-
PDF
- Split View
-
Views
-
Cite
Cite
Anna Franzone, Thomas Pilgrim, Nicolas Arnold, Dik Heg, Bettina Langhammer, Raffaele Piccolo, Eva Roost, Fabien Praz, Lorenz Räber, Marco Valgimigli, Peter Wenaweser, Peter Jüni, Thierry Carrel, Stephan Windecker, Stefan Stortecky, Rates and predictors of hospital readmission after transcatheter aortic valve implantation, European Heart Journal, Volume 38, Issue 28, 21 July 2017, Pages 2211–2217, https://doi.org/10.1093/eurheartj/ehx182
Close - Share Icon Share
Abstract
To analyse reasons, timing and predictors of hospital readmissions after transcatheter aortic valve implantation (TAVI).
Patients included in the Bern TAVI Registry between August 2007 and June 2014 were analysed. Fine and Gray competing risk regression was used to identify factors predictive of hospital readmission within 1 year after TAVI with bootstrap analysis for internal validation. Of 868 patients alive at discharge, 221 (25.4%) were readmitted within 1 year. Compared with patients not requiring readmission, those with at least one readmission more frequently were male and more often had atrial fibrillation and higher creatinine values (P < 0.05 for all cases). For overall 308 readmissions, cardiovascular causes accounted for 46.1% with heart failure as the most frequent indication; non-cardiovascular readmissions occurred for surgery (11.7%), gastrointestinal disorders (9.7%), malignancy (4.9%), respiratory diseases (4.6%) and chronic kidney failure (2.6%). Male gender (subhazard ratio, SHR, 1.33, 95% confidence intervals, CI, 1.02–1.73, P = 0.035) and stage 3 kidney injury (SHR 2.04, 95% CI 1.12–3.71, P = 0.021) were found independent risk factors for any hospital readmission, whereas previous myocardial infarction (SHR 1.88, 95% CI 1.22–2.90, P = 0.004) and in-hospital life-threatening bleeding (SHR 2.18, 95%CI 1.24–3.85, P = 0.007) were associated with cardiovascular readmissions. The event rate for mortality was significantly increased after readmissions for any cause (RR 4.29, 95% CI 2.86–6.42, P < 0.001).
Hospital readmission was observed in one out of four patients during the first year after TAVI and was associated with a significant increase in mortality.
Introduction
Transcatheter aortic valve implantation (TAVI) is the treatment of choice for patients with symptomatic, severe aortic valve stenosis (AS) deemed inoperable or at high risk for surgery.1 Reproducible rates of procedural success, favourable clinical results and the restoration of valve-related quality of life have been consistently documented,2 prompting the extension of its application to lower risk patients.3 Notwithstanding, the burden of comorbid conditions and frailty encountered in routine clinical practice of contemporary TAVI populations impacts on short- and long-term outcomes.
Unplanned hospital readmissions after the index hospitalization are considered an indicator for hospital performance and quality of care,4 and are associated with a significant increase in the economic burden of health care. Most frequently, unplanned hospital readmissions are the result of pre-existing patient frailty, comorbidities and peri-procedural complications and have a relevant impact on quality of life, thereby counteracting the beneficial effects of the procedure. Thus, a systematic appraisal of causes and predictors of readmissions after TAVI can be useful to identify patients at increased risk for repeat unplanned hospital admissions and identify preventive strategies. Only few previous reports have elaborated on this issue to date,5–8 and we therefore explored the frequency, the reasons and predictors of hospital readmission within the first year after TAVI.
Methods
Study population
The Bern TAVI Registry is part of the Swiss TAVI Registry (NCT01368250) and prospectively collects clinical and procedural data of consecutive patients undergoing TAVI at Bern University Hospital. The registry was approved by the local ethics committee and all patients provided written informed consent to participate. The present study complies with the declaration of Helsinki. Procedure and data collection are described in Supplemental Material.
Definition of hospital readmission
Hospital readmission was defined as any new hospitalization with a length of stay of at least one day occurring at our institution or at other hospitals. Unplanned consultation in outpatient clinical setting were not considered in this category. During follow-up visits, patients were questioned about the eventual occurrence of new hospital admission since the last contact and asked to provide original hospitalization records. To ensure accurate collection of causes, length and course of rehospitalization, general practitioners or referral institutions could be contacted.
Statistical analysis
The main objectives of this analysis were: (i) to assess the frequency and reasons of hospital readmissions that occurred within the first year after the procedure among patients included in the Bern TAVI Registry; (ii) to determine the predictive factors of hospital readmission; and (iii) to evaluate the association between hospital readmission and mortality. Baseline clinical characteristics of the study population were described using frequencies with percentages for categorical variables and means with standard deviation for continuous variables.
