making significant contact with the fibula (0); flaring laterally and backing the fibula (1).
314. Shape of the distal articular end of the tibia in distal view: ovoid (0); subrectangular (1).
315. Shape of the anteromedial corner of the distal articular surface of the tibia: forming a right angle (0); forming an acute angle (1).
316. Position of the lateral margin of descending caudoventral process of the distal end of the tibia: protrudes laterally at least as far as the anterolateral corner of the distal tibia (0); set well back from the anterolateral corner of the distal tibia (1).
317. A triangular rugose area on the medial side of the fibula: absent (0); present (1).
318. Transverse width of the mid-shaft of the fibula: greater than 0.75 of the transverse width of the mid-shaft of the tibia (0); between 0.5 and 0.75 of the transverse width of the mid-shaft of the tibia (1); less than 0.5 of the transverse width of the mid-shaft of the tibia (2). Ordered.
319. Position of fibula trochanter: on anterior surface of fibula (0); laterally facing (1); anteriorly facing but with strong lateral bulge (2).
320. Depth of the medial end of the astragalar body in cranial view: roughly equal to the lateral end (0); much shallower creating a wedge-shaped astragalar body (1).
321. Shape of the posteromedial margin of the astragalus in dorsal view: forming a moderately sharp corner of a subrectangular astragalus (0); evenly rounded without formation of a caudomedial corner (1).
322. Dorsally facing horizontal shelf forming part of the fibular facet of the astragalus: present (0); absent with a largely vertical fibular facet (1).
323. Pyramidal dorsal process on the posteromedial corner of the astragalus: absent (0); present (1).
324. Shape of the ascending process of the astragalus: anteroposteriorly deeper than transversely wide (0); transversely wider than anteroposteriorly deep (1).
325. Posterior extent of ascending process of the astragalus: positioned anteriorly upon the astragalus (0); close to the posterior margin of the astragalus (1).
326. Sharp medial margin around the depression posterior to the ascending process of the astragalus: absent (0); present (1).
327. Buttress dividing posterior fossa of astragalus and supporting ascending process: absent (0); present (1).
328. Vascular foramina set in a fossa at the base of the ascending process of the astragalus: present (0); absent (1).
329. Distal articular surface of astragalus: relatively flat or weakly convex (0); extremely convex and roller-shaped (1).
330. Transverse width of the calcaneum: greater than 30% of the transverse width of the astragalus (0); less than 30% of the transverse width of the astragalus (1).
331. Lateral surface of calcaneum: simple (0); with a fossa (1).
332. Medial peg of calcaneum fitting into astragalus: present, even if rudimentary (0); absent (1).
333. Calcaneal tuber: large and well developed (0); highly reduced to absent (1).
334. Shape of posteromedial heel of distal tarsal four (lateral distal tarsal): proximodistally deepest part of the bone (0); no deeper than the rest of the bone (1).
335. Shape of posteromedial process of distal tarsal four in proximal view: rounded (0); pointed (1).
336. Ossified distal tarsals: present (0); absent (1).
337. Proximal width of the first metatarsal: less than the proximal width of the second metatarsal (0); at least as great as the proximal width of the second metatarsal (1).
338. Size of first metatarsal: maximum proximal breadth less than 0.4 times its proximodistal length (0); maximum proximal breadth between 0.4 and 0.7 times its proximodistal length (1); maximum proximal breadth greater than 0.7 times its proximodistal length (2). Ordered.
339. Orientation of proximal articular surface of metatarsal one: horizontal (0); sloping proximolaterally relative to the long axis of the bone (1).
340. Shaft of metatarsal I: closely appressed to metatarsal II throughout its length (0); only closely appressed proximally, with a space between metatarsals I and II distally (1).
341. Orientation of the transverse axis of the distal end of metatarsal one: horizontal (0); angled proximomedially (1).
342. Shape of the medial margin of the proximal surface of the second metatarsal: straight (0); concave (1).
343. Shape of the lateral margin of the proximal surface of the second metatarsal: straight (0); concave (1).
344. Projection of ventral flange on proximal surface of second metatarsal: neither corner appreciably more developed than the other (0); laterally flaring (1); medially flaring (2).
345. Well-developed facet on proximolateral corner of plantar ventrolateral flange of metatarsal II for articulation with medial distal tarsal: absent (0); present (1).
346. Length of the third metatarsal: greater than 40% of the length of the tibia (0); less than 40% of the length of the tibia (1).
