-
PDF
- Split View
-
Views
-
Cite
Cite
Chance M. Witt, Luciana Bolona, Michelle O. Kinney, Christopher Moir, Michael J. Ackerman, Suraj Kapa, Samuel J. Asirvatham, Christopher J. McLeod, Denervation of the extrinsic cardiac sympathetic nervous system as a treatment modality for arrhythmia, EP Europace, Volume 19, Issue 7, July 2017, Pages 1075–1083, https://doi.org/10.1093/europace/eux011
Close - Share Icon Share
Abstract
Denervation of the extrinsic cardiac sympathetic nervous system is a method of altering the autonomic tone experienced by the heart and vasculature. It has been studied and employed as a therapy for cardiac disease for decades. Currently, there is a high level of interest in using cardiac denervation for treatment of arrhythmias. This review describes the anatomy and physiology of the cardiac autonomic nervous system followed by a discussion of the mechanistic studies which provide a basis for the therapeutic use of sympathetic denervation. The clinical research supporting its use in human arrhythmias is then appraised, covering the standard indications, such as long QT syndrome, as well as future possibilities. Last, a detailed account of the methods for performing surgical cardiac denervation and percutaneous stellate ganglion anesthetic block is provided, including the complications of each procedure. An understanding of the anatomy and physiology of the cardiac autonomic nervous system along with the techniques of surgical denervation and percutaneous anesthetic block will allow the clinician to effectively discuss and implement these therapies.
Introduction
Modulation of the cardiac autonomic nervous system is increasingly a consideration in the management of cardiac arrhythmias. Over the last several decades, various approaches have been employed to influence sympathetic and parasympathetic function–either with medications or via operative intervention. Cardiac sympathetic denervation is typically accomplished through surgical resection or pharmacologic infiltration of the extrinsic sympathetic chain. Such denervation therapy, particularly left cardiac sympathetic denervation (LCSD), mitigates against ventricular arrhythmia in specific patients with long QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT), and may also benefit select patients with other channelopathies and cardiomyopathies. In addition, there is limited evidence of an antiarrhythmic effect on other rhythm disturbances such as atrial fibrillation and inappropriate sinus tachycardia. This review updates the clinician on the relevant anatomy and physiology of the cardiac autonomic nervous system and examines the current indications for and efficacy of denervation of the extrinsic cardiac sympathetic nervous system in the context of cardiac arrhythmia. While several modern electrophysiology procedures cause a form of cardiac sympathetic denervation, this term will be used in this review to refer only to denervation of the extrinsic cardiac sympathetic nervous system.
Background
The utility of pharmacologic sympathetic modification by beta-blockade for the reduction of arrhythmia and mortality is well proven.1–3 Surgical sympathetic blockade, in the form of cardiac denervation surgery has been embraced for specific syndromes, but its more widespread application in arrhythmia management is still evolving.
Historically, cardiac denervation surgery was performed as a therapy for angina and arrhythmia by Jonnesco almost a century ago,4 and by the 1960’s was being considered as a therapy for ventricular arrhythmia.5–7 Multiple studies over the next few decades by Schwartz and others examined the potential mechanism underlying its beneficial effect,8–12 and although not entirely understood, larger studies have demonstrated clear benefit in patients with cardiac channelopathies.13–21 Primarily reported in the pediatric community for use in life-threatening ventricular arrhythmias after failure of medical therapy, sympathetic denervation is now considered more frequently in other patients with ventricular tachycardia and other arrhythmias.
