-
PDF
- Split View
-
Views
-
Cite
Cite
Laura Milazzo, Manuela Piazza, Ornella Sangaletti, Nadia Gatti, Anna Cappelletti, Fulvio Adorni, Spinello Antinori, Massimo Galli, Mauro Moroni, Agostino Riva, [13C]Methionine breath test: a novel method to detect antiretroviral drug-related mitochondrial toxicity, Journal of Antimicrobial Chemotherapy, Volume 55, Issue 1, January 2005, Pages 84–89, https://doi.org/10.1093/jac/dkh497
Close - Share Icon Share
Abstract
Objectives: A major side effect of antiretroviral drugs is nucleoside reverse transcriptase inhibitor (NRTI)-related mitochondrial toxicity, the in vivo diagnosis of which is difficult and not yet standardized. We used the [13C]methionine breath test to investigate hepatic mitochondrial oxidation in HIV-1-infected patients receiving antiretroviral therapy.
Patients and methods: The [13C]methionine breath test was performed in healthy subjects (n=10), HIV-infected patients on antiretroviral therapy with (n=6) and without (n=15) hyperlactataemia and naive HIV-infected patients (n=11). After oral administration of [13C]methionine (2 mg/kg body weight), hepatic methionine metabolism was measured by breath 13CO2 enrichment, expressed as δ over baseline (DOB) every 15 min for 120 min by mass spectrometry.
Results: The four study groups showed a significant difference in 13CO2 exhalation (P=0.001). HIV-infected patients on antiretroviral therapy with normal serum lactate had reduced exhalation of 13CO2 compared with healthy subjects (DOB mean peak: 8.82±0.62 versus 11±0.9, P<0.05). HIV patients with hyperlactataemia had even lower values when compared with patients with normal lactataemia (DOB mean peak: 4.98±0.68 versus 8.82±0.62, P<0.05).
Conclusions: The [13C]methionine breath test possibly showed mitochondrial impairment in antiretroviral-treated HIV-positive patients, particularly with hyperlactataemia. This non-invasive test can be used to monitor drug-related mitochondrial toxicity in vivo and to discover early and asymptomatic damage of the respiratory chain.
Introduction
A main issue in the management of HIV-infected patients is long-term toxicity of antiretroviral therapy (ART). Several adverse events due to mitochondrial toxicity, such as hepatic steatosis, myopathy, cardiomyopathy, peripheral neuropathy, pancreatitis, diabetes mellitus, lipid metabolic dysfunction and lipodystrophy syndrome, are associated with the use of nucleoside reverse transcriptase inhibitors (NRTIs).1–3 A rare adverse effect of NRTIs, also related to mitochondrial impairment, is lactic acidosis, which is a dramatic and potentially fatal condition. It is noteworthy that hyperlactataemia in patients receiving ART may present as a chronic, asymptomatic feature.1,4,5
NRTIs could induce mitochondrial toxicity by inhibiting human DNA polymerase γ leading to depletion of mitochondrial DNA and damage of the respiratory chain.6,7 Mitochondrial DNA levels are significantly decreased in peripheral blood cells of HIV-infected patients with symptomatic hyperlactataemia,2,8 which is usually associated with the use of stavudine, with or without didanosine, rather than with zidovudine-based regimens.1
Among the available techniques for in vivo diagnosis of mitochondrial toxicity, the measurement of lactataemia and the quantification of mitochondrial DNA levels in peripheral blood cells or tissues are still under investigation and are hampered by technical difficulties and lack of specificity.9,10 Moreover, the direct monitoring of mitochondrial DNA in target tissues requires repeated biopsies and cannot be considered for ethical and practical reasons.
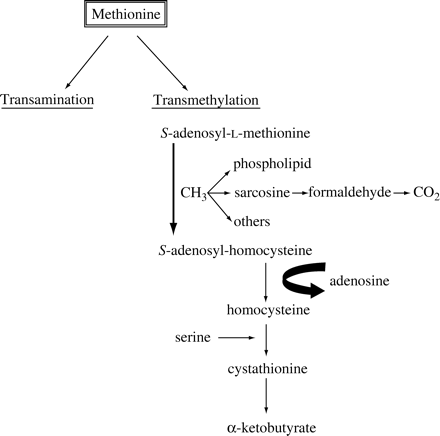
Methionine is an essential amino acid mostly metabolized by the liver through two major pathways (see Figure 1). Transamination to α-keto-γ-methiolbutyric acid occurs mainly in the liver, but not under normal metabolic conditions;11–13 under physiological conditions, transmethylation by methionine adenosyltranferase into homocysteine is the major metabolic pathway and occurs only in the liver, as most other tissues lack one or more enzymes of the cycle.14S-adenosyl-L-methionine is converted into S-adenosyl-homocysteine by the mainly hepatic enzyme N-methyltransferase. The function of this enzyme is to remove methyl groups leading to different products; the major pathway to remove excess methionine methyl groups is via sarcosine production.15,16 Sarcosine is oxidized by sarcosine dehydrogenase to produce a one-carbon fragment at the oxidation level of formaldehyde; such carbon fragments can be subsequently converted into CO2. Sarcosine dehydrogenase is oxidized by a mitochondrial oxidation system,17–19 and it has been shown that the sarcosine oxidase system of rat liver is present exclusively in the mitochondria.20 Therefore, methyl-13C-labelled methionine could probably be used to evaluate the oxidative capacity of liver mitochondria.21
The breath test with 13C-labelled methionine is a non-invasive, non-radioactive technique, and has been used to investigate drug-related acute liver toxicity,22 ethanol-induced liver oxidative stress23 and impaired hepatic mitochondrial oxidation in liver steatosis and cirrhosis.24 We have previously described profound impairment of 13CO2 excretion in four HIV-1-infected patients on antiretroviral treatment presenting hyperlactataemia. Such impairment rapidly improved after therapy withdrawal or modification.25 These data prompted us to extend the study wider and to different groups of patients.
The aim of our study was to evaluate the validity and feasibility of the [13C]methionine breath test for the diagnosis of liver mitochondrial impairment in antiretroviral-treated and -untreated HIV-1-infected patients with or without symptoms of hyperlactataemia. Given the low frequency of patients with symptomatic hyperlactataemia, we included in this group four patients that we had previously described.
Patients and methods
We consecutively enrolled 15 ART multiexperienced HIV-infected patients on NRTI-containing antiretroviral combinations and six NRTI-treated patients presenting symptomatic hyperlactataemia. We also included 11 HIV-infected patients naive for antiretroviral treatment and 10 healthy subjects as controls. Exclusion criteria were hepatitis B virus (HBV) or hepatitis C virus (HCV) co-infection, alcohol consumption >80 g/week, use of any drug other than antiretrovirals and a serum lactate level >2.67 mmol/L, except for hyperlactataemic patients who had serum lactate ≥3 mmol/L. All the treated patients had been exposed to thymidine analogues in their therapeutic history.
The study design was approved by the ethical committee of our institution and written informed consent was obtained from all the patients.
The following parameters were considered for both patients and controls: body mass index (BMI; calculated as weight in kilograms divided by squared height in metres); serum transaminases; triglycerides; cholesterol. Viral load (HIV-RNA), CD4 cell count and serum lactate were also evaluated in all HIV-positive subjects. The blood sample for measurement of lactate was collected in sodium fluoride potassium oxalate tubes, without the use of tourniquet. HIV-infected patients underwent a liver ultrasound examination.
The [13C]methionine breath test was performed after an overnight fast and with rest for 30 min before and during the test. All the subjects drank 200 mL of orange juice and 30 min later received 2 mg/kg body weight of methyl-13C-labelled methionine (L-[13C]methionine; Isotec Inc., Miamisburg, OH, USA) dissolved in 100 mL of water. A deep breath was exhaled into glass tubes at baseline, 30 min after methionine consumption and thereafter every 15 min up to 120 min. Breath 13CO2 enrichment was measured with a gas isotope mass spectrometer (BreathMat; FinniganMat, Bremen, Germany).
Results were expressed as: change (δ) over baseline (DOB) CO2 enrichment; dose of 13CO2/h (percentage of the dose of administered 13C recovered per hour); and cumulative percentage of the dose of 13C recovered over the testing period (13C cumulative dose).
Data are expressed as means ± S.E.M. The results were analysed by ANOVA for repeated measures, the Kruskal–Wallis non-parametric test and the ANOVA test for multiple comparisons.
Results
The general characteristics of the patients and healthy controls are summarized in Table 1. No statistically significant differences emerged among the studied groups regarding age, sex, CDC stage, BMI and CD4 cell count. Naive patients, as expected, presented higher HIV plasma viraemia compared with patients on treatment (P=0.004). Six of the patients (three in the naive group and three in the treated asymptomatic group) presented a slight elevation in aminotransferase values [mean value: 101 IU/L (range 63–160); normal value: <50 IU/L], with an ultrasound pattern of liver steatosis in three subjects. Additionally, 10 HIV-positive patients with normal aminotransferases showed liver steatosis at ultrasound examination. A clear difference emerged between treated and untreated subjects with regard to triglyceride levels (P=0.04) without any correlation with high serum lactate; meanwhile, no difference was found for cholesterol levels. Of the six patients with hyperlactataemia, two reported nausea, vomiting, intense fatigue and symptoms of peripheral neuropathy; the remaining four reported myalgias and peripheral neuropathy. The mean value of serum lactate was 6.3 mmol/L with a range of 3–8.4 mmol/L (normal values: 0.67–2.67) in this group.
The six subjects with hyperlactataemia had been treated for a mean of 2.2 years versus 6.8 years for the asymptomatic patients (P=0.003). Overall, 14 patients were on treatment with a thymidine analogue-containing regimen and seven with a thymidine analogue-sparing regimen. Of the 14 patients on treatment with thymidine analogues, seven were on zidovudine and seven on stavudine (Table 1). All of the patients on thymidine analogue NRTI-sparing regimens had previously received zidovudine or stavudine or both and had discontinued the drugs for at least 3 months before the test.
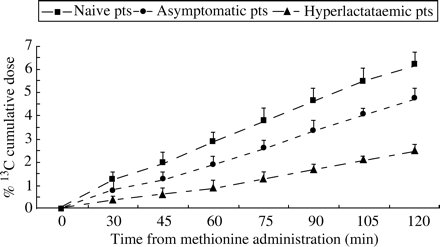
The 13CO2 breath exhalation curves (expressed as DOB) are shown in Figure 2. 13CO2 excretion by healthy controls increased early after the oral administration of the isotope, reaching the maximum value after 60 min with a mean peak DOB value of 11 ± 0.9. A similar slope was obtained from HIV-infected subjects naive for antiretroviral therapy, with no difference between the two curves.
Asymptomatic HIV-positive patients on antiretroviral treatment with normal blood lactate values showed reduced 13CO2 exhalation compared with healthy subjects. The 13CO2 excretion rate in these subjects increased more slowly, with a delayed 13CO2 peak at 75 min, instead of the 60 min in healthy controls and naive patients. The peak was also significantly decreased (peak DOB 8.82 ± 0.62, P < 0.05).
Finally, the 13CO2 breath exhalation curve of HIV-positive patients receiving ART and presenting symptoms of hyperlactataemia showed a very low and delayed profile, and cumulative excretion values were significantly reduced compared with both HIV patients without hyperlactataemia and healthy controls (P=0.001 and <0.05 respectively) (Figures 2 and 3).
The cumulative 13CO2 excretion, expressed as a percentage of 13C cumulative dose, of the three groups of HIV-infected patients, is reported in Figure 3; the 13CO2 excretion rate of treated patients was reduced compared with naive subjects, particularly in patients with hyperlactataemia (P < 0.05) (Figure 3).
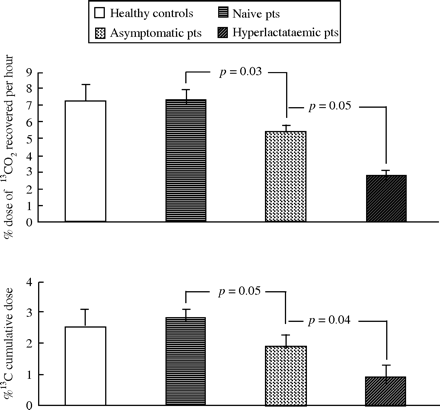
The 13C cumulative dose at 60 min and the percentage of 13C dose recovered per hour was significantly different among the four groups of subjects (P=0.02 and 0.001, respectively) (Figure 4).
In a multivariate analysis, the DOB at 60 min did not correlate with age, gender, BMI or aminotransferase values, and if only patients on treatment (with or without hyperlactataemia) are considered, the DOB did not correlate with the duration of antiretroviral therapy. A non-significant inverse relationship was found between DOB at 60 min and triglyceride values (Pearson's correlation coefficient: −0.26).
The DOB values at 60 min were not significantly different between the patients receiving thymidine analogues and those with a thymidine analogue-sparing regimen.
Interestingly, one of the normolactataemic patients presented evidence of severe lipoatrophy and had a dramatically low curve with a DOB at 60 min of 3.87.
Discussion
We previously described the first application of the [13C]methionine breath test on four HIV-infected patients with ART-related symptomatic hyperlactataemia.25 The sensitivity of the test in our preliminary report prompted us to verify whether it might be useful in the detection of early and asymptomatic derangement of mitochondrial function in patients on ART. We demonstrate here the potential usefulness of this test in detecting drug-related mitochondrial toxicity, even in the absence of hyperlactataemia.
The low percentage of 13C recovered over the test period confirms the incomplete recovery of the tracer with breath and the dispersion of methyl groups to other pathways.23 Our data show that even this incomplete recovery is sufficient to detect significant differences among the studied groups.
Although there are no conclusive data demonstrating that the transfer of methyl groups via sarcosine oxidation occurs predominantly in mitochondria, such a pathway seems to be at least partially dependent on mitochondrial function. Moreover, the sensitivity of the test to ethanol oxidative stress,23 and to acute NRTI-related lactic acidosis with known mitochondrial pathogenesis,25 suggest an important role of mitochondria in [methyl-13C]methionine metabolism.
In this pilot study, we compared 13CO2 breath excretion in four different groups of subjects: HIV-infected patients on a long-term antiretroviral treatment with or without symptoms of hyperlactataemia, patients never treated with ART and healthy controls. We demonstrated that patients receiving ART showed decreased 13CO2 excretion compared with healthy controls or naive patients; even lower values were observed in patients with drug-related hyperlactataemia. In this subset of patients, our previous report showed that drug withdrawal or modification rapidly improved 13CO2 breath excretion in parallel with hyperlactataemia, demonstrating the sensitivity of the test for detecting potential mitochondrial impairment.25 It is noteworthy that one of the hyperlactataemic patients, not previously described, presented a severe lactic acidosis accompanied by liver necrosis [with a peak of alanine aminotransferase (ALT) of 2775 IU/L, normal value <50 IU/L]. The patient underwent three 13CO2 breath tests: the first at admission with a normal ALT value and serum lactate of 7.5 mmol/L reached a DOB peak of 4.5; the second immediately after ALT and lactate normalization reached a DOB peak of 25.9; and the last test performed 20 days after clinical resolution reached a DOB peak of 11.5. The peak value of 25.9 is much higher than any other peak observed in our cohort of patients and possibly mirrors the intense hepatic regeneration with recovery of liver function.
We decided to exclude HBV and HCV co-infected patients in this preliminary study because we wanted to address the issue of NRTI-related mitochondrial toxicity and, as literature data have demonstrated that liver impairment alters the 13CO2 breath test, we decided that including patients with chronic hepatitis and liver damage could be a confounding factor; therefore none of our patients was affected by chronic viral liver disease, had reported alcohol abuse or was receiving any drug other than antiretrovirals. However, many of the patients showed a sonographic pattern of liver steatosis that is known to be associated with mitochondrial impairment.6 But even asymptomatic subjects with normal liver tests and no ultrasonographic signs of steatosis presented a reduction in 13CO2 excretion, if compared with untreated patients and healthy controls (data not shown). This was confirmed by the multivariate analysis that found no correlation between liver enzymes and DOB at 60 min.
Furthermore, the identical 13CO2 excretion pattern observed in untreated HIV-infected patients and healthy controls suggests that mitochondrial impairment is definitively drug-related rather than HIV-related, at least in patients without advanced disease.
It is noteworthy that the presence of hyperlactataemia and the higher impairment of 13CO2 excretion are not related to the years of exposure to antiretroviral drugs. These data confirm previous observations that hyperlactataemia emerges more frequently during the first 12 months of NRTI-containing ART1,4,26,27 and is probably dependent on individual predisposing factors.
Mitochondrial toxicity, and in particular hyperlactataemia, are reported to be associated, more probably, with the use of stavudine with or without didanosine compared with zidovudine-based regimens,1 although both stavudine and zidovudine are known to exert mitochondrial toxicity.26,27 In our study group, different NRTI regimens were involved in the impairment of hepatic mitochondrial function. The small size of our population and the current or previous exposure of all the patients to thymidine analogues do not allow us to draw a definitive conclusion about the role of each single NRTI in the impairment of 13CO2 excretion by breath test.
As thymidine analogues are relatively strong inhibitors of γ-polymerase, they determine a time- and dose-dependent decrease in the intracellular levels of mitochondrial DNA. Therefore, patients in our study receiving a thymidine analogue-sparing regimen might have an impaired breath test because of carryover toxicity. Alternatively, it cannot be excluded that mitochondrial damage is due to persistent exposure to NRTI other than thymidine analogues. Nevertheless, the time needed for complete recovery from NRTI-induced mitochondrial toxicity—even if such a recovery is possible—are unknown.
In our experience, the [13C]methionine breath test appears as a sensitive test to evaluate early drug-related mitochondrial toxicity in HIV-positive patients on antiretroviral treatment, even before the appearance of liver biochemical abnormalities, alterations of serum lactate or symptoms of hyperlactataemia. Serum lactate determination has been proposed as a routine screening in HIV-infected patients receiving ART, particularly in the presence of neurological or metabolic symptoms.1 Further studies for a definitive validation on a larger population could confirm if this test is more sensitive for the detection of drug-related mitochondrial impairment than serum lactate levels in the long-term management of treated patients.
The major limits of the present study are the small sample size and its cross-sectional nature. Since the test is not yet standardized, a range of normal values is not currently available. A longitudinal study on naive patients starting ART with a breath test performed at timed intervals will provide more information on the possible clinical applications of the test.
Interestingly, we detected a high prevalence of ultrasound pattern of liver steatosis and also hypertriglyceridaemia in patients receiving ART, suggesting a possible link between drug mitochondrial toxicity and metabolic disorders.
The difficulty of obtaining tissue samples such as liver, muscle or adipose tissue biopsies for the quantification of mitochondrial DNA or the ultrastructural analysis of mitochondria makes it necessary to find easier and non-invasive tests. The search for surrogate markers of mitochondrial toxicity has focused, in particular, on mitochondrial DNA content in peripheral blood mononuclear cells (PBMC), but data are controversial.8,28–30 In this report, we suggest that [13C]methionine excretion may be less complicated and less invasive than determination of mitochondrial DNA in PBMC, even if further validation is needed.
Further studies are required to define the validity of the [13C]methionine breath test in predicting and preventing systemic and organ related mitochondrial toxicity and establishing the relationship between liver mitochondrial impairment and the complex metabolic alterations observed in patients receiving NRTIs.
Schematic representation of the metabolism of the essential amino acid, methionine, via the transamination and transmethylation pathways.
Mean values of 13CO2 breath excretion rate after administration of [13C]methionine (given as DOB) in antiretroviral-naive HIV-infected patients (pts), patients on treatment with normal serum lactate, patients on treatment with hyperlactataemia and healthy controls. P=0.001 by ANOVA of the four curves from 30–90 min. P < 0.05 for DOB at 60 min of (*) healthy controls and naive patients versus asymptomatic patients and (**) asymptomatic patients versus hyperlactataemic patients.
Cumulative 13CO2 breath excretion in HIV-infected patients naive for ART, on treatment with normal serum lactate and on treatment with hyperlactataemia. Results are expressed as mean ± S.E.M.P < 0.05 by ANOVA.
13CO2 dose/h and cumulative dose measured by breath test at 60 min in healthy controls, antiretroviral-naive HIV-infected patients (pts), patients on treatment with normal serum lactate and patients on treatment with hyperlactataemia. P=0.001 (% dose of 13CO2 recovered per hour) by ANOVA. P=0.02 (% 13C cumulative dose) by ANOVA. The comparison by Kruskall-Wallis test is reported in the figure.
Characteristics of the patients
| Characteristic . | Naive patients (11) . | Treated asymptomatic (15) . | Patients with hyperlactataemia (6) . | Healthy controls (10) . | ||||
|---|---|---|---|---|---|---|---|---|
| Male sex (%) | 8 (72.7) | 9 (60) | 4 (66) | 5 (50) | ||||
| Age, years (mean ± S.E.M.) | 38 ± 3 | 42.4 ± 2.1 | 45.6 ± 3.8 | 39.7 ± 1.8 | ||||
| CDC group | ||||||||
| A | 6 | 9 | 3 | |||||
| B | 4 | 5 | 0 | |||||
| C | 1 | 1 | 3 | |||||
| BMI (mean ± S.E.M.) | 22.3 ± 0.7 | 22.8 ± 0.8 | 25.7 ± 1.9 | 21.8 ± 0.7 | ||||
| CD4 cell count, median cells/mm3 (range) | 218 (49–605) | 423 (128–776) | 280 (75–632) | |||||
| HIV-RNA, median copies/mL (range) | 63600 (70–157888)* | 49 (49–3080) | 49 (49–1187) | |||||
| ALT, IU/L (mean ± S.E.M.) | 42 ± 11 | 54.6 ± 12.6 | 26.2 ± 6.5 | 25 ± 2.8 | ||||
| Triglycerides, mg/dL (mean ± S.E.M.) | 93 ± 8.9 | 256 ± 54.5 | 170 ± 22.8** | 85 ± 6.9 | ||||
| Cholesterol, mg/dL (mean ± S.E.M.) | 146 ± 6 | 204 ± 11.4 | 220 ± 24.2 | 172 ± 5.7 | ||||
| Years on ARV (mean ± S.E.M.) | 6.8 ± 0.5*** | 2.2 ± 0.6 | ||||||
| NRTI regimen including | ||||||||
| stavudine | 4 | 3 | ||||||
| zidovudine | 4 | 3 | ||||||
| other | 7 | |||||||
| Characteristic . | Naive patients (11) . | Treated asymptomatic (15) . | Patients with hyperlactataemia (6) . | Healthy controls (10) . | ||||
|---|---|---|---|---|---|---|---|---|
| Male sex (%) | 8 (72.7) | 9 (60) | 4 (66) | 5 (50) | ||||
| Age, years (mean ± S.E.M.) | 38 ± 3 | 42.4 ± 2.1 | 45.6 ± 3.8 | 39.7 ± 1.8 | ||||
| CDC group | ||||||||
| A | 6 | 9 | 3 | |||||
| B | 4 | 5 | 0 | |||||
| C | 1 | 1 | 3 | |||||
| BMI (mean ± S.E.M.) | 22.3 ± 0.7 | 22.8 ± 0.8 | 25.7 ± 1.9 | 21.8 ± 0.7 | ||||
| CD4 cell count, median cells/mm3 (range) | 218 (49–605) | 423 (128–776) | 280 (75–632) | |||||
| HIV-RNA, median copies/mL (range) | 63600 (70–157888)* | 49 (49–3080) | 49 (49–1187) | |||||
| ALT, IU/L (mean ± S.E.M.) | 42 ± 11 | 54.6 ± 12.6 | 26.2 ± 6.5 | 25 ± 2.8 | ||||
| Triglycerides, mg/dL (mean ± S.E.M.) | 93 ± 8.9 | 256 ± 54.5 | 170 ± 22.8** | 85 ± 6.9 | ||||
| Cholesterol, mg/dL (mean ± S.E.M.) | 146 ± 6 | 204 ± 11.4 | 220 ± 24.2 | 172 ± 5.7 | ||||
| Years on ARV (mean ± S.E.M.) | 6.8 ± 0.5*** | 2.2 ± 0.6 | ||||||
| NRTI regimen including | ||||||||
| stavudine | 4 | 3 | ||||||
| zidovudine | 4 | 3 | ||||||
| other | 7 | |||||||
P=0.004 versus treated patients with or without hyperlactataemia.
P=0.04 between treated and untreated patients.
P=0.003 versus patients with hyperlactataemia.
Characteristics of the patients
| Characteristic . | Naive patients (11) . | Treated asymptomatic (15) . | Patients with hyperlactataemia (6) . | Healthy controls (10) . | ||||
|---|---|---|---|---|---|---|---|---|
| Male sex (%) | 8 (72.7) | 9 (60) | 4 (66) | 5 (50) | ||||
| Age, years (mean ± S.E.M.) | 38 ± 3 | 42.4 ± 2.1 | 45.6 ± 3.8 | 39.7 ± 1.8 | ||||
| CDC group | ||||||||
| A | 6 | 9 | 3 | |||||
| B | 4 | 5 | 0 | |||||
| C | 1 | 1 | 3 | |||||
| BMI (mean ± S.E.M.) | 22.3 ± 0.7 | 22.8 ± 0.8 | 25.7 ± 1.9 | 21.8 ± 0.7 | ||||
| CD4 cell count, median cells/mm3 (range) | 218 (49–605) | 423 (128–776) | 280 (75–632) | |||||
| HIV-RNA, median copies/mL (range) | 63600 (70–157888)* | 49 (49–3080) | 49 (49–1187) | |||||
| ALT, IU/L (mean ± S.E.M.) | 42 ± 11 | 54.6 ± 12.6 | 26.2 ± 6.5 | 25 ± 2.8 | ||||
| Triglycerides, mg/dL (mean ± S.E.M.) | 93 ± 8.9 | 256 ± 54.5 | 170 ± 22.8** | 85 ± 6.9 | ||||
| Cholesterol, mg/dL (mean ± S.E.M.) | 146 ± 6 | 204 ± 11.4 | 220 ± 24.2 | 172 ± 5.7 | ||||
| Years on ARV (mean ± S.E.M.) | 6.8 ± 0.5*** | 2.2 ± 0.6 | ||||||
| NRTI regimen including | ||||||||
| stavudine | 4 | 3 | ||||||
| zidovudine | 4 | 3 | ||||||
| other | 7 | |||||||
| Characteristic . | Naive patients (11) . | Treated asymptomatic (15) . | Patients with hyperlactataemia (6) . | Healthy controls (10) . | ||||
|---|---|---|---|---|---|---|---|---|
| Male sex (%) | 8 (72.7) | 9 (60) | 4 (66) | 5 (50) | ||||
| Age, years (mean ± S.E.M.) | 38 ± 3 | 42.4 ± 2.1 | 45.6 ± 3.8 | 39.7 ± 1.8 | ||||
| CDC group | ||||||||
| A | 6 | 9 | 3 | |||||
| B | 4 | 5 | 0 | |||||
| C | 1 | 1 | 3 | |||||
| BMI (mean ± S.E.M.) | 22.3 ± 0.7 | 22.8 ± 0.8 | 25.7 ± 1.9 | 21.8 ± 0.7 | ||||
| CD4 cell count, median cells/mm3 (range) | 218 (49–605) | 423 (128–776) | 280 (75–632) | |||||
| HIV-RNA, median copies/mL (range) | 63600 (70–157888)* | 49 (49–3080) | 49 (49–1187) | |||||
| ALT, IU/L (mean ± S.E.M.) | 42 ± 11 | 54.6 ± 12.6 | 26.2 ± 6.5 | 25 ± 2.8 | ||||
| Triglycerides, mg/dL (mean ± S.E.M.) | 93 ± 8.9 | 256 ± 54.5 | 170 ± 22.8** | 85 ± 6.9 | ||||
| Cholesterol, mg/dL (mean ± S.E.M.) | 146 ± 6 | 204 ± 11.4 | 220 ± 24.2 | 172 ± 5.7 | ||||
| Years on ARV (mean ± S.E.M.) | 6.8 ± 0.5*** | 2.2 ± 0.6 | ||||||
| NRTI regimen including | ||||||||
| stavudine | 4 | 3 | ||||||
| zidovudine | 4 | 3 | ||||||
| other | 7 | |||||||
P=0.004 versus treated patients with or without hyperlactataemia.
