-
PDF
- Split View
-
Views
-
Cite
Cite
Denis D. G. Mater, Philippe Langella, Gérard Corthier, María José Flores, Evidence of vancomycin resistance gene transfer between enterococci of human origin in the gut of mice harbouring human microbiota, Journal of Antimicrobial Chemotherapy, Volume 56, Issue 5, November 2005, Pages 975–978, https://doi.org/10.1093/jac/dki336
Close - Share Icon Share
Abstract
Objectives: Potential intra- and inter-species transfers of vancomycin resistance genes (vanA gene cluster) between Enterococcus strains were evaluated in the gut of heteroxenic mice harbouring a human microbiota.
Methods: Mice colonized with a stable population of E. faecium 64/3 or E. faecalis JH2-2 recipient strain and harbouring an enterococci-free human microbiota were obtained. Donor strain E. faecium HC-VI2 of clinical origin was administered orogastrically to these mice and transfers were evaluated over time in faecal samples.
Results: Only intraspecies transfers were detected in the digestive tract (DT) of mice harbouring a human microbiota. E. faecium 64/3 transconjugants were detected at several sampling times over the 60 day experiment to levels up to 103 cfu/g of faeces, but they did not steadily colonize the DT.
Conclusions: Here, we show for the first time that transfer of the vanA gene cluster can occur between Enterococcus strains in the DT colonized with a human microbiota and in the absence of selective pressure. The colonization properties of other enterococci transconjugants and the influence of vancomycin intake should be further investigated since transfers in the DT of animals and humans might contribute to emergence and dissemination of new vancomycin-resistant bacteria.
Introduction
The Enterococcus genus naturally occurs in the digestive tract (DT) of healthy humans.1 Nevertheless, enterococci can also be pathogenic bacteria and have recently become one of the most important causes of nosocomial infections due to intrinsic and acquired resistances to most therapeutically relevant antibiotics, including vancomycin.2 Since vancomycin is often the ultimate resource against infections with multiresistant Gram-positive bacteria, resistance to this antibiotic is considered as a major clinical problem.2 In enterococci, the most commonly encountered resistance genotype is the vanA type, and the vanA gene cluster is carried on Tn1546-related non-conjugative transposons.3 These transposable elements are frequently integrated in conjugative plasmids which may contribute to dissemination of resistance.
Epidemiological and clinical studies support the idea that the commensal microbiota of some animals and humans constitute a reservoir of vancomycin-resistant (VanR) enterococci and an ecological niche where resistance could spread.2,4 To date, few studies have investigated in vivo transfer of vancomycin resistance,5–7 and data from animal models are still needed to determine transfer potentialities between distinct enterococci species, including strains of clinical origin. Moreover, the presence of a complex resident microbiota in the human DT is a feature that must also be considered since it may affect transferability and influence gut colonization by transconjugant cells.
The goal of this study was to investigate the possibility of intra- and interspecies transfer of vancomycin resistance genes (vanA gene cluster) from a human E. faecium isolate of clinical origin to resident enterococci in the gut. For this purpose, we developed heteroxenic mice models colonized with a stable population of defined E. faecium or E. faecalis recipient strains and harbouring an enterococci-free human microbiota (EFHM).
Materials and methods
Bacterial strains and culture media
Enterococcus faecium HC-VI2 is a clinical VanR strain isolated from a hospitalized patient in Italy and was used as the donor in transfer experiments. Vancomycin resistance is conferred by the vanA gene cluster carried by a Tn1546-like transposon integrated in a conjugative plasmid (F. Biavasco, Università delle Marche, Ancona, Italy, personal communication). Recipient strains of human origin were E. faecium 64/3,8 and E. faecalis JH2-2,9 which are both fusidic acid- and rifampicin-resistant. Both strains conjugate efficiently with HC-VI2 donor in vitro.
Enterococci were routinely grown aerobically at 37°C in Brain-Heart Infusion (BHI) medium supplemented with the appropriate antibiotic (10 mg/L vancomycin for strain HC-VI2; 25 mg/L fusidic acid for strains 64/3 and JH2-2).
Animals and microbiota associations
For each experiment, five germ-free C3H/He mice were reared in sterile isolators as previously described.10 To obtain heteroxenic mice harbouring both the recipient strain and an enterococci-free human microbiota (EFHM), germ-free mice were first colonized with the recipient strain and then orogastrically inoculated twice (2 × 250 µL at intervals of 24 h) with a 10-fold dilution of pooled faeces from five EFHM mice. EFHM mice were first obtained by two orogastric inoculations of germ-free mice with a 108-fold dilution of faeces from a healthy human whose faecal enterococci population did not exceed 3 × 106 cfu/g of faeces. EFHM mice were checked to be enterococci-free by plating 10-fold diluted mice faeces on bile-aesculin-azide selection medium (Biokar Diagnostics, Allonne, France). Animal experiments were performed according to the guidelines of the French Ethics Committee.
