-
PDF
- Split View
-
Views
-
Cite
Cite
Edward J. Cone, George E. Bigelow, Evan S. Herrmann, John M. Mitchell, Charles LoDico, Ronald Flegel, Ryan Vandrey, Non-Smoker Exposure to Secondhand Cannabis Smoke. I. Urine Screening and Confirmation Results, Journal of Analytical Toxicology, Volume 39, Issue 1, January/February 2015, Pages 1–12, https://doi.org/10.1093/jat/bku116
Close - Share Icon Share
Abstract
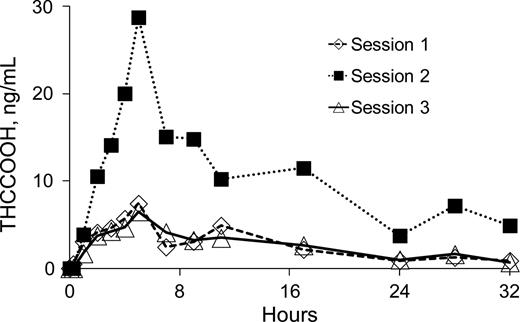
Increased cannabis potency has renewed concerns that secondhand exposure to cannabis smoke can produce positive drug tests. A systematic study was conducted of smoke exposure on drug-free participants. Six experienced cannabis users smoked cannabis cigarettes (5.3% THC in Session 1 and 11.3% THC in Sessions 2 and 3) in a sealed chamber. Six non-smokers were seated with smokers in an alternating manner. Sessions 1 and 2 were conducted with no ventilation and ventilation was employed in Session 3. Non-smoking participant specimens (collected 0–34 h) were analyzed with four immunoassays at different cutoff concentrations (20, 50, 75 and 100 ng/mL) and by GC-MS (LOQ = 0.75 ng/mL). No presumptive positives occurred for non-smokers at 100 and 75 ng/mL; a single positive occurred at 50 ng/mL; and multiple positives occurred at 20 ng/mL. Maximum THCCOOH concentrations by GC-MS for non-smokers ranged from 1.3 to 57.5 ng/mL. THCCOOH concentrations generally increased with THC potency, but room ventilation substantially reduced exposure levels. These results demonstrate that extreme cannabis smoke exposure can produce positive urine tests at commonly utilized cutoff concentrations. However, positive tests are likely to be rare, limited to the hours immediately post-exposure, and occur only under environmental circumstances where exposure is obvious.
Introduction
Cannabis is the most widely produced and illicitly consumed drug globally. The number of cannabis users has been estimated to be as high as 224 million worldwide, and prevalence of use has remained stable in recent years (1). Over the last decade, indoor cultivation of cannabis has proliferated. Increased indoor growing has been mirrored by an increase in shops and Internet sites that provide information, supplies, equipment and seeds for production. The increased support system for cannabis cultivation together with availability of high-quality seeds has greatly expanded access to high-yielding and highly potent cannabis varieties (1). These plants have high levels of Δ9-tetrahydrocannabinol (THC), the primary cannabinoid responsible for psychoactive effects, and, most commonly, negligible levels of cannabidiol (CBD) and other trace cannabinoids that may have therapeutic potential and may counteract some of the effects of THC (2). Use of cannabis preparations containing high potency THC/low CBD cannabinoid ratios has been linked to a number of putative outcomes (3) including increased risk of psychosis (4) and cannabis dependence (5).
Cannabis terminology varies considerably and numerous terms are in use referring to similar or related cannabis products. The term ‘marijuana’ is generally used to refer to the Cannabis plant (leaves, stems, seeds and flowering tops); whereas, the term ‘sinsemilla’ refers to the flowering tops of unfertilized female plants with no seeds. Globally, two main products are produced from cannabis: cannabis herb and cannabis resin. Cannabis resin, also known as ‘Hashish’ is composed of the resinous parts of the flowering tops of cannabis and is mixed with some plant particles and shaped into a variety of forms, e.g., balls, sticks or slabs. ‘Hash oil’ is a liquid or semi-solid concentrated extract of cannabis plant material.
Cannabis herb is produced and consumed in almost all countries of the world, whereas cannabis resin is produced primarily in North Africa, the Near and Middle East and South-West Asia (6). More recently, there has been a proliferation of alternative cannabis preparations and routes of administration with the advent of more relaxed laws regarding cannabis use and the introduction of a commercial market in some areas. These include various forms of cannabis extracts and oils sold as ‘dab’, ‘wax’, ‘shatter’ and a multitude of cannabis-infused food products (e.g., brownies, candy, butter, granola, beverages), commonly referred to as ‘edibles'. Cannabis is often consumed for the psychoactive and physiological effects produced following use including heightened mood or euphoria, relaxation and an increase in appetite, though the use of cannabis for purported medical/health benefit has gained prominence in the past several decades.
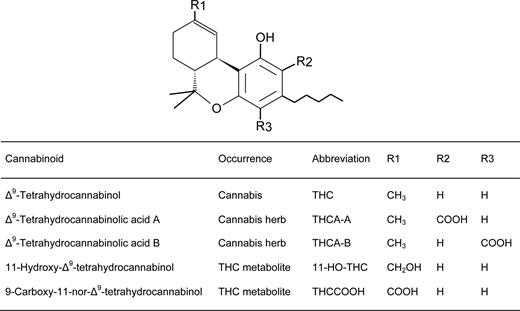
Molecular Structures of THC, THCA-A, THCA-B, 11-HO-THC and THCCOOH.
The shift in cultivation practices over the last 30 years toward production of higher potency THC cannabis with negligible levels of CBD has raised concerns that current cannabis is ‘somehow a different drug than that consumed in previous decades' (3). Indeed, the THC potency of confiscated cannabis in the US was 2.83% for marijuana and 7.28% for sinsemilla in 1985 (8). THC potency of federal seizures increased steadily over the ensuing years, reaching 6.73% for marijuana and 16.19% for sinsemilla in 2012, with the average potency for all cannabis types being 13.19% (9).
Given these consistent potency increases, the possible impact of higher potency cannabis on various drug-testing programs prompted renewed study and evaluation. Concerns have been expressed for decades that a non-smoker in the vicinity of cannabis smokers are exposed, in much the same way as non-smokers are exposed to tobacco smoke, to vaporized constituents of cannabis. A variety of studies have documented the extent of secondhand cannabis smoke exposure to non-smokers under varying condition such as enclosed unventilated rooms [six non-smokers exposed to 16 smoked cannabis cigarettes, 2.8% THC (10–12); a single non-smoker in close proximity to four smokers smoking 0, 0.86, 1.54 or 1.83% THC (13); four non-smokers exposed to six smokers smoking a cannabis cigarette containing 17.2 mg THC (∼1.5% THC) (14); two or three non-smokers exposed to four smokers smoking a cannabis cigarette containing 26.2 mg (∼2.6% THC) (15); and three non-smokers exposed to four cannabis cigarettes containing 27 mg THC (∼2.7% THC) (16)], non-smoker exposure to hashish smoking (17), exposure in a small, closed car in which five non-smokers were exposed to three smokers smoking 1.5% THC cannabis or two smokers smoking 1.5% THC hashish cigarettes (18) and exposure in a non-ventilated room or medium-sized station wagon to 2.5% THC or 2.8% THC cannabis cigarettes (19).
Two more recent exposure studies have been conducted with higher potency cannabis cigarettes. In one study, four non-smokers were exposed to cannabis smoke generated by four smokers who consumed a single cannabis cigarette containing either 5.4% or 10.4% THC cannabis in an unventilated eight-passenger van (20). The second study was conducted as a field experiment in a ventilated coffee shop (establishment where cannabis and hashish can be openly consumed) in the Netherlands (21). In this study, eight non-smokers remained in the shop for 3 h in the presence of numerous smokers who consumed cannabis primarily by smoking, but some individuals used hashish pipes or water pipes. The exact cannabis potency, number of cigarettes, or amount of cannabis consumed in this study is not known. As with the prior studies, the two studies involving high-potency cannabis exposure to non-smokers resulted in the detection of THC and metabolites in urine, blood and oral fluid specimens collected from non-smokers after exposure.
The goal of the current study was to extend research involving high-potency environmental cannabis smoke exposure (commonly referred to as ‘passive exposure’) to non-smokers. Specifically, the study was designed to ascertain the effects of cannabis potency and room ventilation on both pharmacokinetic and pharmacodynamic outcomes when non-smokers were exposed to concentrated cannabis smoke. Urine and other biological fluids and tissues were collected before and after smoke exposure to determine if exposure under any of these conditions would produce positive drug tests based on commonly used drug-testing standards. This report provides a detailed description of the experimental conditions employed in the study and provides complete initial screening and confirmatory data derived from urine specimens.
Experimental
Participants
Volunteer participants were recruited through newspaper advertisements, flyers posted on campus and community bulletin boards and word-of-mouth. Two types of volunteers were recruited: (i) current cannabis users (self-reported use of cannabis at least two times per week during the prior 90 days) who were not currently trying to quit; and (ii) healthy volunteers who had a history of lifetime cannabis exposure, but had not used cannabis or other illicit substances within the previous 6 months (self-report). Current cannabis users were required to test positive for cannabis (>50 ng/mL THCCOOH) and negative for recent use of other commonly used psychoactive substances (amphetamines, benzodiazepines, cocaine, MDMA, opioids, PCP and alcohol) at the screening visit and upon admission to the research unit for each experimental session. Non-smokers were required to test negative for all of the above substances at screening and at admission to experimental sessions. Only non- or minimal tobacco users were enrolled; no tobacco use was permitted during study participation.
Eight current cannabis users participated in three experimental cannabis smoking sessions. The eight subjects (three females and five males) had an average [standard deviation (SD), range] age of 29 (6, 24–40) years, weighed an average of 75 (20, 50–108) kg and had an average body mass index of 25.6 (5.1, 20.3–34.2). These eight subjects self-identified their race/ethnicity as follows: four White, non-Hispanic, two Black or African American, one Hispanic or Latino and one as Middle Eastern (Turkish). The smokers self-reported an average of 12 (7, 2–25) years smoking cannabis. They reported an average of 28 (2, 25–30) days of cannabis use in the previous month and consumed an average of 1.5 (2, 0.4–5.2) grams of cannabis per day. The 18 non-smoker participants (nine females and nine males) had an average age of 28 (7, 20–45) years, weighed an average of 74 (12, 55–98) kg and had an average body mass index of 24.7 (3.6, 18.7–33.0). These subjects self-identified as follows: 12 White, non-Hispanic, three African American, two Hispanic or Latino and one Asian.
To the extent possible, conditions were standardized across sessions. Consequently, the six cannabis smokers who participated in Session 1 were invited to continue their participation in Sessions 2 and 3. Four smokers (2M/2F) participated in all three sessions. Two smokers (1M/1F) participated in two sessions and two (2M) participated in a single session. Each of the 18 non-smoking subjects participated in only a single session.
Written informed consent was obtained prior to study participation. The study was approved by the Johns Hopkins Medicine Institutional Review Board and conducted in accordance with the ethical standards of the Helsinki Declaration. All subjects were compensated for their participation.
Chamber construction and layout
A specially designed smoking chamber, made of plexi-glass walls with aluminum supports and measuring 10 ft. × 13 ft. (3.05 m × 3.96 m) with a 7 ft. (2.13 m) ceiling, was constructed for this experiment. It was situated in a larger room that allowed direct observation of all parts of the chamber from three sides. The door to the exposure chamber was also constructed of plexi-glass and aluminum and was fitted with magnets that ran the entire perimeter to create a seal when closed. The door remained closed during each session with two exceptions. One non-smoker smoker (Session 2) and one smoker (Session 3) were allowed a brief bathroom break that lasted ∼5 min. Consequently, the door was opened briefly for exit and entry in those sessions. A metal/plastic utility table (30 in × 6 ft.) was located in the center of the chamber and 12 metal/plastic folding chairs were situated equidistant around the table. During each exposure session, six smokers and six non-smokers were seated in alternate seating positions around the table.
All participants donned disposable paper clothing including booties over their own clothing before entering the experimental chamber for each session. Smokers also wore disposable head coverings to prevent deposition of cannabinoids on their hair during the session. Non-smokers did not wear head coverings because deposition of cannabinoids on hair during naturalistic secondhand exposure might affect hair drug test results, a secondary study outcome. All participants were supplied with goggles for use as needed for reduction of eye irritation from the smoke. During each session, participants remained in their assigned seats and played games, conversed, or engaged in other activities (e.g., listened to music, used cell phone). Smokers were allowed to drink from bottles of water (supplied at the start of the session). Non-smokers were not allowed to eat or drink during the session or after the session until after the first oral fluid specimen was collected. As a safety measure, pulse oximeter readings were collected pre-session and at 15-min intervals during each session to ensure that an adequate oxygen supply was maintained within the chamber.
Cannabis cigarettes
Cannabis for research purposes was obtained through the US federal drug supply program. Two types of Mississippi-grown cannabis with varying cannabinoid content were supplied for the study and were characterized and rolled into cigarettes by staff at Research Triangle Institute, International. The lower potency cannabis cigarettes were machine rolled and were 85 mm in length × 25 mm circumference and weighed a mean weight (SD) of 0.92 (0.06) g/cigarette; the cigarettes had an assayed mean content of cannabinoids as follows: 5.3% (0.48%) total THC; 0.01% (0.0%) CBD and 0.35% (0.04%) cannabinol (CBN). The higher potency cigarettes were hand-rolled and were 70 mm in length (24.5 mm) and had a mean weight (SD) of 1.0 (0.04) g/cigarette; the cigarettes had an assayed mean content (n = 12 for THC; n = 4 for other cannabinoids) of cannabinoids as follows: 11.3% (0.29%) total THC; 0.08% (0.12%) CBD and 0.76% (0.06%) CBN.
Experimental cannabis exposure conditions
Three experimental cannabis sessions were conducted at weekly or greater intervals. Cannabis exposure sessions lasted 1 h, during which smokers consumed cannabis ad-libitum in the presence of non-smokers inside the closed chamber. The primary goal of these sessions was to conduct a pharmacokinetic evaluation of cannabinoids in biological fluids of non-smokers following extreme exposure to secondhand cannabis smoke. Across the three experimental sessions, cannabis potency and room ventilation conditions were manipulated: Session 1 was conducted without air ventilation and cannabis cigarettes containing 5.3% THC were smoked; Session 2 was conducted without air ventilation and cannabis cigarettes containing 11.3% THC were smoked; Session 3 was conducted with active air ventilation comparable to home air-conditioning (11.2 air changes per hour) and cannabis cigarettes containing 11.3% THC were smoked. Each smoker received a pre-weighed individual supply of cannabis cigarettes at the start of each session, and residues and unused portions were collected for weighing at session end.
Experimental procedures following cannabis smoke exposure
At the end of each 1-h cannabis exposure session, participants exited the room and immediately discarded their disposable clothing and washed their hands and face with soap and water. After drying, they proceeded to a cannabis-free room (investigative area) for participation in specimen collections and behavioral and physiological assessments.
Urine, whole blood and oral fluid specimens were collected prior to each session (baseline) and at timed intervals following each session. Coincident with biological specimen collection, vital signs (heart rate, blood pressure), subjective ratings of intoxication and measures of cognitive performance were also obtained. Hair specimens were collected before and after each session from non-smokers. A single pre-study baseline hair specimen was collected from each cannabis smoker and an additional hair specimen was after Session 1 (or subsequent session for smokers who did not participate in all 3 sessions). Experimental measures were obtained every 30 min for the first 2 h, hourly during hours 2–4 and every 2 h during hours 6–8. Smokers were discharged after the 8-h post-exposure time point. Non-smokers remained in the study under supervision over-night and biological specimens and pharmacodynamic measures were obtained through the 34-h post exposure time point. Assessments and outcomes, other than urine testing results, will be reported elsewhere.
Urine collections
Baseline urine specimens were collected ∼1 h prior to each cannabis session. Following the end of each 1-h cannabis exposure period (designated zero time), participants were asked to void at 0.25, 1, 2, 3 and 4 h. Thereafter, urine specimens were pooled for each subject for the following time intervals: 4–6, 6–8, 8–10, 10–12, 12–22, 22–26, 26–30 and 30–34 h. If multiple specimens were produced by an individual during a pool period, they were combined into an individual pool. Each participant was asked to empty their bladder at the end of each pool period for inclusion in that period. No mixing of specimens between participants occurred.
Because of the logistics involved in collecting multiple types of measures from 12 participants, the exact timing of early specimen collections was somewhat variable; consequently, all specimen times should be considered as nominal values (i.e., ±10 min).
Urine specimens were collected in clean, plastic containers labeled with the participant's identification number, date and collection time. Specimens to be pooled were transferred to a labeled plastic pooling vessel and kept refrigerated during the collection period. The volumes of each individual specimen (first 4 h) and of each pooled sample were measured and two aliquots (minimum of 30 mL each) of each were transferred to polypropylene bottles (bottles ‘A’ and ‘B’). If the specimen volume was <60 mL, the specimen was divided into aliquots of approximately equal volume. All aliquots were stored frozen (≤−20°C) and shipped frozen by overnight express to a designated laboratory for analysis.
Analytical methods
Initial analyses of urine specimens were conducted by Clinical Reference Laboratory (CRL), Lenexa, KS. Bottle A specimens were thawed and aliquots were analyzed by immunoassay and gas chromatography–mass spectrometry (GC–MS). Initial analyses of Bottle A specimens by immunoassays were conducted according to manufacturer's procedure with the Microgenics DRI assay on a Bayer ADVAI 2,400 analyzer for cannabinoids in urine at both 20 ng/mL and 50 ng/mL cutoff concentrations. Creatinine was determined with Siemens modified Jaffe reagent. Specific gravity was determined with a Rudolph J57 refractometer. Determinations of pH were made with Axiom pH reagents (Axiom Diagnostics, Tampa, FL, USA).
THCCOOH concentrations were measured by a current, validated GC-MS method by CRL. Briefly, 40 ng/mL of internal standard (THCCOOH-d9, Cerillant Corp., Round Rock, TX) was added to 1 mL of specimen and the sample was hydrolyzed with 0.2 mL of 5N NaOH. After hydrolysis, 1.5 mL of glacial acetic acid (pH 4) was added and THCOOH was extracted with a solid phase column (3 mL J-65 cation exchange, Biochemical Diagnostics, Edgewood, NY, USA). The column was eluted with 1.5 mL of n-butyl chloride/triethylamine (80/20, v/v) and the eluate was evaporated and derivatized with bistrimethylsilyltrifluoroacetamide (BSTFA). The specimen was transferred to an injection vial and analyzed on an Agilent 5,975 GC/MS. Ions (m/z) monitored were 380 and 479 for the internal standard and 371, 473 and 488 for THCCOOH. The calibration standard (single point calibration) contained 15 ng/mL of THCCOOH and 40 ng/mL of THCCOOH-d9. Four controls (negative, 6 ng/mL, 18.5 ng/mL, pooled positive urine for hydrolysis control) were assayed with each batch. The method had a limit of detection (LOD) and limit of quantification (LOQ) for THCCOOH of 0.75 ng/mL and an upper limit of linearity (ULOL) of 600 ng/mL. Specimens with concentrations ≥ULOL were diluted to provide accurate quantitation. Criteria for acceptance of results included the following: retention times of analyte and internal standard within ±2% of the calibrator; ion ratios within ±20% of the calibrator; and positive control concentrations ±20% of established concentrations.
Following analyses of Bottle A by CRL, Bottle B specimens were thawed for additional immunoassay determinations, aliquoted, and frozen aliquots were immediately shipped to three additional laboratories. Bottle B aliquots were sent to selected laboratories with differing types of immunoassays. They were analyzed as follows (laboratory, location, immunoassay type, cutoff concentrations): CRL, Lenexa, Microgenics DRI, 20 ng/mL, 50 ng/mL; MEDTOX Laboratories, St Paul, MN, KIMS, 20 ng/mL, 50 ng/mL; MetroLab-Legacy Laboratory Services, Portland, OR, EMIT II, 20 ng/mL, 50 ng/mL; and One Source Toxicology Laboratory, Pasadena, TX, CEDIA, 20 ng/mL, 50 ng/mL, 75 ng/mL, 100 ng/mL.
Sensitivity, specificity and agreement
The sensitivity, specificity and agreement of the immunoassays for detection of cannabinoids in urine were calculated by comparison of the qualitative immunoassay response at different cutoff concentrations to the quantitative GC–MS result for THCCOOH. The confirmation cutoff concentration of ≥15 ng/mL for THCCOOH, as utilized in the Substance Abuse and Mental Health Services Administration (SAMHSA) Mandatory Guidelines for Federal Workplace Drug Testing Programs (22), was used to determine if a specimen was positive. True-positive (TP) (immunoassay response ≥ cutoff concentration and GC–MS positive), true-negative (TN) (immunoassay response < cutoff concentration and GC–MS negative), false-positive (FP) (immunoassay response ≥ cutoff concentration and GC–MS negative), and false-negative (FN) (immunoassay response < cutoff concentration and GC–MS positive) were calculated versus GC–MS at the 15 ng/mL cutoff concentration. Diagnostic sensitivity, 100 × [TP/(TP + FN)]; diagnostic specificity, 100 × [TN/(TN + FP)]; and agreement, 100 × [(TP + TN)/(TP + TN + FP + FN)] were calculated at multiple screening cutoff concentrations.
Results
Cannabis use and reported smoke effects
The total, overall amount of cannabis material smoked (determined by weighing cannabis cigarettes provided to all six smokers prior to smoking and weighing remaining cannabis cigarettes and ‘butts' at the end of each 1-h exposure period) by session was as follows: Session 1, total of 10.3 g of 5.3% THC cannabis; Session 2, total of 14.4 g of 11.3% THC cannabis; and Session 3, total of 16.5 g of 11.3% THC cannabis. The mean (range) and median amount of cannabis consumed per smoker was as follows: Session 1, 1.7 g (1.1–2.5 g), 1.6 g; Session 2, 2.4 g (1.6–2.9 g), 2.48 g; and Session 3, 2.8 g (2.1–3.4 g), 2.9 g.
Participants were supplied with goggles to wear during sessions to keep smoke from their eyes and reduce eye irritation. In Session 1, most participants elected not to wear the goggles initially, but then experienced substantial eye irritation. As a result, some smokers reported that they stopped cannabis consumption to avoid adding more smoke to the room at a point where they would have otherwise continued to smoke. Other smokers reported stopping cannabis use during Session 1 because they felt social pressure to do so because others had stopped smoking (due to eye irritation). After Session 1, all participants (both smokers and non-smokers) utilized the goggles part-time or full-time and no longer had issues with eye irritation. The combined effect of these occurrences and possibly other factors was that total cannabis consumption in Session 1, at the lower THC potency, was less than in Sessions 2 and 3.
Photograph from outside the exposure room approximately mid-way through Session 2 in which six cannabis smokers and six non-smokers participated in cannabis smoke studies.
