-
PDF
- Split View
-
Views
-
Cite
Cite
Marija Stojadinovic, Raymond Pieters, Joost Smit, Tanja Cirkovic Velickovic, Cross-Linking of β-Lactoglobulin Enhances Allergic Sensitization Through Changes in Cellular Uptake and Processing, Toxicological Sciences, Volume 140, Issue 1, July 2014, Pages 224–235, https://doi.org/10.1093/toxsci/kfu062
Close - Share Icon Share
Abstract
Cross-linking of proteins has been exploited by the food industry to change food texture and functionality but the effects of these manipulations on food allergenicity still remain unclear. To model the safety assessment of these food biopolymers, we created cross-linked bovine β-lactoglobulin (CL-BLG) by laccase treatment. The purpose of the present study was to compare the immunogenicity and allergenicity of CL-BLG with native BLG in a mouse model of food allergy. First, BALB/c mice were intragastrically sensitized and orally challenged with BLG or CL-BLG and BLG-specific serum antibodies and splenic leukocyte cytokine production and cell proliferation were measured. Hereafter, epithelial protein uptake was monitored in vitro and in vivo and the effects of BLG cross-linking on interactions with dendritic cells were analyzed in vitro. Sensitization of mice with CL-BLG resulted in higher levels of IgE, IgG1, and IgG2a. In contrast, a subsequent oral challenge with CL-BLG resulted in lower mast cell degranulation. Cross-linking of BLG reduced its epithelial uptake but promoted sampling through Peyer's patches. Differences in endocytosis by dendritic cells (DCs) and in vitro endolysosomal processing were observed between BLG and CL-BLG. CL-BLG primed DCs induced higher Th2 response in vitro. Cross-linking of BLG increased its sensitizing capacity, implying that the assessment of highly polymerized food proteins is of clinical importance in food allergy. Moreover, manufacturers of foods or therapeutic proteins should pay considerate attention to the health risk of protein aggregation.
Disclaimer: The EC does not share responsibility for the content of the article. The authors are members of the COST Action FA 1005 Infogest.
Food taste determines our eating habits since early age. Taste is the outcome of all food sensory properties: color, flavor, and texture. Food texture is related to structure, mechanical, and rheological properties of food matrix (ISO 5492:2008). Improvement of food structure can be achieved in many ways, such as by creation of covalent cross-links between food components (e.g., proteins and carbohydrates). Cross-linking of proteins has been exploited in the food industry for cereal, dairy, meat, and fish processing. Enzymes as agents for protein cross-linking have been suggested as more useful than chemical or physical means due to their high specificity, mild conditions and therefore low risk of formation of toxic products. Cross-linking enzymes used for this purpose are transglutaminases, peroxidases, phenol oxidases (tyrosinases and laccase), and sulfhydryl oxidases (Buchert et al., 2010). Although transglutaminases are the only cross-linking enzymes approved in the current European Union (EU) legislation on food enzymes (EU regulation 1332/2008), it seems only a matter of time before other cross-linking enzymes, especially multifunctional phenol oxidases, are allowed as technological aid.
Although tailoring of protein structure is necessary for creation of new functional properties, there is a risk of creating foods with increased allergic potential. Therefore, all new proteins and protein derivatives need to be tested for allergenicity before they are released into the food market (FAO/WHO, 2001). Considerations in the assessment of allergic potential of novel foods are: (1) prevention of development of de novo hypersensitivity/allergy and (2) prevention of increase of allergenic potential of allergens already present in foods (Food Directorate, Health Products and Food Branch, Health Canada, 2006). The effect of enzymatic cross-linking on food protein allergenicity has been investigated before, mainly by in vitro tests. For instance, transglutaminase treatment of peanut proteins, wheat flower, β-casein, and ω-5 gliadin showed variable effects on the allergenicity of proteins (Clare et al., 2006; Leszczyńska et al., 2006; Monogioudi et al., 2011; Palosuo et al., 2003), but polyphenol oxidases reduced the IgE binding capacity of peanut Ara h 1 and 2, milk β-casein and β-lactoglobulin (BLG) (Chung et al., 2005; Stanic et al., 2010; Tantoush et al., 2011). Variable observations between these studies suggest that assessment of allergenicity, let alone of immunogenicity, of cross-linked proteins is not straightforward and is influenced by features inherent to protein structure (complexity of tertiary structure, linear vs. conformational epitopes), extent of cross-linking (polymerization efficiency, polymer size, utilization of mediators) and type of enzymes used (reactivity toward different amino acid side chains).
