-
PDF
- Split View
-
Views
-
Cite
Cite
Per Ewing, Bo Blomgren, Åke Ryrfeldt, Per Gerde, Increasing Exposure Levels Cause an Abrupt Change in the Absorption and Metabolism of Acutely Inhaled Benzo(a)pyrene in the Isolated, Ventilated, and Perfused Lung of the Rat, Toxicological Sciences, Volume 91, Issue 2, June 2006, Pages 332–340, https://doi.org/10.1093/toxsci/kfj104
Close - Share Icon Share
Abstract
The carcinogenic polycyclic aromatic hydrocarbons (PAHs) are active primarily at the site of entry to the body. Lung cancer following inhalation of PAH-containing aerosols such as tobacco smoke is one likely example. A suggested mechanism for this site preference is a slow passage of the highly lipophilic PAHs through the thicker epithelia of the conducting airways, accompanied by substantial local metabolism in airway epithelium. However, it is likely that the airway epithelium will become saturated with PAHs at surprisingly low exposure levels. The purpose of this research was to quantify the level of saturation for inhaled benzo(a)pyrene (BaP) in the isolated, perfused lung (IPL) of the rat. BaP was coated onto carrier particles of silica 3.5 μm diameter at three different levels. The DustGun aerosol generator was then used to deliver respectively 2.2, 36, and 8400 ng of BaP to the IPL with the carrier particles in less than 1 min. For 77 min after the exposure, single-pass perfusate was collected from the lungs. Lungs were then removed and, with the perfusate, analyzed for BaP and metabolites. Results show that the absorption and metabolism of inhaled BaP in the lungs was highly dose dependent. At low exposure levels absorption of BaP in the mucosa was proportional to the concentration in the air/blood barrier and proceeded with substantial local metabolism. At higher exposure levels the capacity of the epithelium to dissolve and metabolize BaP became saturated, and the absorption rate remained constant until crystalline BaP had dissolved, and the process proceeded with much smaller fractions of BaP metabolites produced in the mucosa. This phenomenon may explain the well-known difficulties of inducing lung cancer in laboratory animals with inhalants containing carcinogenic PAHs, where similar lifespan exposures are used as humans may experience but with much higher dose rates.
The risk of lung cancer is elevated following exposures to several toxic inhalants, the most notable being tobacco smoke (Doll et al., 1994), and some occupational (Armstrong et al., 2004) and urban exposure atmospheres (Boström et al., 2002). In tobacco smoke most of the carcinogenic activity seems to be linked to the polycyclic aromatic hydrocarbons (PAHs) and the tobacco-specific nitrosamines (Hecht, 1999). While molecular and genetic evidence can be advanced against either substance group, inhalation exposures in laboratory animals generally fail to induce respiratory tract tumors at cumulative doses even much higher than those required to induce lung tumors in humans (Coggins, 2001). Thus, a coherent relation between these exposure scenarios would require a supralinear dose-response relationship in the low dose direction. Yet few if any mechanisms have been described to explain such a phenomenon. However, in the present paper we will describe a mechanism by which a steeper, albeit saturable exposure–target dose relationship in the airway epithelium at low exposure levels may explain such nonlinear kinetics.
Lung cancer is a disease of the site of entry of inhaled toxicants in the lungs. The neoplasms arise in the epithelial cells directly facing the inhaled carcinogens. Inhaled toxicants may therefore induce the disease, either (1) first-pass while being absorbed through the epithelium to the circulating blood in the capillaries of the subepithelium, or (2) in a second exposure following the distribution of the toxicants with the systemic circulation (Gerde and Scott, 2001). While the systemic exposure level can be easily assessed through a blood sample, the first-pass component of exposure is much more difficult to measure and can be considerably higher than the systemic levels. Our research aims at describing the mechanisms underlying the first-pass component of exposure for general classes of soluble inhalants. Previous work has shown that, whereas less lipophilic carcinogens, such as the nitrosamines, absorb rapidly from all regions of the respiratory tract (Gerde et al., 1998a), highly lipophilic carcinogens, such as the PAHs, absorb rapidly from the alveolar type I epithelium (Gerde et al., 1993b) but much slower from other airway regions (Gerde et al., 1997). During the slow absorption from the tracheobronchial region, the highly lipophilic carcinogens distribute only within the first few cell layers encountered, which is the epithelium. The result is a selective exposure of the cell layers where most lung cancers are thought to originate (Marchevsky, 1990). The relation between the rate of deposition of soluble inhalants and local concentration in epithelial cells is fundamental to the understanding of the overall dose response, particularly for known site-of-entry toxicants such as the PAHs. One important consequence of the low mobility of lipophilic toxicants in tissues is a likely saturation of the tracheobronchial epithelium at relatively low exposure levels (Gerde et al., 1991). The saturation will include metabolic saturation, which will limit the amount of activated metabolites generated, and a physicochemical saturation, which will limit the amount of soluble substrate at all available for metabolic activation. The most profound consequence of saturation in the airway mucosa is that the relative role of the lungs in activating inhaled procarcinogens will begin to decrease with increasing dose rates above saturation. In contrast, the liver is likely to maintain its metabolic capacity for the systemically distributed component of inhaled procarcinogens to very high exposure levels (Monteith et al., 1987; Wiersma and Roth, 1983). Because it can be suspected that most experiments to induce lung cancer in laboratory animals are made above the likely saturation interval for the airway mucosa, it is critically important to understand the mechanisms of saturation in the airway mucosa in order to improve the risk assessment for inhaled PAHs.
The isolated, ventilated, and perfused lung of rodents (IPL) has been used to study the disposition and metabolism of toxicants as well as pharmaceutical agents in the lungs (Ryrfeldt and Nilsson, 1977). The studied agents including BaP have been added either via the circulation, by intratracheal instillation (Bond et al., 1988), or as nebulized suspensions (Tronde et al., 2002). Few options have been available for exposing the IPL to dry powder aerosols in a controlled manner. However, recently we have developed a new aerosol generator based on the DustGun technology which can expose the IPL to respirable dry powder aerosols (Gerde et al., 2004). The combined system has two advantages that make it suitable for studying lung-specific effects of inhaled procarcinogens: (1) A complete control of the mass balance over the lungs allows accurate determination of toxicant disposition. (2) In the isolated and perfused rat lung lies an opportunity to study the endogenous capacity of the lung to metabolize PAHs without any interference from liver metabolism. Little is known about the capacity of the lung to bioactivate PAHs at the site of entry at very different exposure levels.
We sought to answer the following questions regarding metabolic activation of BaP at widely different exposure levels: (1) What is the relation between solute absorption, tissue concentration, and rate of metabolic activation of BaP at very different substrate concentrations? (2) Is it possible to identify the local limit of physicochemical saturation in the airway mucosa? (3) What are the consequences of physicochemical as well as metabolic saturation in the airway mucosa? Should there be a reappraisal of the method of high-to-low dose extrapolation for inhaled, highly lipophilic toxicants?
MATERIAL AND METHODS
Experimental design.
Inhalation exposures to typical polydisperse carrier aerosols of soluble inhalants give rise to a wide distribution of parallel absorption and disposition processes in the pulmonary air/blood barrier that preclude detailed observation of the absorption kinetics in typical target regions of the respiratory tract. We therefore undertook several steps to allow study of the kinetics of absorption and saturation in a sufficiently uniform segment of the air/blood barrier. This was done by exposing the lungs to a short-duration bolus of a highly concentrated aerosol of uniform particle size. Four conditions for the experiments were desired:
A high degree of deposition in the metabolically active epithelium of the peripheral bronchi and bronchioles.
An inhalation exposure with uniformly sized carrier particles, but with varying amounts of BaP adsorbed or precipitated.
The BaP should be readily available for desorption to reveal resistance to diffusion within air/blood barrier rather than within the carrier particles.
Particles should be deposited sufficiently scattered to minimize interference between deposited particles.
The DustGun dry powder generator was used to deagglomerate and aerosolize a fine powder of silica coated with BaP at three different levels (Table 1). The IPL of the rat was exposed for approximately 1 min to the aerosolized BaP/silica particles. Single-pass perfusate was collected and fractionated for 77 min after exposure. Immediately after the perfusate collection period, lungs and perfusate were frozen and stored for later analysis of BaP and metabolites.
Some Critical Properties of the Exposures for Studying the Deposition and Disposition of BaP in the Lungs
Parameter/exposure level of BaP . | Low . | Medium . | High . |
|---|---|---|---|
| Amount of BaP on coated silica powder (mg/g) | 0.096 ± 0.002 | 1.30 ± 0.06 | 167 ± 2 |
| Amount of BaP per silica particle (fg) | 3.4 | 45 | 3500 |
| Percent of initial powder load on end filter | 6.8 | 12.6 | 30.3 |
| Powder concentration in aerosol (μg/l) | 680 | 1300 | 3000 |
| Theoretical deposition of powder in lung (μg) | 12.7 | 22.2 | 53.2 |
| Measured deposition of powder in lung (μg) | 23 ± 4 | 27 ± 3 | 53 ± 5 |
| Measured/theoretical deposition | 1.81 | 1.22 | 1.00 |
| Number of deposited particles in the lungs | 610,000 | 790,000 | 2,400,000 |
| Initial deposition of BaP in lung (ng) | 2.2 ± 0.4 | 36 ± 4 | 8400 ± 900 |
Parameter/exposure level of BaP . | Low . | Medium . | High . |
|---|---|---|---|
| Amount of BaP on coated silica powder (mg/g) | 0.096 ± 0.002 | 1.30 ± 0.06 | 167 ± 2 |
| Amount of BaP per silica particle (fg) | 3.4 | 45 | 3500 |
| Percent of initial powder load on end filter | 6.8 | 12.6 | 30.3 |
| Powder concentration in aerosol (μg/l) | 680 | 1300 | 3000 |
| Theoretical deposition of powder in lung (μg) | 12.7 | 22.2 | 53.2 |
| Measured deposition of powder in lung (μg) | 23 ± 4 | 27 ± 3 | 53 ± 5 |
| Measured/theoretical deposition | 1.81 | 1.22 | 1.00 |
| Number of deposited particles in the lungs | 610,000 | 790,000 | 2,400,000 |
| Initial deposition of BaP in lung (ng) | 2.2 ± 0.4 | 36 ± 4 | 8400 ± 900 |
Note. The lungs were each exposed to a loaded amount in the aerosol generator of 3.02 mg powder.
Some Critical Properties of the Exposures for Studying the Deposition and Disposition of BaP in the Lungs
Parameter/exposure level of BaP . | Low . | Medium . | High . |
|---|---|---|---|
| Amount of BaP on coated silica powder (mg/g) | 0.096 ± 0.002 | 1.30 ± 0.06 | 167 ± 2 |
| Amount of BaP per silica particle (fg) | 3.4 | 45 | 3500 |
| Percent of initial powder load on end filter | 6.8 | 12.6 | 30.3 |
| Powder concentration in aerosol (μg/l) | 680 | 1300 | 3000 |
| Theoretical deposition of powder in lung (μg) | 12.7 | 22.2 | 53.2 |
| Measured deposition of powder in lung (μg) | 23 ± 4 | 27 ± 3 | 53 ± 5 |
| Measured/theoretical deposition | 1.81 | 1.22 | 1.00 |
| Number of deposited particles in the lungs | 610,000 | 790,000 | 2,400,000 |
| Initial deposition of BaP in lung (ng) | 2.2 ± 0.4 | 36 ± 4 | 8400 ± 900 |
Parameter/exposure level of BaP . | Low . | Medium . | High . |
|---|---|---|---|
| Amount of BaP on coated silica powder (mg/g) | 0.096 ± 0.002 | 1.30 ± 0.06 | 167 ± 2 |
| Amount of BaP per silica particle (fg) | 3.4 | 45 | 3500 |
| Percent of initial powder load on end filter | 6.8 | 12.6 | 30.3 |
| Powder concentration in aerosol (μg/l) | 680 | 1300 | 3000 |
| Theoretical deposition of powder in lung (μg) | 12.7 | 22.2 | 53.2 |
| Measured deposition of powder in lung (μg) | 23 ± 4 | 27 ± 3 | 53 ± 5 |
| Measured/theoretical deposition | 1.81 | 1.22 | 1.00 |
| Number of deposited particles in the lungs | 610,000 | 790,000 | 2,400,000 |
| Initial deposition of BaP in lung (ng) | 2.2 ± 0.4 | 36 ± 4 | 8400 ± 900 |
Note. The lungs were each exposed to a loaded amount in the aerosol generator of 3.02 mg powder.
Preparation and characterization of the exposure particles.
Silica particles were chosen as carriers for the BaP into the lungs due to their inert properties and weak adsorptive binding of the hydrocarbon. The Waters Spherisorb C1, 3μm (Milford, MA) was found particularly suitable with a rather high internal surface area provided by pores of 80 A diameter. These particles are available with different covalently bound surface groups that allow a suitable polarity of the surface to be chosen. Tritium-labeled B(a)P in toluene solution was used (463.3 dpm/pg uniformly labeled, TRK 662 from batches 0.8× SP2 + 0.2× B99A, 98% purity; Amersham). For the two highest coating levels the labeled BaP was diluted with unlabeled BaP (SIGMA). BaP in toluene solution and silica powder were transferred and mixed in a glass ampoule. After evaporation to dryness with argon gas the ampoule was sealed under a reduced-argon atmosphere. The ampoule was heated for 5 h at 260°C to allow the BaP to distribute evenly over the silica surface. The BaP/silica powder was tested for three critical properties: (1) The concentration of BaP on the silica was measured by extracting triplicate portions of the powder in excess toluene followed by liquid scintillation counting (LSC) (Table 1). (2) The purity of the particle-associated BaP after the preparation procedure was measured in the toluene extract using HPLC. For all three preparations the purity was found to be >94%. (3) The simulated bioavailability in lung surfactant was measured in vitro in 1-n-octanol as previously described (Gerde et al., 2001). About 100 mg of the BaP/silica powder was added to a stirred reactor containing 17 ml 1-octanol at 37°C. Samples of the stirred suspension were repeatedly withdrawn for measuring the fraction of BaP released from the particles as a function of time.
The aerosols were generated with the DustGun dry powder generator as previously described in detail (Gerde et al., 2004). The particle size distribution of the aerosolized silica particles was measured with a MOUDI model 110 cascade impactor (MSP Corp., Minneapolis, MN). Three mg silica particles were loaded to the powder chamber of the dry powder generator. The mass mean aerodynamic diameter (MMAD) was measured to be 3.5 μm with a geometric standard deviation of 1.73 (Gerde et al., 2004).
Exposure of the isolated and perfused rat lung to silica particles.
Ten female Sprague-Dawley rats weighing 325 ± 19 g (SD, n = 10) were used in this experiment. The protocol was approved by the local ethics committee (Stockholms Norra Djurförsöksetiska Nämnd). The surgical procedure and lung mechanical measurements were performed as described in detail elsewhere (Gerde et al., 2004). Briefly, each animal was anesthetized by injecting pentobarbital intraperitoneally (40–50 mg/kg, Mebumal Vet. Nordvacc, Stockholm, Sweden), and the chest was opened. A tracheotomy was performed, and the lungs and heart were excised and placed in a well-humidified, artificial thoracic chamber. The lungs were perfused with Krebs-Ringer bicarbonate buffer at 37°C and ventilated at 75 breaths/min by creating an alternating negative pressure. The tracheal airflow was measured with a heated pneumotachograph, and the thoracic pressure changes were monitored with a pressure sensor. The following data were sampled by an EMKA lung monitoring system: lung conductance (Gaw), dynamic compliance (Cdyn), and tidal volume (TV). The lungs were allowed to stabilize for 20 min with recirculating perfusion of buffer. Only lung preparations with the following stable baseline values were used: perfusate flow rate, 19.8 ± 3.2 ml/min; Gaw, 69.3 ± 14.5 ml/s/kPa; Cdyn, 3.2 ± 0.5 ml/kPa; and TV, 1.13 ± 0.11 ml (SD, n = 9). The average weight of the lungs after the perfusate collection period was 1.73 ± 0.13 g (SD, n = 9).
The aerosol generator was integrated into an exposure system where the IPL of the rat, perfused by an albumin buffer, was exposed to a bolus of aerosolized silica for approximately 1 min. The exposure aerosol was passed over the tracheal catheter of the IPL using an exposure line without non-rebreathing valves. Rebreathing of exhausted exposure atmosphere was prevented by maintaining a constant total flow rate of 430 ml/min downstream of the lungs. The negative pressure driving the exposure flow was obtained by use of a precision-controlled vacuum source, and all particles exiting or passing the lungs in the exposure stream were collected on a total filter (Whatman GF/F, 25 mm) immediately downstream the lungs. The deposition of aerosol on the filter was measured either gravimetrically or by transferring the filter to 10 ml toluene, vortexing, and shaking vigorously before 0.5 ml toluene was counted using LSC.
Two types of exposures were done: one deposition exposure, and the main series of absorption exposures. During the deposition experiment the lungs were perfused with recirculating Krebs-Ringer buffer for 20 min in order to establish baseline values. The IPL was then exposed in rapid succession to 10 × 4 mg loaded portions of the pure silica carrier particles in repetitive minute-long exposure cycles. The cumulative amount of silica in the exposure air stream collected on one total filter downstream of the lungs was 4 mg. This level of deposition was more than 10 times that of the absorption experiments and was used to obtain a sufficient number of particles counted in stereological estimates for quantitation of both the total number of carrier particles and the ratio of bronchial versus alveolar deposition. Immediately following the last exposure cycle, the lungs were perfused with a Krebs-Ringer buffer with 2% albumin 4% w/v of formaldehyde with single-pass perfusion, and the experiment was stopped. The lungs were then stored in 4% w/v of formaldehyde until they were prepared for determination of the regional deposition of silica particles.