Urinalyses of non-smoker specimens
Analyses of non-smokers urine specimens following exposure to concentrated secondhand cannabis smoke
| Subject # . | Time, h . | THCCOOH GC/MS, ng/mL . | Volume, mL . | Creatinine, mg/dL . | Specific gravity . | pH . | CRL1 DRI, Cutoff = 20 ng/mL, (Equivalent IA response = 20) . | Med Tox KIMS 20 Cutoff = 20 ng/mL, (Equivalent IA response = 0) . | MetroLab EMIT II Plus 20 Cutoff = 20 ng/mL, (Equivalent IA response = 100) . | One Source CEDIA 20 Cutoff = 20 ng/mL . |
|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | ||||||||||
| 7 | −1 | 0 | NA | 56.0 | 1.0055 | 6.7 | −2 | −151 | 10 | NEG |
| 7 | 0.25 | 0 | 230 | 64.5 | 1.0066 | 7.1 | 0 | −149 | 15 | NEG |
| 7 | 1 | 3.8 | 49 | 187.7 | 1.0155 | 6.5 | 28 | −36 | 110 | NEG |
| 7 | 2 | 7.0 | 50 | 177.8 | 1.0162 | 6.3 | 34 | 13 | 158 | POS |
| 7 | 3 | 2.6 | 190 | 52.6 | 1.0056 | 6.2 | −3 | −133 | 42 | NEG |
| 7 | 4 | 1.3 | 220 | 24.1 | 1.0028 | 5.9 | 1 | −138 | 21 | NEG |
| 7 | 4–6 | 1.1 | 450 | 22.2 | 1.0027 | 7.0 | −2 | −145 | 24 | NEG |
| 7 | 6–8 | 2.0 | 380 | 50.4 | 1.0062 | 7.1 | 3 | −119 | 37 | NEG |
| 7 | 8–10 | 3.9 | 195 | 106.3 | 1.0132 | 6.4 | 8 | −91 | 50 | NEG |
| 7 | 10–12 | 2.6 | 455 | 37.2 | 1.0051 | 6.8 | 0 | −139 | 24 | NEG |
| 7 | 12–22 | 1.9 | 440 | 82.5 | 1.0114 | 6.2 | 2 | −112 | 35 | NEG |
| 7 | 22–26 | 1.3 | 640 | 50.7 | 1.0084 | 6.9 | −2 | −147 | 23 | NEG |
| 7 | 26–30 | 1.7 | 305 | 124.5 | 1.0155 | 7.1 | −3 | −133 | 30 | NEG |
| 7 | 30–34 | 1.0 | 670 | 40.1 | 1.0076 | 7.3 | −3 | −146 | 15 | NEG |
| 11 | −1 | 0 | NA | 122.0 | 1.0153 | 6.2 | −8 | −167 | 7 | NEG |
| 11 | 0.25 | 0 | 122 | 64.9 | 1.0109 | 6.8 | 2 | −113 | 40 | NEG |
| 11 | 1 | 3.1 | 69 | 86.5 | 1.0121 | 5.5 | 28 | −47 | 102 | NEG |
| 11 | 2 | 1.8 | 140 | 53.1 | 1.0073 | 5.6 | 9 | −94 | 60 | NEG |
| 11 | 3 | 1.3 | 170 | 41.2 | 1.0048 | 5.4 | 4 | −112 | 33 | NEG |
| 11 | 4 | 3.8 | 35 | 151.7 | 1.0161 | 5.7 | 25 | −36 | 100 | NEG |
| 11 | 4–6 | 6.8 | 71 | 142.1 | 1.0173 | 5.5 | 27 | 6 | 112 | NEG |
| 11 | 6–8 | 2.2 | 140 | 65.1 | 1.0094 | 6.1 | 7 | −84 | 50 | NEG |
| 11 | 8–10 | 2.1 | 140 | 96.1 | 1.0139 | 6.8 | 5 | −88 | 60 | NEG |
| 11 | 10–12 | 1.8 | 210 | 52.3 | 1.0070 | 5.8 | 1 | −141 | 27 | NEG |
| 11 | 12–22 | 1.5 | 730 | 54.4 | 1.0073 | 7.3 | −1 | −142 | 32 | NEG |
| 11 | 22–26 | 1.0 | 300 | 86.4 | 1.0142 | 7.1 | −4 | −126 | 36 | NEG |
| 11 | 26–30 | 1.1 | 280 | 51.6 | 1.0093 | 7.3 | −2 | −129 | 42 | NEG |
| 11 | 30–34 | 1.3 | 180 | 130.6 | 1.0174 | 6.2 | −2 | −106 | 38 | NEG |
| 13 | −1 | 0.0 | NA | 56.2 | 1.0126 | 7.1 | −6 | −163 | 22 | NEG |
| 13 | 0.25 | 0.8 | 218 | 19.6 | 1.0041 | 7.2 | 0 | −142 | 18 | NEG |
| 13 | 1 | 4.0 | 190 | 46.8 | 1.0094 | 7.4 | 10 | −110 | 65 | NEG |
| 13 | 2 | 13.1 | 54 | 100.7 | 1.0179 | 7.4 | 34 | 9 | 170 | POS |
| 13 | 3 | 15.6 | 36 | 91.1 | 1.0154 | 7.1 | 36 | 35 | 174 | POS |
| 13 | 4 | 14.1 | 48 | 100.6 | 1.0148 | 6.2 | 30 | 14 | 139 | POS |
| 13 | 4–6 | 9.6 | 170 | 90.0 | 1.0135 | 5.8 | 19 | −63 | 80 | NEG |
| 13 | 6–8 | 4.7 | 140 | 52.2 | 1.0089 | 5.8 | 7 | −113 | 51 | NEG |
| 13 | 8–10 | 7.0 | 140 | 115.5 | 1.0197 | 6.0 | 11 | −56 | 68 | NEG |
| 13 | 10–12 | 19.3 | 210 | 142.2 | 1.0234 | 5.8 | 33 | 83 | 188 | POS |
| 13 | 12–22 | 3.3 | 480 | 98.9 | 1.0195 | 5.7 | −1 | −102 | 41 | NEG |
| 13 | 22–26 | 0.9 | 510 | 28.7 | 1.0076 | 6.5 | −2 | −153 | 24 | NEG |
| 13 | 26–30 | 2.8 | 180 | 87.3 | 1.0177 | 6.2 | −2 | −127 | 30 | NEG |
| 13 | 30–34 | 1.6 | 250 | 55.9 | 1.0110 | 5.4 | −2 | −131 | 27 | NEG |
| 14 | −1 | 0 | NA | 28.9 | 1.0062 | 7.2 | −3 | −157 | 23 | NEG |
| 14 | 0.25 | 1.1 | 94 | 82.6 | 1.0144 | 7.3 | −2 | −139 | 43 | NEG |
| 14 | 1 | 1.4 | 155 | 42.0 | 1.0077 | 7.2 | 2 | −127 | 42 | NEG |
| 14 | 2 | 1.6 | 200 | 31.7 | 1.0057 | 7.1 | 2 | −140 | 32 | NEG |
| 14 | 3 | 2.6 | 140 | 48.0 | 1.0079 | 6.9 | 3 | −106 | 37 | NEG |
| 14 | 4 | 12.2 | 45 | 119.1 | 1.0161 | 6.3 | 26 | −25 | 103 | NEG |
| 14 | 4–6 | 5.2 | 160 | 98.5 | 1.0148 | 6.8 | 4 | −83 | 63 | NEG |
| 14 | 6–8 | 2.9 | 170 | 70.8 | 1.0120 | 6.5 | 1 | −110 | 35 | NEG |
| 14 | 8–10 | 1.5 | 200 | 46.4 | 1.0079 | 7.2 | 0 | −117 | 38 | NEG |
| 14 | 10–12 | 1.7 | 220 | 67.2 | 1.0109 | 7.0 | 1 | −132 | 30 | NEG |
| 14 | 12–22 | 1.9 | 830 | 89.1 | 1.0128 | 6.4 | −2 | −138 | 26 | NEG |
| 14 | 22–26 | 0 | 260 | 17.4 | 1.0037 | 7.0 | −3 | −152 | 22 | NEG |
| 14 | 26–30 | 0 | 220 | 75.2 | 1.0129 | 7.0 | −3 | −125 | 31 | NEG |
| 14 | 30–34 | 0 | 140 | 23.5 | 1.0060 | 6.9 | −4 | −147 | 17 | NEG |
| 15 | −1 | 0 | NA | 21.9 | 1.0046 | 7.3 | −3 | −161 | 15 | NEG |
| 15 | 0.25 | 0 | 112 | 16.2 | 1.0037 | 7.2 | −1 | −167 | 20 | NEG |
| 15 | 1 | 0 | 136 | 10.0 | 1.0021 | 7.0 | −1 | −147 | 17 | NEG |
| 15 | 2 | 0 | 140 | 10.4 | 1.0020 | 6.6 | −2 | −138 | 13 | NEG |
| 15 | 3 | 1.0 | 100 | 14.5 | 1.0029 | 6.1 | 0 | −148 | 23 | NEG |
| 15 | 4 | 1.7 | 94 | 30.7 | 1.0057 | 6.3 | −1 | −113 | 31 | NEG |
| 15 | 4–6 | 1.7 | 110 | 35.8 | 1.0069 | 7.0 | −1 | −155 | 38 | NEG |
| 15 | 6–8 | 0 | 130 | 12.8 | 1.0027 | 6.1 | −2 | −147 | 19 | NEG |
| 15 | 8–10 | 1.0 | 130 | 24.9 | 1.0057 | 7.2 | −1 | −143 | 28 | NEG |
| 15 | 10–12 | 1.9 | 165 | 44.0 | 1.0099 | 7.2 | −1 | −122 | 46 | NEG |
| 15 | 12–22 | 0.8 | 360 | 54.7 | 1.0085 | 6.0 | −2 | −147 | 30 | NEG |
| 15 | 22–26 | 0.8 | 280 | 38.1 | 1.0082 | 6.0 | −2 | −158 | 25 | NEG |
| 15 | 26–30 | 0 | 335 | 18.4 | 1.0044 | 7.0 | −4 | −142 | 22 | NEG |
| 15 | 30–34 | 1.3 | 420 | 49.4 | 1.0104 | 6.5 | −4 | −131 | 22 | NEG |
| 16 | −1 | 0 | NA | 105.9 | 1.0108 | 7.4 | −6 | −147 | 26 | NEG |
| 16 | 0.25 | 1.0 | 98 | 57.1 | 1.0065 | 7.2 | −1 | −134 | 38 | NEG |
| 16 | 1 | 6.1 | 28 | 225.8 | 1.0197 | 7.4 | 33 | −51 | 182 | NEG |
| 16 | 2 | 1.1 | 120 | 30.0 | 1.0033 | 7.0 | 1 | −140 | 28 | NEG |
| 16 | 3 | 4.5 | 92 | 105.5 | 1.0102 | 6.4 | 13 | −69 | 89 | NEG |
| 16 | 4 | 1.0 | 89 | 21.1 | 1.0025 | 6.2 | 0 | −142 | 24 | NEG |
| 16 | 4–6 | 20.1 | 103 | 19.7 | 1.0028 | 7.0 | 38 | −43 | 103 | POS |
| 16 | 6–8 | 2.8 | 105 | 77.3 | 1.0090 | 7.1 | 3 | −124 | 56 | NEG |
| 16 | 8–10 | 2.9 | 100 | 141.8 | 1.0127 | 6.9 | 4 | −103 | 68 | NEG |
| 16 | 10–12 | 2.1 | 130 | 122.2 | 1.0112 | 6.5 | 2 | −78 | 52 | NEG |
| 16 | 12–22 | 3.5 | 170 | 171.8 | 1.0185 | 7.2 | 3 | −86 | 65 | NEG |
| 16 | 22–26 | 1.2 | 330 | 50.0 | 1.0086 | 7.5 | −3 | −134 | 36 | NEG |
| 16 | 26–30 | 1.8 | 190 | 113.8 | 1.0160 | 7.4 | −2 | −148 | 53 | NEG |
| 16 | 30–34 | 0 | 120 | 249.4 | 1.0230 | 6.3 | −1 | −91 | 59 | NEG |
| Session 2 | ||||||||||
| 8 | −1 | 0 | 257 | 12.0 | 1.0017 | 6.0 | 1 | −157 | 17 | NEG |
| 8 | 0.25 | 0 | 440 | 15.4 | 1.0027 | 6.9 | 2 | −165 | 19 | NEG |
| 8 | 1 | 5.6 | 125 | 42.9 | 1.0063 | 6.2 | 22 | −81 | 79 | NEG |
| 8 | 2 | MS | MS | MS | MS | MS | MS | MS | MS | MS |
| 8 | 3 | 10.8 | 190 | 39.7 | 1.0063 | 5.1 | 25 | −45 | 93 | NEG |
| 8 | 4 | 3.1 | 420 | 10.1 | 1.0016 | 5.6 | 6 | −148 | 30 | NEG |
| 8 | 4–6 | MS | MS | MS | MS | MS | MS | MS | MS | MS |
| 8 | 6–8 | 6.2 | 375 | 32.3 | 1.0053 | 6.6 | 8 | −114 | 53 | NEG |
| 8 | 8–10 | 4.8 | 300 | 32.6 | 1.0062 | 7.2 | 7 | −127 | 48 | NEG |
| 8 | 10–12 | 9.5 | 180 | 64.7 | 1.0127 | 7.1 | 13 | −90 | 83 | NEG |
| 8 | 12–22 | 6.3 | 530 | 80.5 | 1.0117 | 6.0 | 7 | −109 | 54 | NEG |
| 8 | 22–26 | 1.9 | 520 | 24.9 | 1.0054 | 7.3 | 2 | −149 | 32 | NEG |
| 8 | 26–30 | 2.6 | 440 | 40.5 | 1.0067 | 7.3 | 1 | −146 | 35 | NEG |
| 8 | 30–34 | 4.3 | 220 | 86.4 | 1.0129 | 7.2 | 2 | −127 | 44 | NEG |
| 23 | −1 | 0 | 40 | 262.5 | 1.0263 | 5.6 | −7 | −128 | 16 | NEG |
| 23 | 0.25 | 0 | 610 | 22.4 | 1.0029 | 6.2 | 1 | −135 | 21 | NEG |
| 23 | 1 | 3.6 | 790 | 21.4 | 1.0026 | 6.3 | 8 | −139 | 34 | NEG |
| 23 | 2 | 6.9 | 270 | 17.0 | 1.0021 | 6.1 | 10 | −103 | 52 | NEG |
| 23 | 3 | 27.3 | 160 | 61.6 | 1.0079 | 5.4 | 40 | −4 | 144 | POS |
| 23 | 4 | 18.8 | 340 | 42.4 | 1.0054 | 5.6 | 26 | −43 | 100 | NEG |
| 23 | 4–6 | 57.5 | 100 | 168.7 | 1.0212 | 5.6 | 47 | 108 | 226 | POS |
| 23 | 6–8 | 36.8 | 90 | 139.1 | 1.0198 | 5.6 | 44 | 48 | 179 | POS |
| 23 | 8–10 | 32.7 | 220 | 162.4 | 1.0219 | 5.6 | 40 | 69 | 196 | POS |
| 23 | 10–12 | 17.4 | 270 | 98.3 | 1.0157 | 6.0 | 22 | −50 | 98 | NEG |
| 23 | 12–22 | 25.2 | 510 | 175.6 | 1.0226 | 5.8 | 29 | −6 | 108 | POS |
| 23 | 22–26 | 5.3 | 970 | 36.3 | 1.0062 | 7.3 | 4 | −135 | 45 | NEG |
| 23 | 26–30 | 12.4 | 350 | 121.3 | 1.0178 | 7.1 | 6 | −71 | 90 | NEG |
| 23 | 30–34 | 9.3 | 400 | 112.8 | 1.0179 | 6.4 | 4 | −89 | 59 | NEG |
| 37 | −1 | 0 | 138 | 42.3 | 1.0066 | 5.4 | 0 | −163 | 22 | NEG |
| 37 | 0.25 | 0 | 395 | 31.0 | 1.0045 | 5.5 | 3 | −147 | 35 | NEG |
| 37 | 1 | 4.7 | 110 | 34.4 | 1.0044 | 5.4 | 13 | −99 | 61 | NEG |
| 37 | 2 | 17.0 | 60 | 109.3 | 1.0138 | 5.5 | 45 | 47 | 170 | POS |
| 37 | 3 | 23.6 | 40 | 161.2 | 1.0206 | 5.5 | 42 | 106 | 218 | POS |
| 37 | 4 | 46.3 | 40 | 149.7 | 1.0218 | 6.9 | 46 | 118 | 285 | POS |
| 37 | 4–6 | 36.9 | 75 | 157.3 | 1.0218 | 7.1 | 45 | 86 | 243 | POS |
| 37 | 6–8 | 24.1 | 130 | 110.1 | 1.0197 | 7.2 | 33 | 2 | 170 | NEG |
| 37 | 8–10 | 21.3 | 150 | 88.8 | 1.0194 | 6.8 | 27 | −24 | 123 | NEG |
| 37 | 10–12 | 19.3 | 170 | 85.5 | 1.0198 | 6.9 | 24 | −33 | 121 | POS |
| 37 | 12–22 | 20.4 | 550 | 99.5 | 1.0194 | 6.2 | 23 | −48 | 114 | NEG |
| 37 | 22–26 | 10.4 | 360 | 55.1 | 1.0128 | 7.2 | 9 | −87 | 79 | NEG |
| 37 | 26–30 | 15.6 | 170 | 108.3 | 1.0208 | 7.3 | 13 | −74 | 108 | NEG |
| 37 | 30–34 | 10.3 | 180 | 103.1 | 1.0219 | 7.1 | 4 | −91 | 73 | NEG |
| 38 | −1 | 0 | 114 | 102.5 | 1.017 | 5.8 | −5 | −166 | 14 | NEG |
| 38 | 0.25 | 0 | 750 | 11.0 | 1.0018 | 6.7 | 1 | −163 | 16 | NEG |
| 38 | 1 | 1.9 | 117 | 10.8 | 1.0016 | 6.3 | 5 | −138 | 33 | NEG |
| 38 | 2 | 2.9 | 625 | 10.5 | 1.0016 | 6.4 | 5 | −145 | 38 | NEG |
| 38 | 3 | 7.8 | 380 | 23.7 | 1.0039 | 6.0 | 13 | −109 | 55 | NEG |
| 38 | 4 | 20.1 | 123 | 62.7 | 1.0097 | 5.6 | 32 | −33 | 117 | POS |
| 38 | 4–6 | 19.7 | 220 | 66.1 | 1.0119 | 6.1 | 26 | −47 | 105 | NEG |
| 38 | 6–8 | 8.3 | 475 | 39.2 | 1.0073 | 6.0 | 9 | −97 | 62 | NEG |
| 38 | 8–10 | 8.1 | 670 | 43.8 | 1.0085 | 6.2 | 10 | −110 | 49 | NEG |
| 38 | 10–12 | 4.5 | 390 | 27.8 | 1.0053 | 6.1 | 5 | −145 | 39 | NEG |
| 38 | 12–22 | 4.4 | 1,480 | 40.7 | 1.0063 | 5.8 | 6 | −141 | 22 | NEG |
| 38 | 22–26 | 1.2 | 1,860 | 12.7 | 1.0024 | 5.9 | 2 | −167 | 20 | NEG |
| 38 | 26–30 | 2 | 1,120 | 31.1 | 1.0056 | 6.9 | 0 | −153 | 27 | NEG |
| 38 | 30–34 | 1.8 | 790 | 34.8 | 1.007 | 7.2 | −2 | −165 | 31 | NEG |
| 40 | −1 | 0 | 150 | 44.9 | 1.0052 | 6.6 | −2 | −136 | 21 | NEG |
| 40 | 0.25 | 0 | 190 | 66.8 | 1.0076 | 6.7 | 1 | −132 | 30 | NEG |
| 40 | 1 | 1.6 | 162 | 96.6 | 1.0099 | 6.5 | 9 | −105 | 61 | NEG |
| 40 | 2 | 5.5 | 60 | 128.8 | 1.0155 | 6.3 | 19 | −66 | 100 | NEG |
| 40 | 3 | 3 | 122 | 58.8 | 1.0091 | 6.5 | 5 | −106 | 58 | NEG |
| 40 | 4 | 2.8 | 165 | 48.5 | 1.0083 | 6.7 | 3 | −124 | 44 | NEG |
| 40 | 4–6 | 0 | 500 | 28.0 | 1.0048 | 6.9 | 1 | −150 | 36 | NEG |
| 40 | 6–8 | 3.1 | 710 | 19.5 | 1.0034 | 6.7 | 2 | −132 | 28 | NEG |
| 40 | 8–10 | 1.3 | 350 | 33.2 | 1.0065 | 7.2 | −1 | −143 | 40 | NEG |
| 40 | 10–12 | 1.1 | 750 | 25.9 | 1.0056 | 7.2 | −1 | −137 | 27 | NEG |
| 40 | 12–22 | 1.5 | 750 | 63.6 | 1.0094 | 7.1 | 0 | −136 | 35 | NEG |
| 40 | 22–26 | 3.6 | 480 | 22.8 | 1.0049 | 6.8 | 1 | −143 | 32 | NEG |
| 40 | 26–30 | 1.4 | 405 | 76.9 | 1.0118 | 7.2 | −2 | −131 | 43 | NEG |
| 40 | 30–34 | 0 | 550 | 37.8 | 1.0065 | 7.2 | −1 | −143 | 27 | NEG |
| 41 | −1 | 0 | 290 | 16.7 | 1.0032 | 5.8 | −2 | −135 | 12 | NEG |
| 41 | 0.25 | 0 | 605 | 18.4 | 1.0031 | 5.6 | 0 | −144 | 18 | NEG |
| 41 | 1 | 5.8 | 350 | 22.1 | 1.0036 | 6.6 | 10 | −103 | 50 | NEG |
| 41 | 2 | 20.4 | 240 | 37.0 | 1.0078 | 7.3 | 27 | −52 | 121 | NEG |
| 41 | 3 | 12.2 | 335 | 21.0 | 1.0049 | 7.1 | 11 | −100 | 75 | NEG |
| 41 | 4 | 29.1 | 115 | 52.1 | 1.0108 | 7.3 | 28 | −27 | 138 | POS |
| 41 | 4–6 | 29.7 | 215 | 56.5 | 1.0108 | 7.2 | 28 | −37 | 127 | NEG |
| 41 | 6–8 | 12 | 560 | 24.5 | 1.0052 | 7.2 | 8 | −103 | 67 | NEG |
| 41 | 8–10 | 20.7 | 170 | 52.0 | 1.0105 | 7.4 | 19 | −45 | 126 | NEG |
| 41 | 10–12 | 9.6 | 590 | 28.8 | 1.0064 | 7.2 | 7 | −98 | 60 | NEG |
| 41 | 12–22 | 11.1 | 1,000 | 50.5 | 1.0082 | 6.5 | 8 | −99 | 67 | NEG |
| 41 | 22–26 | 0 | 1,260 | 56.0 | 1.0081 | 7.2 | −2 | −150 | 24 | NEG |
| 41 | 26–30 | 9 | 350 | 68.5 | 1.0114 | 7.2 | 4 | −120 | 68 | NEG |
| 41 | 30–34 | 3.7 | 920 | 34.1 | 1.0070 | 7.3 | −1 | −161 | 35 | NEG |
| Session 3 | ||||||||||
| 25 | −1 | 0 | 72 | 109.5 | 1.0084 | 5.8 | 1 | −151 | 12 | NEG |
| 25 | 0.25 | 0 | 190 | 56.7 | 1.0064 | 5.9 | 2 | −150 | 23 | NEG |
| 25 | 1 | 0 | 350 | 18.2 | 1.0029 | 7.0 | 5 | −149 | 26 | NEG |
| 25 | 2 | 1.8 | 210 | 25.4 | 1.0039 | 7.2 | 4 | −146 | 26 | NEG |
| 25 | 3 | 1.1 | 440 | 18.1 | 1.0026 | 7.1 | 4 | −148 | 18 | NEG |
| 25 | 4 | 1.2 | 460 | 18.3 | 1.0025 | 7.1 | 4 | −144 | 19 | NEG |
| 25 | 4–6 | 2.2 | 560 | 39.0 | 1.0057 | 7.3 | 3 | −119 | 24 | NEG |
| 25 | 6–8 | 1.1 | 610 | 25.3 | 1.0037 | 7.1 | 2 | −150 | 18 | NEG |
| 25 | 8–10 | 0.8 | 320 | 27.2 | 1.0030 | 6.9 | 4 | −141 | 25 | NEG |
| 25 | 10–12 | 2.4 | 200 | 88.6 | 1.0089 | 6.4 | 4 | −134 | 31 | NEG |
| 25 | 12–22 | 0 | 1,950 | 38.5 | 1.0041 | 6.7 | 3 | −145 | 21 | NEG |
| 25 | 22–26 | 0 | 2,150 | 13.3 | 1.0030 | 7.2 | 3 | −164 | 15 | NEG |
| 25 | 26–30 | 0 | 880 | 36.4 | 1.0054 | 7.3 | 0 | −165 | 18 | NEG |
| 25 | 30–34 | 0 | 680 | 43.6 | 1.0079 | 6.7 | 1 | −146 | 21 | NEG |
| 26 | −1 | 0 | 30 | 168.7 | 1.0228 | 6.1 | −6 | −148 | 13 | NEG |
| 26 | 0.25 | 0 | 150 | 136.7 | 1.0196 | 6.1 | −5 | −157 | 19 | NEG |
| 26 | 1 | 1.7 | 50 | 146.0 | 1.0199 | 6.1 | −2 | −147 | 26 | NEG |
| 26 | 2 | 4.6 | 40 | 153.6 | 1.0209 | 6.1 | 1 | −129 | 33 | NEG |
| 26 | 3 | 5.2 | 60 | 149.2 | 1.0208 | 6.2 | 2 | −125 | 41 | NEG |
| 26 | 4 | 6.6 | 100 | 156.7 | 1.0213 | 6.2 | 3 | −117 | 46 | NEG |
| 26 | 4–6 | 8.3 | 130 | 170.4 | 1.0220 | 6.9 | 5 | −121 | 56 | NEG |
| 26 | 6–8 | 8.0 | 200 | 152.2 | 1.0222 | 7.1 | 2 | −105 | 56 | NEG |
| 26 | 8–10 | 8.7 | 270 | 160.1 | 1.0227 | 7.1 | 2 | −123 | 51 | NEG |
| 26 | 10–12 | 6.8 | 170 | 143.0 | 1.0240 | 7.0 | 0 | −126 | 50 | NEG |
| 26 | 12–22 | 3.4 | 630 | 109.2 | 1.0178 | 6.5 | 0 | −141 | 33 | NEG |
| 26 | 22–26 | 2.7 | 170 | 112.9 | 1.0159 | 7.1 | −1 | −139 | 33 | NEG |
| 26 | 26–30 | 2.5 | 350 | 96.7 | 1.0143 | 7.2 | 0 | −129 | 32 | NEG |
| 26 | 30–34 | 2.0 | 550 | 88.6 | 1.0152 | 7.1 | 0 | −146 | 23 | NEG |
| 27 | −1 | 0 | 360 | 27.1 | 1.0057 | 6.2 | 2 | −137 | 14 | NEG |
| 27 | 0.25 | 0 | 460 | 20.2 | 1.0047 | 7.3 | 3 | −178 | 19 | NEG |
| 27 | 1 | 2.6 | 150 | 48.3 | 1.0091 | 7.2 | 9 | −122 | 54 | NEG |
| 27 | 2 | 8.2 | 110 | 82.0 | 1.0141 | 7.1 | 17 | −78 | 77 | NEG |
| 27 | 3 | 7.3 | 50 | 130.9 | 1.0183 | 6.1 | 15 | −73 | 79 | NEG |
| 27 | 4 | 1.8 | 230 | 24.3 | 1.0046 | 6.3 | 5 | −148 | 25 | NEG |
| 27 | 4–6 | 3.3 | 150 | 43.7 | 1.0080 | 6.9 | 4 | −128 | 35 | NEG |
| 27 | 6–8 | 7.0 | 95 | 127.6 | 1.0193 | 7.1 | 8 | −110 | 67 | NEG |
| 27 | 8–10 | 5.1 | 290 | 180.1 | 1.0230 | 5.6 | 4 | −108 | 47 | NEG |
| 27 | 10–12 | 5.1 | 90 | 146.5 | 1.0234 | 6.1 | 4 | −86 | 42 | NEG |
| 27 | 12–22 | 1.4 | 710 | 74.0 | 1.0128 | 5.9 | 1 | −142 | 23 | NEG |
| 27 | 22–26 | 0.8 | 480 | 39.6 | 1.0100 | 7.2 | 0 | −157 | 20 | NEG |
| 27 | 26–30 | 0.8 | 540 | 47.2 | 1.0100 | 7.4 | −1 | −150 | 29 | NEG |
| 27 | 30–34 | 0 | 550 | 49.0 | 1.0093 | 7.2 | 1 | −132 | 24 | NEG |
| 28 | −1 | 0 | 330 | 28.6 | 1.0107 | 6.9 | −1 | −161 | 16 | NEG |
| 28 | 0.25 | 0 | 315 | 32.3 | 1.0107 | 6.7 | 1 | −147 | 24 | NEG |
| 28 | 1 | 4.3 | 60 | 64.7 | 1.0162 | 5.8 | 5 | −111 | 47 | NEG |
| 28 | 2 | 5.5 | 95 | 60.7 | 1.0145 | 6.1 | 7 | −132 | 43 | NEG |
| 28 | 3 | 3.5 | 210 | 23.7 | 1.0065 | 6.5 | 4 | −140 | 30 | NEG |
| 28 | 4 | 2.2 | 270 | 15.3 | 1.0043 | 6.7 | 3 | −151 | 27 | NEG |
| 28 | 4–6 | 9.0 | 80 | 87.8 | 1.0182 | 6.3 | 4 | −105 | 50 | NEG |
| 28 | 6–8 | 3.8 | 290 | 52.0 | 1.0125 | 7.0 | 2 | −138 | 34 | NEG |
| 28 | 8–10 | 1.3 | 330 | 21.8 | 1.0054 | 6.7 | 3 | −148 | 21 | NEG |
| 28 | 10–12 | 2.6 | 170 | 53.9 | 1.0118 | 6.7 | 2 | −145 | 32 | NEG |
| 28 | 12–22 | 2.5 | 340 | 130.8 | 1.0197 | 5.8 | −1 | −130 | 29 | NEG |
| 28 | 22–26 | 0.9 | 730 | 27.7 | 1.0084 | 7.2 | 2 | −149 | 31 | NEG |
| 28 | 26–30 | 1.0 | 460 | 33.3 | 1.0087 | 7.3 | 1 | −159 | 33 | NEG |
| 28 | 30–34 | 1.0 | 450 | 40.3 | 1.0105 | 7.3 | −1 | −151 | 34 | NEG |
| 29 | −1 | 0 | 170 | 32.9 | 1.0031 | 6.2 | 0 | −143 | 17 | NEG |
| 29 | 0.25 | 0 | 390 | 10.3 | 1.0016 | 6.8 | 2 | −148 | 33 | NEG |
| 29 | 1 | 0 | 300 | 9.7 | 1.0018 | 7.2 | 2 | −148 | 26 | NEG |
| 29 | 2 | 0 | 340 | 20.2 | 1.0034 | 6.5 | 3 | −127 | 29 | NEG |
| 29 | 3 | 1.3 | 170 | 18.9 | 1.0041 | 7.3 | 4 | −148 | 20 | NEG |
| 29 | 4 | 1.1 | 320 | 15.6 | 1.0032 | 7.3 | 3 | −157 | 24 | NEG |
| 29 | 4–6 | 0.8 | 570 | 13.1 | 1.0025 | 7.3 | 4 | −132 | 27 | NEG |
| 29 | 6–8 | 0 | 250 | 19.1 | 1.0033 | 7.2 | 2 | −154 | 23 | NEG |
| 29 | 8–10 | 0 | 600 | 20.0 | 1.0032 | 7.4 | 3 | −131 | 19 | NEG |
| 29 | 10–12 | 0 | 400 | 21.1 | 1.0040 | 7.4 | 3 | −158 | 30 | NEG |
| 29 | 12–22 | 1.3 | 660 | 73.2 | 1.0086 | 6.9 | 1 | −151 | 27 | NEG |
| 29 | 22–26 | 0 | 1,510 | 12.5 | 1.0025 | 7.4 | 3 | −155 | 18 | NEG |
| 29 | 26–30 | 0 | 540 | 26.1 | 1.0048 | 7.3 | 3 | −153 | 24 | NEG |
| 29 | 30–34 | 0 | 1,550 | 13.6 | 1.0031 | 6.8 | 3 | −150 | 22 | NEG |
| 36 | −1 | 0 | 40 | 164.0 | 1.0233 | 6.1 | −8 | −139 | 27 | NEG |
| 36 | 0.25 | 0 | 380 | 25.3 | 1.0045 | 6.7 | 1 | −139 | 23 | NEG |
| 36 | 1 | 1.6 | 230 | 24.4 | 1.0044 | 6.9 | 4 | −130 | 21 | NEG |
| 36 | 2 | 2.4 | 310 | 22.2 | 1.0042 | 6.6 | 3 | −143 | 25 | NEG |
| 36 | 3 | 7.1 | 80 | 58.9 | 1.0092 | 6.2 | 6 | −111 | 44 | NEG |
| 36 | 4 | 15.0 | 50 | 124.7 | 1.0174 | 6.9 | 14 | −59 | 94 | NEG |
| 36 | 4–6 | 15.5 | 100 | 147.8 | 1.0209 | 7.4 | 14 | −57 | 107 | NEG |
| 36 | 6–8 | 4.8 | 230 | 46.5 | 1.0102 | 7.4 | 4 | −127 | 38 | NEG |
| 36 | 8–10 | 3.4 | 170 | 39.2 | 1.0089 | 7.4 | 3 | −144 | 38 | NEG |
| 36 | 10–12 | 3.9 | 250 | 55.7 | 1.0112 | 7.2 | 2 | −127 | 46 | NEG |
| 36 | 12–22 | 7.1 | 380 | 145.4 | 1.0198 | 6.7 | 1 | −114 | 56 | NEG |
| 36 | 22–26 | 1.5 | 850 | 29.3 | 1.0077 | 7.4 | 2 | −150 | 32 | NEG |
| 36 | 26–30 | 5.3 | 220 | 103.1 | 1.0168 | 7.4 | 1 | −120 | 61 | NEG |
| 36 | 30–34 | 1.0 | 1,010 | 26.7 | 1.0058 | 7.4 | 2 | −172 | 34 | NEG |
| Subject # . | Time, h . | THCCOOH GC/MS, ng/mL . | Volume, mL . | Creatinine, mg/dL . | Specific gravity . | pH . | CRL1 DRI, Cutoff = 20 ng/mL, (Equivalent IA response = 20) . | Med Tox KIMS 20 Cutoff = 20 ng/mL, (Equivalent IA response = 0) . | MetroLab EMIT II Plus 20 Cutoff = 20 ng/mL, (Equivalent IA response = 100) . | One Source CEDIA 20 Cutoff = 20 ng/mL . |
|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | ||||||||||
| 7 | −1 | 0 | NA | 56.0 | 1.0055 | 6.7 | −2 | −151 | 10 | NEG |
| 7 | 0.25 | 0 | 230 | 64.5 | 1.0066 | 7.1 | 0 | −149 | 15 | NEG |
| 7 | 1 | 3.8 | 49 | 187.7 | 1.0155 | 6.5 | 28 | −36 | 110 | NEG |
| 7 | 2 | 7.0 | 50 | 177.8 | 1.0162 | 6.3 | 34 | 13 | 158 | POS |
| 7 | 3 | 2.6 | 190 | 52.6 | 1.0056 | 6.2 | −3 | −133 | 42 | NEG |
| 7 | 4 | 1.3 | 220 | 24.1 | 1.0028 | 5.9 | 1 | −138 | 21 | NEG |
| 7 | 4–6 | 1.1 | 450 | 22.2 | 1.0027 | 7.0 | −2 | −145 | 24 | NEG |
| 7 | 6–8 | 2.0 | 380 | 50.4 | 1.0062 | 7.1 | 3 | −119 | 37 | NEG |
| 7 | 8–10 | 3.9 | 195 | 106.3 | 1.0132 | 6.4 | 8 | −91 | 50 | NEG |
| 7 | 10–12 | 2.6 | 455 | 37.2 | 1.0051 | 6.8 | 0 | −139 | 24 | NEG |
| 7 | 12–22 | 1.9 | 440 | 82.5 | 1.0114 | 6.2 | 2 | −112 | 35 | NEG |
| 7 | 22–26 | 1.3 | 640 | 50.7 | 1.0084 | 6.9 | −2 | −147 | 23 | NEG |
| 7 | 26–30 | 1.7 | 305 | 124.5 | 1.0155 | 7.1 | −3 | −133 | 30 | NEG |
| 7 | 30–34 | 1.0 | 670 | 40.1 | 1.0076 | 7.3 | −3 | −146 | 15 | NEG |
| 11 | −1 | 0 | NA | 122.0 | 1.0153 | 6.2 | −8 | −167 | 7 | NEG |
| 11 | 0.25 | 0 | 122 | 64.9 | 1.0109 | 6.8 | 2 | −113 | 40 | NEG |
| 11 | 1 | 3.1 | 69 | 86.5 | 1.0121 | 5.5 | 28 | −47 | 102 | NEG |
| 11 | 2 | 1.8 | 140 | 53.1 | 1.0073 | 5.6 | 9 | −94 | 60 | NEG |
| 11 | 3 | 1.3 | 170 | 41.2 | 1.0048 | 5.4 | 4 | −112 | 33 | NEG |
| 11 | 4 | 3.8 | 35 | 151.7 | 1.0161 | 5.7 | 25 | −36 | 100 | NEG |
| 11 | 4–6 | 6.8 | 71 | 142.1 | 1.0173 | 5.5 | 27 | 6 | 112 | NEG |
| 11 | 6–8 | 2.2 | 140 | 65.1 | 1.0094 | 6.1 | 7 | −84 | 50 | NEG |
| 11 | 8–10 | 2.1 | 140 | 96.1 | 1.0139 | 6.8 | 5 | −88 | 60 | NEG |
| 11 | 10–12 | 1.8 | 210 | 52.3 | 1.0070 | 5.8 | 1 | −141 | 27 | NEG |
| 11 | 12–22 | 1.5 | 730 | 54.4 | 1.0073 | 7.3 | −1 | −142 | 32 | NEG |
| 11 | 22–26 | 1.0 | 300 | 86.4 | 1.0142 | 7.1 | −4 | −126 | 36 | NEG |
| 11 | 26–30 | 1.1 | 280 | 51.6 | 1.0093 | 7.3 | −2 | −129 | 42 | NEG |
| 11 | 30–34 | 1.3 | 180 | 130.6 | 1.0174 | 6.2 | −2 | −106 | 38 | NEG |
| 13 | −1 | 0.0 | NA | 56.2 | 1.0126 | 7.1 | −6 | −163 | 22 | NEG |
| 13 | 0.25 | 0.8 | 218 | 19.6 | 1.0041 | 7.2 | 0 | −142 | 18 | NEG |
| 13 | 1 | 4.0 | 190 | 46.8 | 1.0094 | 7.4 | 10 | −110 | 65 | NEG |
| 13 | 2 | 13.1 | 54 | 100.7 | 1.0179 | 7.4 | 34 | 9 | 170 | POS |
| 13 | 3 | 15.6 | 36 | 91.1 | 1.0154 | 7.1 | 36 | 35 | 174 | POS |
| 13 | 4 | 14.1 | 48 | 100.6 | 1.0148 | 6.2 | 30 | 14 | 139 | POS |
| 13 | 4–6 | 9.6 | 170 | 90.0 | 1.0135 | 5.8 | 19 | −63 | 80 | NEG |
| 13 | 6–8 | 4.7 | 140 | 52.2 | 1.0089 | 5.8 | 7 | −113 | 51 | NEG |
| 13 | 8–10 | 7.0 | 140 | 115.5 | 1.0197 | 6.0 | 11 | −56 | 68 | NEG |
| 13 | 10–12 | 19.3 | 210 | 142.2 | 1.0234 | 5.8 | 33 | 83 | 188 | POS |
| 13 | 12–22 | 3.3 | 480 | 98.9 | 1.0195 | 5.7 | −1 | −102 | 41 | NEG |
| 13 | 22–26 | 0.9 | 510 | 28.7 | 1.0076 | 6.5 | −2 | −153 | 24 | NEG |
| 13 | 26–30 | 2.8 | 180 | 87.3 | 1.0177 | 6.2 | −2 | −127 | 30 | NEG |
| 13 | 30–34 | 1.6 | 250 | 55.9 | 1.0110 | 5.4 | −2 | −131 | 27 | NEG |
| 14 | −1 | 0 | NA | 28.9 | 1.0062 | 7.2 | −3 | −157 | 23 | NEG |
| 14 | 0.25 | 1.1 | 94 | 82.6 | 1.0144 | 7.3 | −2 | −139 | 43 | NEG |
| 14 | 1 | 1.4 | 155 | 42.0 | 1.0077 | 7.2 | 2 | −127 | 42 | NEG |
| 14 | 2 | 1.6 | 200 | 31.7 | 1.0057 | 7.1 | 2 | −140 | 32 | NEG |
| 14 | 3 | 2.6 | 140 | 48.0 | 1.0079 | 6.9 | 3 | −106 | 37 | NEG |
| 14 | 4 | 12.2 | 45 | 119.1 | 1.0161 | 6.3 | 26 | −25 | 103 | NEG |
| 14 | 4–6 | 5.2 | 160 | 98.5 | 1.0148 | 6.8 | 4 | −83 | 63 | NEG |
| 14 | 6–8 | 2.9 | 170 | 70.8 | 1.0120 | 6.5 | 1 | −110 | 35 | NEG |
| 14 | 8–10 | 1.5 | 200 | 46.4 | 1.0079 | 7.2 | 0 | −117 | 38 | NEG |
| 14 | 10–12 | 1.7 | 220 | 67.2 | 1.0109 | 7.0 | 1 | −132 | 30 | NEG |
| 14 | 12–22 | 1.9 | 830 | 89.1 | 1.0128 | 6.4 | −2 | −138 | 26 | NEG |
| 14 | 22–26 | 0 | 260 | 17.4 | 1.0037 | 7.0 | −3 | −152 | 22 | NEG |
| 14 | 26–30 | 0 | 220 | 75.2 | 1.0129 | 7.0 | −3 | −125 | 31 | NEG |
| 14 | 30–34 | 0 | 140 | 23.5 | 1.0060 | 6.9 | −4 | −147 | 17 | NEG |
| 15 | −1 | 0 | NA | 21.9 | 1.0046 | 7.3 | −3 | −161 | 15 | NEG |
| 15 | 0.25 | 0 | 112 | 16.2 | 1.0037 | 7.2 | −1 | −167 | 20 | NEG |
| 15 | 1 | 0 | 136 | 10.0 | 1.0021 | 7.0 | −1 | −147 | 17 | NEG |
| 15 | 2 | 0 | 140 | 10.4 | 1.0020 | 6.6 | −2 | −138 | 13 | NEG |
| 15 | 3 | 1.0 | 100 | 14.5 | 1.0029 | 6.1 | 0 | −148 | 23 | NEG |
| 15 | 4 | 1.7 | 94 | 30.7 | 1.0057 | 6.3 | −1 | −113 | 31 | NEG |
| 15 | 4–6 | 1.7 | 110 | 35.8 | 1.0069 | 7.0 | −1 | −155 | 38 | NEG |
| 15 | 6–8 | 0 | 130 | 12.8 | 1.0027 | 6.1 | −2 | −147 | 19 | NEG |
| 15 | 8–10 | 1.0 | 130 | 24.9 | 1.0057 | 7.2 | −1 | −143 | 28 | NEG |
| 15 | 10–12 | 1.9 | 165 | 44.0 | 1.0099 | 7.2 | −1 | −122 | 46 | NEG |
| 15 | 12–22 | 0.8 | 360 | 54.7 | 1.0085 | 6.0 | −2 | −147 | 30 | NEG |
| 15 | 22–26 | 0.8 | 280 | 38.1 | 1.0082 | 6.0 | −2 | −158 | 25 | NEG |
| 15 | 26–30 | 0 | 335 | 18.4 | 1.0044 | 7.0 | −4 | −142 | 22 | NEG |
| 15 | 30–34 | 1.3 | 420 | 49.4 | 1.0104 | 6.5 | −4 | −131 | 22 | NEG |
| 16 | −1 | 0 | NA | 105.9 | 1.0108 | 7.4 | −6 | −147 | 26 | NEG |
| 16 | 0.25 | 1.0 | 98 | 57.1 | 1.0065 | 7.2 | −1 | −134 | 38 | NEG |
| 16 | 1 | 6.1 | 28 | 225.8 | 1.0197 | 7.4 | 33 | −51 | 182 | NEG |
| 16 | 2 | 1.1 | 120 | 30.0 | 1.0033 | 7.0 | 1 | −140 | 28 | NEG |
| 16 | 3 | 4.5 | 92 | 105.5 | 1.0102 | 6.4 | 13 | −69 | 89 | NEG |
| 16 | 4 | 1.0 | 89 | 21.1 | 1.0025 | 6.2 | 0 | −142 | 24 | NEG |
| 16 | 4–6 | 20.1 | 103 | 19.7 | 1.0028 | 7.0 | 38 | −43 | 103 | POS |
| 16 | 6–8 | 2.8 | 105 | 77.3 | 1.0090 | 7.1 | 3 | −124 | 56 | NEG |
| 16 | 8–10 | 2.9 | 100 | 141.8 | 1.0127 | 6.9 | 4 | −103 | 68 | NEG |
| 16 | 10–12 | 2.1 | 130 | 122.2 | 1.0112 | 6.5 | 2 | −78 | 52 | NEG |