BLG is a well-studied small globular protein present at around 50% in bovine whey (Kontopidis et al., 2004). Because of high nutritional value and desirable techno-functional properties, this protein is frequently used as an additive in many food products (Stanić-Vučinić and Ćirković Veličković, 2013). On the other hand, BLG is one of major milk allergens, recognized by ∼50% of cow's milk allergic patients (Nakano et al., 2010; Natale et al., 2004). Enzymatic cross-linking was investigated for the improvement of the functionality of BLG (Eissa et al., 2006), but because of its compact tertiary structure (target amino acid residues are hindered for the enzyme) reaction efficiency is often low. However, by using high intensity ultrasound (to alter protein tertiary structure) prior to enzymatic reaction with laccase, we efficiently cross-linked BLG into high molecular weight aggregates (Stanic-Vucinic et al., 2012). The proposed process is economical and environmentally friendly, offers a good end-product that can be used in many types of foods and beverages. The obtained product (CL-BLG) possesses different structures and physicochemical properties than native protein (solubility is decreased, tertiary and secondary structures are altered, size is increased) and will presumably exhibit different biological behavior.
Because BLG sensitizes through gastrointestinal tract (GIT), we used an established mouse model of food allergy in which proteins are administered orally to address both the sensitizing and allergenic potency of CL-BLG. To explain the results observed in this model, we followed (1) protein digestibility and transport in the GIT and (2) processing and presentation by dendritic cells. Our data demonstrate that cross-linking of BLG promoted both immunogenicity and allergenicity in mice by changing protein digestibility and transport in the GIT, as well as the processing by dendritic cells.
MATERIALS AND METHODS
Protein sample preparation and characterization
BLG was isolated from raw bovine milk and CL-BLG was prepared as published before (Stanic-Vucinic et al., 2012), brief description is included in Supplementary Data. Proteins were conjugated to pHrodo Green succinimidyl ester (Invitrogen, Life Technologies, Breda, The Netherlands) and FITC-succinimidyl ester (Sigma-Aldrich, Taufkirchen, Germany) according to the manufacturer's instructions. For in vitro cell experiments, lipopolysaccharide (LPS) content in protein samples was reduced by affinity chromatography on Polymyxin B-agarose raisins following manufacturer's instructions (Bio-Rad Laboratories, Richmond, CA). Protein concentration was determined with bicinchoninic acid (BCA)-assay (Pierce, Amsterdam, The Netherlands). Agarose gel electrophoresis and scanning electron microscopy (SEM) were performed to determine CL-BLG molecular weight and morphology (Supplementary Data).
Cell cultures
Conditions for cell culture media used in the paper are listed in the Supplementary Data.
Mice
Five-week-old specific pathogen-free female BALB/c and C3H/HeOuJ mice were purchased from Charles River (Sulzfeld, Germany). All mice were housed under specific pathogen-free conditions within the animal care facility at the Utrecht University. Experiments have been approved by the Animal Experiments Committee of the Utrecht University.
Sensitization model
Murine sensitization protocol was similar to previously described one (Kanjarawi et al., 2011). BALB/c mice were intragastrically (ig) sensitized with 5 mg of BLG or CL-BLG with 15 μg of Cholera Toxin (CT, List Biological Laboratories, Inc., CA) on days 0, 7, 14, and 21 (n = 8/group). Control group received PBS instead of protein (n = 6). All mice received a challenge dose of 15 mg of BLG or CL-BLG on day 28. Levels of BLG-specific IgE, IgG1, and IgG2a were determined in sera before the challenge (from day 24) by in-house ELISA (Supplementary Data). Concentration of mouse mast cell protease-1 (mMCP-1) was determined in sera obtained on day 28 within 60 min after the oral challenge, using a commercially available ELISA (Moredun Scientific Ltd, Midlothian, UK). Splenic cells were incubated with 50 μg/ml of BLG for 72 h. Subsequently, in the culture supernatants, levels of IFN-γ, IL-5, and IL-13 were determined by commercially available ELISA (e-Bioscience, Austria). Cell proliferation was assessed by 3H-thymidine incorporation (Supplementary Data).
Transport through Caco-2 cells
For transport studies, Caco-2 cells were seeded at 6.8 × 104 cells/cm2 on polycarbonate membranes with a pore size of 0.4 μm in 12-well plates (Transwell, Corning Costar, Cambridge, MA). Cells at passages of 59–68 were used in experiments at the age of 21–24 days and the cultures were fed three times per week. Prior to the transport studies and at the end of experiment, transepithelial electrical resistance (TEER) was measured using Millicell-ERS Volt-Ohmmeter (Millipore, Amsterdam, The Netherlands). Only cell monolayers with TEER >500 Ω were used. Transport studies were done with 50 or 500 μg of BLG and CL-BLG in Dulbecco's modified eagle's medium (DMEM) culture medium in 500 μl (0.1 or 1 mg/ml). Aliquots were withdrawn from the receiver compartment at indicated time points and transported BLG and CL-BLG were detected by commercially available ELISA kit (Bethyl Laboratories, Inc., Montgomery).