Rates of hospital readmission within 1-year after TAVI (objective 1) were assessed using competing risks models that considered hospital readmission, using death as the competing event.9 Cumulative incidence plots were constructed to show the cumulative probability of hospital readmission in presence of mortality as competing event. The competing risk models were also used to assess the predictors of readmission (objective 2) by reporting the subhazard ratios (SHRs) that measure the strength of association between each predictor variable and hospital readmission. Multivariable analysis was performed through a forward stepwise selection with inclusion set at P = 0.05. Candidate predictors were: age, gender, body mass index, diabetes, dyslipidaemia, hypertension, history of myocardial infarction, history of cardiac surgery, history of PCI, history of cerebrovascular event, peripheral artery disease, chronic obstructive pulmonary disease, chronic renal failure, CAD, logistic EuroSCORE, STS score, and non-fatal in-hospital events after TAVI (adjudicated using VARC criteria): stroke, bleeding, acute kidney injury. The following echocardiographic parameters obtained after the procedure were also assessed as potential predictors: mean transprosthetic gradient, indexed aortic valve area, left ventricular ejection fraction, any degree of aortic regurgitation, and moderate or severe mitral regurgitation. The internal validity of the final models was tested using 100 bootstrap re-samples. Finally, to assess the impact of early readmission (within 30 days from TAVI) on mortality (from 31 days to 1 year), Cox’s regression was performed using a landmark at 30 days (objective 3).
All analyses were performed by a statistician at the academic clinical trial unit (D.H.) using Stata (version 14; StataCorp LP, College Station, TX). Statistical significance was determined by a 2-sided P < 0.05.
Results
Baseline characteristics
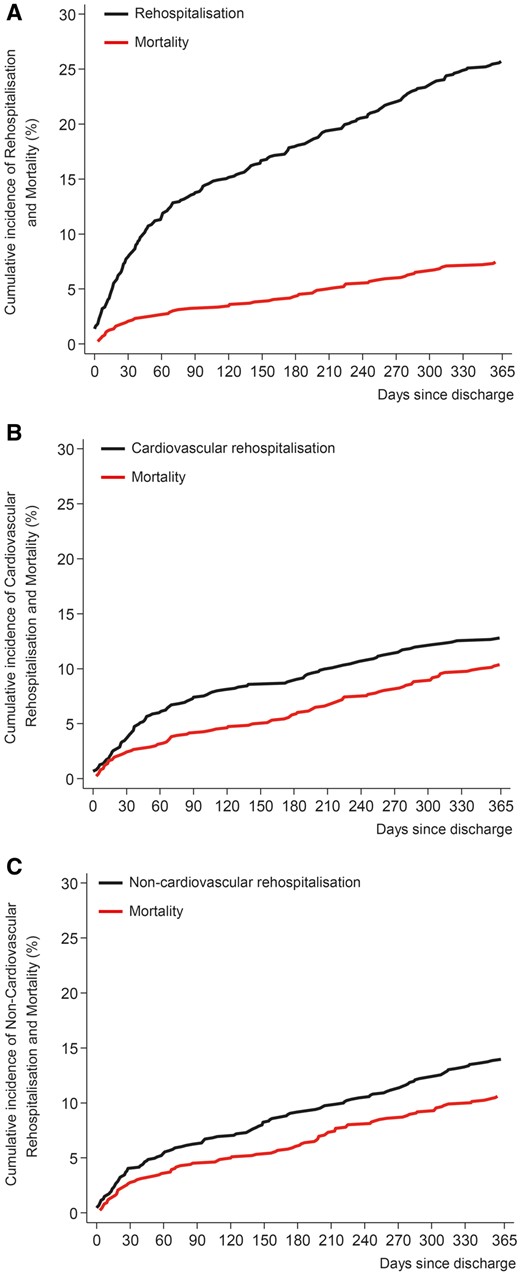
From August 2007 through June 2014, 900 consecutive patients with severe aortic stenosis underwent TAVI and 868 patients (96.5) were alive at discharge. Of these, 221 (25.4%) experienced at least one hospital readmission during the first year after the index procedure. Supplementary material online, Figure S1 shows the number of patients available for 1-year follow-up among those who were or not readmitted after TAVI. Cumulative incidence functions for competing risks models of readmission and mortality are shown Figure 1. Table 1 provides the baseline clinical characteristics. Compared with patients not requiring hospital readmission, those with at least one readmission were more frequently male, presented more often with history of atrial fibrillation requiring oral anticoagulation and had higher levels of creatinine (P < 0.05 for all cases) at baseline. Procedural characteristics are detailed in Supplementary material online, Table S1.
Cumulative incidence function of hospital readmission by causes and accounting for competing risk of mortality. The graphs represent the cumulative incidence of death and any hospital readmission (panel A), hospital readmission for cardiovascular causes (panel B) and hospital readmission for non-cardiovascular causes (panel C) in a competing risk setting. Black line indicates hospital readmission; red line indicates death.