| 16 | 12–22 | 3.5 | 170 | 171.8 | 1.0185 | 7.2 | 3 | −86 | 65 | NEG |
| 16 | 22–26 | 1.2 | 330 | 50.0 | 1.0086 | 7.5 | −3 | −134 | 36 | NEG |
| 16 | 26–30 | 1.8 | 190 | 113.8 | 1.0160 | 7.4 | −2 | −148 | 53 | NEG |
| 16 | 30–34 | 0 | 120 | 249.4 | 1.0230 | 6.3 | −1 | −91 | 59 | NEG |
| Session 2 | ||||||||||
| 8 | −1 | 0 | 257 | 12.0 | 1.0017 | 6.0 | 1 | −157 | 17 | NEG |
| 8 | 0.25 | 0 | 440 | 15.4 | 1.0027 | 6.9 | 2 | −165 | 19 | NEG |
| 8 | 1 | 5.6 | 125 | 42.9 | 1.0063 | 6.2 | 22 | −81 | 79 | NEG |
| 8 | 2 | MS | MS | MS | MS | MS | MS | MS | MS | MS |
| 8 | 3 | 10.8 | 190 | 39.7 | 1.0063 | 5.1 | 25 | −45 | 93 | NEG |
| 8 | 4 | 3.1 | 420 | 10.1 | 1.0016 | 5.6 | 6 | −148 | 30 | NEG |
| 8 | 4–6 | MS | MS | MS | MS | MS | MS | MS | MS | MS |
| 8 | 6–8 | 6.2 | 375 | 32.3 | 1.0053 | 6.6 | 8 | −114 | 53 | NEG |
| 8 | 8–10 | 4.8 | 300 | 32.6 | 1.0062 | 7.2 | 7 | −127 | 48 | NEG |
| 8 | 10–12 | 9.5 | 180 | 64.7 | 1.0127 | 7.1 | 13 | −90 | 83 | NEG |
| 8 | 12–22 | 6.3 | 530 | 80.5 | 1.0117 | 6.0 | 7 | −109 | 54 | NEG |
| 8 | 22–26 | 1.9 | 520 | 24.9 | 1.0054 | 7.3 | 2 | −149 | 32 | NEG |
| 8 | 26–30 | 2.6 | 440 | 40.5 | 1.0067 | 7.3 | 1 | −146 | 35 | NEG |
| 8 | 30–34 | 4.3 | 220 | 86.4 | 1.0129 | 7.2 | 2 | −127 | 44 | NEG |
| 23 | −1 | 0 | 40 | 262.5 | 1.0263 | 5.6 | −7 | −128 | 16 | NEG |
| 23 | 0.25 | 0 | 610 | 22.4 | 1.0029 | 6.2 | 1 | −135 | 21 | NEG |
| 23 | 1 | 3.6 | 790 | 21.4 | 1.0026 | 6.3 | 8 | −139 | 34 | NEG |
| 23 | 2 | 6.9 | 270 | 17.0 | 1.0021 | 6.1 | 10 | −103 | 52 | NEG |
| 23 | 3 | 27.3 | 160 | 61.6 | 1.0079 | 5.4 | 40 | −4 | 144 | POS |
| 23 | 4 | 18.8 | 340 | 42.4 | 1.0054 | 5.6 | 26 | −43 | 100 | NEG |
| 23 | 4–6 | 57.5 | 100 | 168.7 | 1.0212 | 5.6 | 47 | 108 | 226 | POS |
| 23 | 6–8 | 36.8 | 90 | 139.1 | 1.0198 | 5.6 | 44 | 48 | 179 | POS |
| 23 | 8–10 | 32.7 | 220 | 162.4 | 1.0219 | 5.6 | 40 | 69 | 196 | POS |
| 23 | 10–12 | 17.4 | 270 | 98.3 | 1.0157 | 6.0 | 22 | −50 | 98 | NEG |
| 23 | 12–22 | 25.2 | 510 | 175.6 | 1.0226 | 5.8 | 29 | −6 | 108 | POS |
| 23 | 22–26 | 5.3 | 970 | 36.3 | 1.0062 | 7.3 | 4 | −135 | 45 | NEG |
| 23 | 26–30 | 12.4 | 350 | 121.3 | 1.0178 | 7.1 | 6 | −71 | 90 | NEG |
| 23 | 30–34 | 9.3 | 400 | 112.8 | 1.0179 | 6.4 | 4 | −89 | 59 | NEG |
| 37 | −1 | 0 | 138 | 42.3 | 1.0066 | 5.4 | 0 | −163 | 22 | NEG |
| 37 | 0.25 | 0 | 395 | 31.0 | 1.0045 | 5.5 | 3 | −147 | 35 | NEG |
| 37 | 1 | 4.7 | 110 | 34.4 | 1.0044 | 5.4 | 13 | −99 | 61 | NEG |
| 37 | 2 | 17.0 | 60 | 109.3 | 1.0138 | 5.5 | 45 | 47 | 170 | POS |
| 37 | 3 | 23.6 | 40 | 161.2 | 1.0206 | 5.5 | 42 | 106 | 218 | POS |
| 37 | 4 | 46.3 | 40 | 149.7 | 1.0218 | 6.9 | 46 | 118 | 285 | POS |
| 37 | 4–6 | 36.9 | 75 | 157.3 | 1.0218 | 7.1 | 45 | 86 | 243 | POS |
| 37 | 6–8 | 24.1 | 130 | 110.1 | 1.0197 | 7.2 | 33 | 2 | 170 | NEG |
| 37 | 8–10 | 21.3 | 150 | 88.8 | 1.0194 | 6.8 | 27 | −24 | 123 | NEG |
| 37 | 10–12 | 19.3 | 170 | 85.5 | 1.0198 | 6.9 | 24 | −33 | 121 | POS |
| 37 | 12–22 | 20.4 | 550 | 99.5 | 1.0194 | 6.2 | 23 | −48 | 114 | NEG |
| 37 | 22–26 | 10.4 | 360 | 55.1 | 1.0128 | 7.2 | 9 | −87 | 79 | NEG |
| 37 | 26–30 | 15.6 | 170 | 108.3 | 1.0208 | 7.3 | 13 | −74 | 108 | NEG |
| 37 | 30–34 | 10.3 | 180 | 103.1 | 1.0219 | 7.1 | 4 | −91 | 73 | NEG |
| 38 | −1 | 0 | 114 | 102.5 | 1.017 | 5.8 | −5 | −166 | 14 | NEG |
| 38 | 0.25 | 0 | 750 | 11.0 | 1.0018 | 6.7 | 1 | −163 | 16 | NEG |
| 38 | 1 | 1.9 | 117 | 10.8 | 1.0016 | 6.3 | 5 | −138 | 33 | NEG |
| 38 | 2 | 2.9 | 625 | 10.5 | 1.0016 | 6.4 | 5 | −145 | 38 | NEG |
| 38 | 3 | 7.8 | 380 | 23.7 | 1.0039 | 6.0 | 13 | −109 | 55 | NEG |
| 38 | 4 | 20.1 | 123 | 62.7 | 1.0097 | 5.6 | 32 | −33 | 117 | POS |
| 38 | 4–6 | 19.7 | 220 | 66.1 | 1.0119 | 6.1 | 26 | −47 | 105 | NEG |
| 38 | 6–8 | 8.3 | 475 | 39.2 | 1.0073 | 6.0 | 9 | −97 | 62 | NEG |
| 38 | 8–10 | 8.1 | 670 | 43.8 | 1.0085 | 6.2 | 10 | −110 | 49 | NEG |
| 38 | 10–12 | 4.5 | 390 | 27.8 | 1.0053 | 6.1 | 5 | −145 | 39 | NEG |
| 38 | 12–22 | 4.4 | 1,480 | 40.7 | 1.0063 | 5.8 | 6 | −141 | 22 | NEG |
| 38 | 22–26 | 1.2 | 1,860 | 12.7 | 1.0024 | 5.9 | 2 | −167 | 20 | NEG |
| 38 | 26–30 | 2 | 1,120 | 31.1 | 1.0056 | 6.9 | 0 | −153 | 27 | NEG |
| 38 | 30–34 | 1.8 | 790 | 34.8 | 1.007 | 7.2 | −2 | −165 | 31 | NEG |
| 40 | −1 | 0 | 150 | 44.9 | 1.0052 | 6.6 | −2 | −136 | 21 | NEG |
| 40 | 0.25 | 0 | 190 | 66.8 | 1.0076 | 6.7 | 1 | −132 | 30 | NEG |
| 40 | 1 | 1.6 | 162 | 96.6 | 1.0099 | 6.5 | 9 | −105 | 61 | NEG |
| 40 | 2 | 5.5 | 60 | 128.8 | 1.0155 | 6.3 | 19 | −66 | 100 | NEG |
| 40 | 3 | 3 | 122 | 58.8 | 1.0091 | 6.5 | 5 | −106 | 58 | NEG |
| 40 | 4 | 2.8 | 165 | 48.5 | 1.0083 | 6.7 | 3 | −124 | 44 | NEG |
| 40 | 4–6 | 0 | 500 | 28.0 | 1.0048 | 6.9 | 1 | −150 | 36 | NEG |
| 40 | 6–8 | 3.1 | 710 | 19.5 | 1.0034 | 6.7 | 2 | −132 | 28 | NEG |
| 40 | 8–10 | 1.3 | 350 | 33.2 | 1.0065 | 7.2 | −1 | −143 | 40 | NEG |
| 40 | 10–12 | 1.1 | 750 | 25.9 | 1.0056 | 7.2 | −1 | −137 | 27 | NEG |
| 40 | 12–22 | 1.5 | 750 | 63.6 | 1.0094 | 7.1 | 0 | −136 | 35 | NEG |
| 40 | 22–26 | 3.6 | 480 | 22.8 | 1.0049 | 6.8 | 1 | −143 | 32 | NEG |
| 40 | 26–30 | 1.4 | 405 | 76.9 | 1.0118 | 7.2 | −2 | −131 | 43 | NEG |
| 40 | 30–34 | 0 | 550 | 37.8 | 1.0065 | 7.2 | −1 | −143 | 27 | NEG |
| 41 | −1 | 0 | 290 | 16.7 | 1.0032 | 5.8 | −2 | −135 | 12 | NEG |
| 41 | 0.25 | 0 | 605 | 18.4 | 1.0031 | 5.6 | 0 | −144 | 18 | NEG |
| 41 | 1 | 5.8 | 350 | 22.1 | 1.0036 | 6.6 | 10 | −103 | 50 | NEG |
| 41 | 2 | 20.4 | 240 | 37.0 | 1.0078 | 7.3 | 27 | −52 | 121 | NEG |
| 41 | 3 | 12.2 | 335 | 21.0 | 1.0049 | 7.1 | 11 | −100 | 75 | NEG |
| 41 | 4 | 29.1 | 115 | 52.1 | 1.0108 | 7.3 | 28 | −27 | 138 | POS |
| 41 | 4–6 | 29.7 | 215 | 56.5 | 1.0108 | 7.2 | 28 | −37 | 127 | NEG |
| 41 | 6–8 | 12 | 560 | 24.5 | 1.0052 | 7.2 | 8 | −103 | 67 | NEG |
| 41 | 8–10 | 20.7 | 170 | 52.0 | 1.0105 | 7.4 | 19 | −45 | 126 | NEG |
| 41 | 10–12 | 9.6 | 590 | 28.8 | 1.0064 | 7.2 | 7 | −98 | 60 | NEG |
| 41 | 12–22 | 11.1 | 1,000 | 50.5 | 1.0082 | 6.5 | 8 | −99 | 67 | NEG |
| 41 | 22–26 | 0 | 1,260 | 56.0 | 1.0081 | 7.2 | −2 | −150 | 24 | NEG |
| 41 | 26–30 | 9 | 350 | 68.5 | 1.0114 | 7.2 | 4 | −120 | 68 | NEG |
| 41 | 30–34 | 3.7 | 920 | 34.1 | 1.0070 | 7.3 | −1 | −161 | 35 | NEG |
| Session 3 | ||||||||||
| 25 | −1 | 0 | 72 | 109.5 | 1.0084 | 5.8 | 1 | −151 | 12 | NEG |
| 25 | 0.25 | 0 | 190 | 56.7 | 1.0064 | 5.9 | 2 | −150 | 23 | NEG |
| 25 | 1 | 0 | 350 | 18.2 | 1.0029 | 7.0 | 5 | −149 | 26 | NEG |
| 25 | 2 | 1.8 | 210 | 25.4 | 1.0039 | 7.2 | 4 | −146 | 26 | NEG |
| 25 | 3 | 1.1 | 440 | 18.1 | 1.0026 | 7.1 | 4 | −148 | 18 | NEG |
| 25 | 4 | 1.2 | 460 | 18.3 | 1.0025 | 7.1 | 4 | −144 | 19 | NEG |
| 25 | 4–6 | 2.2 | 560 | 39.0 | 1.0057 | 7.3 | 3 | −119 | 24 | NEG |
| 25 | 6–8 | 1.1 | 610 | 25.3 | 1.0037 | 7.1 | 2 | −150 | 18 | NEG |
| 25 | 8–10 | 0.8 | 320 | 27.2 | 1.0030 | 6.9 | 4 | −141 | 25 | NEG |
| 25 | 10–12 | 2.4 | 200 | 88.6 | 1.0089 | 6.4 | 4 | −134 | 31 | NEG |
| 25 | 12–22 | 0 | 1,950 | 38.5 | 1.0041 | 6.7 | 3 | −145 | 21 | NEG |
| 25 | 22–26 | 0 | 2,150 | 13.3 | 1.0030 | 7.2 | 3 | −164 | 15 | NEG |
| 25 | 26–30 | 0 | 880 | 36.4 | 1.0054 | 7.3 | 0 | −165 | 18 | NEG |
| 25 | 30–34 | 0 | 680 | 43.6 | 1.0079 | 6.7 | 1 | −146 | 21 | NEG |
| 26 | −1 | 0 | 30 | 168.7 | 1.0228 | 6.1 | −6 | −148 | 13 | NEG |
| 26 | 0.25 | 0 | 150 | 136.7 | 1.0196 | 6.1 | −5 | −157 | 19 | NEG |
| 26 | 1 | 1.7 | 50 | 146.0 | 1.0199 | 6.1 | −2 | −147 | 26 | NEG |
| 26 | 2 | 4.6 | 40 | 153.6 | 1.0209 | 6.1 | 1 | −129 | 33 | NEG |
| 26 | 3 | 5.2 | 60 | 149.2 | 1.0208 | 6.2 | 2 | −125 | 41 | NEG |
| 26 | 4 | 6.6 | 100 | 156.7 | 1.0213 | 6.2 | 3 | −117 | 46 | NEG |
| 26 | 4–6 | 8.3 | 130 | 170.4 | 1.0220 | 6.9 | 5 | −121 | 56 | NEG |
| 26 | 6–8 | 8.0 | 200 | 152.2 | 1.0222 | 7.1 | 2 | −105 | 56 | NEG |
| 26 | 8–10 | 8.7 | 270 | 160.1 | 1.0227 | 7.1 | 2 | −123 | 51 | NEG |
| 26 | 10–12 | 6.8 | 170 | 143.0 | 1.0240 | 7.0 | 0 | −126 | 50 | NEG |
| 26 | 12–22 | 3.4 | 630 | 109.2 | 1.0178 | 6.5 | 0 | −141 | 33 | NEG |
| 26 | 22–26 | 2.7 | 170 | 112.9 | 1.0159 | 7.1 | −1 | −139 | 33 | NEG |
| 26 | 26–30 | 2.5 | 350 | 96.7 | 1.0143 | 7.2 | 0 | −129 | 32 | NEG |
| 26 | 30–34 | 2.0 | 550 | 88.6 | 1.0152 | 7.1 | 0 | −146 | 23 | NEG |
| 27 | −1 | 0 | 360 | 27.1 | 1.0057 | 6.2 | 2 | −137 | 14 | NEG |
| 27 | 0.25 | 0 | 460 | 20.2 | 1.0047 | 7.3 | 3 | −178 | 19 | NEG |
| 27 | 1 | 2.6 | 150 | 48.3 | 1.0091 | 7.2 | 9 | −122 | 54 | NEG |
| 27 | 2 | 8.2 | 110 | 82.0 | 1.0141 | 7.1 | 17 | −78 | 77 | NEG |
| 27 | 3 | 7.3 | 50 | 130.9 | 1.0183 | 6.1 | 15 | −73 | 79 | NEG |
| 27 | 4 | 1.8 | 230 | 24.3 | 1.0046 | 6.3 | 5 | −148 | 25 | NEG |
| 27 | 4–6 | 3.3 | 150 | 43.7 | 1.0080 | 6.9 | 4 | −128 | 35 | NEG |
| 27 | 6–8 | 7.0 | 95 | 127.6 | 1.0193 | 7.1 | 8 | −110 | 67 | NEG |
| 27 | 8–10 | 5.1 | 290 | 180.1 | 1.0230 | 5.6 | 4 | −108 | 47 | NEG |
| 27 | 10–12 | 5.1 | 90 | 146.5 | 1.0234 | 6.1 | 4 | −86 | 42 | NEG |
| 27 | 12–22 | 1.4 | 710 | 74.0 | 1.0128 | 5.9 | 1 | −142 | 23 | NEG |
| 27 | 22–26 | 0.8 | 480 | 39.6 | 1.0100 | 7.2 | 0 | −157 | 20 | NEG |
| 27 | 26–30 | 0.8 | 540 | 47.2 | 1.0100 | 7.4 | −1 | −150 | 29 | NEG |
| 27 | 30–34 | 0 | 550 | 49.0 | 1.0093 | 7.2 | 1 | −132 | 24 | NEG |
| 28 | −1 | 0 | 330 | 28.6 | 1.0107 | 6.9 | −1 | −161 | 16 | NEG |
| 28 | 0.25 | 0 | 315 | 32.3 | 1.0107 | 6.7 | 1 | −147 | 24 | NEG |
| 28 | 1 | 4.3 | 60 | 64.7 | 1.0162 | 5.8 | 5 | −111 | 47 | NEG |
| 28 | 2 | 5.5 | 95 | 60.7 | 1.0145 | 6.1 | 7 | −132 | 43 | NEG |
| 28 | 3 | 3.5 | 210 | 23.7 | 1.0065 | 6.5 | 4 | −140 | 30 | NEG |
| 28 | 4 | 2.2 | 270 | 15.3 | 1.0043 | 6.7 | 3 | −151 | 27 | NEG |
| 28 | 4–6 | 9.0 | 80 | 87.8 | 1.0182 | 6.3 | 4 | −105 | 50 | NEG |
| 28 | 6–8 | 3.8 | 290 | 52.0 | 1.0125 | 7.0 | 2 | −138 | 34 | NEG |
| 28 | 8–10 | 1.3 | 330 | 21.8 | 1.0054 | 6.7 | 3 | −148 | 21 | NEG |
| 28 | 10–12 | 2.6 | 170 | 53.9 | 1.0118 | 6.7 | 2 | −145 | 32 | NEG |
| 28 | 12–22 | 2.5 | 340 | 130.8 | 1.0197 | 5.8 | −1 | −130 | 29 | NEG |
| 28 | 22–26 | 0.9 | 730 | 27.7 | 1.0084 | 7.2 | 2 | −149 | 31 | NEG |
| 28 | 26–30 | 1.0 | 460 | 33.3 | 1.0087 | 7.3 | 1 | −159 | 33 | NEG |
| 28 | 30–34 | 1.0 | 450 | 40.3 | 1.0105 | 7.3 | −1 | −151 | 34 | NEG |
| 29 | −1 | 0 | 170 | 32.9 | 1.0031 | 6.2 | 0 | −143 | 17 | NEG |
| 29 | 0.25 | 0 | 390 | 10.3 | 1.0016 | 6.8 | 2 | −148 | 33 | NEG |
| 29 | 1 | 0 | 300 | 9.7 | 1.0018 | 7.2 | 2 | −148 | 26 | NEG |
| 29 | 2 | 0 | 340 | 20.2 | 1.0034 | 6.5 | 3 | −127 | 29 | NEG |
| 29 | 3 | 1.3 | 170 | 18.9 | 1.0041 | 7.3 | 4 | −148 | 20 | NEG |
| 29 | 4 | 1.1 | 320 | 15.6 | 1.0032 | 7.3 | 3 | −157 | 24 | NEG |
| 29 | 4–6 | 0.8 | 570 | 13.1 | 1.0025 | 7.3 | 4 | −132 | 27 | NEG |
| 29 | 6–8 | 0 | 250 | 19.1 | 1.0033 | 7.2 | 2 | −154 | 23 | NEG |
| 29 | 8–10 | 0 | 600 | 20.0 | 1.0032 | 7.4 | 3 | −131 | 19 | NEG |
| 29 | 10–12 | 0 | 400 | 21.1 | 1.0040 | 7.4 | 3 | −158 | 30 | NEG |
| 29 | 12–22 | 1.3 | 660 | 73.2 | 1.0086 | 6.9 | 1 | −151 | 27 | NEG |
| 29 | 22–26 | 0 | 1,510 | 12.5 | 1.0025 | 7.4 | 3 | −155 | 18 | NEG |
| 29 | 26–30 | 0 | 540 | 26.1 | 1.0048 | 7.3 | 3 | −153 | 24 | NEG |
| 29 | 30–34 | 0 | 1,550 | 13.6 | 1.0031 | 6.8 | 3 | −150 | 22 | NEG |
| 36 | −1 | 0 | 40 | 164.0 | 1.0233 | 6.1 | −8 | −139 | 27 | NEG |
| 36 | 0.25 | 0 | 380 | 25.3 | 1.0045 | 6.7 | 1 | −139 | 23 | NEG |
| 36 | 1 | 1.6 | 230 | 24.4 | 1.0044 | 6.9 | 4 | −130 | 21 | NEG |
| 36 | 2 | 2.4 | 310 | 22.2 | 1.0042 | 6.6 | 3 | −143 | 25 | NEG |
| 36 | 3 | 7.1 | 80 | 58.9 | 1.0092 | 6.2 | 6 | −111 | 44 | NEG |
| 36 | 4 | 15.0 | 50 | 124.7 | 1.0174 | 6.9 | 14 | −59 | 94 | NEG |
| 36 | 4–6 | 15.5 | 100 | 147.8 | 1.0209 | 7.4 | 14 | −57 | 107 | NEG |
| 36 | 6–8 | 4.8 | 230 | 46.5 | 1.0102 | 7.4 | 4 | −127 | 38 | NEG |
| 36 | 8–10 | 3.4 | 170 | 39.2 | 1.0089 | 7.4 | 3 | −144 | 38 | NEG |
| 36 | 10–12 | 3.9 | 250 | 55.7 | 1.0112 | 7.2 | 2 | −127 | 46 | NEG |
| 36 | 12–22 | 7.1 | 380 | 145.4 | 1.0198 | 6.7 | 1 | −114 | 56 | NEG |
| 36 | 22–26 | 1.5 | 850 | 29.3 | 1.0077 | 7.4 | 2 | −150 | 32 | NEG |
| 36 | 26–30 | 5.3 | 220 | 103.1 | 1.0168 | 7.4 | 1 | −120 | 61 | NEG |
| 36 | 30–34 | 1.0 | 1,010 | 26.7 | 1.0058 | 7.4 | 2 | −172 | 34 | NEG |
IA, immunoassay; NA, not applicable; MS, missing specimen; NEG, negative; POS, positive.