In vivo transport through mouse intestinal tract
BALB/c mice (n = 3/group) were exposed by oral gavage with 4 mg of FITC labeled BLG or CL-BLG. Subsequently, animals were sacrificed after 15 and 30 min and the whole intestine was thoroughly rinsed with PBS and immediately frozen in liquid nitrogen. Seven micrometers sections were cut, counterstained with 4',6-diamidino-2-phenylindole (DAPI) and anti-M-cell antibody (antimouse glycoprotein-2 antibody (GP-2), MBL International, Woburn, MA). Fluorescent micrographs were obtained with Olympus microscope BX60 equipped with color CCD camera Leica DFC425C and analyzed by LEICA-LAS AF software (Leica Microsystems GmbH, Germany).
Simulation of gastrointestinal degradation of proteins—in vitro biphasal digestion assay
Simulated gastric (SGD) and intestinal digestion (SID) were conducted in accordance with U.S. Pharmacopoeia. SGD was performed according to Thomas et al. (2004), with slight modifications. Briefly, a tube with 0.76 ml of simulated gastric fluid (SGF; 0.084N HCl, 35mM NaCl, pH 1.2, and 4000 U of pepsin A (2540 U/mg, Sigma-Aldrich)) was prewarmed at 37°C and 40 μl of 5 mg/ml BLG or CL-BLG solution were added. Digestion proceeded at 37°C for 60 min under continuous shaking. Subsequently, digestion was stopped by addition of 0.24 ml of 0.3M Na2CO3 to give pH of 8.0. A sample was taken.
Pancreatin from porcine pancreas (Sigma-Aldrich) was dissolved in 200mM sodium phosphate buffer with 1.8% NaCl, pH 7.4. To 0.84 ml of previously incubated SGD mixture, 0.21 ml of pancreatin solution was added to give final pancreatin concentration 1% (wt/vol) and digestion continued at 37°C under continuous shaking. Pancreatic enzymes:protein mass ratio was 13:1 as described elsewhere (Stanic et al., 2010). Aliquots of 0.25 ml were withdrawn after 5, 15, 30, and 60 min of SID and reaction was stopped by boiling for 5 min at 95°C. To the remaining 0.2 ml of SGD mixture, sample of previously incubated, 0.05 ml of heat inactivated pancreatin solution was added. This mixture was marked as the time point 0 for SID. Samples were run on 14% SDS-PAGE under reducing conditions according to Laemmli (1970). Digestion pattern was analyzed after staining with silver nitrate as described by Swain and Ross (1995).
Protein uptake by DCs
Bone marrow cells from BALB/c were isolated and cultured in cell culturing medium supplemented with GM-CSF (10 ng/ml, R&D, Oxon, UK), as previously described (Lutz et al., 1999). Only 6-day-old BMDCs (differentiated from bone marrow cells isolated from BALB/c mice) were used for the following experiments. To access protein uptake and endocytosis, 10 μg of fluorescein isothiocyanate (FITC) or pHrodo Green-labeled BLG or CL-BLG were incubated with 1 × 106 BMDCs in 1 ml of cell culture medium for 0, 15, 30, 60, 120, 180, and 240 min. Percentage of FITC or pHrodo Green-positive DCs was measured using FACSCalibur flow cytometer (BD Biosciences). Flow cytometry data were analyzed in Cyflogic software (CyFlo Ltd, Turku, Finland). Stimulations were conducted in triplicates.
Simulation of protein processing in DCs—in vitro endolysosomal degradation
Endolysosomal fraction was isolated from BMDCs, as published before (Matthias et al., 2011). Holo-HRP was one of the proteins digested by microsomal content in Matthias et al. (2011) study with half-life of 66 min. We digested Holo-HRP (Sigma-Aldrich) with our microsomal preparation under the same conditions and determined protein half-life to be 54 min (Supplementary Data). Because the proteolytical activities of both microsomal preparations were comparable, we performed experiments as previously described by Matthias et al. (2011).
Briefly, 0.25 mg/ml of BLG or CL-BLG were digested by 0.4 mg/ml of microsomal proteins in 20 μl of 100mM citrate buffer pH 4.8 in the presence of 2mM dithiothreitol at 37°C. After 0, 3, 6, 12, 24, 36, and 48 h reactions were stopped by heating at 95°C for 5 min. Digestion mixtures were run on 14% SDS-PAGE under reducing conditions. Protein bands were visualized by silver staining. Qualitative analysis of digested samples was performed by LC-ESI MS/MS (Supplementary Data). For data visualization web-based MS-Tools were used (Kavan and Man, 2011). The major histocompatibility complex class II (MHCII) binding predictions were made on 19 September 2013 using the Immune Epitope Database (IEDB) analysis resource SMM-Align (version 1.1) tool and RANKPEP (Nielsen et al., 2007; Reche and Reinherz, 2007). From SMM-Align analysis only peptides with IC50 values below 5000nM (upper limit for all known T-cell epitopes) were considered as possible binders.
