-
PDF
- Split View
-
Views
-
Cite
Cite
Chris O’Neill, The potential roles for embryotrophic ligands in preimplantation embryo development, Human Reproduction Update, Volume 14, Issue 3, May/June 2008, Pages 275–288, https://doi.org/10.1093/humupd/dmn002
Close - Share Icon Share
Abstract
Identification of the role(s) extracellular ligands play in regulating the development of the mammalian preimplantation embryo is a controversial area. Unequivocal evidence for their role is complicated by the apparent overlapping actions of multiple ligands. The discovery that the embryo also releases its own repertoire of ligands and expresses their corresponding receptors has further constrained analysis of their roles. Conventional ligand ablation strategies have limited utility when the cell responding to multiple ligands also produces them. The application of methods for identifying signal transduction events that occur in the early embryo in response to ligands has allowed direct assessment of the actions of these putative trophic ligands. A range of ligands induce phosphatidylinositol-3-kinase mediated survival signalling, and this is required for normal embryo development. Survival signalling maintains apoptotic pathways in a latent state within normal somatic cells, and they may fulfill the same role in the early embryo. Survival signals can also mitigate the adverse response of embryos to genotoxic and non-genotoxic stressors. Currently, there is no unequivocal evidence for a direct role of these ligands in the induction of mitosis in the early embryo. Embryotrophic ligands, acting via their specific receptors, to activate a network of effectors to create pro-survival, anti-apoptotic settings within the preimplantation embryo and these are required for normal embryo survival.
Introduction
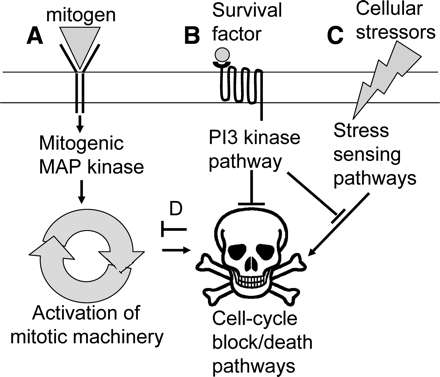
Normal mammalian somatic cells are thought to have an absolute requirement for the presence of external trophic ligands. These ligands have the minimal roles of promoting mitosis (mitogens/growth factors) and/or survival (survival factors) in their target cells. Interestingly, initiation of mitosis generally has the effect of inducing cell death unless there is concomitant activation of survival signalling pathways. In the absence of mitogens, cells remain quiescent within the cell-cycle. In contrast, the absence of survival signals results in a cell defaulting to apoptosis. Fig. 1 summarizes these interactions.
Canonical actions of trophic Ligands.
In somatic cells trophic ligands have the minimal role of inducing mitosis and cell survival by receptor-mediated signalling events. (A) Mitogenic signalling activates the mitogen activated protein kinase signalling pathway leading to the synthesis of proteins required for cell proliferation. (B) The actions of survival signals are commonly mediated by PI3 kinase action leading to activation of a range of pro-survival and anti-apoptotic mediators. (C) The PI3 kinase pathways can also mitigate adverse responses to cellular stressors. (D) Mitogenic signals can cause feedback inhibition which can be mitigated by the survival signalling pathway.
The preimplantation mammalian embryo is characterized by an apparent autonomy of development from exogenous trophic ligands. Upon fertilization (or indeed following metabolic activation of the oocyte by any of a variety of means) the succeeding events of development, up until the blastocyst stage, have the appearance of a programmed, autopoietic process—one with no apparent requirement for external cues. This autonomous pattern of growth is exemplified by the growth of the preimplantation embryo (from the zygote to blastocyst stage) in vitro in simple, defined culture medium.
Despite this apparent autonomy from external ligands, the expression of a surprising number of receptors for signalling ligands has been identified in the preimplantation embryo of a variety of species. Furthermore, the addition of exogenous ligands to embryo culture media influences the metabolism of embryos in vitro via their action on these receptors. The picture is complicated further by the identification of a range of potentially trophic ligands that are produced and released by the embryo itself. This raises the hypothesis that autocrine trophic ligands act on preimplantation embryos. This paper reviews the evidence for the action of trophic ligands in mitogenic and survival signalling during the preimplantation phase of development. It also assesses the potential role of these ligands in mitigating the embryo’s response to stressors. Much of the evidence for the putative action of trophic ligands is derived from mouse studies, thus the mouse embryo will be the basis of discussion, except where otherwise indicated.
The nature of autonomous embryo growth and survival
The metabolic activation of the oocyte that occurs following fertilization induces a cascade of developmental events that lead to the formation of the blastocyst. These events can occur in simple defined culture media containing only balanced salts, carbohydrate nutrients and pH buffering systems (Whitten, 1957). More recent advances in media design include the addition of amino acids (Gardner and Lane, 1993), antioxidants (Nasr-Esfahani et al., 1990; Nasr-Esfahani and Johnson, 1991; Umaoka et al., 1991) and osmolytes (Biggers et al., 1993). The growth of the preimplantation embryo in the absence of exogenous ligands (that can be considered to act via receptor-mediated signalling) provides a strong argument in favour of the developmental autonomy of the embryo.
It is common to add simple protein sources, such as albumin, to embryo culture medium (Whitten, 1957). This facilitates embryo handling in vitro (in the absence of macromolecules, embryos are sticky and readily lost with handling) and absorbs xenobiotics and other toxins, which can potentially contaminate media preparations. Culture performed in media with the notional absence of protein, including albumin, is still successful (Cholewa and Whitten, 1970). Such results should be treated with caution, however, since the processes of collection of embryos generally results in contamination of media with some protein from the donor female. Small amounts of plasma protein contamination (of which albumin is the dominant fraction) is likely to persist. This may be important since trace concentrations (ng/ml) of albumin are more beneficial in embryo culture than the commonly used higher concentrations (mg/ml) (O’Neill, 1997). Albumin readily absorbs and acts as a carrier for lipophyllic vitamins, hormones and bioactive lipids (Kragh-Hansen, 1981; Carter and He, 1990) and these are therefore added to media as passengers with the albumin supplement. Importantly, albumin also serves as an efficient carrier of released autocrine embryotrophins (O’Neill, 1997).
Taken at face value, the culture of embryos in notionally defined media supports the hypothesis that early embryo development is autonomous of an absolute requirement for exogenous trophic ligands. There is some question of whether such embryos possess the same level of viability as embryos cultured in more complete media. Yet, even with some loss of viability, these results support a view that there may be no absolute requirement for exogenous trophic ligands in embryo development (Whitten, 1957). Such evidence, however, does not take account of the potential role of trophic ligands produced by the embryo itself. Where embryos express receptors for such released ligands, the potential for a closed signalling loop exists even in simple defined media. Such factors are classed as autocrine ligands.
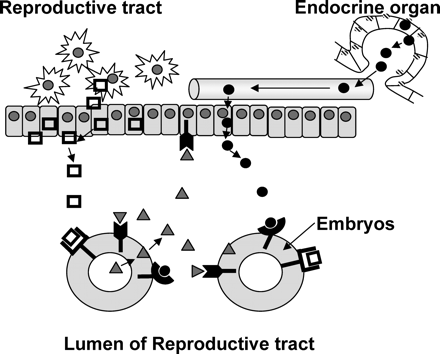
Fig. 2 illustrates the range of potential interactions that may be fulfilled by soluble ligands. A growing body of evidence shows that the preimplantation embryo releases mediators that act back on the embryo itself. An important consideration (and the source of much confusion) is that autocrine mediators may act on the same cell or neighbouring cells of the same type. An autocrine action contrasts with the action of paracrine ligands. Paracrine ligands are produced and released by neighbouring cells of different type. Endocrine ligands are released by distant cells and move through the body via the blood or lymph streams. Thus, embryonic paracrine mediators include ligands produced and released by the reproductive tract (e.g. cytokines (Sanford et al., 1992); calcitonin (Wang et al., 1998)). These form part of the reproductive tract’s luminal fluid that bathe the early embryo. Endocrine factors, such as insulin (Heyner et al., 1993), may form part of the luminal fluid as a consequence of the normal transudation of plasma proteins into the reproductive tract (Aitken, 1977). Of course some mediators may have dual (or triple) modes of action. That is, a given mediator may be produced and released by the embryo and also the reproductive tract (or distant endocrine gland), and can thus potentially act as both autocrine and paracrine/endocrine mediators (O’Neill, 2005). Within the reproductive tract the embryo is likely to be exposed to autocrine, paracrine and endocrine mediators.
