-
PDF
-
Views
-
Cite
Cite
Sang Chul Lee, Won-Joong Kim, Chang-Soon Lee, Jee Youn Moon, Effectiveness of Percutaneous Lumbar Extraforaminotomy in Patients with Lumbar Foraminal Spinal Stenosis: A Prospective, Single-Armed, Observational Pilot Study, Pain Medicine, Volume 18, Issue 10, October 2017, Pages 1975–1986, https://doi.org/10.1093/pm/pnw355
Close - Share Icon Share
Abstract
In lumbar foraminal spinal stenosis (LFSS), numerous ligaments may play an important role in causing radiculopathy by narrowing the exit of the nerve root. In order to achieve effective decompression of lumbar foraminal ligaments, a specially designed instrument for percutaneous lumbar extraforaminotomy (PLEF) was invented. The purpose of this study was to evaluate the effectiveness of PLEF in patients with intractable radiculopathy from LFSS.
A prospective, single-armed, observational pilot study.
A pain center in a tertiary university-based hospital.
The PLEF was performed in patients who suffered from radiculopathy with concordant imaging evidence of a mild to severe degree of LFSS. For each patient, an 11-point numerical rating scale (NRS) pain score, the Oswestry Disability Index (ODI), the Roland Morris Disability Questionnaire (RMDQ) score, and any adverse events were evaluated at three-month follow-ups. Successful responder percentage defined as 40% or greater reduction from baseline NRS score with no increase in ODI, and the RMDQ score was assessed at three months.
Among 26 patients who underwent PLEF, 20 patients completed the study protocol. PLEF was successful in 12 patients (60%). The overall mean pain reduction at three months was 36.3%. Patients who responded well also showed improvement in the ODI (-20%) and RMDQ score (-8.4) at their three-month follow-up. No serious complications were reported in the study.
The PLEF can be an effective and safe treatment option, as well as a minimally invasive procedure, for the management of patients suffering from refractory radiculopathy caused by LFSS.
Introduction
Lumbar foraminal spinal stenosis (LFSS) is defined as the narrowing of the nerve root exit caused by a decrease in the height of an intervertebral disk, osteoarthritic changes in the facet joints, cephalad subluxation of the superior articular process of the inferior vertebra, buckling of the ligamentum flavum, or protrusion of the annulus fibrosus in the lumbar spinal canal [1–3]. Despite a paucity of studies examining the mechanical compression of nerve roots in LFSS, it has been assumed that LFSS results in damage to microvascular structures and continuous compression of nerve roots, subsequently causing ischemia, edema, demyelination, and C-fiber hyperactivation [4,5].
Although various types of minimally invasive procedures have been considered for managing radicular pain caused by LFSS in patients refractory to oral medication and physical therapy, their ability to provide effective pain relief in LFSS is questionable. Lumbar transforaminal epidural steroid injection (TFESI) is the most widely used; however, it shows less effectiveness in lumbar spinal stenosis when compared with disc herniation [6,7] and may not improve average impairment of function [6]. Percutaneous epidural adhesiolysis (PEA) is another option for managing patients with lumbar spinal stenosis [8–10], but previous studies suggest a negative outcome for patients with LFSS, spondylolisthesis, and previous lumbar surgery [8]. Although the spinal cord stimulation may guarantee a positive outcome after a successful trial in lumbar spinal stenosis [11,12], there has been a paucity of information in LFSS and the high initial cost is still burdensome. As a last resort, a surgical decompression such as laminotomy [13], laminectomy [14], medial facetectomy [15], or endoscopic foraminotomy [16] can be considered in a patient refractory to previous conservative treatments; however, a recent review failed to prove both short- and long-term superiority of these lumbar spinal surgeries compared with conservative care at follow-up [17].
One hypothesis of the physiopathology in the LFSS is that numerous lumbar foraminal ligaments cause low back pain and radiculopathy [18–21]. Lumbar foraminal ligaments are composed of superior and inferior transforaminal ligaments and superior and inferior corporotransverse (or corporopedicular) ligaments [4,18]. These structures fix the lumbosacral spinal nerves to the intervertebral foramen and protect the nerve and blood vessels from being damaged. However, if abnormal adhesion or too many transforaminal ligaments exist, they may result in pain through compression of the nerve root [20].
In order to achieve effective decompression of LFSS by resecting foraminal ligaments and to facilitate the spread of medication around the target nerve, a specially designed instrument for percutaneous lumbar extraforaminotomy (PLEF) was invented to allow a minimally invasive procedure. The purpose of this study was to evaluate the effectiveness of PLEF in patients with intractable radiculopathy from LFSS.
Methods
This prospective, single-armed, observational pilot study was approved by the institutional review board at the participating hospital (Seoul National University Hospital IRB No. 1311-067-534) and was conducted in accordance with the ethical principles of the Declaration of Helsinki. This study was registered in ClinicalTrials (NCT02597244) before initiating the study-related procedures. Patient enrollment took place from September 2014 to January 2016. Written informed consent was obtained from all patients before participation in the study.
Patients
Inclusion criteria were: 1) patients between 45 and 85 years of age with chronic lumbar radicular pain at the L4 or 5 dermatome; 2) concordant imaging evidence of LFSS (grade 1–3; grade 1 is mild LFSS, grade 2 is moderate LFSS, and grade 3 is severe LFSS) as demonstrated on preoperative magnetic resonance imaging (MRI) scans [22]; 3) failed pain relief or short-term pain relief for one month or less from previous TFESI; 4) patients with numerical rating scale (NRS) pain scores (with 0 as no pain and 10 as the worst pain possible) of greater than 4 out of 10 despite appropriate conservative treatment including physiotherapy, exercise therapy, or oral medications for at least six months; and 5) patients with a single-side radiculopathy.