In vivo transfer experiments
Mice harbouring both the recipient and EFHM were inoculated orogastrically with a 10-fold concentrated fresh culture of the donor strain (∼2 × 109 cfu per animal). For sampling, faeces from at least three animals were separately collected, homogenized in Liquid Casein-Yeast extract medium (LCY medium) and appropriate dilutions were spread on selective media; BHI-agar plates containing 10 mg/L vancomycin (Van-plates), 25 mg/L fusidic acid (Fus-plates), or both antibiotics (Van-Fus-plates) were used for selection and enumeration of donor, recipient or transconjugant clones, respectively. Data from the different mice were log-transformed and averaged (n ≥ 3).
Transconjugant clones 64/3T and JH2-2T were randomly isolated from Van-Fus-plates during preliminary transfer experiments using gnotoxenic mice (data not shown).
Control of transconjugants
Several clones grown on Van-Fus-plates were randomly picked and checked to verify they were indeed transconjugants: (i) bacterial colonies were streaked on BHI-agar plates containing 25 mg/L rifampicin (Rif-plates), allowing secondary discrimination between recipient/transconjugant strain and donor strain; (ii) DNA of bacterial colonies was purified using the GeneReleaser® kit (BioVentures Inc., Murfreesboro, TN, USA) and was subsequently used for PCR amplification of a 377 bp fragment of the vanA gene, as described by Klare et al.4
Results
Obtaining heteroxenic mice harbouring both recipient strain and enterococci-free human microbiota
When EFHM was inoculated to either 64/3- or JH2-2 mice (data not shown), recipient populations dropped drastically in the following days. In JH2-2 mice harbouring EFHM, the population of the recipient was found to be stable after 13 days (∼3 × 104 cfu/g of faeces). A relative stability (2 × 105 to 2 × 106 cfu/g of faeces) was obtained after 25 days with strain 64/3.
Transfer of vancomycin resistance genes in the gut of heteroxenic mice harbouring both recipient and enterococci-free human microbiota
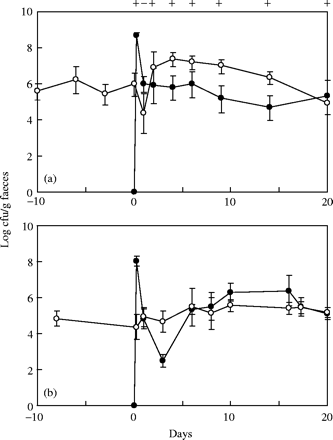
Inoculation of E. faecium HC-VI2 donor cells to either 64/3- or JH2-2 mice harbouring EFHM resulted in a marked decrease in the introduced donor population by 400- to 1700-fold within 24 h (Figure 1). At this time, the levels of donor populations reached those of recipients before inoculation of the donor strain. In 64/3 mice harbouring EFHM (Figure 1a), the donor population level remained almost stable for the next 19 days of the experiment, whereas the recipient population level varied extensively until day 20 to reach a similar level as the donor. In contrast, using JH2-2 mice harbouring EFHM (Figure 1b), the recipient population remained stable from day 1 to day 20, whereas the donor population varied and finally reached the same level as the recipient at the end of the experiment.
Variations in faecal populations of recipient (open circles) and donor (filled circles) in heteroxenic mice. (a) E. faecium 64/3- or (b) E. faecalis JH2-2 mice harbouring EFHM received E. faecium HC-VI2 donor cells at day 0. (+) above graph A indicates sampling times where transconjugants were detected in at least one mouse (Figure 2). For experiment shown in (b), no transconjugant was detected in any mouse. Error bars represent standard deviation for five animals, except for the first two sampling times at which only three mice were analysed.
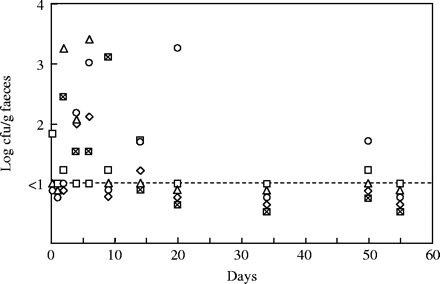
Recovery over a 60 day period of VanR transconjugants from the faeces of E. faecium 64/3 mice harbouring EFHM. Mice were inoculated at day 0 with the donor strain E. faecium HC-VI2. The presence of transconjugants was monitored in five animals, except for the first two sampling times at which only three mice were analysed. Each symbol represents the same animal along the experiment.
After inoculation of mice with HC-VI2 donor cells, faeces were analysed to detect potential transfers. Transconjugants were only recovered from the faeces of the 64/3 mice harbouring EFHM and their population levels varied substantially with a maximum at ∼103 cfu/g of faeces (Figure 2). Surprisingly, they were not detected simultaneously in the five animals used in the experiment: Transconjugants were recovered in the faeces of four mice on days 4 and 6, while they were detected in only two or three mice on days 2, 9 and 14. Moreover, they were found at some sampling times (e.g. day 50) in mice which were devoid of transconjugant before (e.g. day 34) and after (e.g. day 55). When subsequent inoculation of the same group of mice was carried out with a high titre of the transconjugant clone 64/3T, the newly introduced transconjugant population decreased progressively and became undetectable after 14 days (data not shown).