Coculture of stimulated BMDCs with primed T cells
For this experiment, BMDCs were directly exposed to proteins and cytokines secreted by CD4+ T cells were measured upon incubation with stimulated BMDCs as described before (Pochard et al., 2010). C3H/HeOuJ mice were chosen instead of BALB/c due to the establishment of this model in this strain and their higher resistance to endotoxin. BLG-specific CD4+ T cells were obtained from spleens of mice immunized with BLG and aluminum hydroxide (ALUM, Sigma-Aldrich). On days 0 and 7, mice were intraperitoneally injected with 100 μg of BLG and 1 mg of ALUM. Bone marrow cells were cultured and differentiated into BMDC as described above. On day 13, incubations of BMDCs with 50 μg/ml of BLG or CL-BLG were started. On day 14, CD4+ T cells were isolated from splenocyte suspensions by using DynalMouse CD4 Negative Isolation Kit (Invitrogen, Life Technologies). After a 24-h stimulation with BLG or CL-BLG, on day 14, BMDCs were scraped, washed, and transferred to a 96-well plate where they were cocultured (1:10 cell number ratio) with freshly isolated CD4+ T cells for the next 72 h. Subsequently, levels of IFN-γ, IL-5, IL-13, and cell proliferation were measured as mentioned above. Three independent experiments were performed.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) and analyzed using GraphPad Prism software (La Jolla, CA). Data were log-transformed to obtain a normal distribution and then compared using Student's t-test. Differences were considered significant if p-value was <0.05.
RESULTS
Cross-Linking Induced Formation of High Molecular Weight BLG Structures
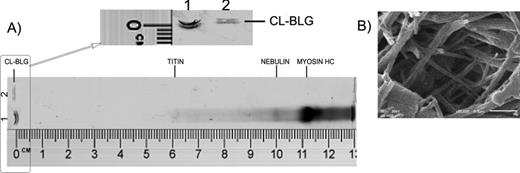
BLG is 18 kDa. To determine the molecular weight of CL-BLG, electrophoresis on agarose gel was carried out. However, as seen in Figure 1A, CL-BLG poorly migrated from the application site. As reference proteins myosine (heavy chain, 220 kDa), nebulin (800 kDa), and titin (around 3000 kDa) from mouse muscle protein extract (MMPE) were used. From the log(Mw) versus retention factors (Rf) curve, molecular weight of CL-BLG was calculated to be ≥50–60 MDa. In addition, SEM was used for the determination of shape and morphology of CL-BLG. Although CL-BLG particles are not uniform in size, their morphology is similar. CL-BLG particles are built from long protein fibers ∼60 nm wide (Fig. 1B). Fibers are intertwined and form a network that may further ensure CL-BLG stability, as known for other biological systems (Maina, 2007).
Characterization of CL-BLG molecular weight and morphology. (A) Agarose gel electrophoresis after staining with CBB. Lane 1: mouse muscle protein extract (MMPE), myosin heavy chain, 220 kDa; nebulin, 800 kDa; titin, ∼3000 kDa. Lane 2: 10 μg of CL-BLG. Ruler is included for comparison with retention factors (Rf) published before for MMPE. (B) Scanning electron microscopy of CL-BLG at ×50,000 magnification. Scale bar corresponds to 0.5 μm.
Cross-Linking of BLG Enhanced Sensitization but Reduced Mast Cell Degranulation
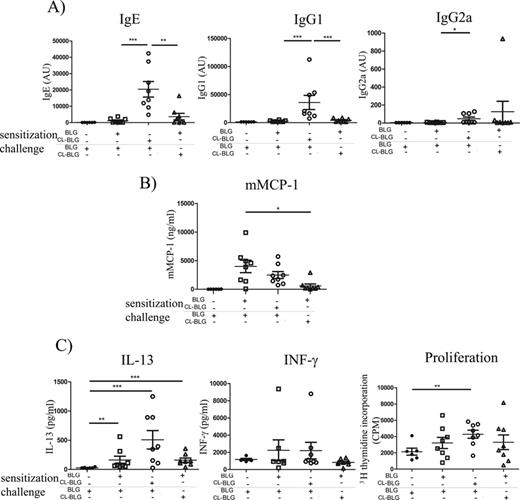
Hereafter, we investigated whether cross-linking of BLG changed its immunogenicity and allergenicity in vivo. Mice sensitized to CL-BLG generated a significantly higher IgE, IgG1, and IgG2a response when compared with mice sensitized to BLG (Fig. 2A). Increase in IgG2a serum levels was not as pronounced as increases in levels of IgE and IgG1. Upon oral challenge with BLG, serum levels of mMCP-1 (as a measure of mast cell degranulation) were similar in mice sensitized with BLG or CL-BLG. However, an oral challenge with CL-BLG induced significantly lower mast cell degranulation in BLG-sensitized mice, compared with the BLG challenge (Fig. 2B).