Exclusion criteria were 1) patients with complaints of dominant back pain rather than leg pain; 2) lack of correlation between patients’ radicular pain and MRI findings; 3) history of prior lumbar spine surgery or any previous extraforaminotomy procedures; 4) iliac crest located higher than the L5 transverse process, disturbing lateral approach to the L5 foramen during the PLEF procedure; 5) progressive neurological deficits, motor weakness, or cauda equina syndrome; 6) allergies to steroids or contrast dyes; 7) clinical signs of spinal cord compression, bleeding disorders, infection, instability, malignancy or other traumatic injuries, poorly controlled coexisting psychiatric conditions, or underlying systemic diseases; and 8) pregnancy.
Administration of oral or transdermal analgesics such as opioids, tramadol, and/or nonsteroidal anti-inflammatory drugs (NSAIDs) was allowed to continue during the study. In addition, dosages of these medications as rescue or regular (around-the-clock) analgesics were modified appropriately in accordance with pain intensity. Adjuvants such as anticonvulsants and antidepressants that were used to manage radicular pain were also continued. In the first month post-PLEF, any additional medication or therapy including physical therapy, trigger point injections, and epidural injections was not allowed. After the first visit, patients were allowed to undergo physical therapy or interventional procedures other than epidural steroid injections such as TFESI and to take additional medication including NSAIDs, antidepressants, anticonvulsants, and/or opioids.
Lumber Extraforaminotomy Procedures
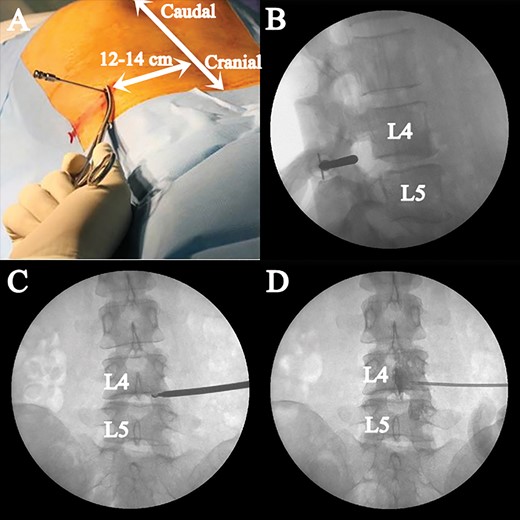
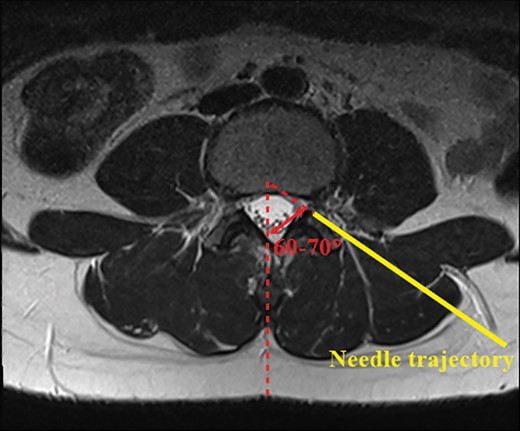
All PLEF procedures were performed by one pain specialist (SCL). A specially designed instrument consisting of a 16G Tuohy needle, 14G trocar, cannula, end mill, curette, and catheter was used for PLEF (Figure 1). Before the intervention, intravenous access was established and antibiotics were administered. The procedure was performed using fluoroscopy under sterile conditions, with simultaneous monitoring of vital signs. After confirming the correlation between the location of the pathology and radicular pain by MRI, a local anesthetic was injected around the entry point with the patient in a prone position. The entry point of the needle was kept 12–14 cm away from the midline of the vertebral body, while the needle trajectory was planned in accordance with the preoperative MRI scan (Figure 2A). The needle trajectory targeted the intervertebral foramen using a posterolateral approach while avoiding any injury to the internal organs (Figure 3). After 8–10 mL of 0.5% lidocaine was administered at the intended needle entry tract, a 15 cm, 16-gauge Tuohy needle was inserted under anteroposterior fluoroscopic guidance (OEC 9800 series; GE Medical Systems, Salt Lake City, UT, USA). In the lateral fluoroscopic view, the needle tip was advanced until it was located at the posterior part of the borderline between the inferior and superior articular processes (Figure 2B). An epidurogram was then obtained after injection of 5 mL contrast medium (iohexol, 300 mg iodine per mL; GE Healthcare, Piscataway, NJ, USA) to confirm that the needle was placed in the epidural space and to avoid intravascular or subarachnoid needle placement. Then, the Tuohy needle was withdrawn slightly to the level of the facet joint capsule. Subsequently, a trocar was inserted adjacent to the Tuohy needle and the Touhy needle was removed. Next, a cannula was inserted through the trocar to guide an end mill, which ultimately replaced the trocar. Finally, the end mill was placed within the epidural space in the intervertebral foramen.
Specially designed instrument for the percutaneous lumbar extraforaminotomy procedure.
Fluoroscopic images during lumber extraforaminotomy procedures. A) Entry point of the needle is 12–14 cm away from the midline of the vertebral body. B) In the lateral fluoroscopic view, the cannula tip is advanced until it is located at the posterior part of the borderline between the inferior and superior articular processes. C) A distraction of the foraminal ligament and mechanical adhesiolysis are performed by the curette through the cannula until the tip of the curette reaches the medial border of the pedicle in the anteroposterior fluoroscopic image. D) Postadhesiolysis epidurogram is obtained before injecting local anesthetics and corticosteroids (Figure 2D).
Needle trajectory to the intervertebral foramen by a posterolateral approach. The needle is advanced to the target foramen while avoiding any injury to the internal organs.