Analyses of non-smokers urine specimens following exposure to concentrated secondhand cannabis smoke
| Subject # . | Time, h . | THCCOOH GC/MS, ng/mL . | Volume, mL . | Creatinine, mg/dL . | Specific gravity . | pH . | CRL1 DRI, Cutoff = 20 ng/mL, (Equivalent IA response = 20) . | Med Tox KIMS 20 Cutoff = 20 ng/mL, (Equivalent IA response = 0) . | MetroLab EMIT II Plus 20 Cutoff = 20 ng/mL, (Equivalent IA response = 100) . | One Source CEDIA 20 Cutoff = 20 ng/mL . |
|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | ||||||||||
| 7 | −1 | 0 | NA | 56.0 | 1.0055 | 6.7 | −2 | −151 | 10 | NEG |
| 7 | 0.25 | 0 | 230 | 64.5 | 1.0066 | 7.1 | 0 | −149 | 15 | NEG |
| 7 | 1 | 3.8 | 49 | 187.7 | 1.0155 | 6.5 | 28 | −36 | 110 | NEG |
| 7 | 2 | 7.0 | 50 | 177.8 | 1.0162 | 6.3 | 34 | 13 | 158 | POS |
| 7 | 3 | 2.6 | 190 | 52.6 | 1.0056 | 6.2 | −3 | −133 | 42 | NEG |
| 7 | 4 | 1.3 | 220 | 24.1 | 1.0028 | 5.9 | 1 | −138 | 21 | NEG |
| 7 | 4–6 | 1.1 | 450 | 22.2 | 1.0027 | 7.0 | −2 | −145 | 24 | NEG |
| 7 | 6–8 | 2.0 | 380 | 50.4 | 1.0062 | 7.1 | 3 | −119 | 37 | NEG |
| 7 | 8–10 | 3.9 | 195 | 106.3 | 1.0132 | 6.4 | 8 | −91 | 50 | NEG |
| 7 | 10–12 | 2.6 | 455 | 37.2 | 1.0051 | 6.8 | 0 | −139 | 24 | NEG |
| 7 | 12–22 | 1.9 | 440 | 82.5 | 1.0114 | 6.2 | 2 | −112 | 35 | NEG |
| 7 | 22–26 | 1.3 | 640 | 50.7 | 1.0084 | 6.9 | −2 | −147 | 23 | NEG |
| 7 | 26–30 | 1.7 | 305 | 124.5 | 1.0155 | 7.1 | −3 | −133 | 30 | NEG |
| 7 | 30–34 | 1.0 | 670 | 40.1 | 1.0076 | 7.3 | −3 | −146 | 15 | NEG |
| 11 | −1 | 0 | NA | 122.0 | 1.0153 | 6.2 | −8 | −167 | 7 | NEG |
| 11 | 0.25 | 0 | 122 | 64.9 | 1.0109 | 6.8 | 2 | −113 | 40 | NEG |
| 11 | 1 | 3.1 | 69 | 86.5 | 1.0121 | 5.5 | 28 | −47 | 102 | NEG |
| 11 | 2 | 1.8 | 140 | 53.1 | 1.0073 | 5.6 | 9 | −94 | 60 | NEG |
| 11 | 3 | 1.3 | 170 | 41.2 | 1.0048 | 5.4 | 4 | −112 | 33 | NEG |
| 11 | 4 | 3.8 | 35 | 151.7 | 1.0161 | 5.7 | 25 | −36 | 100 | NEG |
| 11 | 4–6 | 6.8 | 71 | 142.1 | 1.0173 | 5.5 | 27 | 6 | 112 | NEG |
| 11 | 6–8 | 2.2 | 140 | 65.1 | 1.0094 | 6.1 | 7 | −84 | 50 | NEG |
| 11 | 8–10 | 2.1 | 140 | 96.1 | 1.0139 | 6.8 | 5 | −88 | 60 | NEG |
| 11 | 10–12 | 1.8 | 210 | 52.3 | 1.0070 | 5.8 | 1 | −141 | 27 | NEG |
| 11 | 12–22 | 1.5 | 730 | 54.4 | 1.0073 | 7.3 | −1 | −142 | 32 | NEG |
| 11 | 22–26 | 1.0 | 300 | 86.4 | 1.0142 | 7.1 | −4 | −126 | 36 | NEG |
| 11 | 26–30 | 1.1 | 280 | 51.6 | 1.0093 | 7.3 | −2 | −129 | 42 | NEG |
| 11 | 30–34 | 1.3 | 180 | 130.6 | 1.0174 | 6.2 | −2 | −106 | 38 | NEG |
| 13 | −1 | 0.0 | NA | 56.2 | 1.0126 | 7.1 | −6 | −163 | 22 | NEG |
| 13 | 0.25 | 0.8 | 218 | 19.6 | 1.0041 | 7.2 | 0 | −142 | 18 | NEG |
| 13 | 1 | 4.0 | 190 | 46.8 | 1.0094 | 7.4 | 10 | −110 | 65 | NEG |
| 13 | 2 | 13.1 | 54 | 100.7 | 1.0179 | 7.4 | 34 | 9 | 170 | POS |
| 13 | 3 | 15.6 | 36 | 91.1 | 1.0154 | 7.1 | 36 | 35 | 174 | POS |
| 13 | 4 | 14.1 | 48 | 100.6 | 1.0148 | 6.2 | 30 | 14 | 139 | POS |
| 13 | 4–6 | 9.6 | 170 | 90.0 | 1.0135 | 5.8 | 19 | −63 | 80 | NEG |
| 13 | 6–8 | 4.7 | 140 | 52.2 | 1.0089 | 5.8 | 7 | −113 | 51 | NEG |
| 13 | 8–10 | 7.0 | 140 | 115.5 | 1.0197 | 6.0 | 11 | −56 | 68 | NEG |
| 13 | 10–12 | 19.3 | 210 | 142.2 | 1.0234 | 5.8 | 33 | 83 | 188 | POS |
| 13 | 12–22 | 3.3 | 480 | 98.9 | 1.0195 | 5.7 | −1 | −102 | 41 | NEG |
| 13 | 22–26 | 0.9 | 510 | 28.7 | 1.0076 | 6.5 | −2 | −153 | 24 | NEG |
| 13 | 26–30 | 2.8 | 180 | 87.3 | 1.0177 | 6.2 | −2 | −127 | 30 | NEG |
| 13 | 30–34 | 1.6 | 250 | 55.9 | 1.0110 | 5.4 | −2 | −131 | 27 | NEG |
| 14 | −1 | 0 | NA | 28.9 | 1.0062 | 7.2 | −3 | −157 | 23 | NEG |
| 14 | 0.25 | 1.1 | 94 | 82.6 | 1.0144 | 7.3 | −2 | −139 | 43 | NEG |
| 14 | 1 | 1.4 | 155 | 42.0 | 1.0077 | 7.2 | 2 | −127 | 42 | NEG |
| 14 | 2 | 1.6 | 200 | 31.7 | 1.0057 | 7.1 | 2 | −140 | 32 | NEG |
| 14 | 3 | 2.6 | 140 | 48.0 | 1.0079 | 6.9 | 3 | −106 | 37 | NEG |
| 14 | 4 | 12.2 | 45 | 119.1 | 1.0161 | 6.3 | 26 | −25 | 103 | NEG |
| 14 | 4–6 | 5.2 | 160 | 98.5 | 1.0148 | 6.8 | 4 | −83 | 63 | NEG |
| 14 | 6–8 | 2.9 | 170 | 70.8 | 1.0120 | 6.5 | 1 | −110 | 35 | NEG |
| 14 | 8–10 | 1.5 | 200 | 46.4 | 1.0079 | 7.2 | 0 | −117 | 38 | NEG |
| 14 | 10–12 | 1.7 | 220 | 67.2 | 1.0109 | 7.0 | 1 | −132 | 30 | NEG |
| 14 | 12–22 | 1.9 | 830 | 89.1 | 1.0128 | 6.4 | −2 | −138 | 26 | NEG |
| 14 | 22–26 | 0 | 260 | 17.4 | 1.0037 | 7.0 | −3 | −152 | 22 | NEG |
| 14 | 26–30 | 0 | 220 | 75.2 | 1.0129 | 7.0 | −3 | −125 | 31 | NEG |
| 14 | 30–34 | 0 | 140 | 23.5 | 1.0060 | 6.9 | −4 | −147 | 17 | NEG |
| 15 | −1 | 0 | NA | 21.9 | 1.0046 | 7.3 | −3 | −161 | 15 | NEG |
| 15 | 0.25 | 0 | 112 | 16.2 | 1.0037 | 7.2 | −1 | −167 | 20 | NEG |
| 15 | 1 | 0 | 136 | 10.0 | 1.0021 | 7.0 | −1 | −147 | 17 | NEG |
| 15 | 2 | 0 | 140 | 10.4 | 1.0020 | 6.6 | −2 | −138 | 13 | NEG |
| 15 | 3 | 1.0 | 100 | 14.5 | 1.0029 | 6.1 | 0 | −148 | 23 | NEG |
| 15 | 4 | 1.7 | 94 | 30.7 | 1.0057 | 6.3 | −1 | −113 | 31 | NEG |
| 15 | 4–6 | 1.7 | 110 | 35.8 | 1.0069 | 7.0 | −1 | −155 | 38 | NEG |
| 15 | 6–8 | 0 | 130 | 12.8 | 1.0027 | 6.1 | −2 | −147 | 19 | NEG |
| 15 | 8–10 | 1.0 | 130 | 24.9 | 1.0057 | 7.2 | −1 | −143 | 28 | NEG |
| 15 | 10–12 | 1.9 | 165 | 44.0 | 1.0099 | 7.2 | −1 | −122 | 46 | NEG |
| 15 | 12–22 | 0.8 | 360 | 54.7 | 1.0085 | 6.0 | −2 | −147 | 30 | NEG |
| 15 | 22–26 | 0.8 | 280 | 38.1 | 1.0082 | 6.0 | −2 | −158 | 25 | NEG |
| 15 | 26–30 | 0 | 335 | 18.4 | 1.0044 | 7.0 | −4 | −142 | 22 | NEG |
| 15 | 30–34 | 1.3 | 420 | 49.4 | 1.0104 | 6.5 | −4 | −131 | 22 | NEG |
| 16 | −1 | 0 | NA | 105.9 | 1.0108 | 7.4 | −6 | −147 | 26 | NEG |
| 16 | 0.25 | 1.0 | 98 | 57.1 | 1.0065 | 7.2 | −1 | −134 | 38 | NEG |
| 16 | 1 | 6.1 | 28 | 225.8 | 1.0197 | 7.4 | 33 | −51 | 182 | NEG |
| 16 | 2 | 1.1 | 120 | 30.0 | 1.0033 | 7.0 | 1 | −140 | 28 | NEG |
| 16 | 3 | 4.5 | 92 | 105.5 | 1.0102 | 6.4 | 13 | −69 | 89 | NEG |
| 16 | 4 | 1.0 | 89 | 21.1 | 1.0025 | 6.2 | 0 | −142 | 24 | NEG |
| 16 | 4–6 | 20.1 | 103 | 19.7 | 1.0028 | 7.0 | 38 | −43 | 103 | POS |
| 16 | 6–8 | 2.8 | 105 | 77.3 | 1.0090 | 7.1 | 3 | −124 | 56 | NEG |
| 16 | 8–10 | 2.9 | 100 | 141.8 | 1.0127 | 6.9 | 4 | −103 | 68 | NEG |
| 16 | 10–12 | 2.1 | 130 | 122.2 | 1.0112 | 6.5 | 2 | −78 | 52 | NEG |
| 16 | 12–22 | 3.5 | 170 | 171.8 | 1.0185 | 7.2 | 3 | −86 | 65 | NEG |
| 16 | 22–26 | 1.2 | 330 | 50.0 | 1.0086 | 7.5 | −3 | −134 | 36 | NEG |
| 16 | 26–30 | 1.8 | 190 | 113.8 | 1.0160 | 7.4 | −2 | −148 | 53 | NEG |
| 16 | 30–34 | 0 | 120 | 249.4 | 1.0230 | 6.3 | −1 | −91 | 59 | NEG |
| Session 2 | ||||||||||
| 8 | −1 | 0 | 257 | 12.0 | 1.0017 | 6.0 | 1 | −157 | 17 | NEG |
| 8 | 0.25 | 0 | 440 | 15.4 | 1.0027 | 6.9 | 2 | −165 | 19 | NEG |
| 8 | 1 | 5.6 | 125 | 42.9 | 1.0063 | 6.2 | 22 | −81 | 79 | NEG |
| 8 | 2 | MS | MS | MS | MS | MS | MS | MS | MS | MS |
| 8 | 3 | 10.8 | 190 | 39.7 | 1.0063 | 5.1 | 25 | −45 | 93 | NEG |
| 8 | 4 | 3.1 | 420 | 10.1 | 1.0016 | 5.6 | 6 | −148 | 30 | NEG |
| 8 | 4–6 | MS | MS | MS | MS | MS | MS | MS | MS | MS |
| 8 | 6–8 | 6.2 | 375 | 32.3 | 1.0053 | 6.6 | 8 | −114 | 53 | NEG |
| 8 | 8–10 | 4.8 | 300 | 32.6 | 1.0062 | 7.2 | 7 | −127 | 48 | NEG |
| 8 | 10–12 | 9.5 | 180 | 64.7 | 1.0127 | 7.1 | 13 | −90 | 83 | NEG |
| 8 | 12–22 | 6.3 | 530 | 80.5 | 1.0117 | 6.0 | 7 | −109 | 54 | NEG |
| 8 | 22–26 | 1.9 | 520 | 24.9 | 1.0054 | 7.3 | 2 | −149 | 32 | NEG |
| 8 | 26–30 | 2.6 | 440 | 40.5 | 1.0067 | 7.3 | 1 | −146 | 35 | NEG |
| 8 | 30–34 | 4.3 | 220 | 86.4 | 1.0129 | 7.2 | 2 | −127 | 44 | NEG |
| 23 | −1 | 0 | 40 | 262.5 | 1.0263 | 5.6 | −7 | −128 | 16 | NEG |
| 23 | 0.25 | 0 | 610 | 22.4 | 1.0029 | 6.2 | 1 | −135 | 21 | NEG |
| 23 | 1 | 3.6 | 790 | 21.4 | 1.0026 | 6.3 | 8 | −139 | 34 | NEG |
| 23 | 2 | 6.9 | 270 | 17.0 | 1.0021 | 6.1 | 10 | −103 | 52 | NEG |
| 23 | 3 | 27.3 | 160 | 61.6 | 1.0079 | 5.4 | 40 | −4 | 144 | POS |
| 23 | 4 | 18.8 | 340 | 42.4 | 1.0054 | 5.6 | 26 | −43 | 100 | NEG |
| 23 | 4–6 | 57.5 | 100 | 168.7 | 1.0212 | 5.6 | 47 | 108 | 226 | POS |
| 23 | 6–8 | 36.8 | 90 | 139.1 | 1.0198 | 5.6 | 44 | 48 | 179 | POS |
| 23 | 8–10 | 32.7 | 220 | 162.4 | 1.0219 | 5.6 | 40 | 69 | 196 | POS |
| 23 | 10–12 | 17.4 | 270 | 98.3 | 1.0157 | 6.0 | 22 | −50 | 98 | NEG |
| 23 | 12–22 | 25.2 | 510 | 175.6 | 1.0226 | 5.8 | 29 | −6 | 108 | POS |
| 23 | 22–26 | 5.3 | 970 | 36.3 | 1.0062 | 7.3 | 4 | −135 | 45 | NEG |
| 23 | 26–30 | 12.4 | 350 | 121.3 | 1.0178 | 7.1 | 6 | −71 | 90 | NEG |
| 23 | 30–34 | 9.3 | 400 | 112.8 | 1.0179 | 6.4 | 4 | −89 | 59 | NEG |
| 37 | −1 | 0 | 138 | 42.3 | 1.0066 | 5.4 | 0 | −163 | 22 | NEG |
| 37 | 0.25 | 0 | 395 | 31.0 | 1.0045 | 5.5 | 3 | −147 | 35 | NEG |
| 37 | 1 | 4.7 | 110 | 34.4 | 1.0044 | 5.4 | 13 | −99 | 61 | NEG |
| 37 | 2 | 17.0 | 60 | 109.3 | 1.0138 | 5.5 | 45 | 47 | 170 | POS |
| 37 | 3 | 23.6 | 40 | 161.2 | 1.0206 | 5.5 | 42 | 106 | 218 | POS |
| 37 | 4 | 46.3 | 40 | 149.7 | 1.0218 | 6.9 | 46 | 118 | 285 | POS |
| 37 | 4–6 | 36.9 | 75 | 157.3 | 1.0218 | 7.1 | 45 | 86 | 243 | POS |
| 37 | 6–8 | 24.1 | 130 | 110.1 | 1.0197 | 7.2 | 33 | 2 | 170 | NEG |
| 37 | 8–10 | 21.3 | 150 | 88.8 | 1.0194 | 6.8 | 27 | −24 | 123 | NEG |
| 37 | 10–12 | 19.3 | 170 | 85.5 | 1.0198 | 6.9 | 24 | −33 | 121 | POS |
| 37 | 12–22 | 20.4 | 550 | 99.5 | 1.0194 | 6.2 | 23 | −48 | 114 | NEG |
| 37 | 22–26 | 10.4 | 360 | 55.1 | 1.0128 | 7.2 | 9 | −87 | 79 | NEG |
| 37 | 26–30 | 15.6 | 170 | 108.3 | 1.0208 | 7.3 | 13 | −74 | 108 | NEG |
| 37 | 30–34 | 10.3 | 180 | 103.1 | 1.0219 | 7.1 | 4 | −91 | 73 | NEG |
| 38 | −1 | 0 | 114 | 102.5 | 1.017 | 5.8 | −5 | −166 | 14 | NEG |
| 38 | 0.25 | 0 | 750 | 11.0 | 1.0018 | 6.7 | 1 | −163 | 16 | NEG |
| 38 | 1 | 1.9 | 117 | 10.8 | 1.0016 | 6.3 | 5 | −138 | 33 | NEG |
| 38 | 2 | 2.9 | 625 | 10.5 | 1.0016 | 6.4 | 5 | −145 | 38 | NEG |
| 38 | 3 | 7.8 | 380 | 23.7 | 1.0039 | 6.0 | 13 | −109 | 55 | NEG |
| 38 | 4 | 20.1 | 123 | 62.7 | 1.0097 | 5.6 | 32 | −33 | 117 | POS |
| 38 | 4–6 | 19.7 | 220 | 66.1 | 1.0119 | 6.1 | 26 | −47 | 105 | NEG |
| 38 | 6–8 | 8.3 | 475 | 39.2 | 1.0073 | 6.0 | 9 | −97 | 62 | NEG |
| 38 | 8–10 | 8.1 | 670 | 43.8 | 1.0085 | 6.2 | 10 | −110 | 49 | NEG |
| 38 | 10–12 | 4.5 | 390 | 27.8 | 1.0053 | 6.1 | 5 | −145 | 39 | NEG |
| 38 | 12–22 | 4.4 | 1,480 | 40.7 | 1.0063 | 5.8 | 6 | −141 | 22 | NEG |
| 38 | 22–26 | 1.2 | 1,860 | 12.7 | 1.0024 | 5.9 | 2 | −167 | 20 | NEG |
| 38 | 26–30 | 2 | 1,120 | 31.1 | 1.0056 | 6.9 | 0 | −153 | 27 | NEG |
| 38 | 30–34 | 1.8 | 790 | 34.8 | 1.007 | 7.2 | −2 | −165 | 31 | NEG |
| 40 | −1 | 0 | 150 | 44.9 | 1.0052 | 6.6 | −2 | −136 | 21 | NEG |
| 40 | 0.25 | 0 | 190 | 66.8 | 1.0076 | 6.7 | 1 | −132 | 30 | NEG |
| 40 | 1 | 1.6 | 162 | 96.6 | 1.0099 | 6.5 | 9 | −105 | 61 | NEG |
| 40 | 2 | 5.5 | 60 | 128.8 | 1.0155 | 6.3 | 19 | −66 | 100 | NEG |
| 40 | 3 | 3 | 122 | 58.8 | 1.0091 | 6.5 | 5 | −106 | 58 | NEG |
| 40 | 4 | 2.8 | 165 | 48.5 | 1.0083 | 6.7 | 3 | −124 | 44 | NEG |
| 40 | 4–6 | 0 | 500 | 28.0 | 1.0048 | 6.9 | 1 | −150 | 36 | NEG |
| 40 | 6–8 | 3.1 | 710 | 19.5 | 1.0034 | 6.7 | 2 | −132 | 28 | NEG |
| 40 | 8–10 | 1.3 | 350 | 33.2 | 1.0065 | 7.2 | −1 | −143 | 40 | NEG |
| 40 | 10–12 | 1.1 | 750 | 25.9 | 1.0056 | 7.2 | −1 | −137 | 27 | NEG |
| 40 | 12–22 | 1.5 | 750 | 63.6 | 1.0094 | 7.1 | 0 | −136 | 35 | NEG |
| 40 | 22–26 | 3.6 | 480 | 22.8 | 1.0049 | 6.8 | 1 | −143 | 32 | NEG |
| 40 | 26–30 | 1.4 | 405 | 76.9 | 1.0118 | 7.2 | −2 | −131 | 43 | NEG |
| 40 | 30–34 | 0 | 550 | 37.8 | 1.0065 | 7.2 | −1 | −143 | 27 | NEG |
| 41 | −1 | 0 | 290 | 16.7 | 1.0032 | 5.8 | −2 | −135 | 12 | NEG |
| 41 | 0.25 | 0 | 605 | 18.4 | 1.0031 | 5.6 | 0 | −144 | 18 | NEG |
| 41 | 1 | 5.8 | 350 | 22.1 | 1.0036 | 6.6 | 10 | −103 | 50 | NEG |
| 41 | 2 | 20.4 | 240 | 37.0 | 1.0078 | 7.3 | 27 | −52 | 121 | NEG |
| 41 | 3 | 12.2 | 335 | 21.0 | 1.0049 | 7.1 | 11 | −100 | 75 | NEG |
| 41 | 4 | 29.1 | 115 | 52.1 | 1.0108 | 7.3 | 28 | −27 | 138 | POS |
| 41 | 4–6 | 29.7 | 215 | 56.5 | 1.0108 | 7.2 | 28 | −37 | 127 | NEG |
| 41 | 6–8 | 12 | 560 | 24.5 | 1.0052 | 7.2 | 8 | −103 | 67 | NEG |
| 41 | 8–10 | 20.7 | 170 | 52.0 | 1.0105 | 7.4 | 19 | −45 | 126 | NEG |
| 41 | 10–12 | 9.6 | 590 | 28.8 | 1.0064 | 7.2 | 7 | −98 | 60 | NEG |
| 41 | 12–22 | 11.1 | 1,000 | 50.5 | 1.0082 | 6.5 | 8 | −99 | 67 | NEG |