Immunogenicity and allergenicity of BLG and CL-BLG in vivo. (A) BLG-specific IgE, IgG1 and IgG2a in serum before the challenge. (B) mMCP-1 in serum obtained within 60 min after the oral challenge. (C) T-cell restimulation with BLG: levels of IL-13, INF-γ, and cell proliferation. ***p < 0.001, **p < 0.005, *p < 0.05.
Both BLG and CL-BLG sensitization led to an increase in IL-13 production in spleen cultures restimulated with BLG. The levels of INF-γ were not significantly different than in the control group and IL-5 could not be detected. T-cell proliferation was significantly higher than controls only in CL-BLG-sensitized mice (Fig. 2C).
Cross-Linking Altered BLG Sampling in the Gut and Resistance to Proteolysis
Susceptibility to gastrointestinal degradation and site of antigen absorption are important factors influencing allergenicity and immunogenicity of food allergens (Moreno et al., 2005). Therefore, we investigated BLG and CL-BLG transport across polarized Caco-2 cell monolayer in vitro, a model of intestinal barrier function. Transport of CL-BLG was significantly reduced when compared with BLG, even at a 10 times higher concentration of CL-BLG (Fig. 3A).
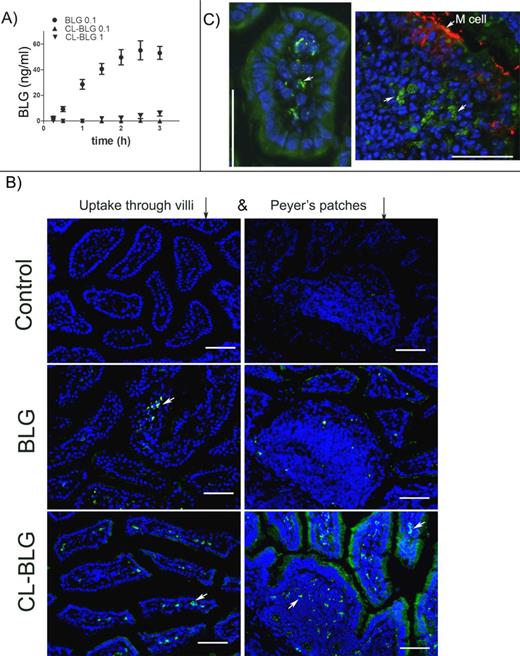
Bioavailability of BLG and CL-BLG in vitro and in vivo. (A) Transcytosis of proteins (0.1 mg/ml BLG, 0.1 or 1 mg/ml CL-BLG) across Caco-2 monolayer. (B) FITC-labeled BLG and CL-BLG uptake through villi 15 min after feeding and Peyer's patches after 30 min. Control-mice fed with PBS. (C) Selected sections showing proteins taken up inside a vilus (BLG 15 min) and part of Peyer's patch (CL-BLG 30 min). M-cells were stained in red by GP-2 immunostain. Scale bars correspond to 100 μm.
The uptake of BLG and CL-BLG was also evaluated in vivo. Both FITC-labeled BLG and CL-BLG were detected in the lamina propria of small intestinal villi already 15 min after ingestion. Consistent with its particulate nature, CL-BLG was detected after 30 min in the follicular and interfollicular areas of Peyer's patches (PPs) (Fig. 3B). BLG was not detected in PPs. Interestingly, after 30 min, enhanced CL-BLG uptake in villi neighboring the PPs was seen. Proteins taken up by the intestinal cells had a dotty and granular appearance at high magnification as shown in Figure 3C on selected sections of villi and PPs.
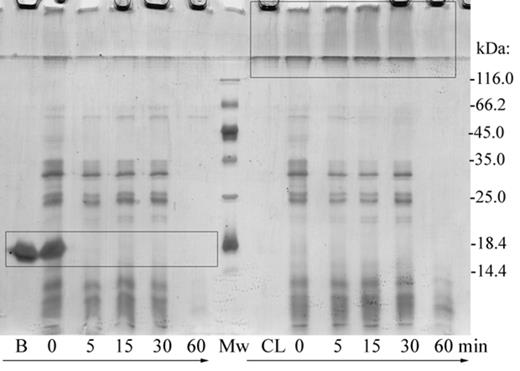
To analyze protein susceptibility to gastrointestinal digestion, we performed simulated gastric digestion with pepsin followed by simulated intestinal digestion with pancreatin (mixture of enzymes from porcine pancreas). Digests were analyzed on 14% SDS-PAGE under reducing conditions (Fig. 4). Longer resistance of CL-BLG was observed as it was completely digested after 60 min whereas BLG was already processed within 5 min of simulated intestinal digestion. CL-BLG degradation proceeded through intermediates >116 kDa observed after 5, 15, and 30 min as smear in the stacking gel. These intermediates were not present in BLG digests indicating that they are originating from CL-BLG only and not from other proteins (present in pepsin and pancreatin preparation).