In the next step, the end mill was removed and a curette was introduced through the cannula while maintaining the bevel of the curette facing to the ventral side to avoid any neurovascular injury (Figure 2C). A distraction of the foraminal ligament and mechanical adhesiolysis were performed by advancing and withdrawing the curette repetitively through the cannula until the tip of the curette reached the medial border in the anteroposterior view of the pedicle. After obtaining an epidurogram postadhesiolysis (Figure 2D), a mixture of 10 mL 0.15% bupivacaine and 5 mg dexamethasone was injected through the catheter, followed by 10 mL normal saline with 3,000 IU hyaluronidase. In the recovery room, patients were monitored closely for any potential complications, including motor weakness over the lower extremities and reduced sphincter tone. Patients were discharged after monitoring for two hours.
Clinical Evaluation
This open-labeled observational study included a baseline visit before PLEF (Visit 0) and thereafter follow-ups at one, two, and three months (Visits 1, 2, and 3, respectively). The NRS pain score at each visit was calculated as the average of NRS pain score with regard to radicular leg pain [23] for the previous week. After the PLEF procedure, enrolled patients were encouraged to record a daily diary during the follow-up period. The Korean versions of the nine-item Oswestry Disability Index (ODI; the range is 0 to 100, where 0 means no disability) [24] and the Roland Morris Disability Questionnaire (RMDQ; the range is 0 to 24, where 0 means no disability) were also used for evaluating the physical function at each visit [25]. One research nurse who was independent of the study conducted all assessments.
The primary end point of the study was the percentage of successful responders at Visit 3. This indicator was determined according to previous studies, with some modifications such as a reduction of 40% or more compared with the baseline NRS pain score and no increase from baseline ODI and RMDQ score at Visit 3 [26–29]. Furthermore, increased dosage of analgesics, prescription of new analgesics, or administration of an additional epidural steroid injection during the follow-up period were all considered treatment failure.
Changes in the 11-point NRS scores of radicular pain, ODI, and RMDQ score at the three-month follow-up (Visit 3) compared with baseline (Visit 0) were analyzed for responding and nonresponding patients. In addition, any overall changes in the NRS pain score, ODI, and RMDQ score over time were compared between responding and nonresponding patients. Patients were prescribed oral analgesics at each visit and were encouraged to record their consumption of medication in the daily diary throughout the study period. At Visit 3, the consumption of oral medication including NSAIDs, opioids, antidepressants, and anticonvulsants was compared with baseline consumption in each patient. Finally, patient satisfaction with the EF procedure was assessed using a five-point Likert satisfaction scale (1 = extremely dissatisfied; 2 = somewhat dissatisfied; 3 = neutral; 4 = somewhat satisfied; and 5 = extremely satisfied) at Visit 3.
Patients were asked to report any adverse events of TFEF during the study period. Reported adverse events were noted and evaluated at each visit.
Statistical Analysis
Efficacy analyses were performed on the per-protocol population for the primary and secondary end points. Safety analysis was performed on the intent-to-treat population.
Differences between responding and nonresponding patients were tested using the Mann-Whitney test for nonparametric data and Fisher’s exact test for parametric data. The Wilcoxon signed-rank test was used to assess the statistical significance of differences between the NRS scores, ODIs, and RMDQ scores at Visit 3 and at the baseline. Subsequently, general linear mixed model analysis for longitudinal data was also performed.
Data were analyzed with SPSS version 22.0 software (IBM Corp, Armonk, NY, USA) for Windows. Outcomes are shown as the mean (interquartile range [IQR] or standard deviation) or frequency (%) as appropriate. A P value of less than 0.05 was considered statistically significant.
Results
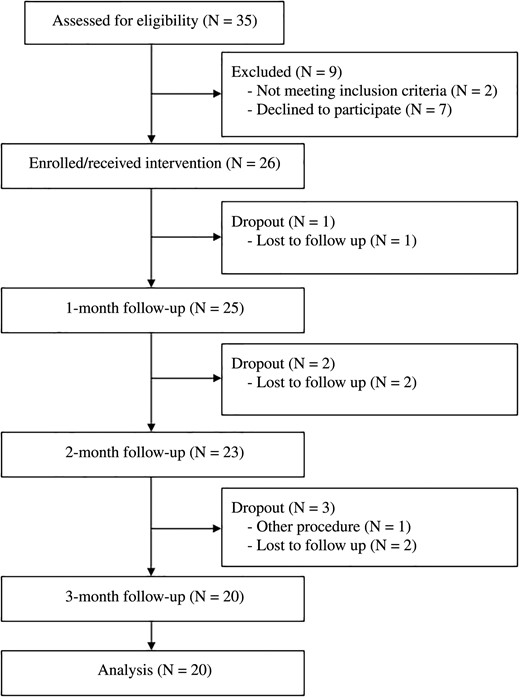
We recruited 35 patients who had been diagnosed with LFSS between September 2014 and September 2015. Of these, a total of 26 patients were enrolled and underwent PLEF. However, six patients dropped out during the three-month follow-up. Hence, 20 patients completed the study protocol, and follow-up data were collected at one, two, and three months (Figure 4).
The baseline patient demographic characteristics, including a comparison between responders and nonresponders, are shown in Table 1. Within the cohort, the majority (N = 14, 70%) underwent PLEF at the L4-5 transforaminal space. The baseline NRS pain score for the lower extremities was 6.60 (IQR = 5.0–8.0), indicating moderate to severe pain. Mean ODI (%) and RMDQ scores were 65.55 (IQR = 53.3–72.8) and 12.60 (IQR = 6.0–15.0), respectively, suggesting that pain affected all aspects of the patient's life [29,30]. No statistically significant differences between responders and nonresponders were observed among the demographics and clinical variables. The only exception was the number of patients receiving opioid medication. Three patients in the nonresponder group were prescribed a weak opioid, tramadol, and acetaminophen mixture and showed a statistical difference compared with the responder group (N = 0, P = 0.049). In the overall cohort, none of the patients had taken strong opioid medication.