| 41 | 22–26 | 0 | 1,260 | 56.0 | 1.0081 | 7.2 | −2 | −150 | 24 | NEG |
| 41 | 26–30 | 9 | 350 | 68.5 | 1.0114 | 7.2 | 4 | −120 | 68 | NEG |
| 41 | 30–34 | 3.7 | 920 | 34.1 | 1.0070 | 7.3 | −1 | −161 | 35 | NEG |
| Session 3 | ||||||||||
| 25 | −1 | 0 | 72 | 109.5 | 1.0084 | 5.8 | 1 | −151 | 12 | NEG |
| 25 | 0.25 | 0 | 190 | 56.7 | 1.0064 | 5.9 | 2 | −150 | 23 | NEG |
| 25 | 1 | 0 | 350 | 18.2 | 1.0029 | 7.0 | 5 | −149 | 26 | NEG |
| 25 | 2 | 1.8 | 210 | 25.4 | 1.0039 | 7.2 | 4 | −146 | 26 | NEG |
| 25 | 3 | 1.1 | 440 | 18.1 | 1.0026 | 7.1 | 4 | −148 | 18 | NEG |
| 25 | 4 | 1.2 | 460 | 18.3 | 1.0025 | 7.1 | 4 | −144 | 19 | NEG |
| 25 | 4–6 | 2.2 | 560 | 39.0 | 1.0057 | 7.3 | 3 | −119 | 24 | NEG |
| 25 | 6–8 | 1.1 | 610 | 25.3 | 1.0037 | 7.1 | 2 | −150 | 18 | NEG |
| 25 | 8–10 | 0.8 | 320 | 27.2 | 1.0030 | 6.9 | 4 | −141 | 25 | NEG |
| 25 | 10–12 | 2.4 | 200 | 88.6 | 1.0089 | 6.4 | 4 | −134 | 31 | NEG |
| 25 | 12–22 | 0 | 1,950 | 38.5 | 1.0041 | 6.7 | 3 | −145 | 21 | NEG |
| 25 | 22–26 | 0 | 2,150 | 13.3 | 1.0030 | 7.2 | 3 | −164 | 15 | NEG |
| 25 | 26–30 | 0 | 880 | 36.4 | 1.0054 | 7.3 | 0 | −165 | 18 | NEG |
| 25 | 30–34 | 0 | 680 | 43.6 | 1.0079 | 6.7 | 1 | −146 | 21 | NEG |
| 26 | −1 | 0 | 30 | 168.7 | 1.0228 | 6.1 | −6 | −148 | 13 | NEG |
| 26 | 0.25 | 0 | 150 | 136.7 | 1.0196 | 6.1 | −5 | −157 | 19 | NEG |
| 26 | 1 | 1.7 | 50 | 146.0 | 1.0199 | 6.1 | −2 | −147 | 26 | NEG |
| 26 | 2 | 4.6 | 40 | 153.6 | 1.0209 | 6.1 | 1 | −129 | 33 | NEG |
| 26 | 3 | 5.2 | 60 | 149.2 | 1.0208 | 6.2 | 2 | −125 | 41 | NEG |
| 26 | 4 | 6.6 | 100 | 156.7 | 1.0213 | 6.2 | 3 | −117 | 46 | NEG |
| 26 | 4–6 | 8.3 | 130 | 170.4 | 1.0220 | 6.9 | 5 | −121 | 56 | NEG |
| 26 | 6–8 | 8.0 | 200 | 152.2 | 1.0222 | 7.1 | 2 | −105 | 56 | NEG |
| 26 | 8–10 | 8.7 | 270 | 160.1 | 1.0227 | 7.1 | 2 | −123 | 51 | NEG |
| 26 | 10–12 | 6.8 | 170 | 143.0 | 1.0240 | 7.0 | 0 | −126 | 50 | NEG |
| 26 | 12–22 | 3.4 | 630 | 109.2 | 1.0178 | 6.5 | 0 | −141 | 33 | NEG |
| 26 | 22–26 | 2.7 | 170 | 112.9 | 1.0159 | 7.1 | −1 | −139 | 33 | NEG |
| 26 | 26–30 | 2.5 | 350 | 96.7 | 1.0143 | 7.2 | 0 | −129 | 32 | NEG |
| 26 | 30–34 | 2.0 | 550 | 88.6 | 1.0152 | 7.1 | 0 | −146 | 23 | NEG |
| 27 | −1 | 0 | 360 | 27.1 | 1.0057 | 6.2 | 2 | −137 | 14 | NEG |
| 27 | 0.25 | 0 | 460 | 20.2 | 1.0047 | 7.3 | 3 | −178 | 19 | NEG |
| 27 | 1 | 2.6 | 150 | 48.3 | 1.0091 | 7.2 | 9 | −122 | 54 | NEG |
| 27 | 2 | 8.2 | 110 | 82.0 | 1.0141 | 7.1 | 17 | −78 | 77 | NEG |
| 27 | 3 | 7.3 | 50 | 130.9 | 1.0183 | 6.1 | 15 | −73 | 79 | NEG |
| 27 | 4 | 1.8 | 230 | 24.3 | 1.0046 | 6.3 | 5 | −148 | 25 | NEG |
| 27 | 4–6 | 3.3 | 150 | 43.7 | 1.0080 | 6.9 | 4 | −128 | 35 | NEG |
| 27 | 6–8 | 7.0 | 95 | 127.6 | 1.0193 | 7.1 | 8 | −110 | 67 | NEG |
| 27 | 8–10 | 5.1 | 290 | 180.1 | 1.0230 | 5.6 | 4 | −108 | 47 | NEG |
| 27 | 10–12 | 5.1 | 90 | 146.5 | 1.0234 | 6.1 | 4 | −86 | 42 | NEG |
| 27 | 12–22 | 1.4 | 710 | 74.0 | 1.0128 | 5.9 | 1 | −142 | 23 | NEG |
| 27 | 22–26 | 0.8 | 480 | 39.6 | 1.0100 | 7.2 | 0 | −157 | 20 | NEG |
| 27 | 26–30 | 0.8 | 540 | 47.2 | 1.0100 | 7.4 | −1 | −150 | 29 | NEG |
| 27 | 30–34 | 0 | 550 | 49.0 | 1.0093 | 7.2 | 1 | −132 | 24 | NEG |
| 28 | −1 | 0 | 330 | 28.6 | 1.0107 | 6.9 | −1 | −161 | 16 | NEG |
| 28 | 0.25 | 0 | 315 | 32.3 | 1.0107 | 6.7 | 1 | −147 | 24 | NEG |
| 28 | 1 | 4.3 | 60 | 64.7 | 1.0162 | 5.8 | 5 | −111 | 47 | NEG |
| 28 | 2 | 5.5 | 95 | 60.7 | 1.0145 | 6.1 | 7 | −132 | 43 | NEG |
| 28 | 3 | 3.5 | 210 | 23.7 | 1.0065 | 6.5 | 4 | −140 | 30 | NEG |
| 28 | 4 | 2.2 | 270 | 15.3 | 1.0043 | 6.7 | 3 | −151 | 27 | NEG |
| 28 | 4–6 | 9.0 | 80 | 87.8 | 1.0182 | 6.3 | 4 | −105 | 50 | NEG |
| 28 | 6–8 | 3.8 | 290 | 52.0 | 1.0125 | 7.0 | 2 | −138 | 34 | NEG |
| 28 | 8–10 | 1.3 | 330 | 21.8 | 1.0054 | 6.7 | 3 | −148 | 21 | NEG |
| 28 | 10–12 | 2.6 | 170 | 53.9 | 1.0118 | 6.7 | 2 | −145 | 32 | NEG |
| 28 | 12–22 | 2.5 | 340 | 130.8 | 1.0197 | 5.8 | −1 | −130 | 29 | NEG |
| 28 | 22–26 | 0.9 | 730 | 27.7 | 1.0084 | 7.2 | 2 | −149 | 31 | NEG |
| 28 | 26–30 | 1.0 | 460 | 33.3 | 1.0087 | 7.3 | 1 | −159 | 33 | NEG |
| 28 | 30–34 | 1.0 | 450 | 40.3 | 1.0105 | 7.3 | −1 | −151 | 34 | NEG |
| 29 | −1 | 0 | 170 | 32.9 | 1.0031 | 6.2 | 0 | −143 | 17 | NEG |
| 29 | 0.25 | 0 | 390 | 10.3 | 1.0016 | 6.8 | 2 | −148 | 33 | NEG |
| 29 | 1 | 0 | 300 | 9.7 | 1.0018 | 7.2 | 2 | −148 | 26 | NEG |
| 29 | 2 | 0 | 340 | 20.2 | 1.0034 | 6.5 | 3 | −127 | 29 | NEG |
| 29 | 3 | 1.3 | 170 | 18.9 | 1.0041 | 7.3 | 4 | −148 | 20 | NEG |
| 29 | 4 | 1.1 | 320 | 15.6 | 1.0032 | 7.3 | 3 | −157 | 24 | NEG |
| 29 | 4–6 | 0.8 | 570 | 13.1 | 1.0025 | 7.3 | 4 | −132 | 27 | NEG |
| 29 | 6–8 | 0 | 250 | 19.1 | 1.0033 | 7.2 | 2 | −154 | 23 | NEG |
| 29 | 8–10 | 0 | 600 | 20.0 | 1.0032 | 7.4 | 3 | −131 | 19 | NEG |
| 29 | 10–12 | 0 | 400 | 21.1 | 1.0040 | 7.4 | 3 | −158 | 30 | NEG |
| 29 | 12–22 | 1.3 | 660 | 73.2 | 1.0086 | 6.9 | 1 | −151 | 27 | NEG |
| 29 | 22–26 | 0 | 1,510 | 12.5 | 1.0025 | 7.4 | 3 | −155 | 18 | NEG |
| 29 | 26–30 | 0 | 540 | 26.1 | 1.0048 | 7.3 | 3 | −153 | 24 | NEG |
| 29 | 30–34 | 0 | 1,550 | 13.6 | 1.0031 | 6.8 | 3 | −150 | 22 | NEG |
| 36 | −1 | 0 | 40 | 164.0 | 1.0233 | 6.1 | −8 | −139 | 27 | NEG |
| 36 | 0.25 | 0 | 380 | 25.3 | 1.0045 | 6.7 | 1 | −139 | 23 | NEG |
| 36 | 1 | 1.6 | 230 | 24.4 | 1.0044 | 6.9 | 4 | −130 | 21 | NEG |
| 36 | 2 | 2.4 | 310 | 22.2 | 1.0042 | 6.6 | 3 | −143 | 25 | NEG |
| 36 | 3 | 7.1 | 80 | 58.9 | 1.0092 | 6.2 | 6 | −111 | 44 | NEG |
| 36 | 4 | 15.0 | 50 | 124.7 | 1.0174 | 6.9 | 14 | −59 | 94 | NEG |
| 36 | 4–6 | 15.5 | 100 | 147.8 | 1.0209 | 7.4 | 14 | −57 | 107 | NEG |
| 36 | 6–8 | 4.8 | 230 | 46.5 | 1.0102 | 7.4 | 4 | −127 | 38 | NEG |
| 36 | 8–10 | 3.4 | 170 | 39.2 | 1.0089 | 7.4 | 3 | −144 | 38 | NEG |
| 36 | 10–12 | 3.9 | 250 | 55.7 | 1.0112 | 7.2 | 2 | −127 | 46 | NEG |
| 36 | 12–22 | 7.1 | 380 | 145.4 | 1.0198 | 6.7 | 1 | −114 | 56 | NEG |
| 36 | 22–26 | 1.5 | 850 | 29.3 | 1.0077 | 7.4 | 2 | −150 | 32 | NEG |
| 36 | 26–30 | 5.3 | 220 | 103.1 | 1.0168 | 7.4 | 1 | −120 | 61 | NEG |
| 36 | 30–34 | 1.0 | 1,010 | 26.7 | 1.0058 | 7.4 | 2 | −172 | 34 | NEG |
| Subject # . | Time, h . | THCCOOH GC/MS, ng/mL . | Volume, mL . | Creatinine, mg/dL . | Specific gravity . | pH . | CRL1 DRI, Cutoff = 20 ng/mL, (Equivalent IA response = 20) . | Med Tox KIMS 20 Cutoff = 20 ng/mL, (Equivalent IA response = 0) . | MetroLab EMIT II Plus 20 Cutoff = 20 ng/mL, (Equivalent IA response = 100) . | One Source CEDIA 20 Cutoff = 20 ng/mL . |
|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | ||||||||||
| 7 | −1 | 0 | NA | 56.0 | 1.0055 | 6.7 | −2 | −151 | 10 | NEG |
| 7 | 0.25 | 0 | 230 | 64.5 | 1.0066 | 7.1 | 0 | −149 | 15 | NEG |
| 7 | 1 | 3.8 | 49 | 187.7 | 1.0155 | 6.5 | 28 | −36 | 110 | NEG |
| 7 | 2 | 7.0 | 50 | 177.8 | 1.0162 | 6.3 | 34 | 13 | 158 | POS |
| 7 | 3 | 2.6 | 190 | 52.6 | 1.0056 | 6.2 | −3 | −133 | 42 | NEG |
| 7 | 4 | 1.3 | 220 | 24.1 | 1.0028 | 5.9 | 1 | −138 | 21 | NEG |
| 7 | 4–6 | 1.1 | 450 | 22.2 | 1.0027 | 7.0 | −2 | −145 | 24 | NEG |
| 7 | 6–8 | 2.0 | 380 | 50.4 | 1.0062 | 7.1 | 3 | −119 | 37 | NEG |
| 7 | 8–10 | 3.9 | 195 | 106.3 | 1.0132 | 6.4 | 8 | −91 | 50 | NEG |
| 7 | 10–12 | 2.6 | 455 | 37.2 | 1.0051 | 6.8 | 0 | −139 | 24 | NEG |
| 7 | 12–22 | 1.9 | 440 | 82.5 | 1.0114 | 6.2 | 2 | −112 | 35 | NEG |
| 7 | 22–26 | 1.3 | 640 | 50.7 | 1.0084 | 6.9 | −2 | −147 | 23 | NEG |
| 7 | 26–30 | 1.7 | 305 | 124.5 | 1.0155 | 7.1 | −3 | −133 | 30 | NEG |
| 7 | 30–34 | 1.0 | 670 | 40.1 | 1.0076 | 7.3 | −3 | −146 | 15 | NEG |
| 11 | −1 | 0 | NA | 122.0 | 1.0153 | 6.2 | −8 | −167 | 7 | NEG |
| 11 | 0.25 | 0 | 122 | 64.9 | 1.0109 | 6.8 | 2 | −113 | 40 | NEG |
| 11 | 1 | 3.1 | 69 | 86.5 | 1.0121 | 5.5 | 28 | −47 | 102 | NEG |
| 11 | 2 | 1.8 | 140 | 53.1 | 1.0073 | 5.6 | 9 | −94 | 60 | NEG |
| 11 | 3 | 1.3 | 170 | 41.2 | 1.0048 | 5.4 | 4 | −112 | 33 | NEG |
| 11 | 4 | 3.8 | 35 | 151.7 | 1.0161 | 5.7 | 25 | −36 | 100 | NEG |
| 11 | 4–6 | 6.8 | 71 | 142.1 | 1.0173 | 5.5 | 27 | 6 | 112 | NEG |
| 11 | 6–8 | 2.2 | 140 | 65.1 | 1.0094 | 6.1 | 7 | −84 | 50 | NEG |
| 11 | 8–10 | 2.1 | 140 | 96.1 | 1.0139 | 6.8 | 5 | −88 | 60 | NEG |
| 11 | 10–12 | 1.8 | 210 | 52.3 | 1.0070 | 5.8 | 1 | −141 | 27 | NEG |
| 11 | 12–22 | 1.5 | 730 | 54.4 | 1.0073 | 7.3 | −1 | −142 | 32 | NEG |
| 11 | 22–26 | 1.0 | 300 | 86.4 | 1.0142 | 7.1 | −4 | −126 | 36 | NEG |
| 11 | 26–30 | 1.1 | 280 | 51.6 | 1.0093 | 7.3 | −2 | −129 | 42 | NEG |
| 11 | 30–34 | 1.3 | 180 | 130.6 | 1.0174 | 6.2 | −2 | −106 | 38 | NEG |
| 13 | −1 | 0.0 | NA | 56.2 | 1.0126 | 7.1 | −6 | −163 | 22 | NEG |
| 13 | 0.25 | 0.8 | 218 | 19.6 | 1.0041 | 7.2 | 0 | −142 | 18 | NEG |
| 13 | 1 | 4.0 | 190 | 46.8 | 1.0094 | 7.4 | 10 | −110 | 65 | NEG |
| 13 | 2 | 13.1 | 54 | 100.7 | 1.0179 | 7.4 | 34 | 9 | 170 | POS |
| 13 | 3 | 15.6 | 36 | 91.1 | 1.0154 | 7.1 | 36 | 35 | 174 | POS |
| 13 | 4 | 14.1 | 48 | 100.6 | 1.0148 | 6.2 | 30 | 14 | 139 | POS |
| 13 | 4–6 | 9.6 | 170 | 90.0 | 1.0135 | 5.8 | 19 | −63 | 80 | NEG |
| 13 | 6–8 | 4.7 | 140 | 52.2 | 1.0089 | 5.8 | 7 | −113 | 51 | NEG |
| 13 | 8–10 | 7.0 | 140 | 115.5 | 1.0197 | 6.0 | 11 | −56 | 68 | NEG |
| 13 | 10–12 | 19.3 | 210 | 142.2 | 1.0234 | 5.8 | 33 | 83 | 188 | POS |
| 13 | 12–22 | 3.3 | 480 | 98.9 | 1.0195 | 5.7 | −1 | −102 | 41 | NEG |
| 13 | 22–26 | 0.9 | 510 | 28.7 | 1.0076 | 6.5 | −2 | −153 | 24 | NEG |
| 13 | 26–30 | 2.8 | 180 | 87.3 | 1.0177 | 6.2 | −2 | −127 | 30 | NEG |
| 13 | 30–34 | 1.6 | 250 | 55.9 | 1.0110 | 5.4 | −2 | −131 | 27 | NEG |
| 14 | −1 | 0 | NA | 28.9 | 1.0062 | 7.2 | −3 | −157 | 23 | NEG |
| 14 | 0.25 | 1.1 | 94 | 82.6 | 1.0144 | 7.3 | −2 | −139 | 43 | NEG |
| 14 | 1 | 1.4 | 155 | 42.0 | 1.0077 | 7.2 | 2 | −127 | 42 | NEG |
| 14 | 2 | 1.6 | 200 | 31.7 | 1.0057 | 7.1 | 2 | −140 | 32 | NEG |
| 14 | 3 | 2.6 | 140 | 48.0 | 1.0079 | 6.9 | 3 | −106 | 37 | NEG |
| 14 | 4 | 12.2 | 45 | 119.1 | 1.0161 | 6.3 | 26 | −25 | 103 | NEG |
| 14 | 4–6 | 5.2 | 160 | 98.5 | 1.0148 | 6.8 | 4 | −83 | 63 | NEG |
| 14 | 6–8 | 2.9 | 170 | 70.8 | 1.0120 | 6.5 | 1 | −110 | 35 | NEG |
| 14 | 8–10 | 1.5 | 200 | 46.4 | 1.0079 | 7.2 | 0 | −117 | 38 | NEG |
| 14 | 10–12 | 1.7 | 220 | 67.2 | 1.0109 | 7.0 | 1 | −132 | 30 | NEG |
| 14 | 12–22 | 1.9 | 830 | 89.1 | 1.0128 | 6.4 | −2 | −138 | 26 | NEG |
| 14 | 22–26 | 0 | 260 | 17.4 | 1.0037 | 7.0 | −3 | −152 | 22 | NEG |
| 14 | 26–30 | 0 | 220 | 75.2 | 1.0129 | 7.0 | −3 | −125 | 31 | NEG |
| 14 | 30–34 | 0 | 140 | 23.5 | 1.0060 | 6.9 | −4 | −147 | 17 | NEG |
| 15 | −1 | 0 | NA | 21.9 | 1.0046 | 7.3 | −3 | −161 | 15 | NEG |
| 15 | 0.25 | 0 | 112 | 16.2 | 1.0037 | 7.2 | −1 | −167 | 20 | NEG |
| 15 | 1 | 0 | 136 | 10.0 | 1.0021 | 7.0 | −1 | −147 | 17 | NEG |
| 15 | 2 | 0 | 140 | 10.4 | 1.0020 | 6.6 | −2 | −138 | 13 | NEG |
| 15 | 3 | 1.0 | 100 | 14.5 | 1.0029 | 6.1 | 0 | −148 | 23 | NEG |
| 15 | 4 | 1.7 | 94 | 30.7 | 1.0057 | 6.3 | −1 | −113 | 31 | NEG |
| 15 | 4–6 | 1.7 | 110 | 35.8 | 1.0069 | 7.0 | −1 | −155 | 38 | NEG |
| 15 | 6–8 | 0 | 130 | 12.8 | 1.0027 | 6.1 | −2 | −147 | 19 | NEG |
| 15 | 8–10 | 1.0 | 130 | 24.9 | 1.0057 | 7.2 | −1 | −143 | 28 | NEG |
| 15 | 10–12 | 1.9 | 165 | 44.0 | 1.0099 | 7.2 | −1 | −122 | 46 | NEG |
| 15 | 12–22 | 0.8 | 360 | 54.7 | 1.0085 | 6.0 | −2 | −147 | 30 | NEG |
| 15 | 22–26 | 0.8 | 280 | 38.1 | 1.0082 | 6.0 | −2 | −158 | 25 | NEG |
| 15 | 26–30 | 0 | 335 | 18.4 | 1.0044 | 7.0 | −4 | −142 | 22 | NEG |
| 15 | 30–34 | 1.3 | 420 | 49.4 | 1.0104 | 6.5 | −4 | −131 | 22 | NEG |
| 16 | −1 | 0 | NA | 105.9 | 1.0108 | 7.4 | −6 | −147 | 26 | NEG |
| 16 | 0.25 | 1.0 | 98 | 57.1 | 1.0065 | 7.2 | −1 | −134 | 38 | NEG |
| 16 | 1 | 6.1 | 28 | 225.8 | 1.0197 | 7.4 | 33 | −51 | 182 | NEG |
| 16 | 2 | 1.1 | 120 | 30.0 | 1.0033 | 7.0 | 1 | −140 | 28 | NEG |
| 16 | 3 | 4.5 | 92 | 105.5 | 1.0102 | 6.4 | 13 | −69 | 89 | NEG |
| 16 | 4 | 1.0 | 89 | 21.1 | 1.0025 | 6.2 | 0 | −142 | 24 | NEG |
| 16 | 4–6 | 20.1 | 103 | 19.7 | 1.0028 | 7.0 | 38 | −43 | 103 | POS |
| 16 | 6–8 | 2.8 | 105 | 77.3 | 1.0090 | 7.1 | 3 | −124 | 56 | NEG |
| 16 | 8–10 | 2.9 | 100 | 141.8 | 1.0127 | 6.9 | 4 | −103 | 68 | NEG |
| 16 | 10–12 | 2.1 | 130 | 122.2 | 1.0112 | 6.5 | 2 | −78 | 52 | NEG |