The sources of embryotrophic ligands.
The preimplantation embryo produces autocrine ligands (▴) that can act on the embryo itself or neighbouring embryos, or act in a paracrine fashion on the reproductive tract. The reproductive tract produces ligands (□) that act as paracrine embryotrophins. Distant endocrine organs can produce hormones (•) that, upon transudation into the uterus, can act as autocrine embryotrophins.
A further potential interaction of ligands is the enhancement of autocrine factor production by paracrine factors (and vice versa). Thus, the co-culture of human embryos with endometrial cells results in the enhanced expression of autocrine insulin-like growth factor (IGF)-1 and IGF-2 and the IGF-1 receptor by the embryos (Liu et al., 1999), while platelet-activating factor (PAF) can induce the expression of prostaglandins by the reproductive tract (Smith and Kelly, 1988). This potential for cooperative interactions between ligands is an area that has received little experimental attention. It is likely that the range of such interactions is much broader than these examples.
The range of putative embryonic paracrine and endocrine mediators has been widely reviewed (Adamson, 1993; Harvey et al., 1995; Kaye and Harvey, 1995; Kane et al., 1997; Hardy and Spanos, 2002), hence this review will focus primarily on the emerging evidence for the actions of autocrine factors. Table I lists some of the putative autocrine ligands identified to date. Since platelet-activating factor (1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine, PAF) was the first of the autocrine mediators to be identified and is to date the most fully characterized, much of the discussion that follows will use PAF as an example.
Ligands released by the preimplantation embryo.
| 1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) (Collier et al., 1988; O’Neill, 1985) |
| Activin and Inhibin sub units (Albano et al., 1993; Ouhibi et al., 1990) |
| α-Interferon (Jones et al., 1992) |
| Growth Hormone (GH) (Pantaleon et al., 1997b) |
| Human Leukocyte Antigen-G (HLA-G) (Sher et al., 2005) |
| Insulin-like growth factor 1 (IGF-1) (Kaye et al., 1992; Lighten et al., 1997) |
| Insulin-like growth factor -2 (IGF-2) (Hemmings et al., 1992; Rappolee et al., 1992) |
| Leukaemia Inhibitory Factor (LIF) (Baker et al., 1993) |
| Transforming Growth factor-α (TGF-α) (Rappolee et al., 1988) |
| Platelet derived growth factor subunits (PDGF) (Osterlund et al., 1996; Rappolee et al., 1988) |
| Prostaglandin E2 (PGE2) (Niimura and Ishida, 1987; Tan et al., 2005) |
| Transforming growth factor-β (TGF-β) (Babalola and Schultz, 1995; Rappolee et al., 1988) |
| 1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) (Collier et al., 1988; O’Neill, 1985) |
| Activin and Inhibin sub units (Albano et al., 1993; Ouhibi et al., 1990) |
| α-Interferon (Jones et al., 1992) |
| Growth Hormone (GH) (Pantaleon et al., 1997b) |
| Human Leukocyte Antigen-G (HLA-G) (Sher et al., 2005) |
| Insulin-like growth factor 1 (IGF-1) (Kaye et al., 1992; Lighten et al., 1997) |
| Insulin-like growth factor -2 (IGF-2) (Hemmings et al., 1992; Rappolee et al., 1992) |
| Leukaemia Inhibitory Factor (LIF) (Baker et al., 1993) |
| Transforming Growth factor-α (TGF-α) (Rappolee et al., 1988) |
| Platelet derived growth factor subunits (PDGF) (Osterlund et al., 1996; Rappolee et al., 1988) |
| Prostaglandin E2 (PGE2) (Niimura and Ishida, 1987; Tan et al., 2005) |
| Transforming growth factor-β (TGF-β) (Babalola and Schultz, 1995; Rappolee et al., 1988) |
Ligands released by the preimplantation embryo.
| 1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) (Collier et al., 1988; O’Neill, 1985) |
| Activin and Inhibin sub units (Albano et al., 1993; Ouhibi et al., 1990) |
| α-Interferon (Jones et al., 1992) |
| Growth Hormone (GH) (Pantaleon et al., 1997b) |
| Human Leukocyte Antigen-G (HLA-G) (Sher et al., 2005) |
| Insulin-like growth factor 1 (IGF-1) (Kaye et al., 1992; Lighten et al., 1997) |
| Insulin-like growth factor -2 (IGF-2) (Hemmings et al., 1992; Rappolee et al., 1992) |
| Leukaemia Inhibitory Factor (LIF) (Baker et al., 1993) |
| Transforming Growth factor-α (TGF-α) (Rappolee et al., 1988) |
| Platelet derived growth factor subunits (PDGF) (Osterlund et al., 1996; Rappolee et al., 1988) |
| Prostaglandin E2 (PGE2) (Niimura and Ishida, 1987; Tan et al., 2005) |
| Transforming growth factor-β (TGF-β) (Babalola and Schultz, 1995; Rappolee et al., 1988) |
| 1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) (Collier et al., 1988; O’Neill, 1985) |
| Activin and Inhibin sub units (Albano et al., 1993; Ouhibi et al., 1990) |
| α-Interferon (Jones et al., 1992) |
| Growth Hormone (GH) (Pantaleon et al., 1997b) |
| Human Leukocyte Antigen-G (HLA-G) (Sher et al., 2005) |
| Insulin-like growth factor 1 (IGF-1) (Kaye et al., 1992; Lighten et al., 1997) |
| Insulin-like growth factor -2 (IGF-2) (Hemmings et al., 1992; Rappolee et al., 1992) |
| Leukaemia Inhibitory Factor (LIF) (Baker et al., 1993) |
| Transforming Growth factor-α (TGF-α) (Rappolee et al., 1988) |
| Platelet derived growth factor subunits (PDGF) (Osterlund et al., 1996; Rappolee et al., 1988) |
| Prostaglandin E2 (PGE2) (Niimura and Ishida, 1987; Tan et al., 2005) |
| Transforming growth factor-β (TGF-β) (Babalola and Schultz, 1995; Rappolee et al., 1988) |
PAF is produced de novo by the embryo soon after fertilization (Wells and O’Neill, 1992, 1994) and is then translocated to the embryo’s membrane. In the presence of extracellular albumin, PAF is released into the embryo’s environment (Ammit and O’Neill, 1997a, b). The released PAF can be detected in embryo culture media for a range of species (mouse (O’Neill, 1985); human (Collier et al., 1988); sheep (Battye et al., 1991); rabbit (Minhas et al., 1993); cow (Stock and Hansel, 1992); and hamster (Velasquez et al., 1995)). PAF exerts a range of paracrine actions within the reproductive tract (O’Neill, 2005) and also acts upon receptors expressed by the embryo itself. It can therefore be classed as a dual autocrine and paracrine mediator. There is some evidence that embryo-derived PAF has an unexpectedly long half-life (Ammit and O’Neill, 1997b), thus the possibility of an endocrine role for PAF also exists, but conclusive evidence of this is awaited.
This range of potential interactions and the multiplicity of putative embryotrophic ligands identified make investigation of their role in vivo difficult. Questions that arise include: (i) do the actions of autocrine, paracrine and endocrine factors overlap and if so why is there overlap; (ii) if their actions differ, in what way; and (iii) are the actions of the various embryotrophins different across the various stages of preimplantation development? Given this complexity, an important priority for the field is to define the actions of some of the embryotrophins in detail. This allows suitable specific hypotheses to be generated for future testing. Currently, the complexity of the range of possible interactions precludes detailed investigation of the action of these mediators within the reproductive tract. As a result, most work to date focuses on the response of selected ligands in vitro.
Evidence for the action of embryotrophic ligands
Since autocrine mediators are released by the embryo in vitro, embryos are exposed to these ligands even in simple defined media. By definition, paracrine and endocrine embryotrophic mediators are excluded from culture in simple defined medium. This means that culture of embryos in simple defined media provides a means of studying the actions of autocrine factors in isolation from other ligands.