Simulated gastric and intestinal digestion. Pepsin digestion was stopped after 60 min and mixture of pancreatic enzymes was added. Digestion continued for 0, 5, 15, 30, and 60 min. Subsequently, digests were analyzed on 14% SDS-PAGE under reducing conditions. Rectangles are marking BLG (B) band, CL-BLG (CL) high molecular weight degradation intermediates. Another bands present in BLG and CL-BLG digests are originating mainly from pepsin and pancreatin. Mw, molecular weight markers.
BLG and CL-BLG Display Distinct Patterns of Uptake by DCs
To better understand T-cell responses elicited by BLG and CL-BLG, we monitored antigen uptake and endocytosis by BMDCs, in vitro endolysosomal degradation and subsequent presentation to CD4+ T cells.
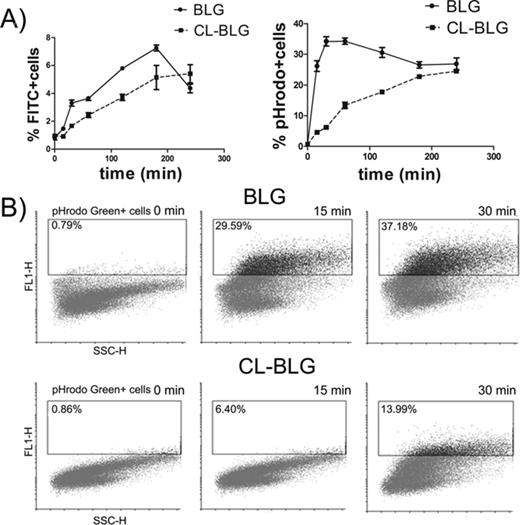
Uptake of fluorescently labeled BLG and CL-BLG by BMDCs was followed in time by flow cytometry. In addition to FITC, pHrodo Green dye was used to label proteins because it fluoresces brightly at the acidic pH and is almost nonfluorescent at neutral pH which makes this dye a good indicator for localization of BLG or CL-BLG in the endolysosomal compartments. FITC-labeled CL-BLG was attached and taken up to a lower extent when compared with FITC-BLG until 240 min when they reached the same level (Fig. 5A). Endocytosis of pHrodo-BLG was fast, reached its maximum within 30–60 min, then slowly decreased and reached saturation after 180 min. pHrodo-labeled CL-BLG was internalized at a much slower rate, which reached saturation between 180 and 240 min, like BLG (Fig. 5A). The biggest differences in protein internalization were observed within the first 30 min, when the percent of pHrodo-positive cells increased from 0.79 to 29.59 and 37.18% for BLG whereas it increased from 0.86 to 6.4 and 13.99% for CL-BLG, after 15 and 30 min, respectively (Fig. 5B).
Uptake of FITC or pHrodo Green labeled BLG and CL-BLG by DCs. (A) Number of positive cells over time. (B) One representative pHrodo positive cell gating profile out of three experiments for 0, 15, and 30 min time points.
CL-BLG Showed Different Susceptibility to Endolysosomal Proteolysis
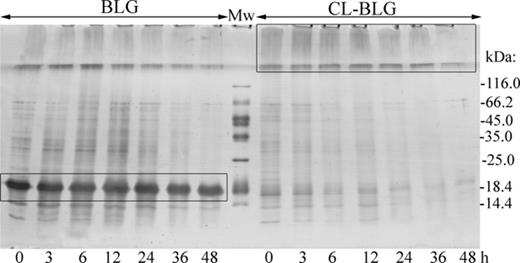
Because efficient antigen processing is of crucial importance for immunogenicity of proteins, we subjected BLG and CL-BLG to proteolysis by endolysosomal enzymes isolated from BMDCs. SDS-PAGE electrophoresis of digested samples revealed that BLG was degraded slowly in time with half-time >48 h, but also that some proteolysis of CL-BLG occurred (Fig. 6). CL-BLG degradation proceeded through intermediates observed as smear in the stacking gel (mostly noticed after 3, 6, and 12 h of digestion).
SDS-PAGE analysis of time dependent endolysosomal degradation of BLG and CL-BLG. Rectangles are marking BLG band, CL-BLG high molecular weight degradation intermediates. Mw, molecular weight protein markers.
