-
PDF
- Split View
-
Views
-
Cite
Cite
Xiaokang Luo, Dong Zhang, Bo Li, Lei Qi, Li Gong, Yue Tang, Hansong Sun, Surgical repair of a ruptured congenital sinus of Valsalva aneurysm: 10-year experience with 286 cases, European Journal of Cardio-Thoracic Surgery, Volume 55, Issue 6, June 2019, Pages 1211–1218, https://doi.org/10.1093/ejcts/ezy437
Close - Share Icon Share
Abstract
Surgical intervention is the main treatment for a ruptured congenital sinus of Valsalva aneurysm (SVA). However, reports on the surgical experience are scarce. We retrospectively analysed the cases of our centre to summarize our 10-year experience.
A total of 286 patients who were diagnosed with a congenital ruptured SVA and underwent surgical repair between 2007 and 2016 were identified for the analysis. Follow-up data (mean ± standard deviation: 49.6 ± 34.9 months) were obtained from outpatient department records and telephone calls.
The SVAs originated from the right coronary sinus (79.7%), the non-coronary sinus (19.6%) and the left coronary sinus (0.7%) but ruptured into the right ventricle (58.4%) and the right atrium (41.3%). The most commonly associated deformities were a ventricular septal defect (46.3%), aortic valve regurgitation (33.2%) and tricuspid regurgitation (20.3%). The SVA defect was closed by direct suturing (9.1%) or patching (90.9%) through an incision in the cardiac chamber involved or a transaortic approach. The mean postoperative hospital stay duration was 7.2 days, and 98.6% of the patients were discharged in New York Heart Association functional class I or II. The incidence rate of short-term complications was 5.7%. There were 4 late deaths, and 9 patients required rehospitalization due to surgery-related events. The estimated 10-year survival rate was greater than 90% according to the Kaplan–Meier survival curve.
Surgical repair is an effective and safe treatment for a ruptured SVA. The majority of patients who undergo surgical repair can survive for a long time.
INTRODUCTION
A congenital sinus of Valsalva aneurysm (SVA) is a rare heart disease caused by the congenital absence of muscular and elastic tissue in the aortic wall of the sinus of Valsalva. An unruptured SVA is often asymptomatic and hard to diagnose. Untreated ruptured SVAs are believed to ultimately develop into heart failure, which affects the life expectancy of the patient [1]. Since the first case of successful surgical repair in 1957 [2], surgical intervention has become the main treatment for ruptured SVAs. However, because of the low incidence of SVA in Western countries, there are few reports on the surgical experience, and the long-term efficacy of surgical management is not very clear. Moreover, the surgical approach remains controversial [3]. In this study, we retrospectively analysed the cases of ruptured SVAs treated with surgical repair in our centre to summarize our 10-year experience.
METHODS
We retrospectively analysed patients in the Fuwai Hospital database between April 2007 and May 2016. The inclusion criteria were a diagnosis of a congenital ruptured SVA and treatment with surgical repair. Patients with an acquired or unruptured SVA were excluded. Patients diagnosed with a ruptured SVA regularly underwent examinations using chest radiography and electrocardiography. Patients older than 50 years routinely underwent preoperative coronary computerized tomography angiography or coronary angiography. The echocardiography was performed before the operation.
Surgical methods
The surgical procedure was performed under cardiopulmonary bypass (CPB) with moderate hypothermia. After the aorta was cross-clamped, the ruptured SVA fistula was exposed, and the aneurysm was clamped if necessary. Crystalloid or blood cardioplegia was infused through the aortic root or directly into the coronary ostia. The sinus of Valsalva, aortic valve and coexisting lesions were carefully inspected. After excision of the aneurysm tissue, the defect in the sinus of Valsalva was closed by direct suturing or patching. At the same time, the aortic valve was repaired or replaced if there were leaflet abnormalities. Any coexisting defects were also repaired. Postoperative echocardiography was performed routinely within 1 week after the operation.
Statistical analysis
The basic information and necessary medical records of each patient were extracted and analysed. Data collection was performed in accordance with regulations and approved by the institutional review board of our hospital. The follow-up data were obtained from the records of the outpatient department and telephone calls. Descriptive data were analysed using the Shapiro–Wilk test for normality. The Student’s t-test and analysis of variance were applied for normal data. The Wilcoxon and Kruskal–Wallis tests were applied for non-normal data. Time-related analyses of survival were performed via the Kaplan–Meier method. No correction for multiple testing was performed. All statistical tests were 2-sided, and P-values of ≤0.05 were considered significant. All analyses were performed using SPSS Statistics 22.0 (IBM, Armonk, NY, USA).
RESULTS
Patient characteristics
In total, 286 patients underwent surgical repair, including 192 (67.1%) males. The age range was 2–64 years, and the mean ± standard deviation (SD) was 33.7 ± 11.1 years. The mean body weight was 64.3 (SD 14.8) kg. The main symptoms of a ruptured SVA were dyspnoea (44.8%), chest congestion (44.4%), palpitation (35.7%) and fatigue (21.3%). Data regarding other symptoms are listed in Table 1; 47.2% of the patients (135/286) complained of a cardiac murmur, which was found on routine physical examination. However, 19.2% (55/286) of the patients were detected only by cardiac murmur without any other symptoms. The typical murmur of a ruptured SVA is always described as loud, harsh, superficial and continuous with either systolic or diastolic accentuation [4]. In our study, 5 patients (1.7%, 5/286) did not have a cardiac murmur on physical examination in the hospital; 69.9% (200/286) and 6.3% (18/286) of the patients had a heart murmur that could only be heard at the left and right sternal borders, respectively. In all, 22.0% (63/286) of the patients had a murmur that could be heard on both sides of the chest. Fifteen percent (43/286) of our patients showed systolic thrill or continuous tremor during cardiac palpation. The median difference between the brachial artery systolic and diastolic blood pressures was 60.0 mmHg (interquartile range 24 mmHg). Only 7 patients had a history of previous cardiovascular interventions, including the repair of a ventricular septal defect (VSD), the repair of tetralogy of Fallot and the aortic valvuloplasty and transcatheter occlusion of a VSD. One patient had a history of the transcatheter occlusion of an SVA.