During the absorption experiments the DustGun generator was loaded with 3.02 ± 0.06 mg powder (SD, n = 9). Three rats were used for each exposure level. The pneumotachograph was removed, and the dry powder aerosol generator was connected directly to the tracheal catheter of the IPL. The aerosol generation cycle was initiated as previously described (Gerde et al., 2004), and the exposure branch of the generator manifold was connected for 2 min. The perfusate sampling scheme was initiated 15 sec before the aerosol generation cycle to obtain two preexposure control samples of the perfusate. For determination of total BaP-equivalent activity (BaP-eq), the single-pass perfusate was then sampled in two regimens; in the first regimen, 5-s samples were taken every 15 s for 13 min and in the second regimen, 5-s samples were taken every 73 s for 64 min, altogether 77 min of perfusion. Between the sampling periods the perfusate was passed to drain. For determination of the gross pattern of metabolites, additional 10-ml samples of the perfusate were taken at 2, 30, and 75 min. These samples were stored under argon at −80°C. Immediately after the perfusate sampling period, the experiment was stopped, and all perfusate samples were weighed. The left lung lobe was weighed wet, then dried overnight at 80°C and weighed again for determination of dry to wet weight ratio. The right caudal lobe was used for assessing the distribution of the BaP-associated activity between its major metabolite groups and was stored at −80°C under argon atmosphere.
Deposition of carrier particles.
- \({\hat{V}}\)= Estimated total lung volume
T = thickness of the slab
a/p = area per grid point
Pi = intersections of points per slab
Three slabs were randomly chosen for vertical uniform random sampling and processing of lung tissue cores. On slabs 1, 4, and 7 out of 8, a plastic holegrid frame was thrown. The holes in the frame were 3 mm in diameter. For every hole falling over lung tissue, the tissue core was sampled using a biopsy punch instrument. Two cores each were sampled from slabs 1 and 4, and one core was sampled from slab 7. The lung tissue cores were subsequently placed in cassettes for processing to wax blocks. The tissue cores were randomly rotated in the cassette, a prerequisite for the generation of vertical uniform random samples. The wax blocks containing the tissue cores were sectioned at a nominal thickness of 5 μm. The specimens were dehydrated and stained with hematoxylin and eosin.
- \({\hat{E}}\)= Estimated deposited silica particles in lung tissue
- \({\hat{V}}\)= Estimated total lung volume
Vs = Volume of the specimen
The total lung volume was estimated to be 4.6 cm3. It was assumed that the density of deposition in the deposition experiment exceeded that of the absorption experiment in proportion to the exposure dose of the two experiments.
Total radioactivity in perfusate and tissues.
The small-volume samples of perfusate was counted using LSC, and the concentration of BaP-eq was calculated. Clearance of BaP in each sampling interval was calculated by scaling the amount of BaP in each vial with the ratio of the time required for each sample to the total time lapsed between the beginning of this sample and the next. The sum of all fractions cleared from interval 1 to 108 gave total clearance with the perfusate.
The dissected and dried lung lobes were combusted using a platinum catalyst to obtain the total radioactivity as tritiated water collected in a liquid nitrogen cold trap (Gerde et al., 1998b). The radioactive water was extracted from the cold trap with UltimaGold liquid scintillation cocktail and counted (Packard Tricarb 2100-TR, Meridien, CT). The amount of BaP-eq deposited in the lung tissues was calculated from the specific activity of each used mixture of labeled/unlabeled BaP. The right caudal lobe was used both for extraction of metabolites and for determination of the total amount of B(a)P. The tissue was cut into 17–20 pieces. One half was aliquoted for drying and combustion, and the measured total radioactivity was then scaled to the total weight of the lobe. The remaining aliquot was prepared for separation of metabolites using HPLC. After the total amount of BaP-eq in lungs and perfusate was determined, the initial deposited dose of the exposure was obtained from the sum of the two measurements.
Determination of metabolism.
The metabolic composition of BaP in the perfusate and tissues was analyzed by organic extraction followed by separation using HPLC (Scott et al., 1998). Five ml perfusate was extracted five times with 5 ml ethyl acetate, and the organic phase was separated and pooled. A 1-ml triplicate of the aqueous phase was counted using LSC, and this fraction was assumed to contain the phase-2 metabolites. The organic phase containing mostly parent compound and the phase-1 metabolites was dried under a stream of nitrogen and redissolved and stored in methanol until HPLC analysis. The lung tissue was ground to a fine powder in liquid nitrogen and extracted five times in 5 ml physiological saline 0.9% and 5 ml ethyl acetate. A 1-ml triplicate sample of the aqueous phase was counted, and this fraction consisted of the phase-2 metabolites. The pellet was dried, combusted, and counted, and this fraction constituted the protein-bound BaP-eq. The pooled organic phase was dried under a stream of nitrogen and redissolved in methanol. BaP and metabolites in all samples were separated using a Beckman Ultrasphere C18 column (5 μm × 4.6 mm × 25 mm) eluted with a methanol/water gradient from 55 to 100% (v/v) together with coeluting metabolite standards from National Cancer Institute (Kansas City, MO). The total recovery of radioactivity from the samples was better than 95% for the perfusate samples and 67 ± 13 % for the lung tissue samples. The lower recovery of radioactivity from the lung samples was caused by the difficulty of completely separating BaP and its lipophilic metabolites from a soluble fraction of endogenous fat (Scott et al., 1998).
RESULTS
Deposition of the Carrier Particles
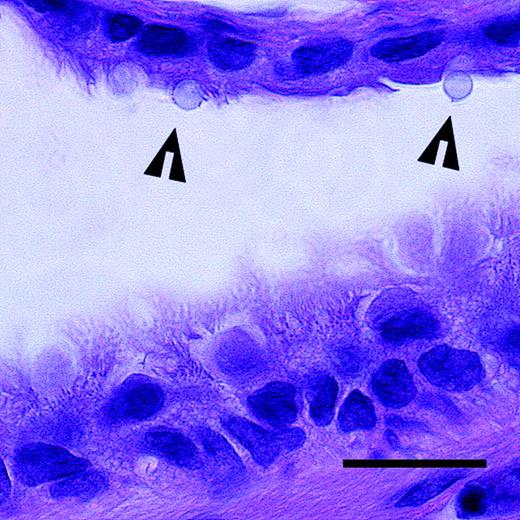
The exposure set up allowed a detailed study of the absorption and metabolism of BaP in the bronchial/bronchiolar airways of the rat. With the particle size chosen, the silica particles were expected to have a high fractional deposition in the peripheral bronchi. The particle deposition study showed that about 4.1 million carrier particles were deposited in the lungs following ten exposure cycles, which would correspond to 410,000 particles per exposure cycle. The microscopic analysis showed that more than 90% of the deposited particles were found in the bronchi/bronchioli, with the rest in the alveolar region. This value for bronchial/bronchiolar deposition was markedly higher than theoretical data on airway deposition. For the used particle size, a typical deposition in the airways of the rat is given as 70% versus 30% alveolar deposition (Hofmann et al., 2000). The most likely explanation for the difference is the effect of electrostatic charge on the generated aerosols in the present study. By induction of mirror charges on the airway walls (Hashish et al., 1994), electrostatic charges on particles would tend to favor deposition in the most narrow passages of the bronchioles. The influence of the electrostatic charge is also seen in the relatively high losses of aerosol between the holding chamber and the end filter downstream of the lungs (Table 1). However, increasing coating of BaP on the silica particles in the absorption study reduced losses and increased the aerosol yield on the end filter as well as in the rat lungs (Table 1). This is probably explained by the less polar properties of crystalline BaP covering the particles at the higher exposure levels. One great advantage with the unusually high deposition in the bronchi was the less complex absorption curves that were easier to interpret. Further contributing to this fact was that, compared to the ten-fold higher exposures used in the deposition study, particles were more likely to be found lodged in the walls of the smaller airways independent of each other (Fig. 1). Once deposited on the airway walls, the model studies of bioavailability in 1-n-octanol in vitro indicated that >85% of the BaP on the silica particles was released within 5 min.