It is argued that the action of autocrine embryotrophins may explain the beneficial effect of culturing embryos communally (in groups) compared to solitary culture. Although the beneficial effects of communal culture are widely considered to be evidence for the action of autocrine factors, others have argued that it may be evidence that the embryo’s metabolic activity creates localized changes in the composition of medium, resulting in a more favourable milieu. If these were so, more embryos in a group would create an even more favourable microclimate. It was suggested that the first observation of the beneficial effects of communal culture (Paria and Dey, 1990) was not reliable since the quality of culture seemed poor (Kane et al., 1997). Yet, this design has been repeated by many groups using a variety of culture and media systems (Lane and Gardner, 1992; Moessner and Dodson, 1995; Salahuddin et al., 1995; Stoddart et al., 1996; O’Neill, 1997, 1998; Stokes et al., 2005). Examples of metabolically derived modifications to the culture environment might include localized depletion of O2 or nutrient concentration, or release of metabolic products. The improvement in development resulting from communal culture of embryos occurred at either 20 or 5% O2 (O’Neill, 1997). Furthermore, it was shown that in microdrops, communal culture of groups of 10 embryos caused virtually no perturbation of the oxygen concentration and groups of 50 embryos produced a drop in oxygen tension that was not large enough to effect development (Baltz and Biggers, 1991). The only metabolic product of the embryo to have been investigated is the accumulation of NH3 in media. Increased NH3 was deleterious to normal development (Gardner and Lane, 1993), so it would seem that communal culture can actually create a deleterious metabolic microenvironment. The beneficial effect of communal culture could be achieved by either changing the number of embryos within a group, while fixing the volume of culture media; or by keeping the number of embryos constant, while changing the volume of medium (O’Neill, 1997). This conclusion is supported by the observation that the beneficial effect of communal culture was governed by the distance between individual embryos in each group (Gopichandran and Leese, 2006). The optimal rate of bovine blastocyst formation occurred when embryos were cultured 165 µm apart. Increasing the distance between embryos resulted in a decline in the rate of blastocyst formation. Similar results have been observed using porcine embryos (Stokes et al., 2005). Supplementation of media with the autocrine embryotrophin PAF could partially overcome the adverse effects of reduced embryo concentration (O’Neill, 1997) or increasing distance between embryos during culture (Gopichandran and Leese, 2006). These findings do not support a role of metabolic conditioning of media. Rather, they argue for a role of released, diffusible trophic factors.
Further associative data that supports the action of diffusible embryotrophins include the following observations: (i) embryos produced by IVF and then cultured are more susceptible to the adverse effects of solitary culture than are corresponding zygotes fertilized in situ (O’Neill, 1997). Embryos produced by IVF had lower levels of production of several autocrine factors compared with embryos fertilized in the reproductive tract and IVF also had an adverse effect of the expression of receptors for some autocrine factors (O’Neill, 1997, 1998; Stojanov and O’Neill, 1999, 2001; Stojanov et al., 1999); (ii) the action of embryotrophins in promoting embryo viability seems most effective if exposure occurs during the first two-cell cycles (O’Neill, 1998). This may also apply to human IVF embryos since improved development occurred if embryos were cultured in groups from the zygote stage (Moessner and Dodson, 1995; Almagor et al., 1996), but not apparently if the co-culture commenced at Day 3 of development (Rijnders and Jansen, 1999); (iii) in human IVF, the addition of an embryo with good development to a group with retarded development resulted in improvement in the development rates and pregnancy potential of the retarded embryos (Lightman et al., 1997). A similar effect has been demonstrated with felid embryos (Spindler and Wildt, 2002); and (iv) The Ped gene is a major determinant of the developmental potential of the preimplantation mouse embryo (Warner et al., 1991, 1993; Xu et al., 1994). In two mouse strains that are genetically identical, except for the Ped gene, the presence of the Ped gene provided embryos with a reproductive advantage over Ped negative embryos. Ped+ embryos developed at twice the rate as Ped− embryos in vitro, and Ped+ embryos released more than twice the amount of the autocrine embryotrophin, PAF, compared with Ped− embryos (Purnell et al., 2006).
It is conceivable that embryos from polytocous species (such as the mouse) have mechanisms for communication between themselves that are not required in monotocous species. If this were so, it would question the general significance of the trophic interactions illustrated by communal culture. Yet, evidence of similar beneficial effects of communal embryo culture has also been demonstrated in normally monotocous species such as the human (Moessner and Dodson, 1995) and bovine (Gopichandran and Leese, 2006).
The available evidence indicates that communal culture of embryos may create both deleterious and beneficial effects for the embryo, with a net beneficial outcome. Currently, the balance of evidence indicates that much of the beneficial effect of communal culture is accounted for by the action of autocrine trophic ligands in defined culture media. This does not exclude the possibility of a subsidiary benefit of metabolically induced changes in the embryo’s microclimate, yet tangible evidence in support of this hypothesis is awaited. This role of autocrine factors does not exclude the likely beneficial actions of trophic ligands present within the reproductive tract (paracrine and endocrine), but these are normally absent during culture in simple defined media. The successful development of embryos in the absence of paracrine or endocrine embryotrophins, may infer that their actions overlap, to a significant degree, the actions of autocrine factors.
An example of the likely beneficial overlap of the actions of the autocrine and paracrine ligands was demonstrated by using mice that lacked the PAF-receptor (Ptafr−/−). Embryo-derived PAF released by the preimplantation embryo acts on this receptor to induce signalling events (further discussion of this point below). Ptafr−/− embryos cultured in vitro in solitary culture had a poor development rate compared to Ptafr+/+ embryos cultured under similar conditions (Lu et al., 2004). The loss of viability of Ptafr−/− embryos in vitro was partially offset by communal culture. Importantly, the viability of the Ptafr−/− embryos in vivo was not apparently adversely affected (Lu et al., 2004). It was concluded that the improved viability of Ptafr−/− embryos in communal culture reflected the likely action of other autocrine factors (e.g. IGF-I and IGF-II), which partially compensate for the absence of PAF signalling. The further enhancement of the viability of Ptafr−/− embryos when they developed within the reproductive tract may indicate the further additional beneficial actions of paracrine or endocrine factors within the reproductive tract (Lu et al., 2004). This example suggests that the combined actions of autocrine ligands and paracrine/endocrine ligands are required for the normal survival and development of the preimplantation embryo. The generality of this conclusion requires wider testing using functional genomic methodology for a wider range of embryotrophins.
Actions of trophic ligands
Defining the actions of a ligand that is constitutively expressed by the cell that also responds to it is an intellectual and experimental challenge. The actions of paracrine or endocrine ligands are classically defined by separation (removal) of the responding cell from the ligand source tissue. In the case of autocrine signalling, this is not readily achieved. This challenge is increased in the early embryo by the apparently overlapping actions of several autocrine factors (Lu et al., 2004). The foregoing discussion of the developmental response of the embryo during solitary culture is one approach to reducing the concentration, and hence action, of autocrine factors. This approach may be complicated by the potential for artifactual consequential (and ill-defined) changes in the embryo’s microclimate (see discussion above). One breakthrough strategy that has facilitated investigation of trophic ligands was the observation of signal transduction events in response their exposure of exogenous ligands (Wang et al., 1998). These techniques were then applied to the investigation of the actions of autocrine ligands (Roudebush et al., 1997; Emerson et al., 2000).
The study of autocrine signalling first became possible by measuring changes in intracellular calcium. It was observed that the addition of albumin to zygotes and two-cell embryos in vitro resulted in spontaneous characteristic transient increases in the embryo’s intracellular calcium concentration [Ca2+]i (Emerson et al., 2000). A range of proofs showed that this response by the embryo was not to albumin itself, but due to albumin’s capacity to induce the release of PAF from the embryo (Emerson et al., 2000). Albumin acts as a carrier for released embryo-derived PAF, facilitating its transfer to embryonic receptors (Ammit and O’Neill, 1997a, b; Emerson et al., 2000) (Fig. 3). It is possible that it also serves this purpose for other ligands, although this has not been adequately investigated. Thus, the measurement of the embryo’s response to albumin was in fact a measure of the embryo’s response to released autocrine PAF. It was further demonstrated that degradation of the embryo’s membrane store of PAF (by treatment with exogenous PAF: acetylhydrolase) allowed systematic study of PAF’s mode of action by exposing embryos to measured concentrations of exogenously applied PAF (Emerson et al., 2000).
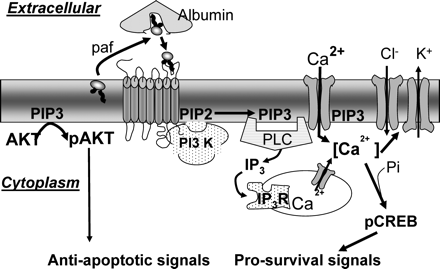
The known pathways of signal transduction by an autocrine embryotrophin.
Platelet-activating factor (PAF) is released by the embryo by its uptake by extracellular albumin. This albumin-bound PAF is then transferred to a high-affinity membrane receptor which leads to activation of PI3 kinase and the production of PIP3. PIP3 acts as a docking site for the plekstrin homology (PH) domain proteins, including threonine/serine specific kinase B (AKT) and phospholipase C (PLC). The net result is the generation of intracellular calcium transients that lead to a transient membrane hyperpolarization and the activation of the cyclic AMP responsive element binding (CREB) transcription factor. AKT can generate a phospho-proteome favouring pro-survival and anti-apoptotic signals. PIP2, phosphatidylinositol (4,5) bisphosphate. PIP3, phosphatidylinositol (3,4,5) trisphosphate.
The signal transduction response to embryo-derived PAF is shown in Fig. 3. The [Ca2+]i signal induced by PAF is receptor-mediated and occurs from the late zygote stage and throughout the two-cell stage. It occurs with an apparent periodicity of approximately 60–90 min (Emerson et al., 2000; Lu et al., 2003, 2004). These calcium transients are necessary for normal embryo development in vitro, since buffering them with BAPTA-AM (1,2-bis-(0-aminophenoxy) ethane-N,N,N1,N1-tetra-acetic acid) blocks development. The adverse effects of buffering [Ca2+]i was partially reversed by addition of excess exogenous PAF to medium (Emerson et al., 2000). Receptor occupancy is transduced in a G-protein and 1-o-phosphatidylinositol-3-kinase (PI3 kinase) dependent manner (Lu et al., 2004). It results in both inositol trisphosphate-induced (IP3) release of internal calcium stores (Emerson et al., 2000) and the activation of a dihydropyridine-sensitive membrane calcium channel (Lu et al., 2003; Li et al., 2007b, c). The generation of a detectable [Ca2+]i transient is dependent upon both these sources of calcium. The [Ca2+]i transient generated by PAF then initiates a transient hyperpolarization of the membrane’s electrical potential. This hyperpolarization involves the activation of a 4,4′-diisothiocyanatostilbene-2,2′-disulfonate-sensitive anion channel (probably a chloride channel) and a tetraethylammonium chloride-sensitive channel (potassium channel) (Li et al., 2007c) (Fig. 3). The membrane hyperpolarization induced by PAF may have a role in limiting the duration of the calcium response. Yet, a range of cellular functions are regulated by changes in membrane potential (e.g. pHi, nutrient transport, mitochondrial function), so it is likely that the combined actions of periodic calcium transients and membrane hyperpolarization results in a generalized pleiotypic response by the embryo to the actions of autocrine PAF.
Some other embryotrophic ligands have also been shown to induce calcium transients. For example, calcitonin induces [Ca2+]i transients in the four-cell to blastocyst stage embryos (Wang et al., 1998) and lysophosphatidic acid-induces [Ca2+]i transients in blastocysts (Liu and Armant, 2004). The lysophosphatidic acid induced [Ca2+]i transient results in transient accumulation of heparin-binding epidermal growth factor (EGF)-like growth factor on the blastocyst surface (Liu and Armant, 2004). Lysophosphatidic acid treatment of zygotes also enhanced development in vitro in a G-protein dependent manner (Kobayashi et al., 1994). It may be that the generation of calcium transients is a common response to several embryotrophins. It is yet to be established whether these ligands also create a transient membrane hyperpolarization, as PAF does.
Signalling events in response to defined ligands provides direct evidence for their actions on the embryo. Several embryotrophic ligands have general metabolic effects on the preimplantation embryo, which result in enhanced rates of metabolism by embryos in vitro (e.g. insulin (Harvey and Kaye, 1988); EGF (Woods and Kaye, 1989); IGF-2 (Harvey and Kaye, 1992), IGF-1 (Pantaleon and Kaye, 1996); growth hormone (Pantaleon et al., 1997b); and PAF (O’Neill et al., 1989; Ryan et al., 1990a). Their actions also result in improvements in the long-term viability of embryos (Ryan et al., 1990b; O’Neill et al., 1992; Sjoblom et al., 2005). The basis of this generalized metabolic effect by a wide range of ligands is not defined, but it is likely that they are a manifestation of broader pleiotrophic responses of the embryo to the ligands.
In somatic cells, the minimal actions of extracellular ligands are the initiation of mitogenesis and the maintenance of cell survival, thus the response of the cell to its repertoire of required ligands determine whether the cell will survival or die, proliferate or become quiescent. The following discussion will consider if these two minimal actions of extracellular ligands in somatic cells are also required in the early embryo.
Ligand-mediated mitogenesis
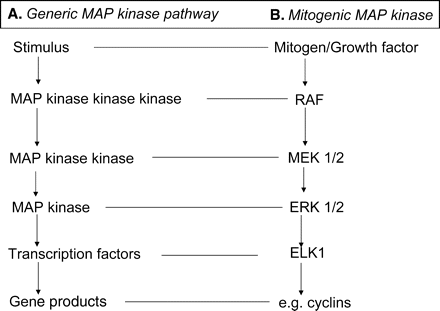
The characteristic feature of the growth and development of the preimplantation stage embryo is a programmed series of reductive mitoses. In somatic cells, mitosis is dependent upon the actions of defined mitogens/growth factors, acting through their receptors to initiate the cell’s mitotic machinery. The canonical actions of mitogens are well reviewed (Meloche and Pouysségur, 2007; Morgan, 2007) and are summarized in Fig. 4. These actions include the receptor-mediated activation of the mitogenic-activated protein kinase (MAP kinase) cascade, which results in the activation of transcription factors. The resulting gene expression triggers the synthesis of the cell-cycle machinery (Morgan, 2007; Roberts and Der, 2007). Activation of this cascade of kinases is required for progression of cells through checkpoints in the cell-cycle (see review, Morgan, 2007).
Canonical mitogenic signalling.
Mitogenic binding to specific receptors activates the MAP kinase cascade. (A) This cascade involves three kinases acting in series. Their action provides a mechanism for amplification of the signal. Active kinase phosphorylates target transcription factors that generate a transcriptome coding for proteins required for cell proliferation. (B) Several classes of MAP kinase exist, members most commonly active in mitogenic responses are shown.
There are several lines of evidence implicating the MAP kinase pathway in preimplantation embryo development. Inhibition of the small G-protein, RAS, (by microinjection of antisense oligonucleotides; a blocking monoclonal antibody; or a dominant negative RAS) caused inhibition of the development of mouse embryo through the two-cell stage in vitro (Yamauchi et al., 1994). This blocking could be overcome by co-treatment with constitutively active RAF (MAP kinase kinase kinase), showing the action of RAS is via this downstream MAP kinase pathway effector (Yamauchi et al., 1994). Activated RAF was found during the cell-cycle in early cleavage stage embryos (Haraguchi et al., 1998). It was not determined whether RAS action in the early embryo occurred at specific cell-cycle checkpoints.
Downstream of RAS/RAF is MEK 1/2 (MAP kinase kinase) and ERK1/2 (MAP kinase) (Roberts and Der, 2007) (Fig. 4). MEK and ERK were expressed in the preimplantation embryo (Liu et al., 2004). Treatment of the late two-cell stage mouse embryo with a ERK inhibitor (U0126) resulted in cell-cycle arrest by the four-cell stage (Maekawa et al., 2007). This study found that the early embryo expressed the phosphorylated form of ERK1/2 and its substrate ELK1 (transcription factor). ERK was active in these early stages of development. Its inhibition resulted in clear changes in the pattern of ERK-dependent gene expression, including a range of cell-cycle regulatory genes (Maekawa et al., 2007). Curiously, however, cell-cycle arrest in these ERK-inhibitor treated embryos did not occur at a G1/S check-point, but at the G2/M transition.
These results present equivocal evidence for a role of ligands in mitogenesis in the early embryo. The strong evidence for a requirement of RAS and ERK in the embryo’s cell-cycle is consistent with a requirement for extracellular signalling, yet blocking the ERK pathway did not create a G1/S block (as is normally the case for blocking of mitogenic ligands in somatic cells). The block at G2/M infers that if the ERK pathway is activated by ligands, then the ligands may be acting in an unconventional manner in the embryo. A major determinant of progression through the G2/M checkpoint is genomic integrity, including the completion of DNA replication. There is some evidence in somatic cells that ligand-induced activation of the RAS/ERK pathway may have a role in progressing cells through G2/M blocks (Hayne et al., 2000), although controversy exists about the role for this pathway in normal G2/M progression (that is, where genomic integrity is not compromised) (Feinstein and Linstedt, 2007). There is evidence for the activation of the ERK pathway during somatic cell M-phase progression, yet this appears to be independent of extracellular mitogens (Feinstein and Linstedt, 2007). In the rabbit blastocyst, insulin stimulated the phosphorylation of ERK1/2 (Navarrete Santos et al., 2004), yet it was not established whether this was associated with cell-cycle progression. Both Erk1−/− (Pages et al., 1999) and Erk2−/− (Hatano et al., 2003) preimplantation embryos are viable, but embryos die at later stages of development. This may suggest that neither of these MAP kinases is essential for early embryonic mitotic events. This is not an unequivocal conclusion, since the necessity to breed from heterozygous parents (due to late embryonic lethality of the homozygous state) does not exclude the possibility that gametic stores of these proteins persist throughout the preimplantation stage. Furthermore, the possibility of extensive redundancy of action between ERK1 and ERK2 cannot be excluded. This is difficult to test experimentally since the compound double knockout cannot be produced due to the lethality of both homozygous states.
In somatic cells, ERK promotes the transcription of D-type cyclins. The synthesis of D-cyclins is a necessary step in G1-phase exit and S-phase entry (Meloche and Pouysségur, 2007). The mitotic D-cyclins are expressed in the mouse embryo’s second and third cell-cycles prior to S-phase, in a pattern similar to that observed in somatic cells (Waclaw and Chatot, 2004). D-type cyclins are generally up-regulated in somatic cells by specific mitogens/growth factors (Meloche and Pouysségur, 2007), thus the apparently controlled expression of D-cyclins in the early embryo may be suggestive of a mitogenic action of exogenous ligands in these early embryos. However, several types of cyclin are expressed during early embryo development and the overlapping actions of these proteins has to date defeated definitive characterization of their relative roles in the regulation of mitosis in the mammalian embryo (Winston, 2001). Thus, while the expression of D-cyclins is suggestive of actions of exogenous mitogenic ligands, definitive proof of this action cannot be claimed at this time.
Direct evidence for a mitotic effect of embryotrophic ligands requires the demonstration that: (i) the ligand specifically induces progression through cell-cycle checkpoints (particularly the G1/S phase checkpoints); and (ii) the withdrawal of the ligand(s) induces cell-cycle quiescence within the embryo. To date, these criteria have not been met experimentally. Indeed, careful analysis of the rate of cell-cycle progression of embryos cultured at different culture densities in vitro (a condition expected to result in relative deprivation of the range of autocrine ligands and exclusion of all paracrine and endocrine ligands) failed to demonstrate a detectable difference in the rate of progression through specific cell-cycles (O’Neill, 1998). The balance of evidence to date does not provide unequivocal support for a role for ligand-induced mitogenesis in the cleavage stages of embryo-development. It is possible that the increased metabolic activity induced by trophic ligands act to support mitotic activity indirectly, but this does not meet the normal definition of a mitogen.
If mitogenesis does not require soluble ligands then it begs the question, what does drive mitosis in the early embryo? One possibility is that the correct experimental design has not yet been devised to detect the mitogenic actions of autocrine embryotrophins. Another possibility is that the mitogenic signalling pathways are constitutively active during these early stages of development and act without a requirement for exogenous stimulation. Constitutive activation of the mitogenic pathways can occur, and is the basis of some types of neoplastic transformation (cancer). Yet such constitutive activation of mitosis in somatic cells generally creates a reflex of cell-cycle blockage or the onset of apoptosis in the responding cells (Meloche and Pouysségur, 2007). If constitutive activation of the mitogenic signalling pathway is the basis of mitogenic activity in the early embryo, then a new set of biological challenges for the embryo exist. How does the embryo prevent the onset of cell-cycle blockage or apoptosis in response to unopposed mitogenic signalling?
Constitutive expression of ERK1/2 pathways in somatic cells commonly results in the up-regulation of anti-proliferative and pro-apoptotic mediators such as P21CIP1 and P16INK4a, respectively (Meloche and Pouysségur, 2007). Cells also may respond by expression of P19ARF, which stabilizes the expression of pro-apoptotic and anti-proliferative mediator tumour suppressor protein, P53 (Sherr and Weber, 2000). The adverse effects of constitutive mitogenic stimulation can be ameliorated by the coincident and countervailing expression of the pro-survival pathways (Fanidi et al., 1992). Thus, a current paradigm in cell biology is that mitogenic signalling needs to be accompanied by survival signalling in order for normal proliferation to proceed. The survival signalling can occur through mitogens having the dual effects of activating both mitogenic and survival signals. The survival response can also be activated by discrete survival factors and/or interaction of the cells with its extracellular matrix.
A systematic assessment of whether the early embryo possesses feedback mechanisms that limit and regulate the proliferative response is yet to be undertaken. If they do exist, the robust mitogenesis that occurs in the early embryo infers a requirement for pro-survival signalling to allow the proliferative response to be maintained.
Ligand-mediated survival signalling
A widely accepted paradigm within cell biology is that cells are programmed to undergo cell death (by apoptosis) if they are deprived of their lineage specific repertoire of survival signals (Raff, 1992). Survival factors act via receptors to induce the latency of action of intracellular mediators. Apoptosis occurs by loss of membrane potential, nuclear fragmentation, cytoplasmic shrinkage and eventual cellular fragmentation. Apoptosis occurs in a manner that does not normally induce inflammatory responses within the body. Specific biochemical mechanisms act to direct apoptosis. In most cells, the components of the apoptotic pathway are constitutively expressed but are maintained in a latent state by the action of survival factors (Raff, 1992). It is reasoned that survival signalling allows cells a means of determining that they exist within their correct temporal-spatial context. Thus, a cell will only be exposed to its correct repertoire of survival factors if it is in the correct context. A requirement for survival signalling in multicellular organisms may facilitate tissue organization and remodeling during development. This signalling may also serve to protect the organism from ectopic or metastatic tissue growth.
As is the case for most cell types studied, the preimplantation embryo appears to constitutively express a wide array of death effectors (Weil et al., 1996; Exley et al., 1999). Since robust mitogenesis occurs following fertilization, and the embryo expresses death effector pathways, it can be inferred that the early embryo has developed a solution to the survival problem. An early study indicated that the preimplantation embryo may be exempt from the requirements for survival factors (Ishizaki et al., 1995; Weil et al., 1996), although it is now argued that this function is fulfilled by autocrine factors (O’Neill, 2005). The requirement for ligand-induced survival signalling therefore seems a likely requirement for normal embryo development.
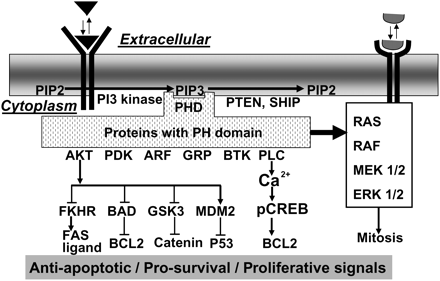
Canonically, survival factors transduce their actions via receptor-mediated activation of PI3 kinase (summarized in Fig. 5). PI3 kinase induces the phosphorylation of membrane inositol phospholipids resulting in the formation of poly-phosphoinositides. The most important reaction may be the conversion of phosphatidylinositol (4,5) bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3) (Marte and Downward, 1997). PIP3 acts as a docking site for a range of proteins containing the pleckstrin homology (PH) domain (Marte and Downward, 1997). The PH domain is one of the most common protein domains in the cell’s proteome, although only a minority of the proteins (∼10%) containing the domain show high specificity binding to poly-phosphoinositides (Lemmon, 2007). The generation of PIP3 facilitates the translocation of proteins containing suitable PH domains to the membrane. This translocation results in their activation and allows the recruitment of signalling complexes that form pro-survival, anti-apoptotic and (in the presence of mitogenic signalling) pro-proliferative signals.
Canonical Actions of PI3 kinase.
Ligand-activated PI3 kinase generates PIP3 which allows binding of proteins containing PH domains. This includes many important signalling proteins which can act on downstream mediators to generate a pro-survival phospho-proteome, or cross-talk with the MAP kinase pathway to maintain the proliferative potential of cells. AKT, thymoma viral proto-oncogene; serine/threonine specific protein kinase B; PDK, 3-phosphoinositide-dependent protein kinase-1; ARF, ADP ribosylation factor; GRP, ARF guanine-nucleotide-exchange factor; BTK, Bruton’s tyrosine kinase; PLC, phospholipase C; PTEN, phosphatase and tensin homolog deleted on chromosome 10; PHD, PH domain; SHIP, inositol polyphosphate-5-phosphatase D; FKHR, forkhead related transcription factor; BAD, Bcl-associated death promoter; GSK3, glycogen synthase kinase 3; MDM2, transformed 3T3 cell double minute p53 binding protein; Bcl-2, B-cell leukemia 2; RAS, small oncogenic G-protein, P21; FAS, TNF receptor superfamily member 6. For other abbreviations see previous legends.
Multiple isoforms of PI3 kinase mRNA (Lu et al., 2004) and PI3 kinase subunit protein (Riley et al., 2005) are expressed in the embryo throughout the preimplantation phase. PI3 kinase inhibitors interfere with normal preimplantation embryo development in vitro (Lu et al., 2004; Gross et al., 2005; Riley et al., 2005), leading to increased rates of cell death and a reduced numbers of cells that populate the embryo (Lu et al., 2004). This effect could be partially reversed by the addition of exogenous PAF in vitro (Lu et al., 2004). It is proposed that embryotrophins (O’Neill, 1998) acting via PI3 kinase are essential for the survival of the early embryo (O’Neill, 2005).
Importantly, when a blocking antibody to PIP3 was infused into the cytoplasm of the two-cell embryo, the signal transduction induced by PAF was blocked (Li et al., 2007b). In comparison, direct injection of PIP3 into the two-cell embryo induced ion-channel activation in two-cell embryos in a manner similar to that induced by PAF (Li et al., 2007b). These results provide the first direct evidence for the formation of PIP3 by activation of PI3 kinase by extracellular ligands such as PAF. Many proteins integral to survival signalling contain PIP3-binding PH domains. Examples of these include thymoma viral proto-oncogene (AKT) (also known as serine/threoine-specific protein kinases B, PKB), phospholipase C delta (PLC delta), 3-phosphoinositide-dependent protein kinase-1 (PDK1), ADP ribosylation factor (ARF), ARF guanine-nucleotide-exchange factor (GRP1) and Bruton’s tyrosine kinase (BTK) (Lemmon, 2007) (Fig. 5).
The direct evidence for the generation of PIP3 in the early embryo (Li et al., 2007b) infers the potential activation of the range of proteins that contain the PH-domain, should they be expressed by the early embryo. This may be indicative of a potentially diverse range of responses by the embryo upon activation of PI3 kinase by ligands. The full extent of the expression of proteins containing PH domains by the preimplantation embryo is yet to be systematically investigated. Two well-known PIP3-sensitive proteins that contain PH domains (AKT and PLC) have had some investigation.
The two-cell embryo expresses the genes that code for AKT (Akt1-3) (Li et al., 2007b) and immunostaining shows that AKT protein is expressed throughout the preimplantation stage (Riley et al., 2005; Li et al., 2007b). It is generally considered that phosphorylation of AKT occurs as a consequence of its translocation to PIP3 in the plasma membrane. Activation of AKT is accompanied by serine 473 phosphorylation (Sarbassov et al., 2005). This phosphorylated form of AKT is extensively expressed in two-cell mouse embryos. PAF-induced the apparent translocation of serine 473 phosphoAKT to the region of the membrane in two-cell embryos in vitro (Li et al., 2007b).
The actions of the three known AKTs overlap to a considerable degree (Brazil and Hemmings, 2001). Genetic deletion of individual Akt genes results in modest phenotypes (Chen et al., 2001; Cho et al., 2001a, b). Akt1−/−Akt2−/− compound mutant mice die at or soon after birth (Peng et al., 2003). Akt1−/−Akt3−/− double mutant mice die in utero (Yang et al., 2005). Triple mutants have not been reported. Since the phenotypes of the compound mutants are more severe than the sum of single null phenotype, a high degree of functional compensation among the three Akt genes is indicated. Given the considerable overlap of AKT function and the lethality of the double mutant, and the presence of multiple isoforms within the early embryo, the selective pharmacological inhibition of AKT provides the best tool for investigation of AKT function in the early embryo at this time. Inhibitors of AKT cause dose-dependent inhibition of normal embryo development in vitro (Li et al., 2007b; Riley et al., 2005), and this could be partially reversed by addition of exogenous PAF (Li et al., 2007b). The similar inhibitory profiles of putative PI3 kinase (Lu et al., 2004) and AKT inhibitors (Li et al., 2007b) are consistent with these agents acting within the same signalling pathway in the early embryo. Activated AKT in turn activates a range of pro-survival effectors. For example (i) AKT-dependent phosphorylation of MDM2 results in the loss of activity of the pro-death mediator P53 (this is discussed further below); (ii) phosphorylation (and inhibition) of GSK-3 (which is a negative regulator of the Wnt/beta-catenin pathway) resulting in the net activation of this pathway; (iii) phosphorylation and inhibition of the pro-apoptotic mediator BAD; and (iv) phosphorylation and inhibition of Forkhead related transcription factor (FKHR/DAF16) (Nishikimi et al., 1999) (Fig. 5). This multiplicity of downstream effectors provides a range of potential mechanisms through which survival ligand activation of PI3 kinase may facilitate embryo survival. With the exception of P53, the potential roles of these effectors have yet to be systematically investigated in the early embryo’s response to putative survival ligands.
The possibility that AKT may play a more direct role in mitosis is suggested by the observations that injection of mRNA coding for a constitutively active myristoylated AKT into zygotes induced cell division more effectively than injection of wild-type AKT (Feng et al., 2007). This result was controlled by the treatment of zygotes with mRNA of kinase-deficient AKT, which delayed the first mitotic division. The study indicated that AKT induced phosphorylation of CDC25B causing the activation of MPF (Feng et al., 2007).
Another important PH-domain containing protein is PLC delta. Immunodetectable PLC delta are expressed in the early embryo (Li et al., 2007b; Wang et al., 2007). PLC delta hydrolyses PIP2 to form IP3 and diacylglycerol. IP3 is a critical component of ligand-mediated [Ca2+]i transients in cells, and its activity is required for PAF-induced [Ca2+]i transients in two-cell embryos (Emerson et al., 2000). Selective inhibition of PLC inhibits PAF-induced calcium transients (Emerson et al., 2000). Since these [Ca2+]i transients were also blocked by PI3 kinase inhibitors and [Ca2+]i signalling can be induced by PIP3 injection into two-cell embryos, the activation of PLC is likely to be a component of PI3 kinase-activated signalling pathway. Importantly, PAF-induced calcium signalling could not be blocked by inhibitors of AKT (Li et al., 2007b) showing that the activation of AKT and calcium signalling were independent responses to PI3 kinase mediated signalling in the two-cell embryo. These studies show that ligand-mediated PIP3 production has the minimum effects of activating calcium-independent (AKT) and calcium-dependent pathways.
One important downstream target of calcium signalling is the cyclic AMP responsive element binding (CREB) family of transcription factors. These transcription factors are activated by phosphorylation, which can be induced by calcium-calmodulin dependent kinases (Shaywitz and Greenberg, 1999; Mayr and Montminy, 2001). In the two-cell embryo, calcium transients induce the phosphorylation and nuclear localization of CREB (Jin and O’Neill, 2007). Nuclear localization of CREB was observed to increase for the first time at the mid two-cell stage and again at the eight-cell stage. Nuclear localization of phosphorylated CREB was also most evident at these stages of development. Phosphorylation of CREB was apparently independent of the actions of cAMP, but required the actions of calcium and calmodulin (Jin and O’Neill, 2007). Two members of the CREB family of transcription factors (CREB and ATF1) are expressed in the preimplantation embryo and are essential for normal preimplantation embryo development (Bleckmann et al., 2002). CREB and ATF1 are closely related by structure and function and show overlapping functions due to their capacity to both homo- and hetero-dimerize to form functional transcription factors. Preimplantation embryos with the compound deletion Creb1−/−Atf1−/− succumb to extensive apoptosis (Bleckmann et al., 2002). The CREB transcriptome is very extensive (Zhang et al., 2005). Importantly, this transcriptome includes many proteins that are necessary for survival signalling, including the anti-apoptotic mediator Bcl-2. The activation of CREB is well recognized to be an important component of the cells survival signalling mechanism (Walton and Dragunow, 2000).
PI3 kinase activity is also implicated in normal glucose homeostasis in the early embryo (Riley et al., 2006). Glucose homeostasis in the embryo is in part regulated by the actions of insulin and IGF-1 acting via the IGF-1 receptor (Pantaleon and Kaye, 1996). Insulin-induced glucose up-take is inhibited by a PI3 kinase inhibitor in mouse blastocysts (Riley et al., 2005). Activation of PI3 kinase downstream of the IGF-receptor is well demonstrated in many settings, although in the rabbit blastocyst insulin does not activate PI3 kinase (Navarrete Santos et al., 2004). Disruptions to normal glucose homeostasis is known to disrupt normal embryo development (Pantaleon et al., 1997a) and may also cause increased rates of embryo cell apoptosis (Moley et al., 1998; Keim et al., 2001).
Results to date show that extracellular trophic ligands PAF can act via their receptors to induce the formation of PIP3 through the actions of PI3 kinase. Both AKT and PLC delta contain the PH domain allowing them to be activated via their interaction with PIP3. These enzymes both have central roles in survival signalling pathways in somatic cells and their activation in the early embryo provides an attractive explanation for the actions of extracellular trophic ligands on the early embryo. It is likely that ligand-induced activation of PI3 kinase exerts pleiotypic functions via the activation of pathways downstream of the range of PH-domain proteins expressed by the early embryo. Characterization of these pathways is required to more fully elucidate the actions of the embryotrophic ligands.
Other potential actions of embryotrophic ligands
Anecdotal evidence indicates that the beneficial effect of communal culture of embryos (or the benefits of addition of exogenous ligands to embryo culture media) is greatest when culture conditions are least optimal (although to date this has not been formally tested). This raises the question of whether an important role of trophic ligands may be to mitigate the embryo’s response to exogenous stressors, be they derived from embryo culture or stressors encountered during normal pregnancy.
While survival signalling pathways are required during mitogenic signalling in somatic cells, they also have important roles in mitigating the cell’s response to environmental stressors. A range of genotoxic and non-genotoxic stressors can induce cell death via the induction of death-promoting signalling pathways. Culture of embryos in vitro from the zygote stage results in a number of cellular stressors including deprivation of trophic ligands (O’Neill, 1997, 1998), metabolic and substrate imbalances (Leese, 2002; Leese et al., 1998), heat and oxidative stresses (Nasr-Esfahani and Johnson, 1991; Raff, 1992), epigenetic defects (Stojanov and O’Neill, 2001; De Rycke et al., 2002) and genetic defects (Delhanty et al., 1997). These stressors are associated with increased rates of apoptotic cell death within embryos due to apoptosis (Jurisicova et al., 1995; Hardy, 1999) and embryotrophins have some role in reversing this (Brison and Schultz, 1997; O’Neill, 1997).
In somatic cells, all such stresses are capable of activating the tumour suppressor protein, P53, (transformation related protein 53; TRP53) stress response pathway (Dobyns, 1992; Agarwal et al., 1998). P53 is a transcription factor that generates a transcriptome capable of reducing the rate of cycle-cell progression (e.g. by the induction of cyclin-dependent kinase inhibitors such as CDKN1A) or induction of cell death (by the synthesis of pro-death mediators, such as BAX) (Agarwal et al., 1998). P53 mRNA and protein is expressed in mouse (Jurisicova et al., 1998; Li et al., 2007a) and human (Wells et al., 2005; Chandrakanthan et al., 2007) preimplantation embryos. The level of expression is increased by the processes of culture in vitro in susceptible embryos, and this increased P53 expression is a major cause of the loss of viability of embryos that result from their culture (Li et al., 2007a). A causal relationship between P53 expression and loss of survivability of embryos following culture is demonstrated by the marked increase in the survival of P53−/− zygotes following culture in vitro compared to their P53+/+ sibling embryos (Chandrakanthan et al., 2006; Li et al., 2007a).
The impairment of embryo development caused by genotoxic stressors can be mitigated by trophic ligands. The addition of either insulin or IGF-1 caused a dose-dependent reduction in the rate of apoptosis that was induced by transient exposure of rabbit embryos to UV light (Herrler et al., 1998). In mice, ligands acting through the IGF-1 receptor, but not the EGF-receptor, were capable of mitigating the adverse consequences of transient exposure to ionizing radiation in vitro (Peters et al., 1996). Although, the mechanisms of ligand action were not defined, it is noteworthy that UV (which induce single DNA strand breaks) and ionizing radiation (double DNA strand breaks) exposure are well known to activate P53-dependent death pathways in somatic cells.
Other evidence supporting a role for P53 expression in the loss of preimplantation embryo survival include: (i) anecdotal evidence that transgenic over-expression of P53 is incompatible with early mouse embryo development; (ii) the early embryopathy that occurs as a consequence of the deletion of Mdm2 is reversed by the simultaneous deletion of P53 (Jones et al., 1995; Montes de Oca Luna et al., 1995; Johnson et al., 2005); and (iii) the early embryopathy that occurs as a consequence of diabetes is partially ameliorated by P53 deletion (Keim et al., 2001).
A role for embryotrophic ligands in maintaining P53 at low levels in the early embryo is indicated by the observation that the reduced development of zygotes that occurs when they are cultured individually compared to communally was ameliorated in embryos lacking the P53 gene (P53−/−) (Chandrakanthan et al., 2006). P53 is an important target for survival factor signalling (Fig. 6). For instance, in somatic cells PIP3 (generated by PI3 kinase) activates AKT which in turn leads to the phosphorylation of MDM2 (Mayo and Donner, 2001; Feng et al., 2004). Phosphorylated MDM2 leads to the ubiquitination and degradation of P53 ensuring that it is maintained at a low intracellular level (Kubbutat et al., 1997). The critical role of MDM2 action in the survival of the embryo is demonstrated by the lethality of early embryos that lack the Mdm2 gene (Mdm2−/−). This lethality is reversed by the double knock-out Mdm2−/−P53−/− (Jones et al., 1995; Montes de Oca Luna et al., 1995).
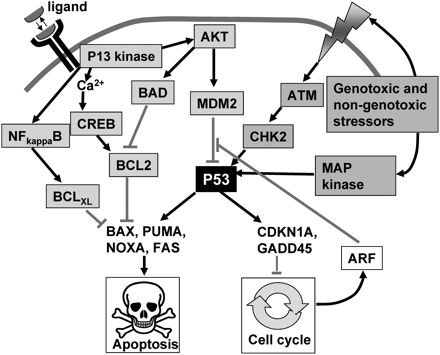
P53 is a critical guardian of cell survival and proliferation.