| 16 | 12–22 | 3.5 | 170 | 171.8 | 1.0185 | 7.2 | 3 | −86 | 65 | NEG |
| 16 | 22–26 | 1.2 | 330 | 50.0 | 1.0086 | 7.5 | −3 | −134 | 36 | NEG |
| 16 | 26–30 | 1.8 | 190 | 113.8 | 1.0160 | 7.4 | −2 | −148 | 53 | NEG |
| 16 | 30–34 | 0 | 120 | 249.4 | 1.0230 | 6.3 | −1 | −91 | 59 | NEG |
| Session 2 | ||||||||||
| 8 | −1 | 0 | 257 | 12.0 | 1.0017 | 6.0 | 1 | −157 | 17 | NEG |
| 8 | 0.25 | 0 | 440 | 15.4 | 1.0027 | 6.9 | 2 | −165 | 19 | NEG |
| 8 | 1 | 5.6 | 125 | 42.9 | 1.0063 | 6.2 | 22 | −81 | 79 | NEG |
| 8 | 2 | MS | MS | MS | MS | MS | MS | MS | MS | MS |
| 8 | 3 | 10.8 | 190 | 39.7 | 1.0063 | 5.1 | 25 | −45 | 93 | NEG |
| 8 | 4 | 3.1 | 420 | 10.1 | 1.0016 | 5.6 | 6 | −148 | 30 | NEG |
| 8 | 4–6 | MS | MS | MS | MS | MS | MS | MS | MS | MS |
| 8 | 6–8 | 6.2 | 375 | 32.3 | 1.0053 | 6.6 | 8 | −114 | 53 | NEG |
| 8 | 8–10 | 4.8 | 300 | 32.6 | 1.0062 | 7.2 | 7 | −127 | 48 | NEG |
| 8 | 10–12 | 9.5 | 180 | 64.7 | 1.0127 | 7.1 | 13 | −90 | 83 | NEG |
| 8 | 12–22 | 6.3 | 530 | 80.5 | 1.0117 | 6.0 | 7 | −109 | 54 | NEG |
| 8 | 22–26 | 1.9 | 520 | 24.9 | 1.0054 | 7.3 | 2 | −149 | 32 | NEG |
| 8 | 26–30 | 2.6 | 440 | 40.5 | 1.0067 | 7.3 | 1 | −146 | 35 | NEG |
| 8 | 30–34 | 4.3 | 220 | 86.4 | 1.0129 | 7.2 | 2 | −127 | 44 | NEG |
| 23 | −1 | 0 | 40 | 262.5 | 1.0263 | 5.6 | −7 | −128 | 16 | NEG |
| 23 | 0.25 | 0 | 610 | 22.4 | 1.0029 | 6.2 | 1 | −135 | 21 | NEG |
| 23 | 1 | 3.6 | 790 | 21.4 | 1.0026 | 6.3 | 8 | −139 | 34 | NEG |
| 23 | 2 | 6.9 | 270 | 17.0 | 1.0021 | 6.1 | 10 | −103 | 52 | NEG |
| 23 | 3 | 27.3 | 160 | 61.6 | 1.0079 | 5.4 | 40 | −4 | 144 | POS |
| 23 | 4 | 18.8 | 340 | 42.4 | 1.0054 | 5.6 | 26 | −43 | 100 | NEG |
| 23 | 4–6 | 57.5 | 100 | 168.7 | 1.0212 | 5.6 | 47 | 108 | 226 | POS |
| 23 | 6–8 | 36.8 | 90 | 139.1 | 1.0198 | 5.6 | 44 | 48 | 179 | POS |
| 23 | 8–10 | 32.7 | 220 | 162.4 | 1.0219 | 5.6 | 40 | 69 | 196 | POS |
| 23 | 10–12 | 17.4 | 270 | 98.3 | 1.0157 | 6.0 | 22 | −50 | 98 | NEG |
| 23 | 12–22 | 25.2 | 510 | 175.6 | 1.0226 | 5.8 | 29 | −6 | 108 | POS |
| 23 | 22–26 | 5.3 | 970 | 36.3 | 1.0062 | 7.3 | 4 | −135 | 45 | NEG |
| 23 | 26–30 | 12.4 | 350 | 121.3 | 1.0178 | 7.1 | 6 | −71 | 90 | NEG |
| 23 | 30–34 | 9.3 | 400 | 112.8 | 1.0179 | 6.4 | 4 | −89 | 59 | NEG |
| 37 | −1 | 0 | 138 | 42.3 | 1.0066 | 5.4 | 0 | −163 | 22 | NEG |
| 37 | 0.25 | 0 | 395 | 31.0 | 1.0045 | 5.5 | 3 | −147 | 35 | NEG |
| 37 | 1 | 4.7 | 110 | 34.4 | 1.0044 | 5.4 | 13 | −99 | 61 | NEG |
| 37 | 2 | 17.0 | 60 | 109.3 | 1.0138 | 5.5 | 45 | 47 | 170 | POS |
| 37 | 3 | 23.6 | 40 | 161.2 | 1.0206 | 5.5 | 42 | 106 | 218 | POS |
| 37 | 4 | 46.3 | 40 | 149.7 | 1.0218 | 6.9 | 46 | 118 | 285 | POS |
| 37 | 4–6 | 36.9 | 75 | 157.3 | 1.0218 | 7.1 | 45 | 86 | 243 | POS |
| 37 | 6–8 | 24.1 | 130 | 110.1 | 1.0197 | 7.2 | 33 | 2 | 170 | NEG |
| 37 | 8–10 | 21.3 | 150 | 88.8 | 1.0194 | 6.8 | 27 | −24 | 123 | NEG |
| 37 | 10–12 | 19.3 | 170 | 85.5 | 1.0198 | 6.9 | 24 | −33 | 121 | POS |
| 37 | 12–22 | 20.4 | 550 | 99.5 | 1.0194 | 6.2 | 23 | −48 | 114 | NEG |
| 37 | 22–26 | 10.4 | 360 | 55.1 | 1.0128 | 7.2 | 9 | −87 | 79 | NEG |
| 37 | 26–30 | 15.6 | 170 | 108.3 | 1.0208 | 7.3 | 13 | −74 | 108 | NEG |
| 37 | 30–34 | 10.3 | 180 | 103.1 | 1.0219 | 7.1 | 4 | −91 | 73 | NEG |
| 38 | −1 | 0 | 114 | 102.5 | 1.017 | 5.8 | −5 | −166 | 14 | NEG |
| 38 | 0.25 | 0 | 750 | 11.0 | 1.0018 | 6.7 | 1 | −163 | 16 | NEG |
| 38 | 1 | 1.9 | 117 | 10.8 | 1.0016 | 6.3 | 5 | −138 | 33 | NEG |
| 38 | 2 | 2.9 | 625 | 10.5 | 1.0016 | 6.4 | 5 | −145 | 38 | NEG |
| 38 | 3 | 7.8 | 380 | 23.7 | 1.0039 | 6.0 | 13 | −109 | 55 | NEG |
| 38 | 4 | 20.1 | 123 | 62.7 | 1.0097 | 5.6 | 32 | −33 | 117 | POS |
| 38 | 4–6 | 19.7 | 220 | 66.1 | 1.0119 | 6.1 | 26 | −47 | 105 | NEG |
| 38 | 6–8 | 8.3 | 475 | 39.2 | 1.0073 | 6.0 | 9 | −97 | 62 | NEG |
| 38 | 8–10 | 8.1 | 670 | 43.8 | 1.0085 | 6.2 | 10 | −110 | 49 | NEG |
| 38 | 10–12 | 4.5 | 390 | 27.8 | 1.0053 | 6.1 | 5 | −145 | 39 | NEG |
| 38 | 12–22 | 4.4 | 1,480 | 40.7 | 1.0063 | 5.8 | 6 | −141 | 22 | NEG |
| 38 | 22–26 | 1.2 | 1,860 | 12.7 | 1.0024 | 5.9 | 2 | −167 | 20 | NEG |
| 38 | 26–30 | 2 | 1,120 | 31.1 | 1.0056 | 6.9 | 0 | −153 | 27 | NEG |
| 38 | 30–34 | 1.8 | 790 | 34.8 | 1.007 | 7.2 | −2 | −165 | 31 | NEG |
| 40 | −1 | 0 | 150 | 44.9 | 1.0052 | 6.6 | −2 | −136 | 21 | NEG |
| 40 | 0.25 | 0 | 190 | 66.8 | 1.0076 | 6.7 | 1 | −132 | 30 | NEG |
| 40 | 1 | 1.6 | 162 | 96.6 | 1.0099 | 6.5 | 9 | −105 | 61 | NEG |
| 40 | 2 | 5.5 | 60 | 128.8 | 1.0155 | 6.3 | 19 | −66 | 100 | NEG |
| 40 | 3 | 3 | 122 | 58.8 | 1.0091 | 6.5 | 5 | −106 | 58 | NEG |
| 40 | 4 | 2.8 | 165 | 48.5 | 1.0083 | 6.7 | 3 | −124 | 44 | NEG |
| 40 | 4–6 | 0 | 500 | 28.0 | 1.0048 | 6.9 | 1 | −150 | 36 | NEG |
| 40 | 6–8 | 3.1 | 710 | 19.5 | 1.0034 | 6.7 | 2 | −132 | 28 | NEG |
| 40 | 8–10 | 1.3 | 350 | 33.2 | 1.0065 | 7.2 | −1 | −143 | 40 | NEG |
| 40 | 10–12 | 1.1 | 750 | 25.9 | 1.0056 | 7.2 | −1 | −137 | 27 | NEG |
| 40 | 12–22 | 1.5 | 750 | 63.6 | 1.0094 | 7.1 | 0 | −136 | 35 | NEG |
| 40 | 22–26 | 3.6 | 480 | 22.8 | 1.0049 | 6.8 | 1 | −143 | 32 | NEG |
| 40 | 26–30 | 1.4 | 405 | 76.9 | 1.0118 | 7.2 | −2 | −131 | 43 | NEG |
| 40 | 30–34 | 0 | 550 | 37.8 | 1.0065 | 7.2 | −1 | −143 | 27 | NEG |
| 41 | −1 | 0 | 290 | 16.7 | 1.0032 | 5.8 | −2 | −135 | 12 | NEG |
| 41 | 0.25 | 0 | 605 | 18.4 | 1.0031 | 5.6 | 0 | −144 | 18 | NEG |
| 41 | 1 | 5.8 | 350 | 22.1 | 1.0036 | 6.6 | 10 | −103 | 50 | NEG |
| 41 | 2 | 20.4 | 240 | 37.0 | 1.0078 | 7.3 | 27 | −52 | 121 | NEG |
| 41 | 3 | 12.2 | 335 | 21.0 | 1.0049 | 7.1 | 11 | −100 | 75 | NEG |
| 41 | 4 | 29.1 | 115 | 52.1 | 1.0108 | 7.3 | 28 | −27 | 138 | POS |
| 41 | 4–6 | 29.7 | 215 | 56.5 | 1.0108 | 7.2 | 28 | −37 | 127 | NEG |
| 41 | 6–8 | 12 | 560 | 24.5 | 1.0052 | 7.2 | 8 | −103 | 67 | NEG |
| 41 | 8–10 | 20.7 | 170 | 52.0 | 1.0105 | 7.4 | 19 | −45 | 126 | NEG |
| 41 | 10–12 | 9.6 | 590 | 28.8 | 1.0064 | 7.2 | 7 | −98 | 60 | NEG |
| 41 | 12–22 | 11.1 | 1,000 | 50.5 | 1.0082 | 6.5 | 8 | −99 | 67 | NEG |
| 41 | 22–26 | 0 | 1,260 | 56.0 | 1.0081 | 7.2 | −2 | −150 | 24 | NEG |
| 41 | 26–30 | 9 | 350 | 68.5 | 1.0114 | 7.2 | 4 | −120 | 68 | NEG |
| 41 | 30–34 | 3.7 | 920 | 34.1 | 1.0070 | 7.3 | −1 | −161 | 35 | NEG |
| Session 3 | ||||||||||
| 25 | −1 | 0 | 72 | 109.5 | 1.0084 | 5.8 | 1 | −151 | 12 | NEG |
| 25 | 0.25 | 0 | 190 | 56.7 | 1.0064 | 5.9 | 2 | −150 | 23 | NEG |
| 25 | 1 | 0 | 350 | 18.2 | 1.0029 | 7.0 | 5 | −149 | 26 | NEG |
| 25 | 2 | 1.8 | 210 | 25.4 | 1.0039 | 7.2 | 4 | −146 | 26 | NEG |
| 25 | 3 | 1.1 | 440 | 18.1 | 1.0026 | 7.1 | 4 | −148 | 18 | NEG |
| 25 | 4 | 1.2 | 460 | 18.3 | 1.0025 | 7.1 | 4 | −144 | 19 | NEG |
| 25 | 4–6 | 2.2 | 560 | 39.0 | 1.0057 | 7.3 | 3 | −119 | 24 | NEG |
| 25 | 6–8 | 1.1 | 610 | 25.3 | 1.0037 | 7.1 | 2 | −150 | 18 | NEG |
| 25 | 8–10 | 0.8 | 320 | 27.2 | 1.0030 | 6.9 | 4 | −141 | 25 | NEG |
| 25 | 10–12 | 2.4 | 200 | 88.6 | 1.0089 | 6.4 | 4 | −134 | 31 | NEG |
| 25 | 12–22 | 0 | 1,950 | 38.5 | 1.0041 | 6.7 | 3 | −145 | 21 | NEG |
| 25 | 22–26 | 0 | 2,150 | 13.3 | 1.0030 | 7.2 | 3 | −164 | 15 | NEG |
| 25 | 26–30 | 0 | 880 | 36.4 | 1.0054 | 7.3 | 0 | −165 | 18 | NEG |
| 25 | 30–34 | 0 | 680 | 43.6 | 1.0079 | 6.7 | 1 | −146 | 21 | NEG |
| 26 | −1 | 0 | 30 | 168.7 | 1.0228 | 6.1 | −6 | −148 | 13 | NEG |
| 26 | 0.25 | 0 | 150 | 136.7 | 1.0196 | 6.1 | −5 | −157 | 19 | NEG |
| 26 | 1 | 1.7 | 50 | 146.0 | 1.0199 | 6.1 | −2 | −147 | 26 | NEG |
| 26 | 2 | 4.6 | 40 | 153.6 | 1.0209 | 6.1 | 1 | −129 | 33 | NEG |
| 26 | 3 | 5.2 | 60 | 149.2 | 1.0208 | 6.2 | 2 | −125 | 41 | NEG |
| 26 | 4 | 6.6 | 100 | 156.7 | 1.0213 | 6.2 | 3 | −117 | 46 | NEG |
| 26 | 4–6 | 8.3 | 130 | 170.4 | 1.0220 | 6.9 | 5 | −121 | 56 | NEG |
| 26 | 6–8 | 8.0 | 200 | 152.2 | 1.0222 | 7.1 | 2 | −105 | 56 | NEG |
| 26 | 8–10 | 8.7 | 270 | 160.1 | 1.0227 | 7.1 | 2 | −123 | 51 | NEG |
| 26 | 10–12 | 6.8 | 170 | 143.0 | 1.0240 | 7.0 | 0 | −126 | 50 | NEG |
| 26 | 12–22 | 3.4 | 630 | 109.2 | 1.0178 | 6.5 | 0 | −141 | 33 | NEG |
| 26 | 22–26 | 2.7 | 170 | 112.9 | 1.0159 | 7.1 | −1 | −139 | 33 | NEG |
| 26 | 26–30 | 2.5 | 350 | 96.7 | 1.0143 | 7.2 | 0 | −129 | 32 | NEG |
| 26 | 30–34 | 2.0 | 550 | 88.6 | 1.0152 | 7.1 | 0 | −146 | 23 | NEG |
| 27 | −1 | 0 | 360 | 27.1 | 1.0057 | 6.2 | 2 | −137 | 14 | NEG |
| 27 | 0.25 | 0 | 460 | 20.2 | 1.0047 | 7.3 | 3 | −178 | 19 | NEG |
| 27 | 1 | 2.6 | 150 | 48.3 | 1.0091 | 7.2 | 9 | −122 | 54 | NEG |
| 27 | 2 | 8.2 | 110 | 82.0 | 1.0141 | 7.1 | 17 | −78 | 77 | NEG |
| 27 | 3 | 7.3 | 50 | 130.9 | 1.0183 | 6.1 | 15 | −73 | 79 | NEG |
| 27 | 4 | 1.8 | 230 | 24.3 | 1.0046 | 6.3 | 5 | −148 | 25 | NEG |
| 27 | 4–6 | 3.3 | 150 | 43.7 | 1.0080 | 6.9 | 4 | −128 | 35 | NEG |
| 27 | 6–8 | 7.0 | 95 | 127.6 | 1.0193 | 7.1 | 8 | −110 | 67 | NEG |
| 27 | 8–10 | 5.1 | 290 | 180.1 | 1.0230 | 5.6 | 4 | −108 | 47 | NEG |
| 27 | 10–12 | 5.1 | 90 | 146.5 | 1.0234 | 6.1 | 4 | −86 | 42 | NEG |
| 27 | 12–22 | 1.4 | 710 | 74.0 | 1.0128 | 5.9 | 1 | −142 | 23 | NEG |
| 27 | 22–26 | 0.8 | 480 | 39.6 | 1.0100 | 7.2 | 0 | −157 | 20 | NEG |
| 27 | 26–30 | 0.8 | 540 | 47.2 | 1.0100 | 7.4 | −1 | −150 | 29 | NEG |
| 27 | 30–34 | 0 | 550 | 49.0 | 1.0093 | 7.2 | 1 | −132 | 24 | NEG |
| 28 | −1 | 0 | 330 | 28.6 | 1.0107 | 6.9 | −1 | −161 | 16 | NEG |
| 28 | 0.25 | 0 | 315 | 32.3 | 1.0107 | 6.7 | 1 | −147 | 24 | NEG |
| 28 | 1 | 4.3 | 60 | 64.7 | 1.0162 | 5.8 | 5 | −111 | 47 | NEG |
| 28 | 2 | 5.5 | 95 | 60.7 | 1.0145 | 6.1 | 7 | −132 | 43 | NEG |
| 28 | 3 | 3.5 | 210 | 23.7 | 1.0065 | 6.5 | 4 | −140 | 30 | NEG |
| 28 | 4 | 2.2 | 270 | 15.3 | 1.0043 | 6.7 | 3 | −151 | 27 | NEG |
| 28 | 4–6 | 9.0 | 80 | 87.8 | 1.0182 | 6.3 | 4 | −105 | 50 | NEG |
| 28 | 6–8 | 3.8 | 290 | 52.0 | 1.0125 | 7.0 | 2 | −138 | 34 | NEG |
| 28 | 8–10 | 1.3 | 330 | 21.8 | 1.0054 | 6.7 | 3 | −148 | 21 | NEG |
| 28 | 10–12 | 2.6 | 170 | 53.9 | 1.0118 | 6.7 | 2 | −145 | 32 | NEG |
| 28 | 12–22 | 2.5 | 340 | 130.8 | 1.0197 | 5.8 | −1 | −130 | 29 | NEG |
| 28 | 22–26 | 0.9 | 730 | 27.7 | 1.0084 | 7.2 | 2 | −149 | 31 | NEG |
| 28 | 26–30 | 1.0 | 460 | 33.3 | 1.0087 | 7.3 | 1 | −159 | 33 | NEG |
| 28 | 30–34 | 1.0 | 450 | 40.3 | 1.0105 | 7.3 | −1 | −151 | 34 | NEG |
| 29 | −1 | 0 | 170 | 32.9 | 1.0031 | 6.2 | 0 | −143 | 17 | NEG |
| 29 | 0.25 | 0 | 390 | 10.3 | 1.0016 | 6.8 | 2 | −148 | 33 | NEG |
| 29 | 1 | 0 | 300 | 9.7 | 1.0018 | 7.2 | 2 | −148 | 26 | NEG |
| 29 | 2 | 0 | 340 | 20.2 | 1.0034 | 6.5 | 3 | −127 | 29 | NEG |
| 29 | 3 | 1.3 | 170 | 18.9 | 1.0041 | 7.3 | 4 | −148 | 20 | NEG |
| 29 | 4 | 1.1 | 320 | 15.6 | 1.0032 | 7.3 | 3 | −157 | 24 | NEG |
| 29 | 4–6 | 0.8 | 570 | 13.1 | 1.0025 | 7.3 | 4 | −132 | 27 | NEG |
| 29 | 6–8 | 0 | 250 | 19.1 | 1.0033 | 7.2 | 2 | −154 | 23 | NEG |
| 29 | 8–10 | 0 | 600 | 20.0 | 1.0032 | 7.4 | 3 | −131 | 19 | NEG |
| 29 | 10–12 | 0 | 400 | 21.1 | 1.0040 | 7.4 | 3 | −158 | 30 | NEG |
| 29 | 12–22 | 1.3 | 660 | 73.2 | 1.0086 | 6.9 | 1 | −151 | 27 | NEG |
| 29 | 22–26 | 0 | 1,510 | 12.5 | 1.0025 | 7.4 | 3 | −155 | 18 | NEG |
| 29 | 26–30 | 0 | 540 | 26.1 | 1.0048 | 7.3 | 3 | −153 | 24 | NEG |
| 29 | 30–34 | 0 | 1,550 | 13.6 | 1.0031 | 6.8 | 3 | −150 | 22 | NEG |
| 36 | −1 | 0 | 40 | 164.0 | 1.0233 | 6.1 | −8 | −139 | 27 | NEG |
| 36 | 0.25 | 0 | 380 | 25.3 | 1.0045 | 6.7 | 1 | −139 | 23 | NEG |
| 36 | 1 | 1.6 | 230 | 24.4 | 1.0044 | 6.9 | 4 | −130 | 21 | NEG |
| 36 | 2 | 2.4 | 310 | 22.2 | 1.0042 | 6.6 | 3 | −143 | 25 | NEG |
| 36 | 3 | 7.1 | 80 | 58.9 | 1.0092 | 6.2 | 6 | −111 | 44 | NEG |
| 36 | 4 | 15.0 | 50 | 124.7 | 1.0174 | 6.9 | 14 | −59 | 94 | NEG |
| 36 | 4–6 | 15.5 | 100 | 147.8 | 1.0209 | 7.4 | 14 | −57 | 107 | NEG |
| 36 | 6–8 | 4.8 | 230 | 46.5 | 1.0102 | 7.4 | 4 | −127 | 38 | NEG |
| 36 | 8–10 | 3.4 | 170 | 39.2 | 1.0089 | 7.4 | 3 | −144 | 38 | NEG |
| 36 | 10–12 | 3.9 | 250 | 55.7 | 1.0112 | 7.2 | 2 | −127 | 46 | NEG |
| 36 | 12–22 | 7.1 | 380 | 145.4 | 1.0198 | 6.7 | 1 | −114 | 56 | NEG |
| 36 | 22–26 | 1.5 | 850 | 29.3 | 1.0077 | 7.4 | 2 | −150 | 32 | NEG |
| 36 | 26–30 | 5.3 | 220 | 103.1 | 1.0168 | 7.4 | 1 | −120 | 61 | NEG |
| 36 | 30–34 | 1.0 | 1,010 | 26.7 | 1.0058 | 7.4 | 2 | −172 | 34 | NEG |
IA, immunoassay; NA, not applicable; MS, missing specimen; NEG, negative; POS, positive.