Baseline characteristics and demographics
| Parameters . | Value (N = 20) . | Responders (N = 12) . | Nonresponders (N = 8) . | P . |
|---|---|---|---|---|
| Age, y | 67.6 (60.3–73.8) | 69.8 (65.3–74.0) | 64.3 (58.5–66.8) | 0.115 |
| Gender, male/female | 7 (35.0)/13 (65.0) | 5/7 | 2/6 | 0.642 |
| Height, cm | 160.7 (153.4–167.6) | 161.5 (153.9–169.0) | 159.5 (149.1–167.1) | 0.624 |
| Weight, kg | 64.6 (58.2–74.3) | 66.3 (55.9–75.8) | 62.2 (58.2–69.5) | 0.343 |
| Hypertension | 13 (65.0) | 9 (75.0) | 4 (50.0) | 0.356 |
| Total duration of pain, mo | 12.0 (8.0–14.0) | 11.2 (8.0–14.0) | 13.1 (8.3–18.0) | 0.305 |
| Level, L4–5/L5–S1 vertebrae | 14 (70.0)/6 (30.0) | 7/5 | 7/1 | 0.325 |
| Side, left/right | 10 (50.0)/10 (50.0) | 6/6 | 4/4 | 0.370 |
| LFSS grade I/II/III | 8(40.0)/9(45)/3(15.0) | 5/6/1 | 3/3/2 | 0.584 |
| Previous epidural injection | 3.7 (2.3–5.0) | 3.1 (1.3–5.0) | 4.5 (3.3–5.0) | 0.157 |
| NRS pain score of leg pain, 0–10 | 6.6 (5.0–8.0) | 7.1 (5.0–8.8) | 5.9 (5.0–6.8) | 0.157 |
| ODI, % | 65.6 (53.3–72.8) | 64.8 (53.3–71.1) | 66.7 (52.8–84.4) | 0.427 |
| RMDQ score, 0–24 | 12.6 (6.0–15.0) | 12.1 (6.0–14.8) | 13.2 (6.5–18.0) | 0.970 |
| Analgesic use | ||||
| Non-NSAIDs* | 8 (40.0) | 4 (33.3) | 4 (50.0) | 0.648 |
| NSAIDs | 10 (50.0) | 5 (41.7) | 5 (62.5) | 0.650 |
| Opioids | 3 (15.0) | 0 (0.0) | 3 (37.5) | 0.049 |
| Parameters . | Value (N = 20) . | Responders (N = 12) . | Nonresponders (N = 8) . | P . |
|---|---|---|---|---|
| Age, y | 67.6 (60.3–73.8) | 69.8 (65.3–74.0) | 64.3 (58.5–66.8) | 0.115 |
| Gender, male/female | 7 (35.0)/13 (65.0) | 5/7 | 2/6 | 0.642 |
| Height, cm | 160.7 (153.4–167.6) | 161.5 (153.9–169.0) | 159.5 (149.1–167.1) | 0.624 |
| Weight, kg | 64.6 (58.2–74.3) | 66.3 (55.9–75.8) | 62.2 (58.2–69.5) | 0.343 |
| Hypertension | 13 (65.0) | 9 (75.0) | 4 (50.0) | 0.356 |
| Total duration of pain, mo | 12.0 (8.0–14.0) | 11.2 (8.0–14.0) | 13.1 (8.3–18.0) | 0.305 |
| Level, L4–5/L5–S1 vertebrae | 14 (70.0)/6 (30.0) | 7/5 | 7/1 | 0.325 |
| Side, left/right | 10 (50.0)/10 (50.0) | 6/6 | 4/4 | 0.370 |
| LFSS grade I/II/III | 8(40.0)/9(45)/3(15.0) | 5/6/1 | 3/3/2 | 0.584 |
| Previous epidural injection | 3.7 (2.3–5.0) | 3.1 (1.3–5.0) | 4.5 (3.3–5.0) | 0.157 |
| NRS pain score of leg pain, 0–10 | 6.6 (5.0–8.0) | 7.1 (5.0–8.8) | 5.9 (5.0–6.8) | 0.157 |
| ODI, % | 65.6 (53.3–72.8) | 64.8 (53.3–71.1) | 66.7 (52.8–84.4) | 0.427 |
| RMDQ score, 0–24 | 12.6 (6.0–15.0) | 12.1 (6.0–14.8) | 13.2 (6.5–18.0) | 0.970 |
| Analgesic use | ||||
| Non-NSAIDs* | 8 (40.0) | 4 (33.3) | 4 (50.0) | 0.648 |
| NSAIDs | 10 (50.0) | 5 (41.7) | 5 (62.5) | 0.650 |
| Opioids | 3 (15.0) | 0 (0.0) | 3 (37.5) | 0.049 |
Data are expressed as number (%) or median (interquartile range).
LFSS = lumbar foraminal spinal stenosis; NRS = numerical rating scale; NSAID = nonsteroidal anti-inflammatory drug; ODI = Oswestry Disability Index; RMDQ = Roland Morris Disability Questionnaire.
Non-NSAIDs include antidepressants and/or anticonvulsants.