347. Proximal outline of metatarsal III: subtriangular with acute or rounded posterior border (0); subtrapezoidal, with posterior border broadly exposed in plantar view (1).
348. Minimum transverse shaft diameters of third and fourth metatarsals: greater than 60% of the minimum transverse shaft diameter of the second metatarsal (0); less than 60% of the minimum transverse shaft diameter of the second metatarsal (1).
349. Transverse width of the proximal end of the fourth metatarsal: less than twice the anteroposterior depth of the proximal end (0); at least twice the anteroposterior depth of the proximal end (1).
350. Angle formed by the anterior and anteromedial borders of metatarsal IV: obtuse (0); right angle, or acute (1).
351. Transverse width of the proximal end of the fifth metatarsal: less than 25% of the length of the fifth metatarsal (0); between 30 and 49% of the length of the fifth metatarsa (1); greater than 50% of the length of the fifth metatarsal (2). Ordered.
352. Transverse width of distal articular surface of metatarsal four in distal view: greater than the anteroposterior depth (0); less than the anteroposterior depth (1).
353. Pedal digit five: reduced, non-weight bearing (0); large (fifth metatarsal at least 70% of fourth metatarsal), robust and weight bearing (1).
354. Length of non-terminal pedal phalanges: all longer than wide (0); proximal-most phalanges longer than wide while more distal phalanges are as wide as long (1); all non-terminal phalanges are as wide, if not wider, than long (2). Ordered.
355. Length of the first phalanx of pedal digit one: greater than the length of the ungual of pedal digit one (0); less than the length of the ungual of pedal digit one (1).
356. Length of the ungual of pedal digit one: less than at least some non-terminal phalanges (0); longer than all non-terminal phalanges but shorter than first metatarsal (1); longer than the first metatarsal (2). Ordered.
357. Shape of the ungual of pedal digit one: shallow, pointed, with convex sides and a broad ventral surface (0); deep, abruptly tapering, with flattened sides and a narrow ventral surface (1).
358. Shape of proximal articular surface of pedal unguals: proximally facing, visible on medial and lateral sides (0); proximomedially facing and visible only in medial view, causing medial deflection of pedal unguals in articulation (1).
359. Penultimate phalanges of pedal digits two and three: well developed (0); reduced disc-shaped elements if they are ossified at all (1).
360. Shape of the unguals of pedal digits two and three: dorsoventrally deep with a proximal articulating surface that is at least as deep as it is wide (0); dorsoventrally flattened with a proximal articulating surface that is wider than deep (1).
361. Length of the ungual of pedal digit two: greater than the length of the ungual of pedal digit one (0); between 90 and 100% of the length of the ungual of pedal digit one (1); less than 90% of the length of the ungual of pedal digit one (2). Ordered.
362. Size of the ungual of pedal digit three: greater than 85% of the ungual of pedal digit two in all linear dimensions (0); less than 85% of the ungual of pedal digit two in all linear dimensions (1).
363. Number of phalanges in pedal digit four: four (0); fewer than four (1).
364. Phalanges of pedal digit five: present (0); absent (1).
365. Femoral length: less than 200 mm (0); between 200 and 399 mm (1); between 400 and 599 mm (2); between 600 and 799 mm (3); between 800 and 1000 mm (4); greater than 1000 mm (5). Ordered. EXCLUDED.
366. Laminae/ridges extending from the basipterygoid process onto the parasphenoid rostrum: extend parallel until they fade into the ventral margin of the cultriform process (0); converge anteromedially on the ventral surface of the cultriform process (1) (this study).
367. Angle between basipterygoid process and cultriform process of the parabasisphenoid: < 90° (0); 90° (1); > 90° (2) (this study – modified fromButler et al., 2008).
368. Length of the basisphenoid (from the basipterygoid process to the basisphenoidal component of the basal tubera) in relation to the length of the basioccipital (from the basioccipital component of the basal tubera to posterior limit of the condyle): longer or equal (0); shorter (1) (this study – modified fromButler et al., 2008).
369. Notch in the posterodorsal margin of the lateral portion of the parabasisphenoid: absent (0); present (1) (this study).
370. Number of foramina in the otoccipital between the exoccipital pillar (excluding the foramina for the hypoglossal nerve) posteriorly and fenestra ovalis anteriorly: one (0), two (1) (this study).
371. Unossified gap between the basioccipital and basisphenoidal component of the basal tubera and ventral ramus of the opistothic: absent (0); present (1) (this study – Modified fromGower, 2002; Yates & Kitching, 2003).