Baseline clinical characteristics
| . | Patients alive at discharge . | Patients with 1-year readmission . | . | |
|---|---|---|---|---|
| None . | Once or more . | P-value . | ||
| n = 868 . | n = 647 . | n = 221 . | . | |
| Age | 82.4 ± 5.8 | 82.4 ± 5.8 | 82.3 ± 5.8 | 0.69 |
| Female gender | 466 (53.7) | 361 (55.8) | 105 (47.5) | 0.035 |
| Body mass index (kg/m2) | 26.3 ± 5.1 | 26.4 ± 5.0 | 26.2 ± 5.3 | 0.73 |
| Diabetes mellitus | 235 (27.1) | 176 (27.2) | 59 (26.7) | 0.93 |
| Hypercholesterolaemia | 550 (63.4) | 401 (62.0) | 149 (67.4) | 0.17 |
| Hypertension | 737 (84.9) | 544 (84.1) | 193 (87.3) | 0.28 |
| Previous myocardial infarction | 140 (16.1) | 96 (14.8) | 44 (19.9) | 0.09 |
| Previous cardiac surgery | 144 (16.6) | 101 (15.6) | 43 (19.5) | 0.21 |
| Previous PCI | 236 (27.2) | 176 (27.2) | 60 (27.1) | 1.00 |
| Previous stroke | 37 (4.4) | 29 (4.6) | 8 (3.8) | 0.70 |
| Chronic obstructive pulmonary disease | 135 (15.6) | 98 (15.2) | 37 (16.7) | 0.59 |
| Coronary artery disease | 556 (64.1) | 414 (64.0) | 142 (64.3) | 1.00 |
| Atrial fibrillation | 246 (31.8) | 167 (29.2) | 79 (39.1) | 0.01 |
| Left ventricular ejection fraction (%) | 53.4 ± 15.2 | 53.9 ± 14.8 | 51.8 ± 16.1 | 0.097 |
| Aortic valve area (cm2) | 0.64 ± 0.24 | 0.64 ± 0.24 | 0.65 ± 0.23 | 0.67 |
| Mean transaortic gradient (mmHg) | 42.6 ± 17.1 | 43.22 ± 17.4 | 40.7 ± 16.2 | 0.08 |
| Mitral regurgitation, moderate or severe | 179 (22.4) | 126 (21.1) | 53 (26) | 0.17 |
| Tricuspid regurgitation, moderate or severe | 69 (13.2) | 56 (13.9) | 13 (10.8) | 0.44 |
| Severe pulmonary arterial hypertension | 94 (24.5) | 71 (24.7) | 23 (24) | 1.00 |
| NYHA III/IV | 589 (68) | 429 (66.5) | 160 (72.4) | 0.11 |
| Logistic EuroScore (%) | 21.4 ± 13.1 | 21.1 ± 12.6 | 22.4 ± 14.4 | 0.19 |
| STS Score (%) | 6.6 ± 4.3 | 6.6 ± 4.5 | 6.5 ± 3.8 | 0.83 |
| Haemoglobin (g/L) | 120.8 ± 16.6 | 121.1 ± 17.0 | 119.7 ± 15.6 | 0.36 |
| Troponin (µg/L) | 0.14 ± 1.2 | 0.15 ± 1.4 | 0.12 ± 0.6 | 0.64 |
| Creatinine (µmol/L) | 102.6 ± 51.4 | 99.7 ± 46.1 | 111.0 ± 63.8 | 0.007 |
| BNP (pg/mL) | 650.8 ± 839.2 | 655.2 ± 848.4 | 637.4 ± 812.8 | 0.89 |
| . | Patients alive at discharge . | Patients with 1-year readmission . | . | |
|---|---|---|---|---|
| None . | Once or more . | P-value . | ||
| n = 868 . | n = 647 . | n = 221 . | . | |
| Age | 82.4 ± 5.8 | 82.4 ± 5.8 | 82.3 ± 5.8 | 0.69 |
| Female gender | 466 (53.7) | 361 (55.8) | 105 (47.5) | 0.035 |
| Body mass index (kg/m2) | 26.3 ± 5.1 | 26.4 ± 5.0 | 26.2 ± 5.3 | 0.73 |
| Diabetes mellitus | 235 (27.1) | 176 (27.2) | 59 (26.7) | 0.93 |
| Hypercholesterolaemia | 550 (63.4) | 401 (62.0) | 149 (67.4) | 0.17 |
| Hypertension | 737 (84.9) | 544 (84.1) | 193 (87.3) | 0.28 |
| Previous myocardial infarction | 140 (16.1) | 96 (14.8) | 44 (19.9) | 0.09 |
| Previous cardiac surgery | 144 (16.6) | 101 (15.6) | 43 (19.5) | 0.21 |
| Previous PCI | 236 (27.2) | 176 (27.2) | 60 (27.1) | 1.00 |
| Previous stroke | 37 (4.4) | 29 (4.6) | 8 (3.8) | 0.70 |
| Chronic obstructive pulmonary disease | 135 (15.6) | 98 (15.2) | 37 (16.7) | 0.59 |
| Coronary artery disease | 556 (64.1) | 414 (64.0) | 142 (64.3) | 1.00 |
| Atrial fibrillation | 246 (31.8) | 167 (29.2) | 79 (39.1) | 0.01 |
| Left ventricular ejection fraction (%) | 53.4 ± 15.2 | 53.9 ± 14.8 | 51.8 ± 16.1 | 0.097 |
| Aortic valve area (cm2) | 0.64 ± 0.24 | 0.64 ± 0.24 | 0.65 ± 0.23 | 0.67 |
| Mean transaortic gradient (mmHg) | 42.6 ± 17.1 | 43.22 ± 17.4 | 40.7 ± 16.2 | 0.08 |
| Mitral regurgitation, moderate or severe | 179 (22.4) | 126 (21.1) | 53 (26) | 0.17 |
| Tricuspid regurgitation, moderate or severe | 69 (13.2) | 56 (13.9) | 13 (10.8) | 0.44 |
| Severe pulmonary arterial hypertension | 94 (24.5) | 71 (24.7) | 23 (24) | 1.00 |
| NYHA III/IV | 589 (68) | 429 (66.5) | 160 (72.4) | 0.11 |
| Logistic EuroScore (%) | 21.4 ± 13.1 | 21.1 ± 12.6 | 22.4 ± 14.4 | 0.19 |
| STS Score (%) | 6.6 ± 4.3 | 6.6 ± 4.5 | 6.5 ± 3.8 | 0.83 |
| Haemoglobin (g/L) | 120.8 ± 16.6 | 121.1 ± 17.0 | 119.7 ± 15.6 | 0.36 |
| Troponin (µg/L) | 0.14 ± 1.2 | 0.15 ± 1.4 | 0.12 ± 0.6 | 0.64 |
| Creatinine (µmol/L) | 102.6 ± 51.4 | 99.7 ± 46.1 | 111.0 ± 63.8 | 0.007 |
| BNP (pg/mL) | 650.8 ± 839.2 | 655.2 ± 848.4 | 637.4 ± 812.8 | 0.89 |
Depicted are means ± SD with P-values from t-tests, or counts (%) with P-values from Fisher's or χ 2 tests.
BNP, B-natriuretic peptide; GFR, glomerular filtration rate; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; PCI, percutaneous coronary intervention.
Baseline clinical characteristics
| . | Patients alive at discharge . | Patients with 1-year readmission . | . | |
|---|---|---|---|---|
| None . | Once or more . | P-value . | ||
| n = 868 . | n = 647 . | n = 221 . | . | |
| Age | 82.4 ± 5.8 | 82.4 ± 5.8 | 82.3 ± 5.8 | 0.69 |
| Female gender | 466 (53.7) | 361 (55.8) | 105 (47.5) | 0.035 |
| Body mass index (kg/m2) | 26.3 ± 5.1 | 26.4 ± 5.0 | 26.2 ± 5.3 | 0.73 |
| Diabetes mellitus | 235 (27.1) | 176 (27.2) | 59 (26.7) | 0.93 |
| Hypercholesterolaemia | 550 (63.4) | 401 (62.0) | 149 (67.4) | 0.17 |
| Hypertension | 737 (84.9) | 544 (84.1) | 193 (87.3) | 0.28 |
| Previous myocardial infarction | 140 (16.1) | 96 (14.8) | 44 (19.9) | 0.09 |
| Previous cardiac surgery | 144 (16.6) | 101 (15.6) | 43 (19.5) | 0.21 |
| Previous PCI | 236 (27.2) | 176 (27.2) | 60 (27.1) | 1.00 |
| Previous stroke | 37 (4.4) | 29 (4.6) | 8 (3.8) | 0.70 |
| Chronic obstructive pulmonary disease | 135 (15.6) | 98 (15.2) | 37 (16.7) | 0.59 |
| Coronary artery disease | 556 (64.1) | 414 (64.0) | 142 (64.3) | 1.00 |
| Atrial fibrillation | 246 (31.8) | 167 (29.2) | 79 (39.1) | 0.01 |
| Left ventricular ejection fraction (%) | 53.4 ± 15.2 | 53.9 ± 14.8 | 51.8 ± 16.1 | 0.097 |
| Aortic valve area (cm2) | 0.64 ± 0.24 | 0.64 ± 0.24 | 0.65 ± 0.23 | 0.67 |
| Mean transaortic gradient (mmHg) | 42.6 ± 17.1 | 43.22 ± 17.4 | 40.7 ± 16.2 | 0.08 |
| Mitral regurgitation, moderate or severe | 179 (22.4) | 126 (21.1) | 53 (26) | 0.17 |
| Tricuspid regurgitation, moderate or severe | 69 (13.2) | 56 (13.9) | 13 (10.8) | 0.44 |
| Severe pulmonary arterial hypertension | 94 (24.5) | 71 (24.7) | 23 (24) | 1.00 |
| NYHA III/IV | 589 (68) | 429 (66.5) | 160 (72.4) | 0.11 |
| Logistic EuroScore (%) | 21.4 ± 13.1 | 21.1 ± 12.6 | 22.4 ± 14.4 | 0.19 |
| STS Score (%) | 6.6 ± 4.3 | 6.6 ± 4.5 | 6.5 ± 3.8 | 0.83 |
| Haemoglobin (g/L) | 120.8 ± 16.6 | 121.1 ± 17.0 | 119.7 ± 15.6 | 0.36 |
| Troponin (µg/L) | 0.14 ± 1.2 | 0.15 ± 1.4 | 0.12 ± 0.6 | 0.64 |
| Creatinine (µmol/L) | 102.6 ± 51.4 | 99.7 ± 46.1 | 111.0 ± 63.8 | 0.007 |
| BNP (pg/mL) | 650.8 ± 839.2 | 655.2 ± 848.4 | 637.4 ± 812.8 | 0.89 |
| . | Patients alive at discharge . | Patients with 1-year readmission . | . | |
|---|---|---|---|---|
| None . | Once or more . | P-value . | ||
| n = 868 . | n = 647 . | n = 221 . | . | |
| Age | 82.4 ± 5.8 | 82.4 ± 5.8 | 82.3 ± 5.8 | 0.69 |
| Female gender | 466 (53.7) | 361 (55.8) | 105 (47.5) | 0.035 |
| Body mass index (kg/m2) | 26.3 ± 5.1 | 26.4 ± 5.0 | 26.2 ± 5.3 | 0.73 |
| Diabetes mellitus | 235 (27.1) | 176 (27.2) | 59 (26.7) | 0.93 |
| Hypercholesterolaemia | 550 (63.4) | 401 (62.0) | 149 (67.4) | 0.17 |
| Hypertension | 737 (84.9) | 544 (84.1) | 193 (87.3) | 0.28 |
| Previous myocardial infarction | 140 (16.1) | 96 (14.8) | 44 (19.9) | 0.09 |
| Previous cardiac surgery | 144 (16.6) | 101 (15.6) | 43 (19.5) | 0.21 |
| Previous PCI | 236 (27.2) | 176 (27.2) | 60 (27.1) | 1.00 |
| Previous stroke | 37 (4.4) | 29 (4.6) | 8 (3.8) | 0.70 |
| Chronic obstructive pulmonary disease | 135 (15.6) | 98 (15.2) | 37 (16.7) | 0.59 |
| Coronary artery disease | 556 (64.1) | 414 (64.0) | 142 (64.3) | 1.00 |
| Atrial fibrillation | 246 (31.8) | 167 (29.2) | 79 (39.1) | 0.01 |
| Left ventricular ejection fraction (%) | 53.4 ± 15.2 | 53.9 ± 14.8 | 51.8 ± 16.1 | 0.097 |
| Aortic valve area (cm2) | 0.64 ± 0.24 | 0.64 ± 0.24 | 0.65 ± 0.23 | 0.67 |
| Mean transaortic gradient (mmHg) | 42.6 ± 17.1 | 43.22 ± 17.4 | 40.7 ± 16.2 | 0.08 |
| Mitral regurgitation, moderate or severe | 179 (22.4) | 126 (21.1) | 53 (26) | 0.17 |
| Tricuspid regurgitation, moderate or severe | 69 (13.2) | 56 (13.9) | 13 (10.8) | 0.44 |
| Severe pulmonary arterial hypertension | 94 (24.5) | 71 (24.7) | 23 (24) | 1.00 |
| NYHA III/IV | 589 (68) | 429 (66.5) | 160 (72.4) | 0.11 |
| Logistic EuroScore (%) | 21.4 ± 13.1 | 21.1 ± 12.6 | 22.4 ± 14.4 | 0.19 |
| STS Score (%) | 6.6 ± 4.3 | 6.6 ± 4.5 | 6.5 ± 3.8 | 0.83 |
| Haemoglobin (g/L) | 120.8 ± 16.6 | 121.1 ± 17.0 | 119.7 ± 15.6 | 0.36 |
| Troponin (µg/L) | 0.14 ± 1.2 | 0.15 ± 1.4 | 0.12 ± 0.6 | 0.64 |
| Creatinine (µmol/L) | 102.6 ± 51.4 | 99.7 ± 46.1 | 111.0 ± 63.8 | 0.007 |
| BNP (pg/mL) | 650.8 ± 839.2 | 655.2 ± 848.4 | 637.4 ± 812.8 | 0.89 |
Depicted are means ± SD with P-values from t-tests, or counts (%) with P-values from Fisher's or χ 2 tests.
BNP, B-natriuretic peptide; GFR, glomerular filtration rate; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; PCI, percutaneous coronary intervention.
In-hospital events and post-discharge care
As reported in Supplementary material online, Table S2, patients with one or more hospital readmission spent a significantly longer time in intermediate care and overall in hospital compared with patients without readmission. Furthermore, they experienced higher rates of acute kidney injury and had higher circulating levels of BNP. Readmitted patients were more frequently discharged on oral anticoagulation therapy.
Frequency, timing, and reasons of hospital readmission
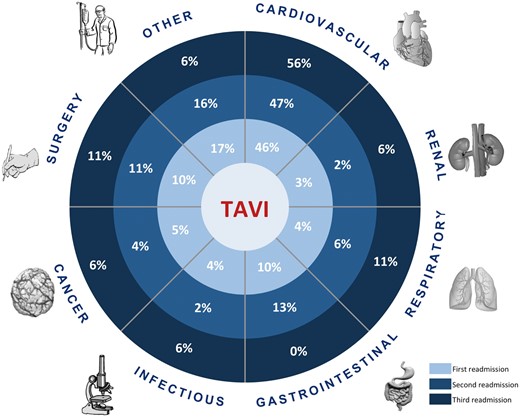
Overall, 308 readmissions occurred in 221 patients during the observational period with a cumulative mean hospital duration of 14.1 ± 16 days. The reasons for hospital readmission are described in Supplementary material online, Table S2. Overall, 142 (46.1%) readmissions were found to be due to cardiovascular causes and 166 (53.8%) for non-cardiovascular causes. Cardiac conditions associated with readmissions included heart failure (39.4%), vascular diseases (33%), cardiac ischaemia (13.3%), arrhythmia (11.2%), and valve-related issues (2.8%). Non-cardiovascular causes of readmission were distributed as follows: 30 (9.7%) for gastrointestinal disorders, 14 (4.6%) for respiratory diseases, 8 (2.6%) for chronic kidney failure, 36 (11.7%) for unplanned non-cardiac surgery (including vascular interventions) and 15 (4.9%) for malignancy-related problems. A total of 12 readmissions (3.9%) were associated with infectious diseases, and 51 (16.6%) with other reasons detailed in Supplementary material online, Table S4. Reasons for subsequent readmissions within the first year after TAVI are illustrated in Figure 2.
Causes of hospital readmission by timing of occurrence. Distribution of causes of hospital readmission according to the timing of their occurrence since discharge after the procedure.
The median time from hospital discharge to the first hospital readmission was 70 days (Supplementary material online, Table S5). While respiratory and infectious diseases led to early readmissions, unplanned surgery and kidney disease were reasons for late readmissions. The time spent in hospital for the first and subsequent hospital readmission is detailed in Supplementary material online, Table S6.
Predictors for hospital readmission
Competing risk regression analysis identified male gender (SHR 1.33, 95% CI 1.02–1.73), and in-hospital acute kidney injury (SHR 2.04, 95% CI 1.12–3.71) as independent risk factors for any hospital readmission. History of myocardial infarction (SHR 1.88, 95% CI 1.22–2.90) and in-hospital life-threatening bleeding (SHR 2.18, 95% CI 1.24–3.85) were associated with a relevant risk increase for readmissions due to cardiovascular causes (Table 2). The addition of post-procedural echocardiographic measures to the model did not show a significant association with the risk of readmission for any or cardiovascular causes within 1 year after TAVI (Supplementary material online, Table S7).
Predictors of hospital readmission within 1 year after TAVI
| . | Multivariate competing risk regression . | Internal validationa . | ||
|---|---|---|---|---|
| . | SHR (95% CI) . | P-value . | Bootstrap SHR (95% CI) . | % P-value <0.05 . |
| Any readmission | ||||
| Gender | 1.33 (1.02–1.73) | 0.035 | 1.28 (1.23–1.33) | 14% |
| In-hospital stroke | 1.77 (0.90–3.51) | 0.099 | 1.30 (0.81–2.08) | 22% |
| In-hospital life-threatening bleeding | 1.40 (0.89–2.19) | 0.143 | 1.38 (1.30–1.48) | 11% |
| In-hospital renal failure (Stage 3) | 2.04 (1.12–3.71) | 0.021 | 2.02 (1.84–2.21) | 24% |
| Cardiovascular readmission | ||||
| History of myocardial infarction | 1.88 (1.22–2.90) | 0.004 | 1.87 (1.77–1.97) | 38% |
| In-hospital stroke | 2.16 (0.88–5.31) | 0.095 | 0.81 (0.39–1.72) | 25% |
| In-hospital life-threatening bleeding | 2.18 (1.24–3.85) | 0.007 | 2.09 (1.93–2.26) | 37% |
| . | Multivariate competing risk regression . | Internal validationa . | ||
|---|---|---|---|---|
| . | SHR (95% CI) . | P-value . | Bootstrap SHR (95% CI) . | % P-value <0.05 . |
| Any readmission | ||||
| Gender | 1.33 (1.02–1.73) | 0.035 | 1.28 (1.23–1.33) | 14% |
| In-hospital stroke | 1.77 (0.90–3.51) | 0.099 | 1.30 (0.81–2.08) | 22% |
| In-hospital life-threatening bleeding | 1.40 (0.89–2.19) | 0.143 | 1.38 (1.30–1.48) | 11% |
| In-hospital renal failure (Stage 3) | 2.04 (1.12–3.71) | 0.021 | 2.02 (1.84–2.21) | 24% |
| Cardiovascular readmission | ||||
| History of myocardial infarction | 1.88 (1.22–2.90) | 0.004 | 1.87 (1.77–1.97) | 38% |
| In-hospital stroke | 2.16 (0.88–5.31) | 0.095 | 0.81 (0.39–1.72) | 25% |
| In-hospital life-threatening bleeding | 2.18 (1.24–3.85) | 0.007 | 2.09 (1.93–2.26) | 37% |
Depicted are subhazard ratios (SHR) with 95% confidence intervals (CI) for the time to a re-hospitalization for any cause and for cardiovascular causes using competing risk regression with mortality.
Subhazard ratios with confidence intervals obtained from 100 Bootstrap samples of 868 patients each, and the percentage of the multivariate competing risk models with each of the individual parameters significant at α of 0.05.
Predictors of hospital readmission within 1 year after TAVI
| . | Multivariate competing risk regression . | Internal validationa . | ||
|---|---|---|---|---|
| . | SHR (95% CI) . | P-value . | Bootstrap SHR (95% CI) . | % P-value <0.05 . |
| Any readmission | ||||
| Gender | 1.33 (1.02–1.73) | 0.035 | 1.28 (1.23–1.33) | 14% |
| In-hospital stroke | 1.77 (0.90–3.51) | 0.099 | 1.30 (0.81–2.08) | 22% |
| In-hospital life-threatening bleeding | 1.40 (0.89–2.19) | 0.143 | 1.38 (1.30–1.48) | 11% |
| In-hospital renal failure (Stage 3) | 2.04 (1.12–3.71) | 0.021 | 2.02 (1.84–2.21) | 24% |
| Cardiovascular readmission | ||||
| History of myocardial infarction | 1.88 (1.22–2.90) | 0.004 | 1.87 (1.77–1.97) | 38% |
| In-hospital stroke | 2.16 (0.88–5.31) | 0.095 | 0.81 (0.39–1.72) | 25% |
| In-hospital life-threatening bleeding | 2.18 (1.24–3.85) | 0.007 | 2.09 (1.93–2.26) | 37% |
| . | Multivariate competing risk regression . | Internal validationa . | ||
|---|---|---|---|---|
| . | SHR (95% CI) . | P-value . | Bootstrap SHR (95% CI) . | % P-value <0.05 . |
| Any readmission | ||||
| Gender | 1.33 (1.02–1.73) | 0.035 | 1.28 (1.23–1.33) | 14% |
| In-hospital stroke | 1.77 (0.90–3.51) | 0.099 | 1.30 (0.81–2.08) | 22% |
| In-hospital life-threatening bleeding | 1.40 (0.89–2.19) | 0.143 | 1.38 (1.30–1.48) | 11% |
| In-hospital renal failure (Stage 3) | 2.04 (1.12–3.71) | 0.021 | 2.02 (1.84–2.21) | 24% |
| Cardiovascular readmission | ||||
| History of myocardial infarction | 1.88 (1.22–2.90) | 0.004 | 1.87 (1.77–1.97) | 38% |
| In-hospital stroke | 2.16 (0.88–5.31) | 0.095 | 0.81 (0.39–1.72) | 25% |
| In-hospital life-threatening bleeding | 2.18 (1.24–3.85) | 0.007 | 2.09 (1.93–2.26) | 37% |
Depicted are subhazard ratios (SHR) with 95% confidence intervals (CI) for the time to a re-hospitalization for any cause and for cardiovascular causes using competing risk regression with mortality.
Subhazard ratios with confidence intervals obtained from 100 Bootstrap samples of 868 patients each, and the percentage of the multivariate competing risk models with each of the individual parameters significant at α of 0.05.
Prognostic impact of hospital readmission
Hospital readmission, occurring at any time point during the first year after TAVI, was associated with an increased risk of all-cause and cardiac mortality: from 10.3 to 37.4%/person years (RR 4.29, 95% CI 2.86–6.42, P < 0.001) and from 7.7 to 22.6%/person years (RR 3.83, 95% CI 2.33–6.28, P < 0.001), respectively. Similarly, rates of cerebrovascular events, myocardial infarction and repeat unplanned intervention were increased after readmission. Early readmissions increased the risk of mortality (HR 2.62, 95% CI 1.40–4.91, P = 0.003) and cardiac mortality (HR 3.00, 95% CI 1.43–6.31, P = 0.004), as reported in Supplementary material online, Table S8.
Discussion
The findings of the present study can be summarized as follows:
– One out of four patients experienced at least one hospital readmission during the first year after TAVI.
– Cardiovascular causes were among the most frequent reasons for hospital readmission.
– Male gender and in-hospital acute kidney injury were found to be independent predictors for any hospital readmission after TAVI.
– Any hospital readmission was associated with an increased risk of mortality and mortality for cardiac causes, and at a larger extent among patients being readmitted during the first 30 days after the index procedure.
Hospital readmission after TAVI
The rate of hospital readmission was comparable to the one reported in the PARTNER A (18.2%) and B population (22.3%),10 , 11 but higher compared with the Austrian TAVI registry (12%).12 Most recently, Nombela-Franco et al. 8 investigated readmissions in 720 patients from two centres and reported a readmission rate of 43.9% during the first year after TAVI. Similarly, the annual rate of readmission due to any cause was as high as 53% for patients included in the large STS/ACC Transcatheter Valve Therapies Registry.7 Several factors could explain the notable difference between the rates of readmission in our cohort and those reported in US centres: (i) differences in the assessment and definition of readmission; (ii) organization of healthcare systems; (iii) procedural protocols; (iv) patients clinical features; (v) length of hospital stay for the index procedure; (vi) management of post-interventional care. Moreover, the majority of patients undergoing TAVI at our institution follow a cardiac rehabilitation program, which may have beneficial effects on functional status and quality of life.
Reasons for hospital readmission after TAVI
Cardiovascular reasons were responsible for more than 40% of unplanned readmissions during the first year after TAVI. Consistent with previous reports, heart failure was the major cause of hospital readmissions with important implications for clinical and functional outcomes.13 Severe heart failure was predominantly observed in more than two thirds of patients with severe AS (68.0% NYHA functional class III and IV), which is considered to be the clinical consequence of left ventricular dysfunction due to the pressure-overload induced cardiac hypertrophy.14 Ventricular unloading after TAVI proved to alleviate heart failure symptoms and result in regression of left ventricular hypertrophy, but this process does not follow a fixed scheme and a number of factors may hinder the recovery of heart function. Indeed, the well-known detrimental impact of concomitant moderate or severe tricuspid or mitral regurgitation and severe pulmonary hypertension15 could play a role in the persistence and progression of heart failure symptoms event after successful TAVI. In addition, the significantly higher prevalence of pre-existing atrial fibrillation among patients experiencing hospital readmission in our analysis supports the notion that this condition is an expression of advanced heart disease and irreversible myocardial remodelling with a substantial impact on cardiovascular morbidity, as previously reported.16
Unplanned surgery and gastrointestinal disorders were identified as the major causes of non-cardiovascular readmissions. Respiratory and infectious diseases were responsible for early hospital readmission, as the risk of infection and exacerbation of chronic pulmonary disease tends to be higher during the early peri-procedural period.17 Of note, a substantial number of patients was readmitted for in-hospital treatment due to a variety of other reasons, that were not captured by our pre-specified category assessment. This observation somewhat mirrors the vulnerability of a geriatric patient population, the fragile clinical status and current limitations in discerning the prognostic impact of peculiar conditions such as frailty, cognitive impairment, and other geriatric disorders.
Risk factors for readmissions after TAVI
Male gender was found to be associated with a relevant increase in rates of hospital readmission for any cause. Determinants of better mid-term survival described for female TAVI patients may explain the lower risk of readmission. Women usually have lower baseline risk and experience a faster reduction of left ventricular hypertrophy after the procedure.18
Post-procedural acute kidney injury was independently associated with the risk for any unplanned hospital readmission. Contrast-induced kidney injury after TAVI has been reported in 12 to 57% of patients with an established impact on dismal prognosis.19 Preventive strategies such as limited use of contrast medium, careful attention to volume status, maintenance of haemodynamic stability, and elimination of nephrotoxic drugs, have been indicated especially for patients with renal impairment at baseline.20 However, at this point in time, standardized approaches or recommendations to avoid acute kidney injury after TAVI are lacking.
In-hospital life-threatening bleeding events were found to increase the risk of readmissions for cardiovascular reasons. A gradient of risk has been described with worse prognosis occurring in patients experiencing major bleeding events after TAVI.21 Peri-procedural blood loss, anaemia, and packed red blood cell transfusion may worsen heart failure and respective symptoms requiring in-hospital management. Moreover, hypoxaemia induced-ischaemia can affect renal function generating a vicious cycle of multi-organ impairment.22 In this context, the prevention of peri-procedural major blood loss is crucial, and the development of TAVI devices and delivery catheters with smaller profiles offer the potential to reduce the burden of access-related complications. Finally, a history of myocardial infarction was identified to predict hospital readmissions due to cardiovascular causes. The impact of concomitant coronary artery disease on outcomes after TAVI is still controversial. However, the development of myocardial stunning and hibernation and chronic neuroendocrine stimulation featuring the progression to chronic heart failure after an acute myocardial infarction may attenuate the beneficial effect of reverse ventricular remodelling triggered by TAVI on cardiac performance.
The field of transcatheter heart valve interventions is not mature enough to adopt the assessment of rehospitalization rates as measure of healthcare efficiency. However, we showed that procedure- rather than patient-related factors increase the risk of readmission after TAVI and this finding has several implications in clinical practice. Peri-procedural events such as acute kidney injury and major bleeding are in part preventable and as such represent pivotal targets for improving TAVI performance.
Impact of readmission on survival after TAVI
Impaired survival has been reported among patients experiencing early readmission after surgical aortic valve replacement. Consistently, we observed a relevant impact of hospital readmissions on the subsequent risk of overall and cardiovascular mortality. In this context, the occurrence of new hospital admission is emerging as a surrogate of adverse prognosis and a measure of cost-effectiveness of the procedure.
We acknowledge the following limitations: the analyses were performed on the basis of the data from a single centre with uncertain generalizability. However, the registry has a prospective design and active follow-up; events were adjudicated by a dedicated event committee and reasons and details of hospital readmissions collected through direct access to hospital records with very low risk of event underreporting by the patients. Moreover, by reporting the experience of a single centre, our study has the potential advantage to assess the performance of healthcare system without bias due to inclusion of patients treated with dissimilar protocols. We did not assess the impact of hospital readmission on other factors (socioeconomic, psychological, quality of life) because they are not captured by our registry. In addition, we were not able to assess the impact of echocardiographic measures of left ventricular diastolic function on the risk of readmission. The analysis was based on events occurring within 1 year after TAVI. Thus, we cannot exclude that the effect of peri-procedural events on outcomes could be diluted at a longer term follow-up in favour of other factors such as comorbidities or myocardial remodelling that is known to occur after several months after TAVI.
Conclusions
Among patients undergoing TAVI, one out of four experienced at least one readmission within the first year after the procedure and was at higher risk for subsequent mortality. The most common cause for cardiovascular readmissions was heart failure, while events driven by valve related issues were rare. Patients undergoing TAVI complicated by in-hospital acute renal failure or bleeding were at increased risk for readmission.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: T.P. has received research grants from Edwards Lifesciences, Symetis, St Jude Medical and lecture fees from Biotronik. RP has received research grants from Veronesi Foundation outside the submitted work. F.P. has received consulting fees from Edwards Lifesciences outside the submitted work. L.R. has received speaker fees from Astra Zeneca and research grants to the institution from Sanofi/Regeneron outside the submitted work. M.V. reports grants from Terumo, and from The Medicines Company, personal fees from Astra Zeneca, Terumo, Bayer, and from Biosensors, outside the submitted work. P.W. has received speaker fees from Medtronic, Edwards Lifesciences and Boston Scientific and research grants from Medtronic outside the submitted work. P.J. has received research grants from Astra Zeneca, Biotronik, Biosensors International, Eli Lilly, The Medicines Company and has served as an unpaid member of the steering group of trials funded by Astra Zeneca, Biotronik, Biosensors, St Jude Medical and The Medicines Company. S.W. has received research grants from Biotronik, Boston Scientific, Bracco Pharmaceutical, Edwards Lifesciences, Terumo, Medtronic and St Jude Medical and speaker fees from Boston Scientific and Daiichi Sankyo outside the submitted work. All the other authors have no conflicts of interest to declare.
References
Author notes
See page 2218 for the editorial comment on this article (doi: 10.1093/eurheartj/ehx252)