Anatomy and physiology
The autonomic nervous system (ANS) at its most basic level conveys electrical impulses between the central nervous system and the end organs, such as the heart. This primarily provides a modulatory effect at a cellular and physiological level on heart rate, blood pressure, electrical conduction, and myocardial contractility in the cardiovascular system. The system incorporates afferent feedback signals which relay information gathered by mechanoreceptors and chemoreceptors throughout the heart and vessels.22
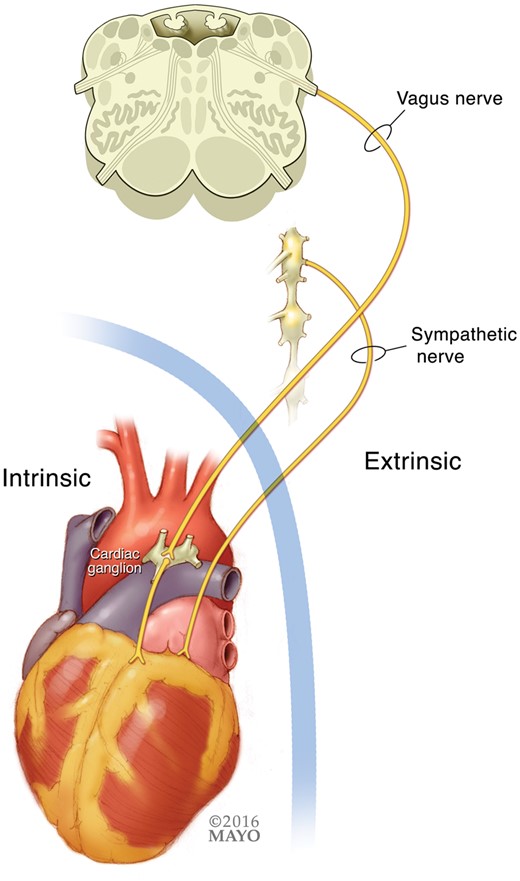
The cardiac ANS itself can be divided anatomically into two parts (Figure 1). The extrinsic section consists of nerves traveling from the central nervous system to the surface of the heart. The intrinsic nervous system, which lies entirely within the pericardium, consists of an interconnected network of nerves covering the heart surface.
Cardiac autonomic innervation can be separated into extrinsic and intrinsic divisions.
Extrinsic cardiac nervous system
The extrinsic portion is further separated into sympathetic and parasympathetic subdivisions. The parasympathetic and sympathetic systems are separate initially with respect to the paths they take to the heart. The parasympathetic nerves arise in the nucleus ambiguus of the medulla oblongata, then leave to form the right and left vagus nerves as they descend through the neck and chest. Prior to continuing into the abdomen, the vagus divides to create the superior, inferior, and thoracic cardiac branches innervating the heart, via a mixed nerve fibre with additional sympathetic function.
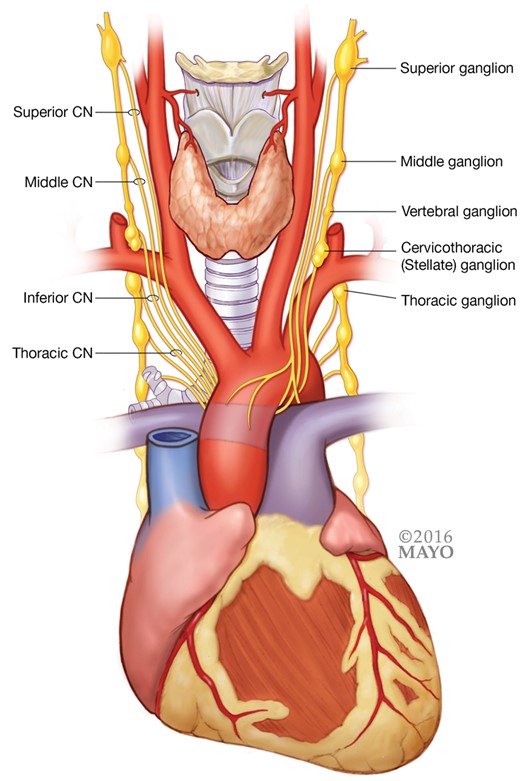
While affected by input from several areas of the brain, pre-ganglionic sympathetic fibres actually originate in the spinal cord and exit via the ventral root. Most synapse in the sympathetic trunk via communicating branches (white rami communicans); however some directly innervate the heart. The sympathetic trunk contains multiple ganglia which lie laterally to the vertebral bodies and connect with spinal nerves. The sympathetic ganglia which supply the heart are: the superior cervical ganglion (C1–C3), middle cervical ganglion (C3–C6), vertebral ganglion (C4–C7), cervicothoracic (or stellate) ganglion (C5–T3), and thoracic ganglia (T2–T6) (Figure 2). The cervicothoracic or stellate ganglion is of particular interest as a site of therapeutic intervention because it provides adequate denervation of the ventricle and avoids complications seen with resection at a more cranial level. This ganglion is composed typically of a fusion of the inferior cervical and first thoracic ganglia, although anatomical variants occur. After synapsing in the sympathetic trunk, the sympathetic fibres connect to the heart through the superior, middle, inferior, and thoracic cardiac nerves. As nerves move closer to the surface of the heart a significant mixture of sympathetic and parasympathetic fibres within discrete nerves occurs.23
Intrinsic cardiac nervous system
The intrinsic ANS forms a network of nerves over the surface of the heart. While the ganglia in this system can be scattered throughout the heart, there are discrete areas of abundant ganglia termed ganglionated plexuses. These occur in common places on the atria and ventricles. There are complex interactions within the intrinsic ANS as well as between the intrinsic and extrinsic ANS.24 While of great importance and a subject of continued vigorous research, the intrinsic ANS will not be discussed in detail in this review which is focused on the extrinsic ANS.