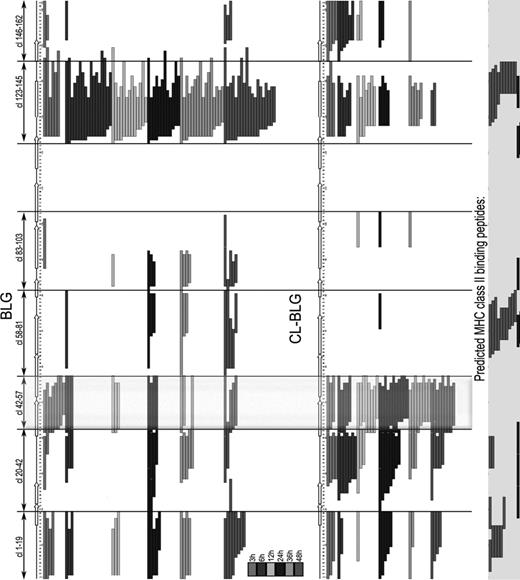
BLG peptides identified in the samples were used for generation of peptide maps that provide insight into peptide profile and identification of potential T-cell determinants (Fig. 7). Peptides from both BLG and CL-BLG appeared in seven nested clusters (start-end amino-acid): 1–19, 20–42, 42–57, 58–81, 83–103, 123–145, and 146–162. Peptides from one cluster share common core and have variable lateral regions. The most abundant peptide cluster for BLG was 123–145, whereas peptides from C-terminus (146–162) were the least present. Peptides from the central region of BLG (58–81, 83–103) appeared mainly later, after 24 h. Although CL-BLG releasing peptides share common clusters with BLG, their overall distribution is different. When compared with BLG, CL-BLG provided higher number of peptides in clusters 42–57 (dominant), 20–42 (abundant), and 146–162 but lower number of peptides in clusters 1–19, 123–145, whereas peptides from the central core region were rarely present.
Chronology chart of peptides released during 3, 6, 12, 24, 36, and 48 h of endolysosomal processing of BLG and CL-BLG. Predicted MHC II binding peptides for IAd and IEd alleles are aligned on the right. Peptides from cluster 42–57 (clx-x) were reported to be immunodominant in T-cell clones from BALB/c mice and cow's milk allergic patients.
For prediction of core sequences of peptides binding to H-2 class II molecules IAd and IEd that are MHC II molecules expressed in BALB/c mice we used RANKPEP and IEDB analysis resource SMM-Align. Peptides from clusters 1–19, 20–42, 58–81 and 123–145 are listed as possible candidates (Fig. 7).
CL-BLG Primed DCs Induced Th2 Response of BLG-specific CD4+ T Cells
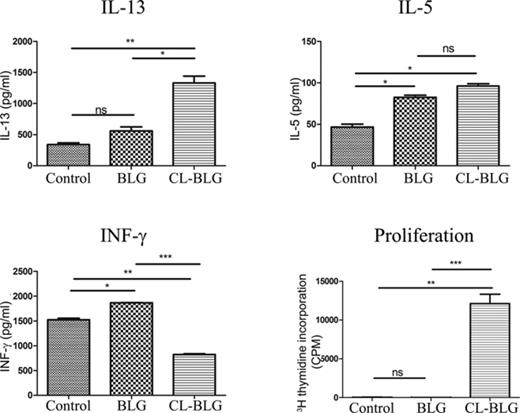
Finally, we investigated whether cross-linking of BLG interfered with antigen presentation by DCs in vitro. CL-BLG loaded DCs induced higher IL-13 and lower INF-γ levels accompanied by elevated T-cell proliferation when compared with naïve or BLG primed DCs. Both BLG- and CL-BLG-pulsed DCs induced IL-5 production but with no significant difference among the groups (Fig. 8). Peptides occurring from CL-BLG endolysosomal digestion when presented with MHC II complex on the surface of DCs induced stronger activation and Th2 response of BLG-specific CD4+ T cells.
Stimulation of BLG-specific CD4+ T cells by BLG or CL-BLG primed BMDCs. Levels of IL-13, IL-5, IFN-γ, and cell proliferation were determined. Control, T cells cocultured with naïve DCs. One experiment out of three independent experiments is shown. ***p < 0.001, **p < 0.005, *p < 0.05.
DISCUSSION
Protein cross-linking in food occurs naturally or during processing (Singh, 1991). By changing type and extent of cross-linking, food industry can modulate food texture and improve food functionality. Although cross-linking of food proteins has been carried out extensively, little is known about the health risks of these new food biopolymers. Having in mind that foods subjected to cross-linking, such as peanut and dairy products, are sources of potent food allergens, it is necessary to perform immunogenicity and allergenicity assessment of these novel protein derivatives. By using laccase cross-linked BLG (CL-BLG) as a model of efficiently polymerized proteins, we have investigated the effect of cross-linking on protein capacity to induce sensitization and allergic response using a mouse model of food allergy with relevant clinical read-out parameters.