Activation of transformation related protein 53 (p53) can be achieved by several means, several of which are some of which are summarized. Mitogenic signalling or cell stress (shown in grey boxes) can activate P53 and this can lead to synthesis of apoptotic mediators (e.g. BAX) or cell-cycle inhibitors (e.g. CDKN1A). Survival factor signalling via PI3 kinase (shown in blue boxes) can mitigate P53 action directly by activating MDM2 or indirectly by blocking BAX by generation of pro-survival mediators such as Bcl-2 or BclXL. Interactions leading to activation of a response are shown with black arrows; inhibitory interactions are shown with red bars. ATM, ataxia telangiectasia mutated protein; CHK2, Checkpoint kinase 2; CDKNIA, cyclin dependent kinase inhibitor; GADD45, growth arrest and DNA-damage-inducible protein 45; PUMA, Bcl-2 binding component 3; NOXA, BH3-only member of Bcl-2 family; BAX, Bcl-2-associated X protein. For other abbreviations see previous legends.
Overexpression of P53 in cells generally leads to the expression of a range of pro-apoptotic mediators, including BAX, PUMA, NOXA and FAS; and/or anti-proliferative mediators including, GADD45 and CDN1A. Culture of zygotes in vitro results in increased P53-dependent expression of BAX (Chandrakanthan et al., 2006). BAX is a mediator of apoptosis, capable of activating the caspase cascade, but its activity can be counteracted by anti-apoptotic mediators such as Bcl-2 (Murphy et al., 2000). In this context, it is noteworthy that Bcl-2 expression is regulated by CREB (Wilson et al., 1996). Extracellular ligands induce calcium signals in embryos, and calcium/calmodulin signalling activates CREB in the embryo (Jin and O’Neill, 2007). Thus, a further plausible pathway through which extracellular ligands could mitigate stress responses is by calcium/calmodulin dependent CREB activation leading to production of a pro-survival transcriptome, including the production of Bcl-2 (Fig. 6).
Some members of the MAP kinase family respond to a range of cell stresses rather than extracellular mitogens. The most important of these are MAP kinase 8 (also known as stress-activated protein kinase or Jun kinase, abbreviated as SAP kinase or JN kinase) and MAP kinase 14 (also known as P38 MAP kinase). These enzymes are expressed in the preimplantation embryo (Wang et al., 2005). As a class, they respond to a wide range of environmental stressors and, like the other MAP kinases, activate a cascade of kinase activities resulting in the activation of transcription factors, including AP-1 (Miller et al., 1996). Activation of this pathway is known to induce the expression of a range of stress response effectors (Ip and Davis, 1998). One important effector of this pathway in many cells is tumour necrosis factor-α (TNF-α), and this ligand is known to induce loss of viability and cell death in the preimplantation embryo (Pampfer et al., 1994). The adverse effects of TNF-α can be reversed by exposure to the exogenous embryotrophin transforming growth factor α (TGF-α), acting via PI 3 kinase (Kawamura et al., 2007).
TNF-α is an important negative regulator of cell survival. It is known to be produced by the reproductive tract, and preimplantation embryos express the TNF-receptor (Kawamura et al., 2007). Treatment of embryos with TNF-α induces increased rates of apoptosis in embryonic cells and this could be overcome by a blocking antibody to TNF-α (Kawamura et al., 2007). Importantly, this effect could also be reversed by TGF-α. TGF-α is a putative autocrine embryotrophin being produced by the embryo (Rappolee et al., 1988; Chia et al., 1995). The beneficial effect of TGF-α was mediated in a PI3 kinase-dependent manner (Kawamura et al., 2007). It was shown that TNF-α resulted in the PI3 kinase-dependent upregulation of survivin expression in embryos (Kawamura et al., 2005). Survivin expression is required for normal preimplantation development since survivin-null (Survinin−/−) preimplantation embryos die (Uren et al., 2000). Survivin appears to act via inhibition of caspase activity, and also has roles in maintaining normal mitotic activity (Altieri, 2003). Survivin activity in the embryo may also be regulated by nuclear factor kappa beta (NF-κB) (Kawamura et al., 2005). An important product of NF-κB activity is BclXL, that (like Bcl-2) acts to inhibit the actions of P53-mediated BAX expression. NF-κB is expressed by the preimplantation embryo and pharmacological inhibition of its activity in zygotes prevents subsequent development of the embryos (Nishikimi et al., 1999). Since increased TNF-α synthesis may occur in the reproductive tract during homeostatic stress (such as diabetes in the rat (Pampfer, 2001) embryotrophins may play a role in mitigating the embryo’s response to stresses in vivo as well as in vitro.
The expression of both P38 MAP kinase phosphorylation and SAP kinase/JN kinase is increased when preimplantation embryos are subjected to sub-optimal culture conditions (Wang et al., 2005). Pharmacological inhibition of SAP kinase/JN kinase (by use of D-JNKI1) resulted in improved embryo development in vitro when embryos were cultured in sub-optimal media, albeit with complex pharmacodynamics (Xie et al., 2006a). Other exogenous severe stresses (such as osmotic stress or shear stress) induced increased levels of phosphorylated SAP kinase/JN kinase. The increased stress protein expression was associated with an increased rate of cellular death due to apoptosis. Embryos could be partially protected from shear stress-induced death by inhibition of SAP kinase/JN kinase (Xie et al., 2006b). Inhibition of P38 MAP kinase reduced the proportion of eight-cell bovine embryos that development to the blastocysts stage, but only if ERK was concurrently inhibited (Madan et al., 2005). Extracellular ligands are known to ameliorate the adverse effects of SAP kinase signalling in some settings but this mode of action in the early embryo has yet to be formally tested. Both SAP kinase (Pluquet and Hainaut, 2001) and P38 MAP kinase (Hamatani et al., 2004) can activate P53. The capacity of extracellular ligands to prevent the activity of P53 (see discussion above) is one plausible mechanism through which ligands could mitigate the effects of the MAP kinase stress sensing pathway.
This discussion indicates that embryotrophins may have a role in mitigating the adverse effects of a range of cell stressors on development of the preimplantation embryo. To date much of the evidence in support of this is circumstantial, so a research priority is to formally test this hypothesis. The early embryo is apparently highly sensitive to a wide range of stressors in vitro and this is also probably also the case in vivo. A detailed understanding of the roles of autocrine, paracrine and endocrine embryotrophins in mitigating these effects may make an important contribution to understanding the nature of early embryopathy following assisted reproductive technologies and in some forms of unexplained infertility.
Conclusion
The current weight of evidence indicates that an important contribution to the beneficial effect of communal growth of preimplantation embryos in simple defined culture media is the action of trophic ligands released by the embryos themselves. Within the reproductive tract, the actions of paracrine and endocrine ligands supplement the actions of the autocrine ligands and may also provide information to the embryo not provided by autocrine factors. The apparent overlapping actions of the range of trophic ligands from various sources may serve to maximize the embryo’s trophic inputs. Any non-overlapping functions of the various trophic ligands will be most practicably defined by the detailed study of the downstream signalling encoded by the various ligand-receptor combinations. It also remains to be established whether the actions of the ligands are developmentally defined, that is, are different actions exerted at different stages of preimplantation development? As an example, one could speculate that a given ligand might have one action on the zygote but a different set of actions at later stages of development, due to the expression of a different repertoire of downstream signalling effectors. The best characterized of the ligands, PAF, shows a pattern of signal transduction capable of activation of pro-survival and anti-apoptotic effector pathways in the early embryo. The actions of these pathways are likely to be necessary for continued cellular proliferation during development, but they may also have important roles in mitigating potentially damaging embryonic responses to exogenous stressors. To date, definitive evidence for a mitogenic role for embryotrophins is lacking. Although embryos exposed to ligands in vitro tend to accumulate more cells over time compared to untreated controls, current evidence suggests that this may owe more to a general increase in metabolic activity and reduced rates of apoptosis than any direct mitogenic effect of the ligands. The application of modern cell biology techniques to the study of the preimplantation embryo provides methods for the careful dissection of the modes of action of its range of trophic ligands. Many questions remain regarding their full range of temporal and developmental actions, yet the systematic application of these tools to the range of active ligands across the various developmental stages will allow many of these extant questions to be addressed.