Laterality of sympathetic effects
Anatomical studies show that the extrinsic cardiac ANS follows typical patterns in most people, however variants are seen frequently.23,25 Furthermore, the areas of ventricular myocardium affected by the left and right stellate ganglion appear to overlap and do not maintain a right and left relationship. While both stellate ganglia likely have some effect on most areas of the heart, it appears that the right stellate ganglion predominately affects the anterior and basal portion of the ventricles whereas the left has more effect on the posterior and apical aspects, although still maintaining effect in the anterior left ventricular wall.8,26,27 The right and left stellate ganglia may have differing functional effects on the ventricles as well. Early studies demonstrated that left stellate ganglion block increased the ventricular fibrillation threshold and protected against ischaemic ventricular arrhythmia whereas block of the right stellate ganglion had an opposite and potentially arrhythmogenic effect.9,10 More recent studies have challenged this understanding by suggesting that right sided, in addition to left-sided, denervation may also have an antiarrhythmic effect.28 Stepwise resection of the stellate ganglia demonstrates that the right stellate ganglion still contributes significant sympathetic effects after complete removal of the left stellate ganglion.29 Notably, studies of this type are typically performed with canine and swine models which may not perfectly replicate human neurophysiology. However, while data are limited, bilateral cardiac sympathetic denervation (CSD) does appear to be at least non-inferior and potentially superior to left CSD for treatment of ventricular arrhythmia in humans.30
Sympathetic and parasympathetic interaction
Even beyond this imprecise understanding of functional laterality, the normal physiology of the cardiac ANS remains complex due to significant interaction between the sympathetic and parasympathetic contribution. For example, the effect of an increase in sympathetic input is dependent on the amount of parasympathetic tone present, and vice versa. Therefore, the classic concept of these two systems having an adversarial relationship is an oversimplification.31 Nonetheless, it can be useful as a way to conceptualize most autonomic physiology.
In general, the sympathetic system provides a positive effect, through release of norepinephrine, leading to increased heart rate, conduction velocity, and contractility. Increased parasympathetic tone, mediated by acetylcholine, leads to the opposite, a negative chronotropic, dromotropic, and inotropic effect. In a normal resting human, the parasympathetic system provides the major influence on cardiac function with the sympathetic tone increasing only in the setting of exercise or other stimulation.32
The afferent component of the system adds further complexity due to constant modulation of sympathetic and parasympathetic tone based on information gathered by receptors in the heart and major vessels.
Anti-adrenergic and anti-arrhythmic effects of extrinsic cardiac sympathetic denervation
Cardiac sympathetic denervation provides its anti-adrenergic effect by a pre-ganglionic interruption in signals to and from the heart. This halts the release of neurotransmitters, primarily norepinephrine, which decreases sympathetic tone.