Baseline characteristics and demographics
| Parameters . | Value (N = 20) . | Responders (N = 12) . | Nonresponders (N = 8) . | P . |
|---|---|---|---|---|
| Age, y | 67.6 (60.3–73.8) | 69.8 (65.3–74.0) | 64.3 (58.5–66.8) | 0.115 |
| Gender, male/female | 7 (35.0)/13 (65.0) | 5/7 | 2/6 | 0.642 |
| Height, cm | 160.7 (153.4–167.6) | 161.5 (153.9–169.0) | 159.5 (149.1–167.1) | 0.624 |
| Weight, kg | 64.6 (58.2–74.3) | 66.3 (55.9–75.8) | 62.2 (58.2–69.5) | 0.343 |
| Hypertension | 13 (65.0) | 9 (75.0) | 4 (50.0) | 0.356 |
| Total duration of pain, mo | 12.0 (8.0–14.0) | 11.2 (8.0–14.0) | 13.1 (8.3–18.0) | 0.305 |
| Level, L4–5/L5–S1 vertebrae | 14 (70.0)/6 (30.0) | 7/5 | 7/1 | 0.325 |
| Side, left/right | 10 (50.0)/10 (50.0) | 6/6 | 4/4 | 0.370 |
| LFSS grade I/II/III | 8(40.0)/9(45)/3(15.0) | 5/6/1 | 3/3/2 | 0.584 |
| Previous epidural injection | 3.7 (2.3–5.0) | 3.1 (1.3–5.0) | 4.5 (3.3–5.0) | 0.157 |
| NRS pain score of leg pain, 0–10 | 6.6 (5.0–8.0) | 7.1 (5.0–8.8) | 5.9 (5.0–6.8) | 0.157 |
| ODI, % | 65.6 (53.3–72.8) | 64.8 (53.3–71.1) | 66.7 (52.8–84.4) | 0.427 |
| RMDQ score, 0–24 | 12.6 (6.0–15.0) | 12.1 (6.0–14.8) | 13.2 (6.5–18.0) | 0.970 |
| Analgesic use | ||||
| Non-NSAIDs* | 8 (40.0) | 4 (33.3) | 4 (50.0) | 0.648 |
| NSAIDs | 10 (50.0) | 5 (41.7) | 5 (62.5) | 0.650 |
| Opioids | 3 (15.0) | 0 (0.0) | 3 (37.5) | 0.049 |
| Parameters . | Value (N = 20) . | Responders (N = 12) . | Nonresponders (N = 8) . | P . |
|---|---|---|---|---|
| Age, y | 67.6 (60.3–73.8) | 69.8 (65.3–74.0) | 64.3 (58.5–66.8) | 0.115 |
| Gender, male/female | 7 (35.0)/13 (65.0) | 5/7 | 2/6 | 0.642 |
| Height, cm | 160.7 (153.4–167.6) | 161.5 (153.9–169.0) | 159.5 (149.1–167.1) | 0.624 |
| Weight, kg | 64.6 (58.2–74.3) | 66.3 (55.9–75.8) | 62.2 (58.2–69.5) | 0.343 |
| Hypertension | 13 (65.0) | 9 (75.0) | 4 (50.0) | 0.356 |
| Total duration of pain, mo | 12.0 (8.0–14.0) | 11.2 (8.0–14.0) | 13.1 (8.3–18.0) | 0.305 |
| Level, L4–5/L5–S1 vertebrae | 14 (70.0)/6 (30.0) | 7/5 | 7/1 | 0.325 |
| Side, left/right | 10 (50.0)/10 (50.0) | 6/6 | 4/4 | 0.370 |
| LFSS grade I/II/III | 8(40.0)/9(45)/3(15.0) | 5/6/1 | 3/3/2 | 0.584 |
| Previous epidural injection | 3.7 (2.3–5.0) | 3.1 (1.3–5.0) | 4.5 (3.3–5.0) | 0.157 |
| NRS pain score of leg pain, 0–10 | 6.6 (5.0–8.0) | 7.1 (5.0–8.8) | 5.9 (5.0–6.8) | 0.157 |
| ODI, % | 65.6 (53.3–72.8) | 64.8 (53.3–71.1) | 66.7 (52.8–84.4) | 0.427 |
| RMDQ score, 0–24 | 12.6 (6.0–15.0) | 12.1 (6.0–14.8) | 13.2 (6.5–18.0) | 0.970 |
| Analgesic use | ||||
| Non-NSAIDs* | 8 (40.0) | 4 (33.3) | 4 (50.0) | 0.648 |
| NSAIDs | 10 (50.0) | 5 (41.7) | 5 (62.5) | 0.650 |
| Opioids | 3 (15.0) | 0 (0.0) | 3 (37.5) | 0.049 |
Data are expressed as number (%) or median (interquartile range).
LFSS = lumbar foraminal spinal stenosis; NRS = numerical rating scale; NSAID = nonsteroidal anti-inflammatory drug; ODI = Oswestry Disability Index; RMDQ = Roland Morris Disability Questionnaire.
Non-NSAIDs include antidepressants and/or anticonvulsants.