Patient characteristics
| Variables . | Patients . |
|---|---|
| Age (years), mean ± SD | 33.7 ± 11.1 |
| Sex, n (%) | |
| Male | 192 (67.1) |
| Female | 95 (32.9) |
| Weight (kg), mean ± SD | 64.3 ± 14.8 |
| BMI (kg/m2), mean ± SD | 23.0 ± 3.9 |
| Brachial artery pressure difference (mmHg) | 62.5 |
| LVEF (%) | 63.3 |
| LVEDD (mm) | 58.0 |
| C/T, mean ± SD | 0.56 ± 0.08 |
| Symptoms, n (%) | |
| Dyspnoea | 128 (44.8) |
| Chest congestion | 127 (44.4) |
| Palpitation | 102 (35.7) |
| Fatigue | 61 (21.3) |
| Oedema | 32 (11.2) |
| Cough | 29 (10.1) |
| Chest pain | 23 (8.0) |
| Fever | 18 (6.3) |
| Paroxysmal nocturnal dyspnoea | 18 (6.3) |
| Abdominal distension | 11 (3.8) |
| Dizziness | 9 (3.1) |
| Haemoptysis | 5 (1.7) |
| Cyanosis | 4 (1.4) |
| Syncope | 1 (0.3) |
| Variables . | Patients . |
|---|---|
| Age (years), mean ± SD | 33.7 ± 11.1 |
| Sex, n (%) | |
| Male | 192 (67.1) |
| Female | 95 (32.9) |
| Weight (kg), mean ± SD | 64.3 ± 14.8 |
| BMI (kg/m2), mean ± SD | 23.0 ± 3.9 |
| Brachial artery pressure difference (mmHg) | 62.5 |
| LVEF (%) | 63.3 |
| LVEDD (mm) | 58.0 |
| C/T, mean ± SD | 0.56 ± 0.08 |
| Symptoms, n (%) | |
| Dyspnoea | 128 (44.8) |
| Chest congestion | 127 (44.4) |
| Palpitation | 102 (35.7) |
| Fatigue | 61 (21.3) |
| Oedema | 32 (11.2) |
| Cough | 29 (10.1) |
| Chest pain | 23 (8.0) |
| Fever | 18 (6.3) |
| Paroxysmal nocturnal dyspnoea | 18 (6.3) |
| Abdominal distension | 11 (3.8) |
| Dizziness | 9 (3.1) |
| Haemoptysis | 5 (1.7) |
| Cyanosis | 4 (1.4) |
| Syncope | 1 (0.3) |
BMI: body mass index; C/T: cardiothoracic ratio; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; SD: standard deviation.
Patient characteristics
| Variables . | Patients . |
|---|---|
| Age (years), mean ± SD | 33.7 ± 11.1 |
| Sex, n (%) | |
| Male | 192 (67.1) |
| Female | 95 (32.9) |
| Weight (kg), mean ± SD | 64.3 ± 14.8 |
| BMI (kg/m2), mean ± SD | 23.0 ± 3.9 |
| Brachial artery pressure difference (mmHg) | 62.5 |
| LVEF (%) | 63.3 |
| LVEDD (mm) | 58.0 |
| C/T, mean ± SD | 0.56 ± 0.08 |
| Symptoms, n (%) | |
| Dyspnoea | 128 (44.8) |
| Chest congestion | 127 (44.4) |
| Palpitation | 102 (35.7) |
| Fatigue | 61 (21.3) |
| Oedema | 32 (11.2) |
| Cough | 29 (10.1) |
| Chest pain | 23 (8.0) |
| Fever | 18 (6.3) |
| Paroxysmal nocturnal dyspnoea | 18 (6.3) |
| Abdominal distension | 11 (3.8) |
| Dizziness | 9 (3.1) |
| Haemoptysis | 5 (1.7) |
| Cyanosis | 4 (1.4) |
| Syncope | 1 (0.3) |
| Variables . | Patients . |
|---|---|
| Age (years), mean ± SD | 33.7 ± 11.1 |
| Sex, n (%) | |
| Male | 192 (67.1) |
| Female | 95 (32.9) |
| Weight (kg), mean ± SD | 64.3 ± 14.8 |
| BMI (kg/m2), mean ± SD | 23.0 ± 3.9 |
| Brachial artery pressure difference (mmHg) | 62.5 |
| LVEF (%) | 63.3 |
| LVEDD (mm) | 58.0 |
| C/T, mean ± SD | 0.56 ± 0.08 |
| Symptoms, n (%) | |
| Dyspnoea | 128 (44.8) |
| Chest congestion | 127 (44.4) |
| Palpitation | 102 (35.7) |
| Fatigue | 61 (21.3) |
| Oedema | 32 (11.2) |
| Cough | 29 (10.1) |
| Chest pain | 23 (8.0) |
| Fever | 18 (6.3) |
| Paroxysmal nocturnal dyspnoea | 18 (6.3) |
| Abdominal distension | 11 (3.8) |
| Dizziness | 9 (3.1) |
| Haemoptysis | 5 (1.7) |
| Cyanosis | 4 (1.4) |
| Syncope | 1 (0.3) |
BMI: body mass index; C/T: cardiothoracic ratio; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; SD: standard deviation.
Preoperative electrocardiogram (ECG) records of only 281 patients were available in our database. Before the operation, conduction block was found in 36 patients (12.8%, 36/281), including incomplete right bundle branch block (14/281), complete right bundle branch block (12/281), first-degree atrioventricular conduction block (9/281), left anterior fascicular block (5/281), left bundle branch block (1/281) and intraventricular block (1/281). Twelve patients (4.2%, 12/281) exhibited abnormal Q waves. Atrial fibrillation was found in 7 patients (2.5%, 7/281). Two patients were also diagnosed with atrial flutter before the operation. The preoperative echocardiographic assessment showed that the median left ventricular end-diastolic dimension (LVEDD) was 58.0 mm (n = 238, interquartile range 10.0) and that the median left ventricular ejection fraction (LVEF) was 63.7% (n = 285, interquartile range 7.7%). The median systolic blood flow velocity of the aortic valve was 1.5 m/s (interquartile range 0.5 m/s). The median transaortic pressure gradient was 9.0 mmHg (interquartile range 6.2 mmHg). Chest radiography showed the mean cardiothoracic ratio (C/T) was 0.56 (SD 0.08). Detailed information regarding patient characteristics is provided in Table 1.
Surgical findings
The SVAs originated from the right coronary sinus (79.7%, 228/286), the non-coronary sinus (19.6%, 56/286) and the left coronary sinus (0.7%, 2/286). In our study, 58.4% (167/286) of the SVA ruptured into the right ventricle and 41.3% (118/286) ruptured into the right atrium. Only 1 patient had a ruptured SVA protruding into the left ventricle (Table 2). The most common accompanying cardiac diseases were a VSD, aortic valve regurgitation (AR) and tricuspid regurgitation, which occurred in 46.3% (132/286), 33.2% (95/286) and 20.3% (58/286) of the patients, respectively. Notably, 20.3% (58/286) of the ruptured SVAs were accompanied by both VSD and AR. In patients with a concomitant VSD, 74.2% (98/132) of the VSDs were supracristal, 12.9% (17/132) were perimembranous, 9.8% (13/132) were intracristal and the others were not recorded. Other accompanying cardiac deformities included right ventricular outlet stenosis, double cavity of right ventricle, pulmonary stenosis and patent foramen ovale.
The origin and chamber of SVA rupture
| Origin . | Chamber of rupture . | Total (%) . | ||
|---|---|---|---|---|
| Right ventricle . | Right atrium . | Left ventricle . | ||
| Right coronary sinus | 161 | 66 | 1 | 228 (79.7) |
| Non-coronary sinus | 4 | 52 | 0 | 56 (19.6) |
| Left coronary sinus | 2 | 0 | 0 | 2 (0.7) |
| Total (%) | 167 (58.4) | 118 (41.3) | 1 (0.3) | 286 (100) |
| Origin . | Chamber of rupture . | Total (%) . | ||
|---|---|---|---|---|
| Right ventricle . | Right atrium . | Left ventricle . | ||
| Right coronary sinus | 161 | 66 | 1 | 228 (79.7) |
| Non-coronary sinus | 4 | 52 | 0 | 56 (19.6) |
| Left coronary sinus | 2 | 0 | 0 | 2 (0.7) |
| Total (%) | 167 (58.4) | 118 (41.3) | 1 (0.3) | 286 (100) |
SVA: sinus of Valsalva aneurysm.
The origin and chamber of SVA rupture
| Origin . | Chamber of rupture . | Total (%) . | ||
|---|---|---|---|---|
| Right ventricle . | Right atrium . | Left ventricle . | ||
| Right coronary sinus | 161 | 66 | 1 | 228 (79.7) |
| Non-coronary sinus | 4 | 52 | 0 | 56 (19.6) |
| Left coronary sinus | 2 | 0 | 0 | 2 (0.7) |
| Total (%) | 167 (58.4) | 118 (41.3) | 1 (0.3) | 286 (100) |
| Origin . | Chamber of rupture . | Total (%) . | ||
|---|---|---|---|---|
| Right ventricle . | Right atrium . | Left ventricle . | ||
| Right coronary sinus | 161 | 66 | 1 | 228 (79.7) |
| Non-coronary sinus | 4 | 52 | 0 | 56 (19.6) |
| Left coronary sinus | 2 | 0 | 0 | 2 (0.7) |
| Total (%) | 167 (58.4) | 118 (41.3) | 1 (0.3) | 286 (100) |
SVA: sinus of Valsalva aneurysm.
Operative characteristics
The operations were performed by 75 different surgeons. Although each surgeon had a preference, all surgeons adhered to the same general principles when choosing the surgical approach. The surgical repair approaches included right atriotomy, aortotomy, right ventriculotomy and pulmonary arteriotomy. Because the structure of the right ventricle can be exposed by pulmonary arteriotomy, incision of the pulmonary artery was commonly applied instead of right ventriculotomy to avoid trauma to the right ventricle. For the convenience of analysis, we divided the ruptured SVAs into 4 groups based on the ruptured chamber and the accompanying deformities: (i) a simple ruptured SVA (without any other deformities); (ii) a ruptured SVA with a VSD (without AR); (iii) a ruptured SVA with AR (without a VSD); and (iv) a ruptured SVA with both AR and a VSD. The detailed information of the approaches is shown in Table 3.
The approaches of surgical repair
| Approaches . | Simple ruptured SVA, n (%) . | SVA with a VSD (without AR), n (%) . | SVA with AR (without a VSD), n (%) . | SVA with both AR and a VSD, n (%) . | |||
|---|---|---|---|---|---|---|---|
| Protruding into the RA . | Protruding into the RV . | Supracristal VSD . | Perimembranous VSD . | Protruding into the RA . | Protruding into the RV . | . | |
| Right atriotomy | 37 (41.4) | 5 (18.5) | 2 (3.4) | 3 (20.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) |
| Aortotomy | 2 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (12.0) | 2 (16.7) | 5 (8.6) |
| Pulmonary arteriotomy and/or right ventriculotomy | 0 (0) | 1 (3.7) | 11 (18.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Pulmonary arteriotomy and/or right ventriculotomy + right atriotomy | 0 (0) | 7 (26.0) | 12 (20.3) | 4 (26.7) | 0 (0.0) | 1 (8.3) | 7 (12.1) |
| Aortotomy + right atriotomy | 51 (56.7) | 3 (11.1) | 3 (5.1) | 5 (33.3) | 21 (84.0) | 3 (25.0) | 5 (8.6) |
| Aortotomy + pulmonary arteriotomy and/or right ventriculotomy | 0 (0) | 5 (18.5) | 7 (11.9) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 16 (27.6) |
| Aortotomy + pulmonary arteriotomy and/or right ventriculotomy + right atriotomy | 0 (0) | 6 (22.2) | 24 (40.7) | 3 (20.0) | 0 (0.0) | 3 (25.0) | 24 (41.4) |
| Total | 90 (100) | 27 (100) | 59 (100) | 15 (100) | 25 (100) | 12 (100) | 58 (100) |
| Approaches . | Simple ruptured SVA, n (%) . | SVA with a VSD (without AR), n (%) . | SVA with AR (without a VSD), n (%) . | SVA with both AR and a VSD, n (%) . | |||
|---|---|---|---|---|---|---|---|
| Protruding into the RA . | Protruding into the RV . | Supracristal VSD . | Perimembranous VSD . | Protruding into the RA . | Protruding into the RV . | . | |
| Right atriotomy | 37 (41.4) | 5 (18.5) | 2 (3.4) | 3 (20.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) |
| Aortotomy | 2 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (12.0) | 2 (16.7) | 5 (8.6) |
| Pulmonary arteriotomy and/or right ventriculotomy | 0 (0) | 1 (3.7) | 11 (18.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Pulmonary arteriotomy and/or right ventriculotomy + right atriotomy | 0 (0) | 7 (26.0) | 12 (20.3) | 4 (26.7) | 0 (0.0) | 1 (8.3) | 7 (12.1) |
| Aortotomy + right atriotomy | 51 (56.7) | 3 (11.1) | 3 (5.1) | 5 (33.3) | 21 (84.0) | 3 (25.0) | 5 (8.6) |
| Aortotomy + pulmonary arteriotomy and/or right ventriculotomy | 0 (0) | 5 (18.5) | 7 (11.9) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 16 (27.6) |
| Aortotomy + pulmonary arteriotomy and/or right ventriculotomy + right atriotomy | 0 (0) | 6 (22.2) | 24 (40.7) | 3 (20.0) | 0 (0.0) | 3 (25.0) | 24 (41.4) |
| Total | 90 (100) | 27 (100) | 59 (100) | 15 (100) | 25 (100) | 12 (100) | 58 (100) |
AR: aortic valve regurgitation; RA: right atrium; RV: right ventricle; SVA: sinus of Valsalva aneurysm; VSD: ventricular septal defect.
The approaches of surgical repair
| Approaches . | Simple ruptured SVA, n (%) . | SVA with a VSD (without AR), n (%) . | SVA with AR (without a VSD), n (%) . | SVA with both AR and a VSD, n (%) . | |||
|---|---|---|---|---|---|---|---|
| Protruding into the RA . | Protruding into the RV . | Supracristal VSD . | Perimembranous VSD . | Protruding into the RA . | Protruding into the RV . | . | |
| Right atriotomy | 37 (41.4) | 5 (18.5) | 2 (3.4) | 3 (20.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) |
| Aortotomy | 2 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (12.0) | 2 (16.7) | 5 (8.6) |
| Pulmonary arteriotomy and/or right ventriculotomy | 0 (0) | 1 (3.7) | 11 (18.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Pulmonary arteriotomy and/or right ventriculotomy + right atriotomy | 0 (0) | 7 (26.0) | 12 (20.3) | 4 (26.7) | 0 (0.0) | 1 (8.3) | 7 (12.1) |
| Aortotomy + right atriotomy | 51 (56.7) | 3 (11.1) | 3 (5.1) | 5 (33.3) | 21 (84.0) | 3 (25.0) | 5 (8.6) |
| Aortotomy + pulmonary arteriotomy and/or right ventriculotomy | 0 (0) | 5 (18.5) | 7 (11.9) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 16 (27.6) |
| Aortotomy + pulmonary arteriotomy and/or right ventriculotomy + right atriotomy | 0 (0) | 6 (22.2) | 24 (40.7) | 3 (20.0) | 0 (0.0) | 3 (25.0) | 24 (41.4) |
| Total | 90 (100) | 27 (100) | 59 (100) | 15 (100) | 25 (100) | 12 (100) | 58 (100) |
| Approaches . | Simple ruptured SVA, n (%) . | SVA with a VSD (without AR), n (%) . | SVA with AR (without a VSD), n (%) . | SVA with both AR and a VSD, n (%) . | |||
|---|---|---|---|---|---|---|---|
| Protruding into the RA . | Protruding into the RV . | Supracristal VSD . | Perimembranous VSD . | Protruding into the RA . | Protruding into the RV . | . | |
| Right atriotomy | 37 (41.4) | 5 (18.5) | 2 (3.4) | 3 (20.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) |
| Aortotomy | 2 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (12.0) | 2 (16.7) | 5 (8.6) |
| Pulmonary arteriotomy and/or right ventriculotomy | 0 (0) | 1 (3.7) | 11 (18.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Pulmonary arteriotomy and/or right ventriculotomy + right atriotomy | 0 (0) | 7 (26.0) | 12 (20.3) | 4 (26.7) | 0 (0.0) | 1 (8.3) | 7 (12.1) |
| Aortotomy + right atriotomy | 51 (56.7) | 3 (11.1) | 3 (5.1) | 5 (33.3) | 21 (84.0) | 3 (25.0) | 5 (8.6) |
| Aortotomy + pulmonary arteriotomy and/or right ventriculotomy | 0 (0) | 5 (18.5) | 7 (11.9) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 16 (27.6) |
| Aortotomy + pulmonary arteriotomy and/or right ventriculotomy + right atriotomy | 0 (0) | 6 (22.2) | 24 (40.7) | 3 (20.0) | 0 (0.0) | 3 (25.0) | 24 (41.4) |
| Total | 90 (100) | 27 (100) | 59 (100) | 15 (100) | 25 (100) | 12 (100) | 58 (100) |
AR: aortic valve regurgitation; RA: right atrium; RV: right ventricle; SVA: sinus of Valsalva aneurysm; VSD: ventricular septal defect.
Most operations were conducted through median sternotomy. Only 1 patient underwent left posterolateral thoracotomy. The windsock of the SVA was excised in every patient, and the defect was closed. In all, 9.1% (26/286) of the defects were closed by direct suturing; pledgeted interrupted sutures were the most common method. Other defects (90.9%, 260/286) were closed by patching using Dacron or autologous pericardial patches. All other coexisting deformities were repaired. After careful inspection, 66 patients with moderate or severe regurgitation were treated, 10 of whom were treated with valvuloplasty (15.2%, 10/66). In another 56 (84.8%, 56/66) patients who underwent aortic valve replacement (AVR), 54 received a mechanical prosthetic valve and only 2 received a bioprosthetic valve. Surgical intervention was not performed for cases of trivial or mild AR. Other corresponding procedures included tricuspid valvuloplasty, mitral valvuloplasty, subvalvular aortic stenosis corrective surgery, right ventricular outflow tract reconstruction, foramen ovale repair, double cavity of right ventricle corrective surgery, coronary artery bypass grafting and tricuspid valve replacement (Table 4).
Operative characteristics
| Variables . | Patients . |
|---|---|
| Aortic cross-clamp time (min), mean ± SD | 79.0 ± 39.4 |
| CPB time (min), mean ± SD | 111.8 ± 49.9 |
| Blood loss volume (ml), mean ± SD | 506.4 ± 212.5 |
| Urine output (ml), mean ± SD | 1103.1 ± 816.8 |
| Postoperative ICU stay (h; n = 190), mean ± SD | 39.6 ± 34.4 |
| Mechanical ventilation support(h; n = 259), mean ± SD | 14.6 ± 11.0 |
| Repair technique, n (%) | |
| Patching | 260 (90.9) |
| Direct suture | 26 (9.1) |
| Accompanying procedures, n (%) | |
| VSD repair | 132 (46.2) |
| Aortic valve replacement | 56 (19.6) |
| Tricuspid valvuloplasty | 45 (15.7) |
| Aortic valvuloplasty | 10 (3.5) |
| Mitral valvuloplasty | 9 (3.1) |
| Subvalvular aortic stenosis correction | 6 (2.1) |
| RVOT reconstruction | 5 (1.7) |
| Foramen ovale repair | 5 (1.7) |
| Double cavity of right ventricle correction | 4 (1.4) |
| CABG | 2 (0.7) |
| Tricuspid valve replacement | 2 (0.7) |
| Double outlet right ventricle repair | 1 (0.3) |
| Modified Morrow procedure | 1 (0.3) |
| Modified maze procedure | 1 (0.3) |
| Pulmonary arterioplasty | 1 (0.3) |
| Variables . | Patients . |
|---|---|
| Aortic cross-clamp time (min), mean ± SD | 79.0 ± 39.4 |
| CPB time (min), mean ± SD | 111.8 ± 49.9 |
| Blood loss volume (ml), mean ± SD | 506.4 ± 212.5 |
| Urine output (ml), mean ± SD | 1103.1 ± 816.8 |
| Postoperative ICU stay (h; n = 190), mean ± SD | 39.6 ± 34.4 |
| Mechanical ventilation support(h; n = 259), mean ± SD | 14.6 ± 11.0 |
| Repair technique, n (%) | |
| Patching | 260 (90.9) |
| Direct suture | 26 (9.1) |
| Accompanying procedures, n (%) | |
| VSD repair | 132 (46.2) |
| Aortic valve replacement | 56 (19.6) |
| Tricuspid valvuloplasty | 45 (15.7) |
| Aortic valvuloplasty | 10 (3.5) |
| Mitral valvuloplasty | 9 (3.1) |
| Subvalvular aortic stenosis correction | 6 (2.1) |
| RVOT reconstruction | 5 (1.7) |
| Foramen ovale repair | 5 (1.7) |
| Double cavity of right ventricle correction | 4 (1.4) |
| CABG | 2 (0.7) |
| Tricuspid valve replacement | 2 (0.7) |
| Double outlet right ventricle repair | 1 (0.3) |
| Modified Morrow procedure | 1 (0.3) |
| Modified maze procedure | 1 (0.3) |
| Pulmonary arterioplasty | 1 (0.3) |
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; ICU: intensive care unit; RVOT: right ventricular outflow tract; SD: standard deviation; VSD: ventricular septal defect.
Operative characteristics
| Variables . | Patients . |
|---|---|
| Aortic cross-clamp time (min), mean ± SD | 79.0 ± 39.4 |
| CPB time (min), mean ± SD | 111.8 ± 49.9 |
| Blood loss volume (ml), mean ± SD | 506.4 ± 212.5 |
| Urine output (ml), mean ± SD | 1103.1 ± 816.8 |
| Postoperative ICU stay (h; n = 190), mean ± SD | 39.6 ± 34.4 |
| Mechanical ventilation support(h; n = 259), mean ± SD | 14.6 ± 11.0 |
| Repair technique, n (%) | |
| Patching | 260 (90.9) |
| Direct suture | 26 (9.1) |
| Accompanying procedures, n (%) | |
| VSD repair | 132 (46.2) |
| Aortic valve replacement | 56 (19.6) |
| Tricuspid valvuloplasty | 45 (15.7) |
| Aortic valvuloplasty | 10 (3.5) |
| Mitral valvuloplasty | 9 (3.1) |
| Subvalvular aortic stenosis correction | 6 (2.1) |
| RVOT reconstruction | 5 (1.7) |
| Foramen ovale repair | 5 (1.7) |
| Double cavity of right ventricle correction | 4 (1.4) |
| CABG | 2 (0.7) |
| Tricuspid valve replacement | 2 (0.7) |
| Double outlet right ventricle repair | 1 (0.3) |
| Modified Morrow procedure | 1 (0.3) |
| Modified maze procedure | 1 (0.3) |
| Pulmonary arterioplasty | 1 (0.3) |
| Variables . | Patients . |
|---|---|
| Aortic cross-clamp time (min), mean ± SD | 79.0 ± 39.4 |
| CPB time (min), mean ± SD | 111.8 ± 49.9 |
| Blood loss volume (ml), mean ± SD | 506.4 ± 212.5 |
| Urine output (ml), mean ± SD | 1103.1 ± 816.8 |
| Postoperative ICU stay (h; n = 190), mean ± SD | 39.6 ± 34.4 |
| Mechanical ventilation support(h; n = 259), mean ± SD | 14.6 ± 11.0 |
| Repair technique, n (%) | |
| Patching | 260 (90.9) |
| Direct suture | 26 (9.1) |
| Accompanying procedures, n (%) | |
| VSD repair | 132 (46.2) |
| Aortic valve replacement | 56 (19.6) |
| Tricuspid valvuloplasty | 45 (15.7) |
| Aortic valvuloplasty | 10 (3.5) |
| Mitral valvuloplasty | 9 (3.1) |
| Subvalvular aortic stenosis correction | 6 (2.1) |
| RVOT reconstruction | 5 (1.7) |
| Foramen ovale repair | 5 (1.7) |
| Double cavity of right ventricle correction | 4 (1.4) |
| CABG | 2 (0.7) |
| Tricuspid valve replacement | 2 (0.7) |
| Double outlet right ventricle repair | 1 (0.3) |
| Modified Morrow procedure | 1 (0.3) |
| Modified maze procedure | 1 (0.3) |
| Pulmonary arterioplasty | 1 (0.3) |
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; ICU: intensive care unit; RVOT: right ventricular outflow tract; SD: standard deviation; VSD: ventricular septal defect.
The mean aortic clamping time was 79.0 (SD 39.4) min, and the mean CPB time was 111.8 (SD 49.9) min. All patients could be easily weaned from CPB. The mean blood loss volume during the operation was 506.4 (SD 212.5) ml, and the mean urine output was 1103.1 (SD 816.8) ml; 34.3% (98/286) of the patients received blood transfusion. The mean transfusion volumes of condensed erythrocytes, fresh frozen plasma and blood platelets were 0.79 (SD 1.9) units, 124.5 (SD 288.1) ml and 0.04 (SD 0.22) units per person, respectively. The postoperative stay duration in the intensive care unit was 39.6 (SD 34.4) h (n = 190). The duration of mechanical ventilation support ranged from 2 h to 110 h, with a mean of 14.6 (SD 11.0) h (n = 259).
Early results
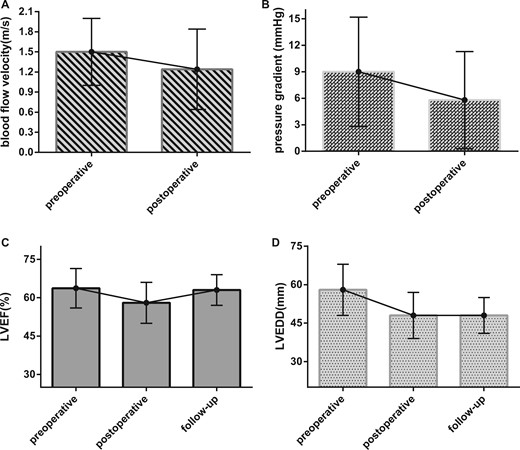
After the operation, the median of peripheral blood pressure differential decreased from 60.0 mmHg to 45.5 mmHg (interquartile range 15 mmHg) with statistical significance (Mann–Whitney U-test, P < 0.001). In all, 284 patients (99.3%, 190/286) underwent a postoperative echocardiographic assessment (within 1 week after the operation). The median LVEF of the postoperative echocardiography was 59.0% (interquartile range 8.0%), and the median LVEDD was 48.0 mm (interquartile range 9.0 mm). Compared with the preoperative echocardiography results, the median systolic blood flow velocity of the aortic valve was significantly reduced (Mann–Whitney U-test, P < 0.001) from 1.5 m/s to 1.2 m/s (interquartile range 0.6 m/s) after the operation (Fig. 1A). The median transaortic pressure gradient also significantly (Mann–Whitney U-test, P < 0.001) decreased from 9.0 mmHg to 5.8 mmHg (interquartile range 5.5 mmHg, Fig. 1B).
Perioperative, postoperative and follow-up echocardiographic results of surgical repair. (A) The median perioperative and postoperative systolic blood flow velocities of the aortic valve was reduced from 1.5 to 1.24 m/s (P < 0.001). (B) The median perioperative and postoperative transaortic pressure gradients decreased from 9.0 to 5.8 mmHg (P < 0.001). (C) The median preoperative, postoperative and follow-up LVEFs were 63.7%, 59.0% and 63.0%, respectively. The postoperative LVEF was significantly lower than the preoperative and follow-up LVEFs (the Kruskal–Wallis test, P < 0.001). However, the difference between the preoperative and follow-up LVEFs was not significant (P = 0.30). (D) The median perioperative, postoperative and follow-up LVEDDs were 58.0 mm, 48.0 mm and 48.0 mm, respectively. The preoperative LVEDD was significantly higher than the postoperative and follow-up LVEDDs (the Kruskal–Wallis test, P < 0.001). However, the difference between the postoperative and follow-up LVEDDs was not significant (P = 0.27). LVEF: left ventricular ejection fraction; LVEDD: left ventricular end-diastolic dimension.
The mean total hospital stay was 14.9 (SD 43.6) days, and the mean postoperative stay duration was 7.2 (SD 2.1) days; 98.6% (282/286) of the patients were discharged as New York Heart Association functional class I or II. Only 3 were class III because of their poor physical condition in the perioperative period. There was no in-hospital death and no instances of low cardiac output syndrome. Seven patients were newly diagnosed with atrial fibrillation. Fifty-one patients were found to have newly emerging conduction block, which was transient in 51.0% (26/51) of the patients. No ventricular arrhythmia or third-degree atrioventricular conduction block developed. One patient developed pneumonia after the operation, which progressed to multiple organ failure; this patient was discharged as New York Heart Association functional class IV. One patient developed a respiratory tract infection postoperatively and recovered in the hospital after 3 weeks of treatment. Two patients underwent a second operation because of massive blood loss secondary to the sternal wound. Another patient underwent a second operation due to sustained haemoglobinuria and large pericardial effusion. Detailed information regarding the short-term complications is provided in Table 5.
Short-term complications
| Complications . | Patients . | Incidence rate (%) . |
|---|---|---|
| Skin wound infection | 5 | 1.7 |
| Massive blood loss needing second operation | 3 | 1.0 |
| Renal insufficiency | 3 | 1.0 |
| Pleural effusion needing thoracentesis | 2 | 0.7 |
| Pneumothorax needing thoracentesis | 2 | 0.7 |
| Respiratory tract infection | 1 | 0.3 |
| Pneumonia | 1 | 0.3 |
| Total | 17 | 5.7 |
| Complications . | Patients . | Incidence rate (%) . |
|---|---|---|
| Skin wound infection | 5 | 1.7 |
| Massive blood loss needing second operation | 3 | 1.0 |
| Renal insufficiency | 3 | 1.0 |
| Pleural effusion needing thoracentesis | 2 | 0.7 |
| Pneumothorax needing thoracentesis | 2 | 0.7 |
| Respiratory tract infection | 1 | 0.3 |
| Pneumonia | 1 | 0.3 |
| Total | 17 | 5.7 |
Short-term complications
| Complications . | Patients . | Incidence rate (%) . |
|---|---|---|
| Skin wound infection | 5 | 1.7 |
| Massive blood loss needing second operation | 3 | 1.0 |
| Renal insufficiency | 3 | 1.0 |
| Pleural effusion needing thoracentesis | 2 | 0.7 |
| Pneumothorax needing thoracentesis | 2 | 0.7 |
| Respiratory tract infection | 1 | 0.3 |
| Pneumonia | 1 | 0.3 |
| Total | 17 | 5.7 |
| Complications . | Patients . | Incidence rate (%) . |
|---|---|---|
| Skin wound infection | 5 | 1.7 |
| Massive blood loss needing second operation | 3 | 1.0 |
| Renal insufficiency | 3 | 1.0 |
| Pleural effusion needing thoracentesis | 2 | 0.7 |
| Pneumothorax needing thoracentesis | 2 | 0.7 |
| Respiratory tract infection | 1 | 0.3 |
| Pneumonia | 1 | 0.3 |
| Total | 17 | 5.7 |
Late results
In all, 190 patients (66.4%, 190/286) underwent a follow-up echocardiographic assessment. The mean follow-up period was 23.0 (SD 28.4) months (range 1–110 months postoperative). The median LVEF was 63.0% (interquartile range 6.0%), and the median LVEDD was 48.0 mm (interquartile range 7.0 mm). The LVEFs assessed by preoperative, postoperative and follow-up echocardiographies were compared using the Kruskal–Wallis test, and the results showed a significant difference (P < 0.001). Further analysis revealed that except for the preoperative LVEF, which was not greater than the follow-up LVEF (P = 0.30), the differences in the LVEF between any other 2 groups were significant (P < 0.001) (Fig. 1C). The preoperative, postoperative and follow-up LVEDDs were also compared using the Kruskal–Wallis test, and the results showed a significant difference (P < 0.001). Further analysis revealed that except for the postoperative LVEDD, which was not greater than the follow-up LVEDD (P = 0.27), the differences in the LVEDD between any other 2 groups were significant (P < 0.001) (Fig. 1D). During the follow-up period, 3 patients were found to have residual shunting from the aorta to the cardiac chamber by echocardiography. One patient had trivial regurgitation in the mechanical prosthetic aortic valve. Twelve patients were found to have newly emerging or aggravating AR; among these patients, 2 underwent reoperation, as mentioned above, and the others were advised to undergo continual observation.
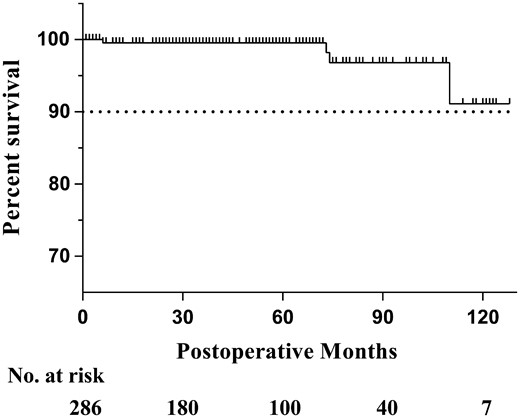
Follow-up data from the outpatient department and telephone calls were available for 92.3% (264/286) of all patients. The follow-up period ranged from 1 to 128 months (mean ± SD 49.6 ± 34.9 months), with a total of 1091.9 patient-years. There were 4 late deaths: 1 was due to bacterial endocarditis 6 years after the surgery, and the other 3 were due to unknown causes. In total, 9 patients required rehospitalization due to events related to the surgery (Table 6); 2 of these patients underwent reoperation with AVR because of severe aortic regurgitation. Another 2 patients underwent radiofrequency ablation in our hospital due to atrial flutter, which occurred postoperatively. One patient developed heart failure and was readmitted 4 years after the operation. Another patient developed bacterial endocarditis a few days after discharge but was cured by prompt treatment. Three patients who had received mechanical prosthetic valve developed accompanying complications requiring hospitalization; 2 patients had a stroke and 1 had gastrointestinal haemorrhage. The Kaplan–Meier survival curve is shown in Fig. 2 and the estimated 10-year survival rate was greater than 90%.