The value of the mechanism by which CSD causes an anti-adrenergic effect can be best illustrated with comparison to other autonomic treatment modalities, most importantly beta adrenergic receptor blocking medications. While CSD has similar treatment roles and clinical outcomes to beta blockers,3 the mechanisms of action are different. The fact that CSD can markedly decrease arrhythmia burden in patients on maximal doses of beta blockers suggests significant mechanistic variation, which has been at least partially explained by the following observations.
First, selective beta blockers only affect the beta receptor. CSD diminishes the release of norepinephrine which, in addition to the beta receptor, can stimulate the alpha adrenergic receptor which may be involved in certain arrhythmias.33 Second, isolated stimulation of the left stellate ganglion has been shown to create pro-arrhythmic conditions, suggesting its specific input may contribute disproportionately to certain diseases compared to the sympathetic system as a whole.34 Lastly, despite complete removal of sympathetic input, an increase in adrenergic receptors is not seen. On the contrary, a decrease in beta receptors has been noted in animal models.35 This last finding may help explain why circulating catecholamines do not negate the effectiveness of denervation.
An added therapeutic benefit of CSD is the relative cardio-selectivity as compared to beta blockers which alter adrenergic receptors throughout the body causing unwanted effects. The surgical outcome is also durable due to the pre-ganglionic resection which removes the ganglion and, with it, the potential for nerve regrowth. This is in contrast to the denervation achieved with cardiac transplantation which may be followed by reinnervation.36
Another potentially important effect is that, similar to vagal nerve stimulators and possibly spinal cord stimulators,37 CSD may cause a reflexive increase in vagal tone due to a decrease in afferent sympathetic signals which typically inhibit parasympathetic tone.38 Although not through CSD, removal of afferent sympathetic effects has been shown to limit autonomic dysfunction and ventricular remodeling in the setting of myocardial infarction in animals.39
Lastly, some of the anti-arrhythmic effect of CSD may be attributed to effects on variations in spatial differences in electrical activity. With stellate ganglion stimulation, there is generally an increase in the dispersion of refractoriness between areas of the ventricles.40,41 Similar effects have been seen in humans with pharmacologic and reflex sympathetic stimulation.42 The functional differences in refractoriness between adjacent areas of myocardium may create an arrythmogenic environment which could be negated by CSD. Other anti-arrhythmic effects relating more to specific arrhythmias are expounded upon in those sections.
Indications
The most extensively implemented and studied use for sympathectomy is for treatment of hyperhidrosis. Sympathetic denervation provides a viable solution for patients with severely symptomatic hyperhidrosis, leading to improved quality of life with minimal complications.43,44 Sympathectomy has been used along these same lines for patients with significant facial blushing.45 In addition, blockade of sympathetic function by local anesthetic injection has been used for sympathetically mediated pain syndromes.46
Ventricular arrhythmia in the setting of inherited diseases
For cardiovascular disease, CSD has been used primarily as a treatment modality in young adults and children with life-threatening arrhythmias due to inherited channelopathies, such as LQTS and CPVT. Recent international guidelines recommend LCSD for LQTS when implantable-cardioverter defibrillator (ICD) or beta blocker therapy are not able to be implemented effectively (class I).47 For CPVT, it may be considered in similar situations (class IIb).47 Most studies have used left CSD although right and bilateral CSD are increasingly being implemented in certain situations.48
The first major demonstration of benefit for LCSD in LQTS revealed a significant reduction in syncope and cardiac arrest.13 More recently, there have been multiple studies showing clear efficacy of LCSD for preventing recurrent arrhythmia and ICD therapies in patients with LQTS and CPVT.14–18,20,21 These small series have shown a greater than 70% success rate in preventing arrhythmia recurrence. It should be noted that while LCSD appears to be highly effective for preventing recurrent arrhythmias, breakthrough events do occur.49 Therefore, patient selection is critical and LCSD is often used as combination therapy. The indications for LCSD monotherapy have not been established fully. Instead, LCSD is more appropriately used as an adjunct therapy in patients who have breakthrough ventricular tachycardia while on medications or to reduce medication requirements in patients who have intolerable side effects. In some cases of severe adverse medication effects, denervation may be used judiciously in place of medications in patients with an ICD in place.
Sympathectomy may be effective in LQTS due to a direct effect on repolarization as evidenced by a decreased QTc after LCSD.50,51 Interestingly, stimulation of sympathetic innervation with nerve growth factor injection of the left stellate ganglion prolongs the QT interval and increases ventricular arrhythmias in canines.52 LCSD may potentially have an opposite, protective effect.
This approach has not only been used for LQTS and CPVT, but also trialed in patients with refractory ventricular arrhythmias on a background of left ventricular noncompaction, hypertrophic cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy as well as with idiopathic ventricular fibrillation. In a study of 27 patients with these disorders (including 13 with CPVT), all of the patients experienced a reduction in arrhythmia burden with 23 having no recurrent events.19
Ventricular arrhythmia in the setting of acquired cardiomyopathy
The utility of CSD for ventricular arrhythmias secondary to ischaemia and ischaemic cardiomyopathy is less well established, but has been effective in some cases. The exact mechanism is not entirely elucidated, but LCSD increases the fibrillatory threshold and prevents ischaemic ectopy in animal models.9,10 Also, chronic ischaemic and non-ischaemic cardiomyopathy are associated with left stellate ganglia containing larger neurons and a higher density of synapses than in normal hearts.53 The dispersion in ventricular refractoriness discussed previously may also play a major role.42
Early data supporting the use of LCSD in patients with ischaemic cardiomyopathy demonstrated a significant reduction in sudden cardiac death rates in high-risk post-myocardial infarction patients (21.3% in the placebo group vs. 3.6% in the cardiac denervation group)–similar to the effect seen with beta-blockade.3 More recently, evidence continues to suggest a significant decrease in ICD shocks in patients with refractory ventricular arrhythmias from acquired heart disease having undergone either left or bilateral CSD with bilateral treatment possibly being superior.30 Efficacy has also been demonstrated for LCSD and/or general anesthesia in the acute management of refractory ventricular arrhythmias,54 and temporary percutaneous sympathetic blockade in patients with ventricular arrhythmia refractory to standard therapy.55 In the context of ventricular fibrillation storm the role of sympathetic blockade with either LCSD or beta-blockade also has been established as a viable therapeutic option if standard therapy has failed.56
Notably, ventricular arrhythmia in the setting of idiopathic non-ischaemic cardiomyopathy is often included with ischaemic cardiomyopathy in these studies. This may be appropriate due to overlapping etiologies and limited patient numbers, although there are likely different levels of response in ischaemic as compared to non-ischaemic cardiomyopathy. Nevertheless, the small numbers of patients in each group in current studies limit comparisons with the necessary statistical rigor to clarify this issue.
Lastly, a specific cause of acquired non-ischaemic cardiomyopathy, Chagas' disease, was the subject of a recent study demonstrating that bilateral CSD reduced ICD shocks from 4 per month to 0 per month in 7 patients with the disease.57
Atrial fibrillation
Although not standard of care, local cardiac denervation therapy has been extensively explored as adjunctive therapy for atrial arrhythmias by targeting ganglionated plexuses.58,59 While this is a very promising ongoing area of research, the ganglionated plexuses are major components of the intrinsic cardiac nervous system which is not the topic of this review.
Extrinsic sympathetic denervation, as discussed above for ventricular arrhythmias, does appear to impact atrial electrical function to some degree. Cryoablation of the bilateral stellate ganglia in a canine heart failure model was associated with a reduced burden of atrial arrhythmia.60 In another canine study, both left and right stellate ganglion stimulation made atrial fibrillation (AF) easier to induce whereas left and right stellate denervation reduced AF inducibility.61 Recently, similar results have been shown in humans. With lidocaine-induced block of the left or right stellate ganglion, AF was less inducible and episodes were of shorter duration. After the block was performed, almost half of the patients (11 out of 24) were not inducible at all.62 If further studies confirm these findings, CSD may be a valuable adjunctive treatment for AF.
Inappropriate sinus tachycardia
Possibly by a more direct effect, amelioration of inappropriate sinus tachycardia syndrome with stellate ganglion block has been seen using percutaneous anesthetic injection.63 The decrease in norepinephrine release produced by blockade of the stellate ganglion could be a very effective method for control of inappropriate sinus tachycardia which may be driven by high sympathetic tone. In this case, given the sidedness of the sinus node, RCSD may be more effective than LCSD, though further study regarding the effectiveness of CSD for treatment of inappropriate sinus tachycardia is still needed.
Procedures
Surgical left cardiac sympathetic denervation
Traditional techniques for left cardiac sympathetic denervation
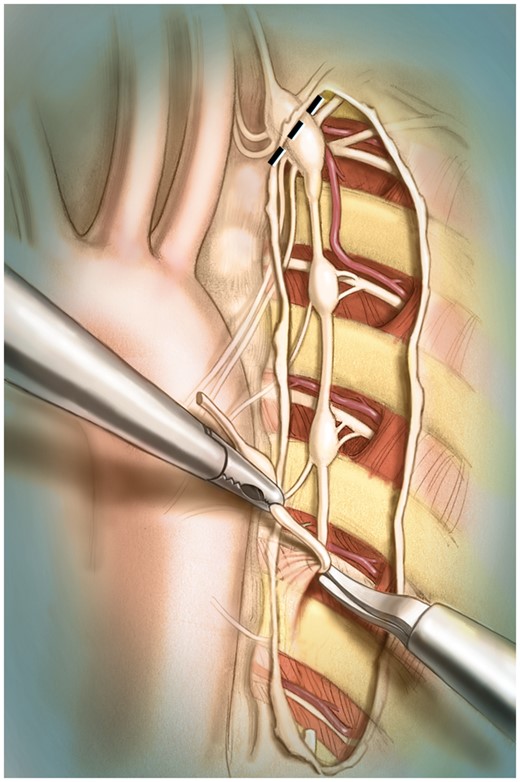
In the late 1960s, left stellectomy, which involves the ablation of the left stellate ganglion, was first introduced to treat LQTS. The procedure was abandoned a few years later because it provided only limited cardiac denervation in humans. The left cervicothoracic sympathectomy was then ushered in–involving total left stellectomy and the removal of the first 4 or 5 left thoracic sympathetic ganglia. This technique produces adequate cardiac sympathetic denervation but is also associated with a Horner’s syndrome.7,13 The modern left cervicothoracic sympathectomy, which we henceforth refer to as left cardiac sympathetic denervation or LCSD, involves removal of the lower half of the left stellate ganglion, together with thoracic ganglia T2 to T4 and their associated rami communicans (Figure 3). This intervention provides adequate cardiac denervation with less than a 5% chance of Horner’s syndrome because most of the sympathetic fibres directed to the ocular region usually cross the upper portion of the left stellate ganglion and thus are spared. LCSD is preganglionic denervation accompanied by removal of synapses that do not regenerate. Therefore, no re-innervation is expected after a properly performed LCSD.13,64,65
Various open chest approaches to the thoracic sympathetic chain have been described.66–68 Open thoracotomy approaches including the anterior transthoracic and the axillary approach ensure full exposure of the sympathetic chain, yet endoscopic approaches now predominate following the first endoscopic sympathetic ablation in 1951.69 The various approaches are described in detail below.
Supraclavicular approach
With denervation by the supraclavicular approach, the incision is placed above the left clavicle, and the platysma and the clavicular head of the sternocleidomastoid muscle are transected. The internal jugular vein is identified just below the muscle and retracted medially; allowing for visualization of the thoracic duct so that it can be avoided. The phrenic nerve coursing along the anterior surface of the scalene muscle is also avoided, and the anterior scalene muscle is transected to expose the subclavian artery. Behind the subclavian artery, and after reflecting apical pleura and lung, the stellate ganglion and the second thoracic ganglion are visualized. The stellate ganglion lies in front of the transverse process of C6 (Chassaignac’s tubercle). This landmark helps to correctly identify the position of the stellate ganglion. When the stellate ganglion has been freed from its bed, the chain can often be followed down as far as the fourth thoracic ganglion. Care must be taken to avoid injury to the intercostal vessels. After local application of lidocaine 1%, which is used to prevent ‘injury currents’ that would produce a significant release of norepinephrine at the ventricular level, the stellate ganglion is dissected in two halves. This separates the first thoracic ganglion from the inferior cervical ganglion which preserves the cephalic portion of the left stellate ganglion preventing Horner’s syndrome. Because LCSD is performed by an extrapleural approach, thoracotomy, and thoracic drainage are unnecessary. For all surgical approaches, the dissected material is sent to the pathology laboratory and read by frozen section to ensure that nerve and ganglia have been removed.65
Transaxillary approach
The transaxillary approach consists of a transaxillary and transpleural approach to reach the upper thoracic sympathetic nerves. The pectoralis major and intercostal muscles are divided, and the pleura is opened, the lungs are retracted thereby exposing the stellate and second ganglion. As above, the stellate ganglion is dissected in two halves. A chest tube is typically inserted.18
Video-assisted thoracoscopic cardiac sympathetic denervation
After anesthetic induction, a bronchus is selectively intubated and the ipsilateral lung collapsed. Through three small mid-axillary and chest wall incisions, the camera and instruments are inserted through the intercostal spaces. The sympathetic ganglia are identified through the pleura, which is then divided to expose the sympathetic chain from T4 to T1. After application of 1% lidocaine, the ganglia, and sympathetic chain are removed by dividing the major rami communicans and fully identifying the much smaller sympathetic nerve branches that travel toward the heart. The stellate ganglion is divided as noted above. Once pathology is confirmed, the endotracheal tube can be pulled back and the lung inflated.17 A chest tube is not routinely used.
Posterior approach
Raskin et al. recently described a ‘minimally-invasive posterior extrapleural thoracic sympathectomy’ in two pediatric patients with ventricular arrhythmias.70 This is performed by using a posterior paraspinal approach with the assistance of a tubular retractor. The sympathetic chain is found next to the spine and removed in sections from T1–T5. This is performed under general anesthesia but does not require collapsing the lungs.
Stellate ganglion block by percutaneous injection
The use of percutaneous local anesthesia injection for temporary sympathetic blockade (stellate ganglion block) for treatment of arrhythmia has not been studied as systematically as the permanent surgical counterpart. It has been used more commonly in the past for sympathetic-related pain syndromes, typically for pain involving the upper extremities. In these cases, the efficacy of the block is assessed by observing an increase in skin temperature of the dorsum of the hand on the ipsilateral side, along with an associated decrease in upper extremity pain
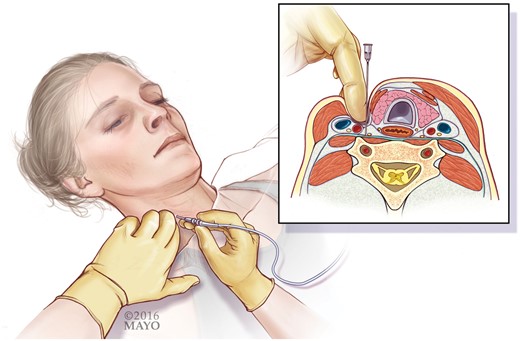
Several approaches to performing the stellate ganglion block have been described. The anterior paratracheal technique was first described in 1934.71 The technique has subsequently undergone several modifications and appears to be associated with a very low serious complication rate72 compared with the anterolateral, lateral, or posterior paravertebral approaches. This technique at the C6 level has the advantage of being farther away from the lung and therefore incurring a reduced incidence of pneumothorax.73–76 This approach utilizes anatomic landmarks and involves palpating the medial edge of the sternocleidomastoid muscle at the level of the cricoid cartilage with the neck extended slightly. The sternocleidomastoid muscle is displaced laterally thereby sweeping the carotid sheath laterally, while the trachea and esophagus are displaced medially. The needle is advanced onto Chasaignac’s tubercle, and is then withdrawn to inject the local anesthetic (Figure 4). Assessment of the block’s efficacy is performed by observation of ptosis of the eyelid and miosis, as well as more subtle signs including enophthalmos, an increase in skin temperature, anhidrosis, and localized vasodilation or nasal stuffiness.
Further technical modifications have involved the use of fluoroscopic imaging and/or ultrasound guidance to assist with identification of the C6 transverse process, proper placement of the needle tip, and avoidance of inadvertent injection into a variety of structures (vertebral artery, carotid artery, dural cuff, epidural space, lung, esophagus, and inferior thyroid artery).73,77,78 The oblique or lateral approaches with fluoroscopic guidance are reasonable techniques for skilled interventionalists.74,79 Fluoroscopy and ultrasound have been shown to be a valid method for anatomic guidance,77–79 and with these imaging guided techniques, the injectate can be visualized going to the correct location. With fluoroscopy the appropriate spread of 2–5 mL of radiographic contrast can be observed prior to injecting the local anesthetic. These techniques may also lead to fewer complications, and, in experienced hands, major adverse effects are rare.80 For a more durable effect, longer acting bupivacaine is typically used at our institution, vs. the short-acting lidocaine counterpart.
The determination of whether a true block of sympathetic outflow has occurred is an ongoing issue with percutaneous sympathetic blockade. As opposed to visual and pathologic confirmation when the ganglia are removed surgically, an anesthetic injection may not affect the exact intended area. Furthermore, some of the changes used to determine effectiveness occur with blockade of nerves cranial to the intended area. One promising area of early research suggests that stellate ganglion activity may be recorded by measuring skin sympathetic activity with specific filtering of ECG recordings.81,82 If reproducible, this could be used to confirm blockade of stellate ganglion activity and monitor for the return of function.
Complications
The most common complication from an extensive CSD surgery that includes a full stellectomy is a permanent Horner’s syndrome, entailing ptosis, anhidrosis, and miosis on the ipsilateral side of the face. Some patient’s will develop only one or two of these features rather than the full syndrome. Temporary Horner’s syndrome effects are used as a marker of effective blockade during local anesthetic injection for stellate ganglion block.77,78 To avoid permanent Horner’s syndrome, most groups recommend dividing the stellate ganglion at the site of fusion and only removing the lower half.20,65,83 With this surgical approach, more recent studies show that this complication occurs rarely and is almost never a permanent condition.13,14,19,20,83
Harlequin facial flushing can be seen in some patients, at least temporarily.14,20,83 With the video-assisted thorascopic approach, a small pneumothorax is common but typically has no chronic sequelae and a chest tube is seldom required (< 5% of the time).17 Excessive sweating in other locations has been noted to be common in the hyperhidrosis patients and is thought to be a compensatory response.44 There are single reports of asystole and bradycardia in patients post bilateral thoracic sympathectomy for hyperhidrosis but this appears to be rare.84–86 While reported infrequently, a neuropathic type pain may occur after the procedure and can be difficult to treat.14,87 The etiology, while seemingly similar to sympathetic dystrophy, is not entirely clear and therefore methods of prevention are not yet known. Despite these potential complications, patient satisfaction with CSD and quality of life post-CSD is rated quite high.88,89
Common adverse effects of stellate ganglion block by injection of local anesthetic include hematoma formation, injury to a blood vessel or other structure, and temporary hoarseness due to recurrent laryngeal nerve block. Less common complications include phrenic nerve block, pneumothorax, osteitis of the transverse process, seizure due to injection of local anesthetic into the vertebral artery, and intradural injection with respiratory embarrassment, bradycardia, and hypotension. Ultrasound-guidance may reduce some of these complications.78,80,90
Conclusions
The sympathetic and parasympathetic nervous systems provide vital coordination of the cardiac rate, rhythm, and responsiveness. A detailed understanding of the anatomy and physiology of the ANS is crucial for further investigational exploration of techniques that can modulate arrhythmogenesis. CSD is an effective therapy for arrhythmia associated with congenital channelopathies and potentially acquired cardiomyopathies. There is good early evidence that modulation of the ANS will be valuable in atrial arrhythmia management. The current procedures for CSD involve a blunt dissection/removal of end-effector inputs to the entire cardiac structures, while finer targeting of elements within the sympathetic and parasympathetic nervous system are likely to be employed in the future for specific effects on rhythm homeostasis.
Conflict of interest: none declared.