No transconjugant was detected throughout the experiment when the donor strain HC-VI2 was introduced in JH2-2 mice harbouring EFHM. In addition, subsequent inoculation of the same group of mice with a high titre of the transconjugant clone JH2-2T showed that the newly introduced transconjugant population was totally eliminated after 4 days (data not shown).
Discussion
Enterococci are common inhabitants of the human DT.1 In this study, we have developed a mouse model harbouring a human microbiota lacking enterococci in order to circumvent the presence of resident enterococci. Consequently, E. faecium 64/3 or E. faecalis JH2-2 were present as the sole enterococci species in mice colonized with the recipient and harbouring EFHM. Moreover, enterococci populations were established at the level usually measured in a conventional human microbiota, indicating the conservation of antagonistic effects produced by the dominant bacterial species.1 Mice harbouring EFHM are thus valuable models for in vivo studies involving enterococci and requiring a human microbiota background.
In vivo transfers were examined in such heteroxenic models. Once administered to the mice, the population of E. faecium HC-VI2 donor immediately decreased due to antagonistic effects of the resident human microbiota on Enterococcus species.1 Then, equilibrium was progressively set up between parental cell populations at levels corresponding to those of enterococci in a conventional human microbiota. Such an evolution towards the equilibrium might be due to specific interactions between parental strains and also to interactions between enterococci and the human microbiota. It is thus a dynamic illustration of variations in bacterial composition of the gut microbiota after the introduction of a foreign strain and an example of bacterial adaptation to the DT. Adaptation should be further studied for enterococci of non-human source such as animal isolates which might differ in colonization abilities.
Transfer of vancomycin resistance in the gut of mice colonized with the recipient and harbouring EFHM was observed between the two E. faecium parental strains, but not between E. faecium and E. faecalis species. VanR 64/3 transconjugants could not establish and stabilize in the gut of 64/3 mice harbouring EFHM. Actually, the transconjugant population levels varied extensively between mice and fluctuated throughout the experiment for a given animal. This strongly suggests that transfer events could occur in the DT at any time during the whole experiment. Inoculation at high titres of transconjugant cells (either clone 64/3T or clone JH2-2T) to mice colonized with the recipient and harbouring EFHM confirmed that VanR transconjugant populations were eliminated and could not colonize the DT. This peculiar feature of transconjugants emphasizes the role of the human microbiota as a potential barrier against emergence of VanR transconjugants. This point should be experimentally examined for different transferable elements and bacteria, including enterococci of animal origin, since it might have an ecological significance in terms of dissemination of the resistance factors. Otherwise, further experiments with vancomycin-treated mice should be performed since the antibiotic may select transconjugants and improve their colonization abilities.
Here, we report for the first time the transfer of vancomycin resistance genes between Enterococcus strains of human origin in the DT of mice in the presence of a human microbiota. Although the transconjugants did not persist in the present mouse model, this finding strengthens the idea that vancomycin resistance gene transfers may occur in the human gut. The colonization properties of other enterococci transconjugants and the influence of vancomycin intake should be further investigated. Transfer events in this ecological niche might indeed contribute to the spread of vancomycin resistance among enterococci and might increase the diversity of VanR bacterial species, leading to the emergence of gut reservoirs for the VanA-type resistance.
We thank Dr F. Biavasco (Università Politecnica delle Marche, Ancona, Italy) and Dr A. Sundsfjord (University and University Hospital of Tromsø, Norway) for the generous gift of strains E. faecium HC-VI2 and E. faecium 64/3, respectively. We also thank Professor A. Collignon (University of Châtenay-Malabry, France) for providing vancomycin. This work has been carried out with financial support from the Commission of the European Union, specific RTD programme ‘Quality of Life and Management of Living Resources’, contract no. QLK2-CT 2002-00843, ARTRADI. It does not necessarily reflect the views of the Commission and in no way anticipates its future policy in this area.
References
Tannock GW, Cook G. Enterococci as members of the intestinal microflora of humans. In: Gilmore MS, ed. The Enterococci: Pathogenesis Molecular Biology and Antibiotic Resistance. Washington, DC, USA: ASM Press,
Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: why are they here, and where do they come from?
Woodford N. Epidemiology of the genetic elements responsible for acquired glycopeptide resistance in enterococci.
Klare I, Heier H, Claus H et al. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry.
Ikeda T, Watanabe T, Matsumoto K et al. Transferability of vanA gene from vancomycin-resistant Enterococcus faecalis in the digestive tract of specific pathogen-free mice.
Johnsen PJ, Simonsen GS, Olsvik O et al. Stability, persistence, and evolution of plasmid-encoded VanA glycopeptide resistance in enterococci in the absence of antibiotic selection in vitro and in gnotobiotic mice.
Moubareck C, Bourgeois N, Courvalin P et al. Multiple antibiotic resistance gene transfer from animal to human enterococci in the digestive tract of gnotobiotic mice.
Werner G, Willems RJ, Hildebrandt B et al. Influence of transferable genetic determinants on the outcome of typing methods commonly used for Enterococcus faecium.
Jacob AE, Hobbs SJ. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes.