-
PDF
- Split View
-
Views
-
Cite
Cite
Sabrina S. Burmeister, Neurobiology of Female Mate Choice in Frogs: Auditory Filtering and Valuation, Integrative and Comparative Biology, Volume 57, Issue 4, October 2017, Pages 857–864, https://doi.org/10.1093/icb/icx098
Close - Share Icon Share
Synopsis
Mate choice is a decision making process with profound implication for the reproductive success of both the sender and the chooser. Preferences for conspecific over heterospecific males and for some conspecifics over others are typically mediated by a female’s response to signals produced by males. And although one can experimentally describe a female’s preference function, there is relatively little understood about the neural mechanisms mediating these preferences. In anurans, mating preferences have often been explained in terms of sensory biases. Indeed, in the túngara frog (Physalaemus pustulosus), the auditory system appears to act as a filter for conspecific calls. However, auditory responses are not good predictors of intraspecific mating preferences in túngara frogs. Rather, neural activity in the preoptic area, which can be gated by estradiol, is a better predictor of mating preferences. A similar pattern holds in spadefoot toads (Spea bombifrons): the preoptic area, but not the auditory midbrain, integrates physiological cues in its response to mating calls in a pattern that predicts preferences. Neuroanatomically, the anuran preoptic area is poised to mediate forebrain influences on auditory response of the midbrain and it has descending projections to the medulla and spinal cord that could directly influence motor responses. Indeed, lesions of the preoptic area abolish phonotaxis. A role for the preoptic area in mating preferences is supported by studies in mammals that show the preoptic area is required for the expression of preferences. Further, activity of the preoptic area correlates with mating preference in fish. This leads to a model for the neurobiological mechanisms of mate choice, in which sensory systems filter relevant signals from irrelevant ones, but the preoptic area assigns value to the range of relevant signals.
Introduction
Mating decisions are among the most consequential decisions any female makes. Not surprisingly, mate choice and its impact on evolutionary processes have garnered intense focus beginning with Darwin (1871). Yet, in spite of significant efforts to understand the neurobiology of copulatory behaviors, such as mounting and lordosis, there is little consensus as to the neurobiology of mate choice per se. In contrast to the robust debate of evolutionary theories of mate choice (Rosenthal 2017), progress on the neurobiology of mate choice generally lacks a cohesive framework.
We can broadly define female mate choice as the biased expression of reproductive behaviors and processes toward preferred males. In mammals, mate choice behaviors may include scent marking and lordosis; in birds, copulation solicitation; in frogs, phonotaxis. The sensory modalities and motor patterns of mate choice differ in each species and, therefore, must involve a set of unique brain regions. Yet, what they all must have in common is some type of mechanism for biasing behavioral responses toward a subset of males. That is, a mechanism for preference. To be clear, while sensation and perception can clearly bias mating decisions, the central questions of interest here are what are the brain region(s) that contribute to the evaluation of potential mating partners? Are those brain regions conserved among vertebrates?
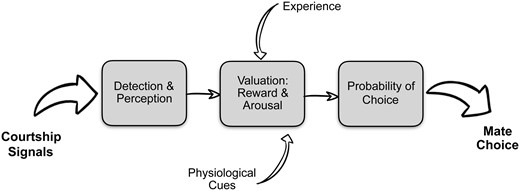
Broadly speaking, we can conceptualize mate choice as a process that begins with detection and perception of potential mates, the integration of that information with physiological cues and past experience, all of which lead to the assignment of a value to mating signals that, in turn, precipitates the preferential expression of behavior (Fig. 1). Fundamentally this process is like any other decision making process and we can expect that the underlying mechanisms, particularly at the computational level, should be shared with other decision making circuits (Rangel etal. 2008; Adams etal. 2012). Yet, because mate choice is fundamental to reproduction, we might expect a conserved mate choice decision making circuit. After all, the circuits for reproductive behavior themselves are highly conserved. If such a circuit exists—that is, one that is fundamental and shared among vertebrates—then we should focus our attention on “integrative” brain regions that contribute to mate choice independent of modality. We might also expect such a circuit to be modulated by physiological conditions and experience, both of which are known to influence mating preferences. Of course, what I have just described is an interrelated network of brain regions called the social behavior network (Newman 1999) that, together with the mesolimbic reward circuitry, form the social decision making network (O’Connell and Hofmann 2012).
Mate choice decision making begins with detection and perception of potential mates. The assigning of value to potential mates, which includes differential reward and arousal, is influenced by past experience and the current physiological state of the female. Preferred signals increase the probability of a mate choice decision.
The social decision making network is a set of interconnected brain regions that are highly conserved among vertebrates (Newman 1999; Goodson 2005; O’Connell and Hofmann 2012). As its name implies, this network is intimately involved in the expression of all social behaviors, from aggression and parental care to reproduction. Most work on the social behavior network has been done on mammals, where a focus has been on the expression of copulatory behavior (mounting and lordosis) (Hull and Dominguez 2007; Pfaff etal. 2008) with less attention paid to how (and whether) they are relevant to the types of mating preferences with which behavioral ecologists are generally concerned. In other vertebrates, studies of mate choice have focused more heavily on the sensory mechanisms of mating preferences (Rosenthal 2017). Yet, there is a body of work emerging from mammals (Martinez and Petrulis 2013), fish (Wong etal. 2012), and frogs (Burmeister etal. 2008; Chakraborty and Burmeister 2015) that points to the preoptic area, the most highly conserved node of the social behavior network (O’Connell and Hofmann 2012) as critical for the expression of mating preferences.
The neurobiology of mate choice in anurans
Mate choice in most anurans is mediated primarily by acoustic signals. There is a rich literature on the acoustic structure of mating calls and their role in sexual selection (Ryan 1985). Thus, the neurobiology of mate choice in anurans has been conceptualized within the context of signal–receiver interactions and has naturally gravitated toward a focus on auditory processing.
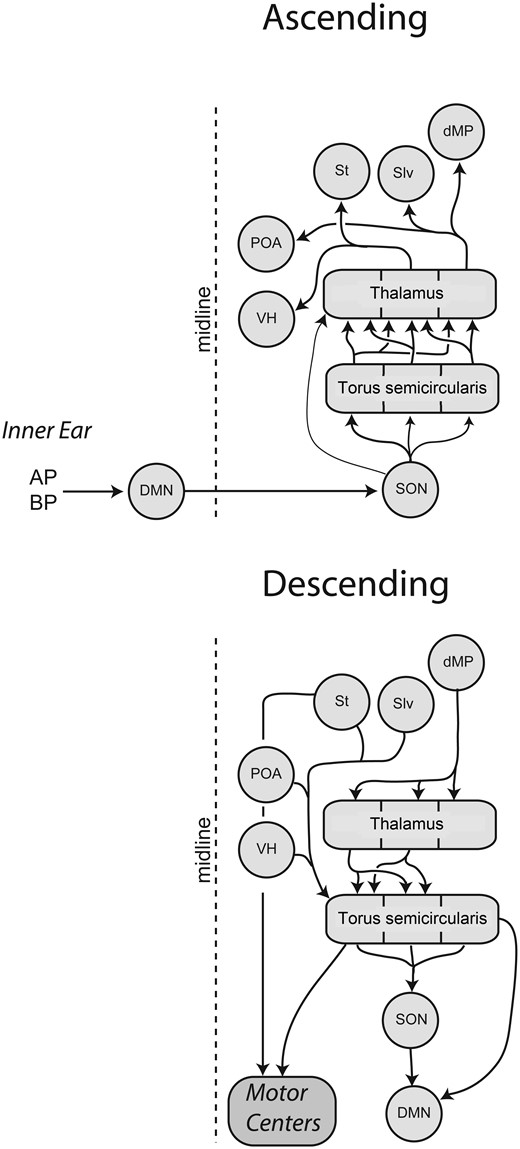
The hodology and function of the anuran auditory system at the level of hindbrain and midbrain follows a pattern similar to mammals and other vertebrates (Wilczynski 1988). Briefly, afferents from peripheral hair cells project to the dorsal medullary nucleus (homolog of the cochlear nucleus); the dorsal medullary nucleus projects to the superior olive; the superior olive projects to the torus semicircularis (homolog of the inferior colliculus) (Wilczynski 1988) (Fig. 2). In both mammals and anurans, the inferior colliculus projects to the forebrain. However, the pattern (and likely the function) of those projections appears to differ substantially. In mammals, there is an auditory cortex that is dedicated primarily to auditory processing. In anurans, no such analogous brain region appears to exist. Rather, all forebrain projections of the torus appear to be combined with other sensory modalities, with the potential exception of the ventromedial thalamus (Wilczynski and Endepols 2006). As a consequence, many have presumed that the torus semicircularis is the highest level of “purely” auditory processing. While the majority of current work support this interpretation, because the function of the anuran forebrain is less well explored, one should remain open minded about the potential contribution of the forebrain to audition.