A section of a small airway in the rat lung with silica particles (arrowheads) deposited on the epithelium. The epithelium in the upper part of the image consists of a single ciliated cell layer. The epithelium in the lower part of the image is pseudostratified. Hematoxylin and eosin. Scale bar = 20 μm.
Desorption and Disposition of BaP
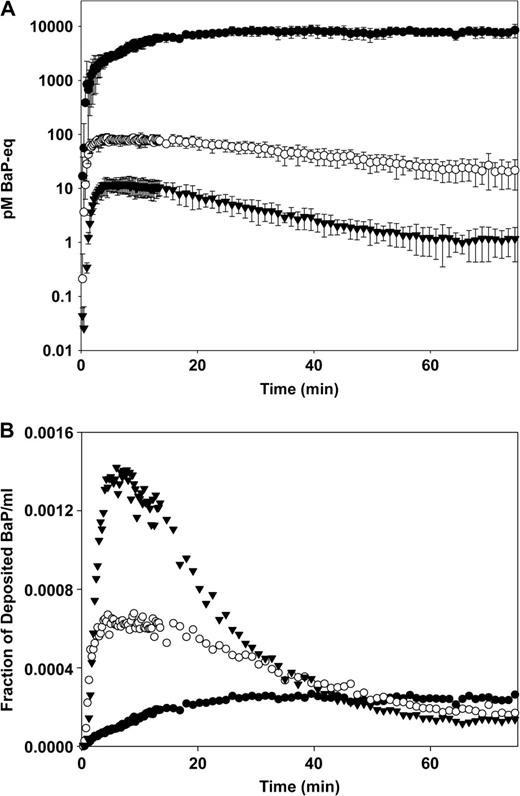
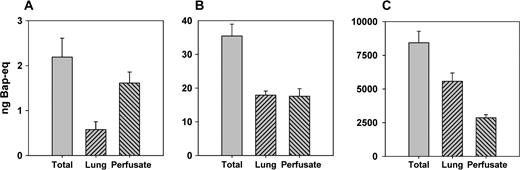
BaP was deposited in the IPL of the rat at three exposure levels ranging almost 4000 times between the low level at 2.2 ng and high level at 8400 ng (Table 1). At all three exposure levels BaP-equivalent activity appeared rapidly in the perfusate (Fig. 2A). However, the onset of physicochemical saturation was indicated both by the levels of BaP in the perfusate and by the fraction of BaP retained in the lungs after the perfusate collection period. At the low exposure level absorption to the perfusate peaked at 3.7 ± 0.4 min (SD, n = 3) and was then a first-order absorption process (Fig. 2B). At the medium exposure level absorption to the perfusate reached a plateau after 3.4 ± 1.4 min (SD, n = 3) that lasted for about 12 min and was then followed by a first-order absorption process. The plateau in the perfusate concentration curve of the medium level exposure is a strong indication that physicochemical saturation was briefly reached in the immediate vicinity of the silica particles at this exposure level (Gerde et al., 1991). At the high exposure level, physicochemical saturation totally dominated the absorption process changing this into a zero-order process for the last two thirds of the experiment. With increasing exposures there was a decrease in the cumulative fraction of BaP-eq that cleared with the perfusate and a corresponding increase in the fraction that was retained in the lungs (Fig. 3).
(A) The concentration of BaP-eq activity (pM) in the perfusate as a function of time. Error bars show standard deviation of the mean, n = 3. (B) The concentration of BaP-eq activity in the perfusate expressed as fraction of the total initial deposition of BaP in the lungs per mL perfusate. Low dose (▾), medium dose (○) and high dose (•).
The total mass balance of BaP-eq over lungs and perfusate (SD, n = 3). (A) Low dose, (B) medium dose, and (C) high dose.