P=0.04 between treated and untreated patients.
P=0.003 versus patients with hyperlactataemia.
We are grateful to Dr Stefano Rusconi for helpful discussions and Mrs Bianca Ghisi for editorial assistance. GlaxoSmithKline and SIAMM (Studio Italiano per le Alterazioni Metaboliche e Morfologiche) partially sponsored the study, with no role other than sponsorship.
References
Boubaker, K., Flepp, M., Sudre, P. et al. (
Cote, H. C., Brumme, Z. L., Craib, K. J. et al. (
Lewis, W. (
John, M., Moore, C. B., James, I. R. et al. (
Ogedegbe, A. E., Thomas, D. L. & Diehl, A. M. (
Johnson, A. A., Ray, A. S., Hanes, J. et al. (
White, E. L., Parker, W. B., Macy, L. J. et al. (
Petit, C., Mathez, D., Barthelemy, C. et al. (
Brinkman, K. (
Harris, M., Chan, K. J., Tesiorowski, A. M. et al. (
Steele, R. D. & Benevenga, N. J. (
Wu, G. Y. & Thompson, J. R. (
Finkelstein, J. D. (
Mudd, S. H. & Poole, J. R. (
Stipanuk, M. H. (
Mato, J. M., Alvarez, L., Ortiz, P. et al. (
Stipanuk, M. H. (
Mudd, S. H., Ebert, M. H. & Scriver, C. R. (
Frisell, W. R., Cronin, J. R. & Mackenzie, C. G. (
Candelli, M., Cazzato, I. A., Zocco, M. A. et al. (
Spahr, L., Negro, F., Rubbia-Brandt, L. et al. (
Armuzzi, A., Marcoccia, S., Zocco, M. A. et al. (
Spahr, L., Negro, F., Leandro, G. et al. (
Milazzo, L., Riva, A., Sangaletti, O. et al. (
Chen, C. H., Vazquez-Padua, M. & Cheng, Y. C. (
Pan-Zhou, X. R., Cui, L., Zhou, X. J. et al. (
Cossarizza, A., Pinti, M., Moretti, L. et al. (
McComsey, G., Tan, D. J., Lederman, M. et al. (
Author notes
1Institute of Infectious and Tropical Diseases, University of Milan, L. Sacco Hospital, Via GB Grassi 74, 20157 Milan; 2 Gastroenterology Unit, L. Sacco Hospital, Milan; 3 Institute of Biomedical Technologies–National Research Council, Milan, Italy



![Mean values of 13CO2 breath excretion rate after administration of [13C]methionine (given as DOB) in antiretroviral-naive HIV-infected patients (pts), patients on treatment with normal serum lactate, patients on treatment with hyperlactataemia and healthy controls. P=0.001 by ANOVA of the four curves from 30–90 min. P < 0.05 for DOB at 60 min of (*) healthy controls and naive patients versus asymptomatic patients and (**) asymptomatic patients versus hyperlactataemic patients.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jac/55/1/10.1093/jac/dkh497/2/m_125386f2.gif?Expires=1716498349&Signature=3ovvBdAMoycZ9XuqOV62z1R18N2pOYA6a7pM042Znli7oURSg4H~9ao7fwHQx41NO~nRlOFu4o4h62syOq9gZoJ3e1MTONhbsgVmAChbxOaKlAWbysXoFdkaNunSqXkOF6zyDpbuZGCnCiIufbTHoPBz1sz1pcQmWXTeptj4PQ4FxdXMVdF0DpY61GGwKwAKuUJQQ297sNYK4S3XEbj2gSBo3EsN-iuw1AdWn3kmRjuZmrxZPTkvRQ0uUDbDxZ97~QR6NaK2DCX8YfcJldjuhd2qepcPO-Yzn60qIyYo-WbJ6bwa8GxU0IwjgzgrTiqSMiyKnz34Z-ZobHPKQGIkjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)