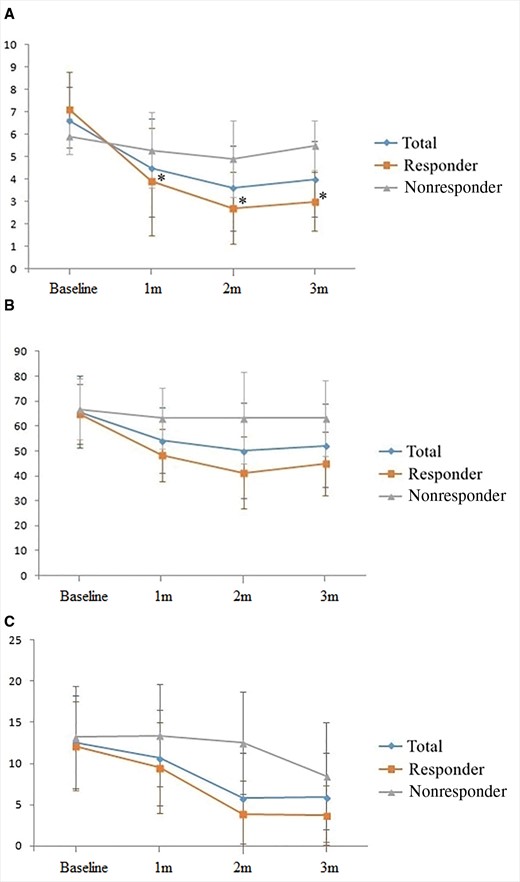
Twelve patients (60%) reported a successful response to the PLEF procedure, with at least 40% pain reduction and no increase from baseline ODI and RMDQ score at Visit 3. Table 2 shows the overall changes in the NRS pain score for the legs, ODI (%), and RMDQ score at three-month follow-up, including a comparison between responders and nonresponders. The mean pain reduction (%) at three months was 36.3% overall. All scores at three months were significantly lower than those at baseline in the responding and nonresponding patients; nonetheless, this was statistically different between the groups. Figure 5 shows that changes in the NRS pain scores, ODI (%), and RMDQ scores decreased significantly over time compared with the baseline score (all P values < 0.001 in general linear mixed model analysis for repeated measures). With regard to patients’ satisfaction, 65% of patients were “extremely satisfied” or “somewhat satisfied” with the PLEF procedure (Table 2), while four patients were “neutral” and two patients were “somewhat dissatisfied.” None of the patients were “extremely dissatisfied” with the PLEF procedure. Although overall the use of analgesics was reduced at three months, there was no statistically significant difference between the responding and nonresponding patients.
Clinical outcomes of responder analysis at three-month follow-up
| Variables . | Total (N = 20) . | Responders (N = 12) . | Nonresponders (N = 8) . | P . |
|---|---|---|---|---|
| NRS pain score at 3 mo | −2.6 (-4.0– -1.0) | −4.1 (-4.8– -3.0) | −0.4 (-1.0–0.0) | <0.001 |
| Changes in NRS from baseline at 3 mo, % | −36.3 (-15.7– -54.2) | −57.0 (-45.8– -71.3) | −5.4 (-20.0–0.0) | <0.001 |
| ODI (%) at 3 mo | −13.3 (-21.7– -5.0) | −19.9 (-25.6– -11.1) | −3.6 (-10.0–3.3) | 0.002 |
| RMDQ score at 3 mo, 0–24 | −6.2 (-8.0– -3.3) | −8.4 (-12.3– -11.1) | −2.9 (-6.8–0.8) | 0.020 |
| Analgesic use | ||||
| Non-NSAIDs* | −3 (5, 20%) | −2 (2, 16.7%) | −1 (3, 37.5%) | 0.347 |
| NSAIDs | −1 (9, 45%) | −2 (3, 25.0%) | +1 (6, 75.0%) | 0.065 |
| Opioids | −1 (2, 10%) | 0 (0, 0.0%) | −1 (2, 25.0%) | 0.147 |
| Satisfaction (1 or 2 at five-point Likert Scale)† | 13 (65) | 10 (83.3) | 3 (37.5) | 0.062 |
| Variables . | Total (N = 20) . | Responders (N = 12) . | Nonresponders (N = 8) . | P . |
|---|---|---|---|---|
| NRS pain score at 3 mo | −2.6 (-4.0– -1.0) | −4.1 (-4.8– -3.0) | −0.4 (-1.0–0.0) | <0.001 |
| Changes in NRS from baseline at 3 mo, % | −36.3 (-15.7– -54.2) | −57.0 (-45.8– -71.3) | −5.4 (-20.0–0.0) | <0.001 |
| ODI (%) at 3 mo | −13.3 (-21.7– -5.0) | −19.9 (-25.6– -11.1) | −3.6 (-10.0–3.3) | 0.002 |
| RMDQ score at 3 mo, 0–24 | −6.2 (-8.0– -3.3) | −8.4 (-12.3– -11.1) | −2.9 (-6.8–0.8) | 0.020 |
| Analgesic use | ||||
| Non-NSAIDs* | −3 (5, 20%) | −2 (2, 16.7%) | −1 (3, 37.5%) | 0.347 |
| NSAIDs | −1 (9, 45%) | −2 (3, 25.0%) | +1 (6, 75.0%) | 0.065 |
| Opioids | −1 (2, 10%) | 0 (0, 0.0%) | −1 (2, 25.0%) | 0.147 |
| Satisfaction (1 or 2 at five-point Likert Scale)† | 13 (65) | 10 (83.3) | 3 (37.5) | 0.062 |
Data are expressed as number (%) or median (interquartile range).
NRS = numerical rating scale; NSAID = nonsteroidal anti-inflammatory drug; ODI = Oswestry Disability Index; RMDQ = Roland Morris Disability Questionnaire.
Non-NSAIDs include antidepressants and/or anticonvulsants.
Patient satisfaction with the extraforaminotomy procedure was assessed using a five-point Likert satisfaction scale (1 = extremely dissatisfied; 2 = somewhat dissatisfied; 3 = neutral; 4 = somewhat satisfied; 5 = extremely satisfied) at Visit 3.
Clinical outcomes of responder analysis at three-month follow-up
| Variables . | Total (N = 20) . | Responders (N = 12) . | Nonresponders (N = 8) . | P . |
|---|---|---|---|---|
| NRS pain score at 3 mo | −2.6 (-4.0– -1.0) | −4.1 (-4.8– -3.0) | −0.4 (-1.0–0.0) | <0.001 |
| Changes in NRS from baseline at 3 mo, % | −36.3 (-15.7– -54.2) | −57.0 (-45.8– -71.3) | −5.4 (-20.0–0.0) | <0.001 |
| ODI (%) at 3 mo | −13.3 (-21.7– -5.0) | −19.9 (-25.6– -11.1) | −3.6 (-10.0–3.3) | 0.002 |
| RMDQ score at 3 mo, 0–24 | −6.2 (-8.0– -3.3) | −8.4 (-12.3– -11.1) | −2.9 (-6.8–0.8) | 0.020 |
| Analgesic use | ||||
| Non-NSAIDs* | −3 (5, 20%) | −2 (2, 16.7%) | −1 (3, 37.5%) | 0.347 |
| NSAIDs | −1 (9, 45%) | −2 (3, 25.0%) | +1 (6, 75.0%) | 0.065 |
| Opioids | −1 (2, 10%) | 0 (0, 0.0%) | −1 (2, 25.0%) | 0.147 |
| Satisfaction (1 or 2 at five-point Likert Scale)† | 13 (65) | 10 (83.3) | 3 (37.5) | 0.062 |
| Variables . | Total (N = 20) . | Responders (N = 12) . | Nonresponders (N = 8) . | P . |
|---|---|---|---|---|
| NRS pain score at 3 mo | −2.6 (-4.0– -1.0) | −4.1 (-4.8– -3.0) | −0.4 (-1.0–0.0) | <0.001 |
| Changes in NRS from baseline at 3 mo, % | −36.3 (-15.7– -54.2) | −57.0 (-45.8– -71.3) | −5.4 (-20.0–0.0) | <0.001 |
| ODI (%) at 3 mo | −13.3 (-21.7– -5.0) | −19.9 (-25.6– -11.1) | −3.6 (-10.0–3.3) | 0.002 |
| RMDQ score at 3 mo, 0–24 | −6.2 (-8.0– -3.3) | −8.4 (-12.3– -11.1) | −2.9 (-6.8–0.8) | 0.020 |
| Analgesic use | ||||
| Non-NSAIDs* | −3 (5, 20%) | −2 (2, 16.7%) | −1 (3, 37.5%) | 0.347 |
| NSAIDs | −1 (9, 45%) | −2 (3, 25.0%) | +1 (6, 75.0%) | 0.065 |
| Opioids | −1 (2, 10%) | 0 (0, 0.0%) | −1 (2, 25.0%) | 0.147 |
| Satisfaction (1 or 2 at five-point Likert Scale)† | 13 (65) | 10 (83.3) | 3 (37.5) | 0.062 |
Data are expressed as number (%) or median (interquartile range).
NRS = numerical rating scale; NSAID = nonsteroidal anti-inflammatory drug; ODI = Oswestry Disability Index; RMDQ = Roland Morris Disability Questionnaire.
Non-NSAIDs include antidepressants and/or anticonvulsants.
Patient satisfaction with the extraforaminotomy procedure was assessed using a five-point Likert satisfaction scale (1 = extremely dissatisfied; 2 = somewhat dissatisfied; 3 = neutral; 4 = somewhat satisfied; 5 = extremely satisfied) at Visit 3.
Changes in the numerical rating scale pain score for the legs (A), Oswestry Disability Index (%) (B), and the Roland Morris Disability Questionnaire score (C). *P < 0.05 compared with baseline.
All adverse events that occurred during the study period were minor and temporary. A majority (N = 13, 65%) of patients reported temporary pain during the procedure, but it was tolerable and did not require additional medication or discontinuation of the procedure. Nine patients complained of procedure-related pain for two to three days in the postprocedural period, but this spontaneously resolved without any neurological sequelae. Transient paresthesia in the lower extremity was reported in three patients; however, this resolved within a week. No other adverse events, such as dural puncture, hematoma formation, persistent motor or sensory impairment, severe pain, paresthesia, or infection were reported.
Discussion
This prospective pilot study showed that the PLEF procedure was successful in 60% (N = 12) of appropriately selected patients with radicular pain from LFSS. Additionally, patients who responded well also showed function improvement as assessed by the ODI (%) and RMDQ score at three-month follow-up. No serious complications were reported in the study. Presumably, the PLEF procedure alleviates LFSS symptoms by mechanically eliminating adhesions of ligamentous structures, which may compress exiting nerve roots, reducing venous stasis, decreasing perineural edema, and eventually promoting spread of injectate [18,20]. PLEF targets adhesiolysis, particularly at the posterior and inferior quadrant of the neural foramen using the rounded rim of a cannula, blunt edge portion of the end mill, and cup-shaped curette. These objects are designed to minimize damage to the surrounding tissue, such as the dura, dorsal root ganglion, and blood vessels. Although a few patients experienced temporary dysesthesia after the procedure, perhaps due to irritation of the exiting nerve root, this was completely relieved in a period of two to three days.
A recent study showed that the success rate of a ventral epidural spread of contrast medium is relatively lower in LFSS than in disc bulging or herniation of the nucleus pulposus [31], and this might be related to a poor outcome. TFESI may allow a high concentration of injectate including corticosteroid to be delivered to the ventral epidural space, where mechanical and chemical irritation to the nerve root generally occurs [32,33]. However, previous studies reported various rates in successful outcomes following TFESI in LFSS. One study presented that about 30% of the patients showed at least 50% or more pain relief at three-month follow-up [34]. The other study suggested that 61.4% of the patients had a reduction of at least two points on the NRS score at two months [6]. These broad success rates might not only be due to the use of different definitions for successful responders, various periods for follow-ups, or different volumes of injectate, but they may also be associated with the diverse causes of LFSS, such as epidural scars and hypertrophy of bony structures, ligaments, and joint capsules, which are contained within a small-sized neural foramen [1–3]. Alternatively, PEA can be used for managing pain from LFSS. Several studies have reported that PEA has a superior efficacy when compared with TFESI in LFSS [9,10,35]. Nonetheless, poor outcomes of PEA are relatively common in LFSS in comparison with central or subarticular stenosis [8,36,37]. We assume that the central canal or subarticular area narrowed by stenosis may be able to provide space for a catheter to be advanced or placed at target sites because of its relatively large diameter compared with the neural foramen. However, even a one-third reduction in the diameter of the neural foramen could seriously hinder the advancement of the catheter to the target neural foramen, consequently precluding the elimination of adhesion [8]. In such cases, the PLEF procedure, a minimally invasive technique, may be considered for detaching the adhesions first. This procedure could also improve the effectiveness of subsequent TFESI or PEA by promoting the spread of the injectate and making a space for inserting the catheter into the neural foramen. Nonetheless, although all adverse events that occurred in the study period were minor and temporary, the mechanically detaching procedure with repetitive advancement and withdrawal of the instrument may have caused procedure-related pain with a bleeding risk during the PLEF procedure. Therefore, in a treatment algorithm of spinal stenosis, PLEF may be suggested as an important procedure as part of the interventional repertoire for the treatment of LFSS that does not need surgical intervention but has been refractory to more conventional interventions such as TFESI or PEA.
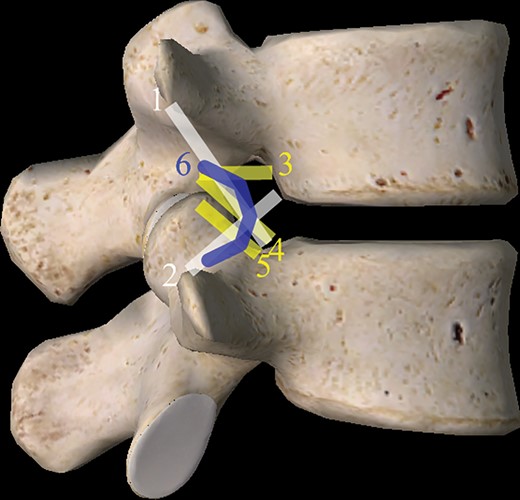
Previous studies have reported that 35% of the foramen area is occupied by the nerve root based on the transverse diameter of the neural compartment [38,39]. Ligaments associated with spinal discs and adjacent articular surfaces can proliferate as a part of the degenerative mechanism, narrowing the disc space and potentially causing compression of the exiting nerve roots [38,19]. Additionally, previous anatomic studies found five types of ligaments in the neural foramen: the superior and inferior corporotransverse ligaments and the superior, middle, and inferior transforaminal ligaments (Figure 6) [4,18,39] These foraminal ligaments are strong and unyielding structures, varying in width and thickness in accordance with vertebral levels [4]. By positioning devices in the posterior and lower quadrant of the target neural foramen, which is just anterior to the ventral articular facet, we assumed that the EF procedure can help to detach adhesions of the inferior transforaminal ligament, lower part of the superior corporopedicular ligament, midtransforaminal ligament, and part of the anterior facet joint capsule. Intraforaminal vessels are located at the anterior and lower quadrant of the intervertebral foramen [4]; therefore, they can presumably be preserved during the procedure.
Schematic drawing of ligaments in the lumbar neural foramen. 1 = superior corporotransverse ligament; 2 = inferior corporotransverse ligament; 3 = superior transforaminal ligament; 4 = middle transforaminal ligament; 5 = inferior transforaminal ligament; 6 = posterior transforaminal ligament.
This study has several limitations. First, this was a prospective pilot study with a small number of participants and only a three-month follow-up period, which is relatively shorter than previous clinical trials of PEA or TFESI [27,40]. In addition, we could not include a control or comparison group such as TFESI without adhesiolysis. Epidural injections of steroid and local anesthetics can inhibit prostaglandin synthesis, stabilization of cellular membranes, suppress immune responses, enhance neuronal blood flow, and wash out inflammatory mediators, in addition to blocking nociceptive C fiber conduction [41,42]. In all patients in this study, a local anesthetic with dexamethasone was administrated into the epidural space, following the foraminal adhesiolysis. Therefore, these limitations may affect the superiority of PLEF over TFESI. Second, we recruited patients with radicular pain but without axial pain so that participants had clinical features similar to those in LFSS. As TFESI or transforaminal PEA were also frequently indicated for reduction of axial lower back pain [6–10], further studies in patients with axial pain are required. Finally, although we confirmed a reduction in neural foraminal adhesions by pre- and postepidurography, we did not compare them quantitatively via epidurography or confirm this by obtaining postprocedure MRI. However, transforaminal ligaments are unlikely to be identified by the majority of radiologists, which means there is a 51% chance they could be missed when a trained radiologist identifies ligaments in an intervertebral foramen [43]. Furthermore, although MRI is broadly used in establishing the diagnosis of LSS and for recommending treatment to patients with LSS, the relationship between abnormal MRI findings and severity in pain from lumbar spinal stenosis is still debated [44]. While embracing these limitations, further better-designed randomized controlled studies with long-term follow-up are needed to verify the clinical implications of PLEF.
Despite these limitations, we have demonstrated some important clinical points. This study showed that PLEF can reduce pain intensity and result in functional improvement of LFSS patients who do not respond to other conservative treatments. In conclusion, a PLEF performed with a specially designed instrument can be an effective and safe treatment option as well as a minimally invasive procedure for patients suffering from refractory pain due to LFSS.
Authors’ Contributions
Sang Chul Lee and Won-Joong Kim contributed equally to this study.
Acknowledgment
The authors thank all participating patients, colleagues, and the staff of the institution for their contributions in data collection and Dr. Kyung Woo Park MD, PhD, and Dr. Hyeon Jung Hwang, MD, at Gwanghye Hospital for their help during the study period.
References
Author notes
Funding source: This study was sponsored by Bio-Spine Corp., Seoul, Republic of Korea. The funder was not involved in conducting the study or developing the manuscript.
Conflict interests: There are no conflicts of interest to report.