THCCOOH concentrations (maximum and last) and times in non-smoker's urine specimens following secondhand exposure to concentrated cannabis smoke
| Session . | 1st Subject . | 2nd Subject . | 3rd Subject . | 4th Subject . | 5th Subject . | 6th Subject . | Mean/ Median . |
|---|---|---|---|---|---|---|---|
| Cmax, ng/mL | |||||||
| 1 | 7 | 6.8 | 19.3 | 12.2 | 1.9 | 20.1 | 11.2/9.6 |
| 2 | 10.8 | 57.5 | 46.3 | 20.1 | 5.5 | 29.7 | 28.3/24.9 |
| 3 | 2.4 | 8.7 | 8.2 | 9 | 1.3 | 15.5 | 7.5/8.5 |
| Tmax, ha | |||||||
| 1 | 2 | 5 | 11 | 4 | 11 | 5 | 6.3/5.0 |
| 2 | 3 | 5 | 4 | 4 | 2 | 4 | 3.7/4.0 |
| 3 | 11 | 9 | 2 | 5 | 3 | 4 | 5.7/4.5 |
| Clast, ng/mL | |||||||
| 1 | 1 | 1.3 | 1.6 | 1.9 | 1.3 | 1.8 | 1.5/1.5 |
| 2 | 4.3 | 9.3 | 10.3 | 1.8 | 1.4 | 3.7 | 5.1/4 |
| 3 | 2.4 | 2 | 0.8 | 1 | 1.3 | 1 | 1.4/1.2 |
| Tlast, ha | |||||||
| 1 | 32 | 32 | 32 | 17 | 32 | 28 | 28.8/32 |
| 2 | 32 | 32 | 32 | 32 | 28 | 32 | 31.3/32 |
| 3 | 11 | 32 | 28 | 32 | 17 | 32 | 25.3/30 |
| Session . | 1st Subject . | 2nd Subject . | 3rd Subject . | 4th Subject . | 5th Subject . | 6th Subject . | Mean/ Median . |
|---|---|---|---|---|---|---|---|
| Cmax, ng/mL | |||||||
| 1 | 7 | 6.8 | 19.3 | 12.2 | 1.9 | 20.1 | 11.2/9.6 |
| 2 | 10.8 | 57.5 | 46.3 | 20.1 | 5.5 | 29.7 | 28.3/24.9 |
| 3 | 2.4 | 8.7 | 8.2 | 9 | 1.3 | 15.5 | 7.5/8.5 |
| Tmax, ha | |||||||
| 1 | 2 | 5 | 11 | 4 | 11 | 5 | 6.3/5.0 |
| 2 | 3 | 5 | 4 | 4 | 2 | 4 | 3.7/4.0 |
| 3 | 11 | 9 | 2 | 5 | 3 | 4 | 5.7/4.5 |
| Clast, ng/mL | |||||||
| 1 | 1 | 1.3 | 1.6 | 1.9 | 1.3 | 1.8 | 1.5/1.5 |
| 2 | 4.3 | 9.3 | 10.3 | 1.8 | 1.4 | 3.7 | 5.1/4 |
| 3 | 2.4 | 2 | 0.8 | 1 | 1.3 | 1 | 1.4/1.2 |
| Tlast, ha | |||||||
| 1 | 32 | 32 | 32 | 17 | 32 | 28 | 28.8/32 |
| 2 | 32 | 32 | 32 | 32 | 28 | 32 | 31.3/32 |
| 3 | 11 | 32 | 28 | 32 | 17 | 32 | 25.3/30 |
aTmax for pooled specimens is expressed as the midpoint of the collection period.
THCCOOH concentrations (maximum and last) and times in non-smoker's urine specimens following secondhand exposure to concentrated cannabis smoke
| Session . | 1st Subject . | 2nd Subject . | 3rd Subject . | 4th Subject . | 5th Subject . | 6th Subject . | Mean/ Median . |
|---|---|---|---|---|---|---|---|
| Cmax, ng/mL | |||||||
| 1 | 7 | 6.8 | 19.3 | 12.2 | 1.9 | 20.1 | 11.2/9.6 |
| 2 | 10.8 | 57.5 | 46.3 | 20.1 | 5.5 | 29.7 | 28.3/24.9 |
| 3 | 2.4 | 8.7 | 8.2 | 9 | 1.3 | 15.5 | 7.5/8.5 |
| Tmax, ha | |||||||
| 1 | 2 | 5 | 11 | 4 | 11 | 5 | 6.3/5.0 |
| 2 | 3 | 5 | 4 | 4 | 2 | 4 | 3.7/4.0 |
| 3 | 11 | 9 | 2 | 5 | 3 | 4 | 5.7/4.5 |
| Clast, ng/mL | |||||||
| 1 | 1 | 1.3 | 1.6 | 1.9 | 1.3 | 1.8 | 1.5/1.5 |
| 2 | 4.3 | 9.3 | 10.3 | 1.8 | 1.4 | 3.7 | 5.1/4 |
| 3 | 2.4 | 2 | 0.8 | 1 | 1.3 | 1 | 1.4/1.2 |
| Tlast, ha | |||||||
| 1 | 32 | 32 | 32 | 17 | 32 | 28 | 28.8/32 |
| 2 | 32 | 32 | 32 | 32 | 28 | 32 | 31.3/32 |
| 3 | 11 | 32 | 28 | 32 | 17 | 32 | 25.3/30 |
| Session . | 1st Subject . | 2nd Subject . | 3rd Subject . | 4th Subject . | 5th Subject . | 6th Subject . | Mean/ Median . |
|---|---|---|---|---|---|---|---|
| Cmax, ng/mL | |||||||
| 1 | 7 | 6.8 | 19.3 | 12.2 | 1.9 | 20.1 | 11.2/9.6 |
| 2 | 10.8 | 57.5 | 46.3 | 20.1 | 5.5 | 29.7 | 28.3/24.9 |
| 3 | 2.4 | 8.7 | 8.2 | 9 | 1.3 | 15.5 | 7.5/8.5 |
| Tmax, ha | |||||||
| 1 | 2 | 5 | 11 | 4 | 11 | 5 | 6.3/5.0 |
| 2 | 3 | 5 | 4 | 4 | 2 | 4 | 3.7/4.0 |
| 3 | 11 | 9 | 2 | 5 | 3 | 4 | 5.7/4.5 |
| Clast, ng/mL | |||||||
| 1 | 1 | 1.3 | 1.6 | 1.9 | 1.3 | 1.8 | 1.5/1.5 |
| 2 | 4.3 | 9.3 | 10.3 | 1.8 | 1.4 | 3.7 | 5.1/4 |
| 3 | 2.4 | 2 | 0.8 | 1 | 1.3 | 1 | 1.4/1.2 |
| Tlast, ha | |||||||
| 1 | 32 | 32 | 32 | 17 | 32 | 28 | 28.8/32 |
| 2 | 32 | 32 | 32 | 32 | 28 | 32 | 31.3/32 |
| 3 | 11 | 32 | 28 | 32 | 17 | 32 | 25.3/30 |
aTmax for pooled specimens is expressed as the midpoint of the collection period.
Average THCCOOH concentrations in urine specimens collected from six non-smokers exposed to cannabis smoke (note: data are plotted at the mid-point for pooled specimens collected after 4 h).
A total of 27 specimens (3 in Session 1, 22 in Session 2 and 2 in Session 3) had THCCOOH concentrations ≥15 ng/mL (confirmatory test cutoff concentration recommended by SAMHSA) (22). These specimens were produced by two participants (#13, #16) in Session 1, four participants (#23, #37, #38, #41) in Session 2 and one participant (#36) in Session 3.
Accompanying data (volume, creatinine, specific gravity and pH) for each individual specimen or specimen pool are also included in Table I. In addition, immunoassay data are shown for four different screening assays (DRI, KIMS, EMIT II and CEDIA) at a cutoff concentration of 20 ng/mL. Although CRL laboratory conducted equivalent immunoassays (DRI) on Bottles A and B, only the data for Bottle A are shown in Table I. All non-smoker specimens screened negative for cannabinoids at cutoff concentrations of 100 and 75 ng/mL for all screening assays.
Screening assays for cannabinoids at a 50 ng/mL cutoff concentration produced a single presumptive positive result (0.4% positivity rate) by the Lab Corp EMITII 5B3 THC Assay for Subject # 37 (4 h post exposure, Session 2). This result was the only presumptive positive produced by immunoassay from the five laboratories; the remaining four laboratories reported the same specimen as negative. However, all five laboratories reported this specimen as positive at the 20 ng/mL cutoff concentration. The individual who produced the specimen was a 24-year-old female who weighed 98.1 kg and had a body mass index (BMI) of 29.9. As shown in Table I, the specimen contained 46.3 ng/mL of THCCOOH (GC/MS); creatinine was 149.7 mg/dL; and the specific gravity of the specimen was 1.0218.
Multiple presumptive positive results for non-smokers occurred by immunoassay at the 20 ng/mL cutoff concentration across the three exposure sessions as shown in Table I. The number of positives at the 20 ng/mL cutoff concentration in each session by assay (DRI, KIMS, EMIT II, CEDIA) was as follows, respectively: Session 1, 12, 6, 12, 6; Session 2, 22, 8, 22, 12; and Session 3, 0, 0, 1, 0. The first appearance of a presumptive positive (initial test) result at the 20 ng/mL cutoff concentration occurred in specimens collected within 1–4 h following exposure. Following the appearance of the first presumptive positives, individuals continued to test positive for 2–22 h.
Sensitivity and specificity of immunoassays
Immunoassay responses from the 250 non-smoker urine specimens (18 participants, 3 experimental exposure sessions; 14 specimens per subject; two missing specimens) were compared with GC–MS measures of THCCOOH concentration (Table III). Specimens were designated as TP, TN, FP or FN based on whether the specimen contained ≥15 ng/mL of THCCOOH by GC–MS and demonstrated an appropriate response by the immunoassay at the designated cutoff concentration. Overall, more TPs and FPs and fewer FNs were identified at the 20 ng/mL cutoff concentration than at higher concentrations. Sensitivity and agreement also increased, whereas specificity decreased, at the lower cutoff concentration. There were 27 FNs registered by four immunoassays (CEDIA, DRI, KIMS and EMIT II) and 26 FNs by the EMIT II 5B3 assay at the 50 ng/mL cutoff concentration. The mean (range) THCCOOH concentration of the 27 FNs was 24.6 (15.0–57.5) ng/mL. The corresponding mean (range) creatinine for these specimens was 102.6 (19.7–175.6) mg/dL. The major portion (n = 22, 81.5%) of the FNs were from participants in Session 2. Three (11.1%) FNs came from Session 1 and two (7.4%) came from Session 3.
Comparisons of immunoassay responses to confirmation analyses in non-smoker's urine specimens following secondhand exposure to concentrated cannabis smoke
| . | One Source CEDIA 100 ng/mL . | One Source CEDIA 75 ng/mL . | One Source CEDIA 50 ng/mL . | CRL1 DRI 50 ng/mL . | CRL2 DRI 50 ng/mL . | Med Tox KIMS 50 ng/mL . | MetroLab EMIT II Plus 50 ng/mL . | Lab Corp EMIT II 5B3 50 ng/mL . |
|---|---|---|---|---|---|---|---|---|
| TP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| TN | 223 | 223 | 223 | 223 | 223 | 223 | 223 | 223 |
| FP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FN | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 26 |
| Total | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| Sensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.7 |
| Specificity | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Agreement | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.6 |
| One Source CEDIA 20 ng/mL | CRL1 DRI 20 ng/mL | CRL2 DRI 20 ng/mL | Med Tox KIMS 20 ng/mL | MetroLab EMIT II Plus 20 ng/mL | ||||
| TP | 15 | 23 | 16 | 10 | 25 | |||
| TN | 220 | 212 | 214 | 219 | 213 | |||
| FP | 3 | 11 | 9 | 4 | 10 | |||
| FN | 12 | 4 | 11 | 17 | 2 | |||
| Total | 250 | 250 | 250 | 250 | 250 | |||
| Sensitivity | 55.6 | 85.2 | 59.3 | 37.0 | 92.6 | |||
| Specificity | 98.7 | 95.1 | 96.0 | 98.2 | 95.5 | |||
| Agreement | 94.0 | 94.0 | 92.0 | 91.6 | 95.2 |
| . | One Source CEDIA 100 ng/mL . | One Source CEDIA 75 ng/mL . | One Source CEDIA 50 ng/mL . | CRL1 DRI 50 ng/mL . | CRL2 DRI 50 ng/mL . | Med Tox KIMS 50 ng/mL . | MetroLab EMIT II Plus 50 ng/mL . | Lab Corp EMIT II 5B3 50 ng/mL . |
|---|---|---|---|---|---|---|---|---|
| TP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| TN | 223 | 223 | 223 | 223 | 223 | 223 | 223 | 223 |
| FP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FN | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 26 |
| Total | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| Sensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.7 |
| Specificity | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Agreement | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.6 |
| One Source CEDIA 20 ng/mL | CRL1 DRI 20 ng/mL | CRL2 DRI 20 ng/mL | Med Tox KIMS 20 ng/mL | MetroLab EMIT II Plus 20 ng/mL | ||||
| TP | 15 | 23 | 16 | 10 | 25 | |||
| TN | 220 | 212 | 214 | 219 | 213 | |||
| FP | 3 | 11 | 9 | 4 | 10 | |||
| FN | 12 | 4 | 11 | 17 | 2 | |||
| Total | 250 | 250 | 250 | 250 | 250 | |||
| Sensitivity | 55.6 | 85.2 | 59.3 | 37.0 | 92.6 | |||
| Specificity | 98.7 | 95.1 | 96.0 | 98.2 | 95.5 | |||
| Agreement | 94.0 | 94.0 | 92.0 | 91.6 | 95.2 |
TP, true positive; TN, true negative; FP, false positive; FN, false negative.
Comparisons of immunoassay responses to confirmation analyses in non-smoker's urine specimens following secondhand exposure to concentrated cannabis smoke
| . | One Source CEDIA 100 ng/mL . | One Source CEDIA 75 ng/mL . | One Source CEDIA 50 ng/mL . | CRL1 DRI 50 ng/mL . | CRL2 DRI 50 ng/mL . | Med Tox KIMS 50 ng/mL . | MetroLab EMIT II Plus 50 ng/mL . | Lab Corp EMIT II 5B3 50 ng/mL . |
|---|---|---|---|---|---|---|---|---|
| TP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| TN | 223 | 223 | 223 | 223 | 223 | 223 | 223 | 223 |
| FP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FN | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 26 |
| Total | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| Sensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.7 |
| Specificity | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Agreement | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.6 |
| One Source CEDIA 20 ng/mL | CRL1 DRI 20 ng/mL | CRL2 DRI 20 ng/mL | Med Tox KIMS 20 ng/mL | MetroLab EMIT II Plus 20 ng/mL | ||||
| TP | 15 | 23 | 16 | 10 | 25 | |||
| TN | 220 | 212 | 214 | 219 | 213 | |||
| FP | 3 | 11 | 9 | 4 | 10 | |||
| FN | 12 | 4 | 11 | 17 | 2 | |||
| Total | 250 | 250 | 250 | 250 | 250 | |||
| Sensitivity | 55.6 | 85.2 | 59.3 | 37.0 | 92.6 | |||
| Specificity | 98.7 | 95.1 | 96.0 | 98.2 | 95.5 | |||
| Agreement | 94.0 | 94.0 | 92.0 | 91.6 | 95.2 |
| . | One Source CEDIA 100 ng/mL . | One Source CEDIA 75 ng/mL . | One Source CEDIA 50 ng/mL . | CRL1 DRI 50 ng/mL . | CRL2 DRI 50 ng/mL . | Med Tox KIMS 50 ng/mL . | MetroLab EMIT II Plus 50 ng/mL . | Lab Corp EMIT II 5B3 50 ng/mL . |
|---|---|---|---|---|---|---|---|---|
| TP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| TN | 223 | 223 | 223 | 223 | 223 | 223 | 223 | 223 |
| FP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FN | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 26 |
| Total | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| Sensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.7 |
| Specificity | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Agreement | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.2 | 89.6 |
| One Source CEDIA 20 ng/mL | CRL1 DRI 20 ng/mL | CRL2 DRI 20 ng/mL | Med Tox KIMS 20 ng/mL | MetroLab EMIT II Plus 20 ng/mL | ||||
| TP | 15 | 23 | 16 | 10 | 25 | |||
| TN | 220 | 212 | 214 | 219 | 213 | |||
| FP | 3 | 11 | 9 | 4 | 10 | |||
| FN | 12 | 4 | 11 | 17 | 2 | |||
| Total | 250 | 250 | 250 | 250 | 250 | |||
| Sensitivity | 55.6 | 85.2 | 59.3 | 37.0 | 92.6 | |||
| Specificity | 98.7 | 95.1 | 96.0 | 98.2 | 95.5 | |||
| Agreement | 94.0 | 94.0 | 92.0 | 91.6 | 95.2 |
TP, true positive; TN, true negative; FP, false positive; FN, false negative.
The DRI immunoassay was initially performed at 50 and 20 ng/mL cutoff concentrations upon receipt and thawing of the frozen specimens (bottle A). Additional immunoassays were performed subsequently with Bottle B. Results of these immunoassays are shown in Table III. The first and second set of DRI analyses of bottles A and B are designated as ‘CRL1 DRI’ and ‘CRL2 DRI’, respectively. At the 50 ng/mL cutoff concentration, the results of the repeat analyses for bottle B versus bottle A were identical. At the 20 ng/mL cutoff concentration, two FPs and seven TPs for bottle A were converted to two TNs and seven FNs, respectively.
Discussion
Cannabis is widely used as a drug of abuse, but is also used for purported medical benefits by patients with various disease states such as anorexia, nausea, multiple sclerosis and neuropathic pain (23–25). The widespread prevalence and multi-purpose use of high-potency cannabis has led to renewed concerns regarding the effects of secondhand or ‘passive’ smoke exposure. This study was conducted to re-evaluate the risk of non-smokers testing positive for cannabis because of secondhand exposure to cannabis smoke under extreme conditions. The study used higher potency cannabis material, relative to earlier studies, that is more similar to strengths of THC currently encountered in cannabis in the USA. In addition, cannabis-using participants in the present study were allowed to smoke cannabis cigarettes on an ad libitum basis, simulating actual use patterns, rather than being experimentally limited, as was done in a prior study using higher potency cannabis (20). Non-smokers sat in close proximity to smokers for each 1-h exposure session. In the two unventilated sessions, smoke conditions were noticeably high and caused irritation to eyes and mucous membranes when goggles were not used. Overall study conditions were designed to produce a high-intensity, short-term cannabis smoke environment in which subjects would clearly recognize that they were undergoing cannabis smoke exposure.
The current study demonstrated that short-term extreme cannabis smoke exposure produces absorption of sufficient amounts of THC for some non-smokers to test positive in urine assays employing sensitive cutoff criteria for initial and confirmatory testing. These results are not unlike earlier cannabis smoke exposure studies (10–21), but comprehensively demonstrate the important role that initial immunoassay screening tests play in determining a presumptive positive followed by a secondary confirmatory method. Immunoassays employing a cutoff concentration of 50 ng/mL, as recommended by the SAMHSA Mandatory Guidelines for Federal Workplace Drug Testing Programs produced negative test results (99.6% negative) with the single exception for one assay (EMIT II), whereas initial tests with a 20 ng/mL cutoff concentration produced multiple positive results. This is important because some private non-regulated drug testing programs utilize lower initial screening cutoffs (e.g., 20 ng/mL), which our study shows increases the likelihood of a positive test result in non-smokers exposed to secondhand cannabis smoke. It is important to note that THCCOOH was detectable in all subjects in all exposure sessions by GC–MS at the LOQ of the confirmatory assay. A total of 27 (10.8%) of 250 non-smoker's specimens had THCCOOH concentrations ≥15 ng/mL for confirmation analysis; 17 specimens had concentrations in excess of 20 ng/mL. One participant (#16 at 4–6 h) produced a specimen with a concentration of 20.1 ng/mL of THCCOOH in Session 1 and four participants (#'s 23, 37, 38, 41) produced a total of 16 specimens with >20 ng/mL concentrations of THCCOOH in Session 2. These specimens were excreted 2–22 h following exposure. No participants in Session 3 produced specimens >20 ng/mL of THCCOOH demonstrating the important effect that room air ventilation had upon lowering exposure and intake of THC by non-smokers.
There was considerable variation in the response across the different immunoassay tests to specimens containing ≥15 ng/mL of THCCOOH. The KIMS 20 assay produced the lowest number of TPs (n = 10) and EMIT II produced the highest number (n = 25). Following cannabis exposure, THCCOOH is excreted in urine primarily as a glucuronide conjugate along with small amounts of free metabolite (26, 27). The differences in immunoassay response to specimens containing ≥15 ng/mL of THCCOOH was likely due to differences in cross-reactivity with the glucuronide conjugate of THCCOOH. Package insert information regarding immunoassay cross-reactivity with the glucuronide conjugate at a 20 ng/mL cutoff concentration appear to be only reported for EMIT II (79%) and KIMS (44.1%).
Study limitations
Limitations of the present study include the fixed order of conditions, non-blindness to the ventilation conditions, the single session and small number of participants in each study condition and the possibility of within-session social influences among the smokers. The extent of passive cannabis smoke exposure and absorption is known to vary according to a number of factors including THC potency, amount of cannabis smoked, duration of exposure and environmental factors such as enclosure space, ventilation conditions and proximity of non-smokers to smokers. Additional considerations include assay sensitivity and specificity and administratively designated cutoff concentrations that determine whether a test is reported as positive or negative.
In the present study, non-smokers were seated alongside smokers in very close proximity. Distance of non-smokers from smokers is likely to be an important factor in determining the extent of absorption of aerosolized THC. Also, the study only evaluated the effects of an acute 1-h exposure period to high-intensity smoke conditions. Hence, the conditions of this study were designed to simulate short-term extreme exposure to cannabis smoke. Multiple exposures to cannabis smoke over longer periods and varying intensities could conceivably result in some accumulation of THC and produce different results. However, an earlier study of smoke exposure from lower potency cannabis (2.8% THC) in which subjects were exposed over six consecutive days for 1 h each day produced only suggestive evidence of accumulation or enhancement of positivity rates (12).
The current study employed chronic, daily cannabis smokers to create extreme smoke exposure conditions in the study chamber. Occasional cannabis smokers may produce greater amounts of side stream smoke as a result of fewer inhalation attempts, but it is presumed that they would combust less cannabis overall in a typical smoking session. Other methods of cannabis inhalation, e.g., ‘vaping’ devices that deliver aerosolized oil derived from cannabis, may also alter levels of exposure to non-smokers. Consequently, the current results should be interpreted as being most representative of short-term exposure to extreme, high-intensity cannabis smoke and can only be partially extrapolated to the multiple scenarios of exposure that may occur for non-smokers in other situational and environmental conditions (e.g., ventilation conditions, amount and frequency of exposure).
Conclusions
Cannabis potency and room ventilation were demonstrated to be two major factors in determining the extent of cannabis smoke exposure to non-smokers residing in close proximity to smokers. Short-term exposure to high-intensity smoke from combusted cannabis resulted in non-smoker inhalation of sufficient amounts of THC to produce positive presumptive urine tests by immunoassay with a 20 ng/mL cutoff concentration, but only a single positive occurred at higher cutoff concentrations (50 ng/mL). GC–MS analysis of presumptive positives confirmed the presence of THCCOOH at ≥15 ng/mL in some specimens. Whether test results for non-smokers would be reported as positive or negative will be highly dependent upon the sensitivity of initial and confirmatory tests and related reporting criteria. Overall, these results indicated that extreme smoke exposure can produce positive tests at lower cutoff concentrations, but not generally at the higher initial test cutoff concentration in general use by SAMHSA's Mandatory Guidelines for Federal Workplace Drug Testing Programs.
Acknowledgments
E.J.C. is a consultant to the Division of Workplace Programs, Substance Abuse and Mental Health Services Administration (SAMHSA) and has an Adjunct Professor appointment with Johns Hopkins University School of Medicine, Baltimore, MD, USA. R.V., G.E.B. and E.S.H. are with Johns Hopkins School of Medicine, Baltimore, MD, USA. J.M.M. is an employee of RTI International and C.L. and R.F. are employees of SAMHSA.