Metabolism
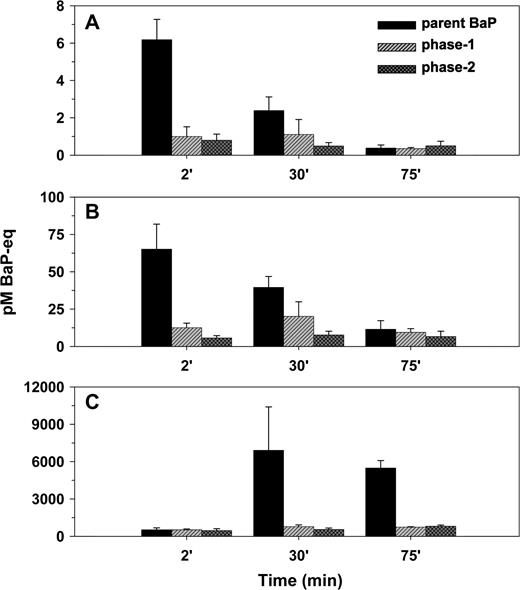
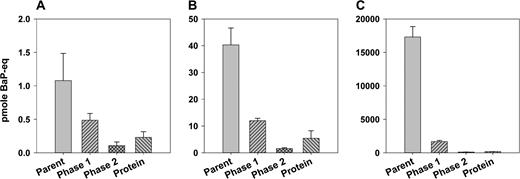
The rate of metabolism of BaP in the lungs increased with increasing exposure levels, but by far not as rapidly as the exposures. When expressed as fraction of the amount of BaP deposited, the rate of metabolism lagged behind the increasing exposure levels in the tissue-retained fraction as well as in the BaP-equivalent activity leaving the lungs with the perfusate (Fig. 4). However, at the early 2-min time point of the high-exposure experiment, we detected a brief pulse of metabolites in the perfusate just before the major breakthrough of parent BaP (Fig. 4). Then after 75 min, the parent compound totally dominated the fraction retained in the mucosa. One part of this retained fraction consisted of BaP dissolved in the tissues, and the other part was lingering as crystalline BaP on the carrier particles. The crystalline BaP was visible through the lung parenchyma as a characteristic yellow-green fluorescence (Lakowicz and Hylden, 1978) in the lung lobes upon visual inspection under UV light. The decreasing relative role of lung metabolism with increasing exposures was also evident from the general level of protein adduction in lung tissues. While the deposited dose increased 4000 times between the low and the high exposure level, the tissue/protein bound fraction only increased with a factor of 800 (Fig. 5). This is a substantial deviation, most of which would have been likely to remain even by the time the entire soluble fraction of BaP had cleared from the lungs of both exposure groups. At the 75-min time point, the metabolic pattern in the perfusate can be compared with that of the tissues (Fig. 5). It was evident that on average the metabolite mix leaving the lungs in the perfusate was much more polar than the fraction remaining in the lungs.
The distribution of BaP-equivalent activity between parent compound, phase-1 metabolites, and phase-2 metabolites in the perfusate as a function of time (SD, n = 3). (A) Low dose, (B) medium dose, and (C) high dose. Note the increasing scale of the y-axis from panel A to C.
The distribution of BaP-equivalent activity between parent compound, phase-1 metabolites, phase-2 metabolites, and the protein-bound fraction in lung tissue at the end of the perfusate collection period (SD, n = 3). (A) Low dose, (B) medium dose, and (C) high dose. Note the increasing scale of the y-axis from panel A to C.
DISCUSSION
The inhaled carcinogen BaP changes its kinetics of absorption at fairly low exposure levels. At low exposure levels absorption of BaP in the airway mucosa is essentially a first-order process accompanied by a substantial metabolic conversion of the carcinogen on its route to the capillary bed of the subepithelium. The metabolic conversion rate at the low exposure level of the present study is similar to that measured in the dog at low exposure levels (Gerde et al., 1997). When increasing exposure levels reach saturation in the epithelial cells nearest to the carrier particles, absorption through the local airway segment abruptly switches to a zero-order process (Fig. 2B). At exposure levels substantially exceeding saturation, only a small fraction of the absorbed BaP was locally metabolized in the mucosa.
The measured kinetics of absorption lends substantial additional support to the previously described mechanism of diffusion-limited absorption of highly lipophilic solutes in the tracheobronchial mucosa (Gerde and Scott, 2001). The driving mechanism behind this process is a much reduced apparent diffusivity in tissues brought about by the ready partitioning of the solute into the lipid membranes of the first cell layer encountered, that is, the epithelium. Transport into the next cell layer is greatly reduced because of the low concentration of solute available for diffusion in the aqueous layers between the cells. The fundamental nature of this absorption mechanism is further indicated by comparison with previous data from the dog. For subsaturation exposures, the absorption half-times of BaP correlate well with air/blood barrier thicknesses between species as different as the rat and the dog (Table 2). Most likely the mechanism is identical in humans too.
First Half-Time of Absorption of BaP in Different Air/Blood Barrier Types of the Rat and Dog following Brief, Low-Level Exposures
Air/blood barrier type . | Approximate thickness (μm) . | Absorption half-time (min) . |
|---|---|---|
| Dog alveolar type-Ia | 1–2 | 2 |
| Rat bronchiolesb | 4–8 | 12 |
| Dog tracheac | 20–30 | 70 |
Air/blood barrier type . | Approximate thickness (μm) . | Absorption half-time (min) . |
|---|---|---|
| Dog alveolar type-Ia | 1–2 | 2 |
| Rat bronchiolesb | 4–8 | 12 |
| Dog tracheac | 20–30 | 70 |
Data from (Gerde et al., 1993a).
Data from the low exposure level of the present study.
Data from (Gerde et al., 1997).
First Half-Time of Absorption of BaP in Different Air/Blood Barrier Types of the Rat and Dog following Brief, Low-Level Exposures
Air/blood barrier type . | Approximate thickness (μm) . | Absorption half-time (min) . |
|---|---|---|
| Dog alveolar type-Ia | 1–2 | 2 |
| Rat bronchiolesb | 4–8 | 12 |
| Dog tracheac | 20–30 | 70 |
Air/blood barrier type . | Approximate thickness (μm) . | Absorption half-time (min) . |
|---|---|---|
| Dog alveolar type-Ia | 1–2 | 2 |
| Rat bronchiolesb | 4–8 | 12 |
| Dog tracheac | 20–30 | 70 |
Data from (Gerde et al., 1993a).
Data from the low exposure level of the present study.
Data from (Gerde et al., 1997).
A first consequence of this exposure mechanism is a highly elevated local dose to the tracheobronchial epithelium at low exposure levels. This central role of local dose to the entrance epithelium for highly lipophilic toxicants may explain the typical greater elevation of cancer incidence in the respiratory tract epithelium than in other tissues following inhalation of aerosols containing highly lipophilic carcinogens such as PAHs (Thyssen et al., 1981). Recently, further evidence has been published indicating that this localized dosimetry also manifest itself in intermediary risk indicators such as DNA adducts. It has been demonstrated that the levels of BaP-related DNA adducts are highly elevated in the airway epithelium of smokers, but not in their lung parenchyma or deeper-lying airway tissues (Rojas et al., 2004). An obvious assumption is that the reactive metabolites of PAHs producing these DNA adducts were generated locally by the activating enzymes of the lung, and not by those of the liver (Gerde et al., 2001).
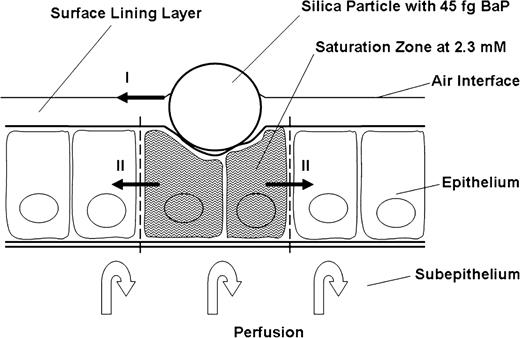
A second consequence of the described absorption mechanism is a great sensitivity of the local dose to the applied exposure rate. As demonstrated herein, the mucosa will reach physicochemical saturation at relatively low exposure rates. Very important to note is that the saturation process is driven primarily by a low mobility in tissues, not a low solubility. The distinction is important because an erroneous conclusion of a limiting solubility would indicate that the substance is not bioavailable and is not likely to reach toxic quantities in the entrance epithelium, whereas the low mobility mechanism clearly indicates high local concentrations in the site-of-entry epithelium (Gerde et al., 1997). With the relatively monophasic absorption process obtained in the present experiments, we can describe the absorption behavior in greater detail. The basis for the description is the single silica particle with its varying content of BaP (Table 1). Following deposition with 3.4 fg BaP on the particle, the hydrocarbon is readily taken up by neighboring cells, and the process is then followed by a slower phase where the solute penetrates to the capillaries of the subepithelium. With 45 fg BaP on the particle, the air/blood barrier surrounding the particle reaches saturation for some 12 min and can therefore be used as a gauge for measuring tissue concentration during the absorption process. At saturation, the phospholipid membranes of cells and airway surfactant contain about 0.02 g BaP/g lipid membrane (Patton et al., 1984). With an estimated lipid content in the tracheobronchial epithelium of 0.03 g lipid/g wet tissue (Gerde and Scott, 2001) and a solubility in saline of 5 μg/l (Mackay et al., 1992), the bronchiolar epithelium facing the deposited carrier particles contains about 0.0006 g BaP/g tissue or 2.3 mM at physicochemical saturation. The silica particles with the medium concentration of BaP can therefore be used to control whether physicochemical saturation is a likely explanation for the plateau signaled by the absorption curve. If the entire content of BaP on the silica particles of the medium exposure would dissolve in a cylindrical section of the air/blood barrier below the particles deposited in the bronchioles, this saturated cylinder would be about 4 μm in diameter and about 5 μm high above the basement membrane (Fig. 6). This is clearly a reasonable size for a saturated section of the air/blood barrier surrounding the silica carrier particles in the bronchioles. Thus, we have good reason to believe that local saturation occurs in a lung the size of that of the rat at acute cumulative inhalation exposures of only 36 ng BaP. Thirty-six ng is about the content of BaP in the smoke from a single cigarette (Hoffmann and Hoffmann, 1998). The detected limit of saturation gives a strong reminder of how uneven the cellular doses are in the body when highly lipophilic toxicants such as BaP are inhaled. The concentration of BaP in the highest exposed cells surrounding the carrier particle lingers at levels around 5 million times higher than the average initial body burden projected for the rat the lungs came from (Table 3).
A schematic drawing indicating the size of the tissue volume surrounding a carrier particle that is likely to be saturated with BaP shortly after deposition of particles from the medium exposure level. The two sets of arrows indicated by roman numerals show the hypothetical routes by which the initial surface area of BaP absorption across the air/blood barrier can be gradually increased following the prolonged state of saturation of the high exposure level. This would explain the slower attainment of steady state in the perfusate level of BaP-eq after the high level exposure.
Unevenness of Dose Following Inhalation of 36 ng BaP in the Rat
Level of dose description . | Concentration (nM) . | Relative concentration . |
|---|---|---|
| Measured maximum in perfusate | 0.085 | 0.2 |
| Whole body average | 0.44 | 1 |
| Lung average | 83 | 200 |
| Saturated entrance epithelium | 2,300,000 | 5,000,000 |
Level of dose description . | Concentration (nM) . | Relative concentration . |
|---|---|---|
| Measured maximum in perfusate | 0.085 | 0.2 |
| Whole body average | 0.44 | 1 |
| Lung average | 83 | 200 |
| Saturated entrance epithelium | 2,300,000 | 5,000,000 |
Note. A likely contributor to the site-of-entry toxicity of highly lipophilic inhalants.
Unevenness of Dose Following Inhalation of 36 ng BaP in the Rat
Level of dose description . | Concentration (nM) . | Relative concentration . |
|---|---|---|
| Measured maximum in perfusate | 0.085 | 0.2 |
| Whole body average | 0.44 | 1 |
| Lung average | 83 | 200 |
| Saturated entrance epithelium | 2,300,000 | 5,000,000 |
Level of dose description . | Concentration (nM) . | Relative concentration . |
|---|---|---|
| Measured maximum in perfusate | 0.085 | 0.2 |
| Whole body average | 0.44 | 1 |
| Lung average | 83 | 200 |
| Saturated entrance epithelium | 2,300,000 | 5,000,000 |
Note. A likely contributor to the site-of-entry toxicity of highly lipophilic inhalants.
For the high exposure level, the concentration continued to increase for almost 20 min before reaching a steady state, which was then maintained for the rest of the perfusion period (Fig. 2B). We hypothesize that the slow attainment of steady state compared with the medium exposure level is caused primarily by a gradual increase in the surface area available for absorption across the epithelium surrounding each particle of the high exposure. Two mechanisms lie near at hand (Fig. 6): (1) the gradual dispersal of BaP from carrier particles with the surfactant lipids of the mucociliary escalator (Gerde et al., 1993c) and (2) the lateral diffusion of BaP from the initially exposed cells around the carrier particles to neighboring cells via the much closer contact of plasma membranes within the epithelium rather than across the basement membrane (Schneeberger, 1991).
The presented inhalation system is a valuable complement to inhalation exposures of whole animals. However, great care must be exercised when interpreting results of the short-duration inhalation exposures of lungs ex vivo, followed by up to a couple of hours of data collection before the experiment must be terminated. Nevertheless, the high resolution of the data collected gives a unique opportunity to assess the site-of-entry dosimetry of typical inhalation exposures. It is particularly important to determine whether the exposures have been performed above or below local saturation. For example, one of the few experiments with inhaled BaP leading to respiratory tract tumors in rodents is the study by Thyssen et al. (1981). Hamsters were chronically exposed to condensation aerosols of BaP at concentrations of 2, 10, and 46 μg/l. It is of great interest to estimate the time it takes for the hamster lung to collect a transient exposure of 36 ng, the amount that caused saturation of the larger rat lung. At the highest exposure concentration and a typical respiration rate in the hamster of 50 ml/min (Mauderly and Tesarek, 1975) with an assumed fractional deposition of a condensation aerosol in rodent lungs of 0.1 (Hofmann et al., 2000), the hamster lung will collect 36 ng BaP within 10 s. This time period is less than 1% of the time it takes for the rat lung to recover from that saturation, or 12 min (Fig. 2B). Even at the middle concentration of the Thyssen study the likely time it takes for the hamster lungs to reach saturation is much shorter than the time to rebound from saturation. It is therefore most likely that both exposure levels of BaP leading to respiratory tract tumors in hamsters were held at levels much higher than that of physicochemical as well as metabolic saturation. In contrast, the human smoker will deposit some 10–20 ng BaP or 500 ng total PAHs after smoking one cigarette (Hinds et al., 1983) in lungs that are at least 100-fold the surface area of rat or hamster lungs. Saturation may occur locally, but is much less likely. The city dweller may inhale the same amount PAHs in one m3 of air (Allen et al., 1996), which will typically take 1 h, so saturation is even less likely to occur. The situation that laboratory animals used to study the risk of inhaled PAHs and people subjected to the same agents under real-life conditions are exposed under two different dosing regimes—supersaturation and subsaturation—calls for a greater awareness that inhaled PAHs may be considerably more potent lung carcinogens when exposing humans at low dose rates over decades than at much higher dose rates provided to laboratory animals for a year or two. This mechanism would help to explain the long-standing contradiction between epidemiological studies in human populations exposed to PAHs and animal experiments, where typically the risk gradient for lung cancer in humans ranges from being 10–100 times steeper than those of animals exposed to PAHs (Boström et al., 2002; Heinrich et al., 1994; Vyskocil et al., 2004).
This research was supported by the Swedish Council for Working Life and Social Research (FAS), Grant No 2001–2621 and the Swedish Agency for Innovation Systems (Vinnova), Grant No 21416–1, with contributing funding from the AstraZeneca Ltd.
References
Allen, J. O., Dookeran, N. M., Smith, K. A., Sarofim, A. F., Taghizadeh, K., and Lafleur, A. L. (
Armstrong, B., Hutchinson, E., Unwin, J., and Fletcher, T. (
Bond, J. A., Medinsky, M. A., Carlini, M. E., and Wolff, R. K. (
Boström, C.-E., Gerde, P., Hanberg, A., Jernström, B., Johansson, C., Kyrklund, T., Rannug, A., Törnqvist, M., Victorin, K., and Westerholm, R. N. (
Coggins, C. R. (
Doll, R., Peto, R., Wheatley, K., Gray, R., and Sutherland, I. (
Gerde, P., Ewing, P., Låstbom, L., Waher, J., Lidén, G., and Ryrfeldt, Å. (
Gerde, P., Medinsky, M. A., and Bond, J. A. (
Gerde, P., Muggenburg, B. A., and Henderson., R. F. (
Gerde, P., Muggenburg, B. A., Hoover, M. D., and Henderson, R. F. (
Gerde, P., Muggenburg, B. A., Lundblad, M., and Dahl, A. R. (
Gerde, P., Muggenburg, B. A., Sabourin, P. J., Harkema, J. R., Hotchkiss, J. A., Hoover, M. D., and Henderson, R. F. (
Gerde, P., Muggenburg, B. A., Stephens, T., Lewis, J. L., Pyon, K. H., and Dahl, A. R. (
Gerde, P., Muggenburg, B. A., Thornton-Manning, J. R., Lewis, J. L., Pyon, K. H., and Dahl, A. R. (
Gerde, P., and Scott, B. R. (
Gerde, P., Stephens, T., and Dahl, A. R. (
Gundersen, H. J., Bendtsen, T. F., Korbo, L., Marcussen, N., Moller, A., Nielsen, K., Nyengaard, J. R., Pakkenberg, B., Sorensen, F. B., Vesterby, A., et al. (
Hashish, A. H., Bailey, A. G., and Williams, T. J. (
Hecht, S. S. (
Heinrich, U., Roller, M., and Pott, F. (
Hinds, W., First, M. W., Huber, G. L., and Shea, J. W. (
Hoffmann, D., and Hoffmann, I. (
Hofmann, W., Asgharian, B., Bergmann, R., Anjilvel, S., and Miller, F. J. (
Lakowicz, J. R., and Hylden, J. L. (
Mackay, D., Shiu, W. Y., and Ma, K. C. (
Marchevsky, A. M. (
Mauderly, J. L., and Tesarek, J. E. (
Monteith, D., Novotny, A., Michalopoulos, G., and Strom, S. (
Patton, J. S., Stone, B., Papa, C., Abramowitz, R., and Yalkowsky, S. H. (
Rojas, M., Marie, B., Vignaud, J. M., Martinet, N., Siat, J., Grosdidier, G., Cascorbi, I., and Alexandrov, K. (
Ryrfeldt, Å., and Nilsson, E. (
Schneeberger, E. E. (
Scott, G. G., Stephens, J. A., Rorbacher, K. D., Thornton-Manning, J. R., Gerde, P., and Dahl, A. R. (
Thyssen, J., Althoff, J., Kimmerle, G., and Mohr, U. (
Tronde, A., Baran, G., Eirefelt, S., Lennernas, H., and Bengtsson, U. H. (
Vyskocil, A., Viau, C., and Camus, M. (
Author notes
*National Institute of Environmental Medicine, Division of Physiology, Karolinska Institutet, Stockholm, Sweden; †Astra Zeneca Ltd, Safety Assessment, Södertälje, Sweden; and ‡Uppsala University, Department of Women's and Children's Health, Uppsala, Sweden




Comments