A summary of the main ascending and descending projections of the auditory system in anuran amphibians. Abbreviations: AP, amphibian papilla; BP, basilar papilla; DMN, dorsal medullary nucleus (homolog of the cochlear nucleus); MP, medial pallium (homolog of the hippocampus); POa, preoptic area; S, septum; SON, superior olivary nucleus; VH, ventral hypothalamus. Modified from Chakraborty etal. (2010).
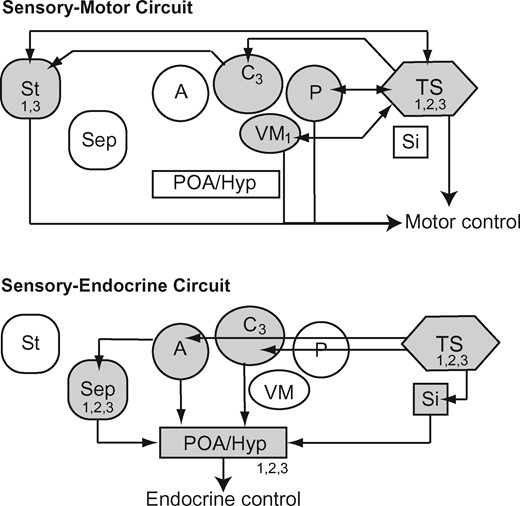
With the contrast between the anuran and the mammalian auditory systems as background, one can appreciate the conceptual framework proposed by Wilczynski and Endepols (2006) for the neural systems mediating sound communication in anurans (Fig. 3). Of those, the sensory-motor system is central to phonotaxis. Here, the torus semicircularis directly integrates ascending auditory information with descending influences on motor control circuits in the brainstem and spinal cord, with some influence from the forebrain (Fig. 3). The idea of the torus semicircularis as a sensory-motor integrator that controls orientation to sounds in the environment is consistent with the conserved function of the inferior colliculus across vertebrates (Casseday and Covey 1996) and it is undoubtedly important in the expression of phonotaxis (Endepols etal. 2003). Further, because mating preferences in female anurans are expressed as differential phonotaxis, some have asked whether activity in the torus can explain mating preferences in females (Hoke etal. 2004; Chakraborty etal. 2010).
The sensory-motor and sensory-endocrine systems proposed by Wilczynski and Endepols (2006), modified from Wilczynski and Burmeister (2016). Parts of these circuits express steroid receptors (1: AR, 2: ER-α, 3: ER-β) at moderate to high levels in túngara frogs, indicating that hormones affect how these systems function. Abbreviations: A, anterior thalamic nucleus; C, central thalamic nucleus; POA/hyp, preoptic area and/or hypothalamus; Sep, septum; Si, secondary isthmal nucleus; St, striatum; TS, torus semicircularis; VM, ventromedial nucleus of the thalamus.
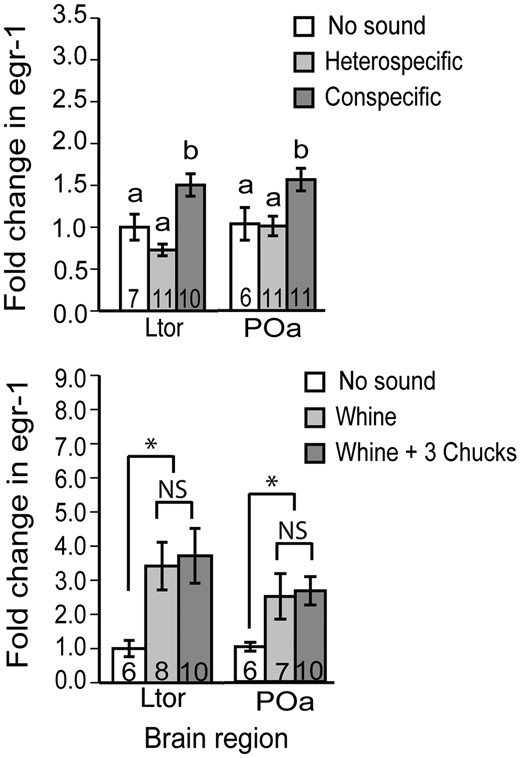
In anurans, the classic example of sensory biases in mate choice is the túngara frog (Physalaemus = Engystomops pustulosus). Males evolved an acoustic ornament (the chuck) that makes the whine much more attractive than the whine alone. However, the preference for the whine-chuck call appears to precede the evolution of the chuck itself, suggesting that there is a preexisting sensory bias for the whine-chuck (Ryan and Rand 1993; Ryan 1998). Chakraborty etal. (2010) asked whether there is evidence of a sensory bias in the auditory system using acoustically evoked egr-1 expression (an immediate early gene) as a marker for neural activation. Interestingly, heterospecific mating calls (whines of Physalaemus enesefae) do not induce egr-1 expression compared with no sound at all (Fig. 4), suggesting that the auditory system applies a strong filter for conspecific mating calls. It is important to recognize that the inner ear of túngara frogs is sensitive to the frequencies contained in the P. enesefae whine, and that the pattern emerges in the superior olivary nucleus and carries forward to the torus semicircularis and beyond (Chakraborty etal. 2010). At the level of egr-1 expression, it is as though the females are deaf to the heterospecific whines.
Egr-1 expression in response to mating calls in the túngara frog suggests that the auditory system acts as a filter for conspecific calls, but do not support the idea of a sensory bias for preferred conspecific mating calls (whine-chucks over whines) (Chakraborty etal. 2010). Patterns of egr-1 expression in the preoptic area are similar to those of the torus semicircularis.
What about preferences among conspecifics? To our surprise, both whine and whine-chuck elicit similar levels of egr-1 in the torus semicircularis, a pattern that carried forward to forebrain targets, including the preoptic area (Fig. 4). In a follow up experiment, L. A. Mangiamele and S. S. Burmeister (unpublished data) presented whine-chucks to female túngara frogs after first habituating them to whines and found that the torus was sensitive to the difference (L. A. Mangiamele and S. S. Burmeister, unpublished data). In contrast, presentation of a novel whine did not release the torus from habituation. Thus, the negative results from Chakraborty etal. (2010) should not be interpreted as an inability of the torus to differentiate the two types of calls, or an inability to measure such a difference using egr-1 expression. Rather, results like these seem to suggest that a simple sensory bias cannot easily explain mating preferences in this system.
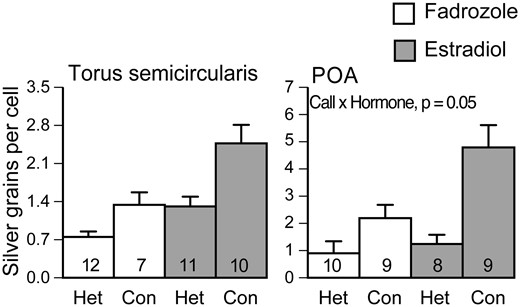
One strategy for identifying the neurobiology of mating preferences is to modulate the behavior and determine which brain regions change their response. In túngara frogs, estradiol is necessary and sufficient for the expression of phonotaxis (Chakraborty and Burmeister 2009). Most likely, estradiol from the maturing eggs acts as a gate for mate choice, ensuring that it occurs when females are ready to oviposit. Estradiol receptors are expressed in a number of brain regions thought to influence phonotaxis, including the torus semicircularis, the preoptic area, and the striatum (Chakraborty and Burmeister 2010). In the torus, expression of ER-α and ER-β is higher in females than males (Chakraborty and Burmeister 2010) and estradiol increases the baseline egr-1 expression levels, appearing to raise the overall level of activity (Fig. 5) (Chakraborty and Burmeister 2015). These findings are consistent with neurophysiological data showing that estradiol increases auditory responses to sounds, including biologically irrelevant sounds (e.g., noise and tones) as well as conspecific calls (Yovanof and Feng 1983). Such steroid effects on audition likely explain, at least in part, seasonal and sex differences in auditory tuning (Wilczynski and Burmeister 2016). In the preoptic area, in contrast, estradiol enhances the difference in auditory responses between the conspecific call and heterospecific call (Fig. 5) (Chakraborty and Burmeister 2015). Although not statistically significant, the septum and nucleus accumbens show similar responses to estradiol as did the preoptic area (Chakraborty and Burmeister 2015), indicating that multiple regions of the social behavior network could be involved in the valuation of mating calls.
Effects of estradiol on auditory responses to mating calls. In the torus semicircularis, both estradiol and hearing mating calls cause an increase in egr-1 expression. In the preoptic area, estradiol only affects egr-1 expression in the presence of conspecific mating calls. Con, conspecific whine-chuck mating call; Het, heterospecific (Physalaemus enesfae) whine. Modified from Chakraborty and Burmeister (2015).
One challenge in studying the neurobiology of mating preferences of túngara frogs is that their preferences are relatively fixed. That is, given a choice between a whine and a whine-chuck, the female will always prefer the whine-chuck (although see Lynch etal. 2005, 2006). This makes it difficult to disentangle the effects of the sensory stimulus from preferences per se. In contrast to túngara frogs, female mating preferences in the Plains spadefoot toad (Spea bombifrons) are plastic (Pfennig 2007). In this species, mating preferences depend on both body condition (mass relative to length) and the depth of the mating pond (Pfennig 2007). Of particular interest here, females with poor body condition are more likely to prefer heterospecifics than females of good body condition, but only in shallow ponds. (While we generally assume that heterospecific matings are maladaptive, in this species heterospecific matings can benefit poor condition females when matings occur in fast-drying ponds (Pfennig 2007).)
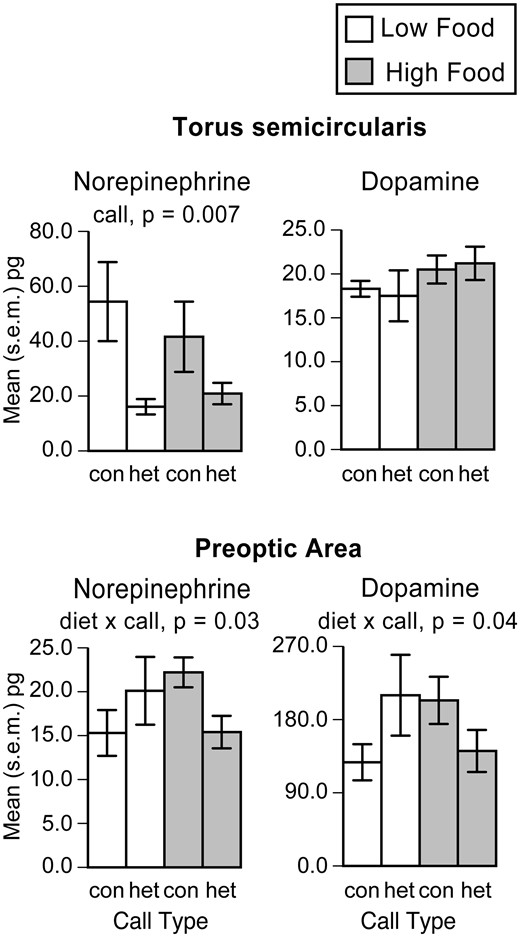
Burmeister etal. (2017) took advantage of this system to disentangle preference from stimulus and, by extension, auditory processing. To do so, they manipulated the diet of juveniles for 6 weeks following metamorphosis and collected their brains 40 min following the presentation of either a conspecific or heterospecific call and measured catecholamine levels using high pressure liquid chromatography. Under these conditions, toads in the low food treatment are predicted to develop a heterospecific mating preference. In the torus semicircularis, levels of norepinephrine were higher in response to the conspecific call, regardless of body condition (Burmeister etal. 2017) (Fig. 6). That is, the torus semicircularis lacked plasticity in its response even though the behavior is predicted to vary. In contrast, diet reversed the response of the preoptic area to call type: norepinephrine and dopamine were higher in response to the “preferred” mating call, whether or not that call was conspecific or heterospecific (Fig. 6) (Burmeister etal. 2017). The results from spadefoot toads are broadly consistent with work showing that estradiol gates acoustic responses in the preoptic area, but not in the torus semicircularis (Chakraborty and Burmeister 2015). Thus, unlike the torus semicircularis, responses of the preoptic area predict mating preferences.
Catecholamine levels in the torus semicircularis and preoptic area in spadefoot toads (Spea bombifrons) when both diet and mating calls were manipulated. In this species, diet and body condition affect preferences for conspecific versus heterospecific mating calls (Pfennig 2007; Pfennig etal. 2013). In the torus semicircularis, mating call type elicited differential catecholamine levels, but those responses showed no plasticity with food treatment. In the preoptic area, food treatment reversed the relationship between mating call and catecholamine levels in a manner that mirrors predicted mating preferences. From Burmeister etal. (2017).
It is important to acknowledge that in the Burmeister et al. study, they were studying juveniles, which limits the ability to extrapolate to mating preferences. However, while it is not for the purposes of mating, both juvenile túngara frogs and spadefoot toads show differential phonotaxis—in other words, preferences. In túngara frogs preferences emerge simultaneously with the expression of phonotaxis early after metamorphosis and gradually increase in frequency until they are expressed in their full form at sexual maturity (Baugh and Ryan 2010). In spadefoot toads, the expression of preferences and the emergence of phonotaxis can be uncoupled, with a low food diet apparently delaying the expression of preferences for conspecifics (Pfennig etal. 2013). Perhaps catecholamines in the preoptic area are a link between diet and the ontogeny of mating preferences in spadefoot toads.
What did Wilczynski and Endepols (2006) have to say about the preoptic area? They conceptualized the preoptic area as part of a sensory-endocrine circuit (Fig. 3). The preoptic area is acoustically sensitive (Allison 1992), receiving a substantial input from the torus semicircularis by way of the thalamus (Allison and Wilczynski 1991). Further, the gonadotropin releasing hormone neurons (GnRH) in the basal forebrain (including the preoptic area) increase production of GnRH in response to mating calls (Burmeister and Wilczynski 2005). This, presumably, is the link between hearing mating calls and increasing gonadal activity that has been widely reported in anurans (Brzoska and Obert 1980; Burmeister and Wilczynski 2000; Chu and Wilczynski 2001; Lynch and Wilczynski 2006). Yet, some intriguing data suggest that the preoptic area of anurans may have a direct influence on phonotaxis. First, the preoptic area has descending projections to the torus semicircularis. Second, while not as robust as the torus, the preoptic area is a source for descending fibers into motor control regions in the brainstem and spinal cord (Sánchez-Camacho etal. 2001). Third, lesions of the preoptic area abolish phonotaxis in female gray treefrogs (Walkowiak etal. 1999). Taken together with our results from spadefoot toads and túngara frogs, it is the preoptic area, and not the torus semicircularis, that is the best candidate for the neurobiological control of female mating preferences in anurans.
In summary, the data from anurans suggest that sensory systems can act as filters to select biologically relevant mating signals that are then assigned a value by the preoptic area, likely in conjunction with other brain regions, such as the septum and nucleus accumbens. This type of circuit is more flexible than one that depends solely on sensory biases for preferences, as it is more amenable to integration with past experiences and physiological state.
Beyond anurans
What of other vertebrates? Evidence from mammals and fish suggest that the preoptic area may play an important role in mating preferences of those species as well. For example, individual variation in preference covaries with gene expression patterns in the POA of swordtails (Wong etal. 2012; Wong and Cummings 2014). Further, the POA of a cichlid fish is sensitive to social information about preferred mates, changing gene expression dramatically depending on whether the preferred mate won or lost a contest (Desjardins etal. 2010).
While mammalian neurobiologists don’t typically think about mating preferences in the same way as do behavioral ecologist, they have made considerable progress in understanding the neurobiology of partner preference (same-sex versus opposite-sex) and the role of learning in the development of preferences (Pfaus etal. 2012). In those studies, the medial preoptic area has been identified as essential for olfactory-mediated preferences. While many of these studies depend on the learning of associations between an arbitrary odor and sexual experience, the medial preoptic area is also required for male- versus female-preferences in Syrian hamsters (Martinez and Petrulis 2013) and ferrets (Paredes and Baum 1995) and preferences for studs over castrated male rats (Xiao etal. 2005). Taken together, the preoptic area clearly has a role beyond the execution of copulatory behaviors. It receives sensory input conveying information about potential mating partners, is modulated by physiological state, is sensitive to rewarding aspects of mating, and may have direct effects on motor control brain regions. Perhaps it is part of a fundamental and conserved circuit for the neurobiology of mate valuation.
Acknowledgments
I thank Kathleen Lynch and Scott MacDougall-Shackleton for inviting me to participate in the symposium and my co-presenters for a stimulating discussion. I also thank the Society of Integrative and Comparative Biology, the Research Coordination Network, and the Company of Biologists for supporting the symposium. Finally, I thank some key collaborators who have shaped my thinking about mate choice and its neurobiology, including Mukta Chakraborty, Lisa Mangiamele, and Karin Pfennig and Michael Ryan.
References
Author notes
From the symposium “Integrating Cognitive, Motivational and Sensory Biases Underlying Acoustic and Multimodal Mate Choice” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 4–8, 2017 at New Orleans, Louisiana.