In our mouse studies, allergic sensitization to CL-BLG was more prominent when compared with BLG, as shown by a significantly higher production of BLG-specific IgG1 and IgE. In contrast, cross-linking of BLG reduced its capacity to induce mast cell degranulation in BLG-sensitized mice. Although CL-BLG-sensitized mice had higher levels of BLG-specific IgE, release of protease-1 during mast cell degranulation did not differ upon oral challenge with BLG when compared with mast cell responses to BLG in BLG-sensitized mice. This higher allergenic capacity (as measured by IgE) but lower actual allergic effector response (as measured by mast cell degranulation) of CL-BLG, suggests that intestinal transport and processing of this protein derivative is altered. This may have led to less protein reaching the mast cells in the intestine. Indeed, changes in intestinal transport and digestibility between BLG and CL-BLG were observed in the following in vitro and in vivo experiments. Soluble BLG, but not CL-BLG was efficiently transported by Caco-2 cells. Remarkably, in vivo transport of BLG occurred through enterocytes only, whereas transport of CL-BLG occurred through both the enterocytes and M cells lining the PPs. Higher resistance of CL-BLG to gastric and intestinal proteolysis, as observed in vitro, may have induced kinetics as well as route of protein's uptake in the gut, as observed in vivo. In accordance with our results, Roth-Walter et al. (2008) reported that pasteurization formed aggregates of whey proteins (BLG and α-lactalbumin) promoted stronger allergic sensitization and triggered lower anaphylactic response in vivo. These aggregates were also preferentially taken up through M cells as opposed to the native proteins which were transported through enterocytes. However, in two recently published studies, treatment of peanut proteins with tyrosinase or milk casein with transglutaminase did not change protein-sensitizing capacity in animal models of food allergy (Radosavljevic et al., 2013; van Esch et al., 2013). From our finding that cross-linking has a profound effect on the route of uptake, we hypothesize that enhancement of immune responses to protein aggregates or polymers will only occur for food proteins that are normally transported through enterocytes, such as BLG. Aggregation of such proteins by processing, such as pasteurization, or by a combination of enzymatic and physicochemical methods, as applied in our study, could result in strong allergic sensitization because their routes of entrance is directed from enterocytes to M cells of PPs. In contrast, in case of proteins such as casein and peanut allergens that are already preferentially transported to PPs (Chambers et al., 2004; Roth-Walter et al., 2008), an increase in immunogenicity will not happen after processing-induced protein polymerization.
To further explain the increased CL-BLG immune-stimulating capacity observed in vivo, we inspected protein interactions with DCs in vitro. Generally, it is considered that uptake, processing and presentation on the surface of antigen presenting cells such as DCs determines the type and severity of immune response (Vyas et al., 2008). Because endocytosis of BLG was impaired by cross-linking, cell-bound CL-BLG was slowly delivered to microsomal enzymes in endolysosomal compartments. Analysis of peptides from endolysosomal digests of CL-BLG revealed a different peptide profile when compared with native BLG. CL-BLG provided less peptides from the central core of protein (accessibility reduced after cross-linking), but provided more peptides from the N- and C-terminus. Because Matthias et al. (2011) showed that the composition, activity and specificity of proteases from endolysosomal preparations of human and murine DCs are similar, proteolysis of BLG and CL-BLG will follow similar kinetics in DCs regardless of their origin. Among the peptides that were identified in the samples after endolysosomal degradation, we identified the major T-cell epitopes of BLG. In agreement with previously published results (Inoue et al., 2001; Sélo et al., 1999; Tsuji et al., 1993), we report that cluster 42–57 for which CL-BLG provided constantly higher number of peptides is probably T-cell immunodominant epitope. Also, after aligning our data with theoretical predictions, we propose that peptides in the cluster 20–42 may (co)determine higher immunogenicity of CL-BLG. Indeed, at the end, CL-BLG-primed DCs induced elevated expansion of BLG-specific T-cell clones and a markedly stronger Th2 response than monomeric protein. From our experience, when examining differences in immunogenicity between two structurally different proteins, except for monitoring resistance to intracellular cleavage by endolysosomal proteases (Kimber and Dearman, 2002), it is important to analyze and identify released peptides. Among those peptides are the ones primarily responsible for T-cell stimulation, type and severity of subsequent immune response (immunodominant T-cell epitopes).
In summary, processing of BLG into high molecular weight particles elicited stronger allergic sensitization in mice due to enhanced resistance to gastrointestinal proteolysis, redirected protein transport to PPs, changed uptake and processing in antigen presenting cells. Thus, antigen delivery to PPs and susceptibility to endolysosomal degradation may be important predictors of dietary protein's immunogenicity. Our findings suggest that BLG aggregates or polymers may elevate the risk of cow's milk allergy in humans, although clinical trials are needed for validation. In addition, although not necessarily translatable to all other soluble proteins, this study underlines the potential risk of their aggregated and/or cross-linked derivatives in the induction of food allergic sensitization.
FUNDING
Ministry of Education and Science of the Republic of Serbia (172024); FP7 RegPot project FCUB ERA (256716).
We thank Rob Bleumink for his assistance in in vivo transport study.
REFERENCES




Comments