Specific reasons for rehospitalization
| Reasons . | Patients . |
|---|---|
| Severe aortic regurgitation needing valve replacement | 2 |
| Atrial flutter needing radiofrequency ablation | 2 |
| Stroke after concomitant mechanical valve replacement | 2 |
| Gastrointestinal haemorrhage after concomitant mechanical valve replacement | 1 |
| Severe heart failure | 1 |
| Bacterial endocarditis a few days after the operation | 1 |
| Total | 9 |
| Reasons . | Patients . |
|---|---|
| Severe aortic regurgitation needing valve replacement | 2 |
| Atrial flutter needing radiofrequency ablation | 2 |
| Stroke after concomitant mechanical valve replacement | 2 |
| Gastrointestinal haemorrhage after concomitant mechanical valve replacement | 1 |
| Severe heart failure | 1 |
| Bacterial endocarditis a few days after the operation | 1 |
| Total | 9 |
Specific reasons for rehospitalization
| Reasons . | Patients . |
|---|---|
| Severe aortic regurgitation needing valve replacement | 2 |
| Atrial flutter needing radiofrequency ablation | 2 |
| Stroke after concomitant mechanical valve replacement | 2 |
| Gastrointestinal haemorrhage after concomitant mechanical valve replacement | 1 |
| Severe heart failure | 1 |
| Bacterial endocarditis a few days after the operation | 1 |
| Total | 9 |
| Reasons . | Patients . |
|---|---|
| Severe aortic regurgitation needing valve replacement | 2 |
| Atrial flutter needing radiofrequency ablation | 2 |
| Stroke after concomitant mechanical valve replacement | 2 |
| Gastrointestinal haemorrhage after concomitant mechanical valve replacement | 1 |
| Severe heart failure | 1 |
| Bacterial endocarditis a few days after the operation | 1 |
| Total | 9 |
The Kaplan–Meier survival curve after surgical repair of ruptured SVAs. SVA: sinus of Valsalva aneurysm.
DISCUSSION
This study summarized the surgical experience of ruptured SVAs in 286 patients. We reviewed the characteristics of the patients and analysed the surgical and follow-up data. The results indicate that the surgical repair of a ruptured SVA has a satisfactory efficacy and safety. Our study provides new evidence regarding the evaluation of this procedure and proposes some suggestions for choosing a surgical approach.
SVAs are uncommon in western countries, and the incidence has been reported to be 5 times greater among Asians than Westerners [5]. SVAs occur more in males than females [6], and the majority of patients are young or middle aged [7], which is consistent with our data. The SVA is pathologically supposed to result from the absence of normal aortic elastic tissue and media in the aortic root [8]. The causes of this change can be either acquired or congenital. Acquired causes include trauma, atherosclerosis, syphilis and Behcet’s disease. However, most ruptured SVAs are congenital [3]. Ruptured SVAs are frequently symptomatic with an acute onset in most patients but few are diagnosed by routine check-ups. The symptoms depend on the degree of shunting and accompanying lesions. In our study, a cardiac murmur could be found in 47.2% of the patients, suggesting that careful physical examination may provide clues for early diagnosis.
According to previous studies and the current study, ruptured SVAs mostly originate from the right coronary sinus and the non-coronary sinus and can rupture into any cardiac chamber but usually protrude into the right ventricle or right atrium [7, 9–11]. Several types of classifications currently exist to describe SVAs such as Sakakibara and Konno [12] and Guo et al. [13] systems. However, these classification methods provide limited guidance for surgical procedures. In our study, we divided ruptured SVAs into 4 groups to summarize the principles of the surgical approach applied, which has a topic of debate in recent years. In addition, this simple classification may provide guidance for the choice of surgical approach.
For simple ruptured SVAs or ruptured SVAs with AR (without a VSD), the approach is predominantly based on the chamber of termination. Our data show that almost all SVAs rupturing into the right atrium can be repaired by a single right atriotomy (Supplementary Material, Fig. S1). Part of the ruptured SVA protruding into the right ventricle could also be exposed by an incision in the right atrium, but in the event of difficulties, pulmonary arteriotomy and/or ventriculotomy is required. Different studies have reported different rates of aortotomy, varying from 43% to 77% [10, 11, 14–17]. In our study, aortotomy was performed for almost all of the ruptured SVAs with AR, and more than half of the simple ruptured SVA patients underwent aortotomy associated with an incision in the involved chamber. Undoubtedly, aortotomy should be performed in clear cases of AR. However, whether to routinely perform aortotomy for simple ruptured SVAs remains a controversial issue. Sarikaya et al. [7] noted that routine aortotomy can provide a good view of the aortic valve to check the pathology of the aneurysm and allow accurate suture placement without fear of damaging the aortic cusps. Murashita et al. [18] also agreed that aortotomy is useful for inspecting the aortic root and valve to avoid distortion of the valve and a residual fistula. However, Jung et al. [19] argued that transaortic repair may cause postoperative AR. For ruptured SVAs with a supracristal VSD, pulmonary arteriotomy and/or right ventriculotomy is necessary because supracristal VSDs can hardly be exposed by a single atriotomy (Supplementary Material, Fig. S2). Although part of a perimembranous VSD can be easily viewed and handled through the tricuspid orifice, an incision in the right atrium is still the first choice for patients with a ruptured SVA accompanied by a perimembranous VSD. Additionally, pulmonary arteriotomy and/or right ventriculotomy is needed in cases with problems related to surgical exposure. A ruptured SVA combined with a VSD and AR is the most complex type, and the approach should provide good exposure of the ventricular septum and aortic valve. The use of multiple incisions for aortotomy, right atriotomy and pulmonary arteriotomy and/or right ventriculotomy is reasonable and appropriate for repairing the defect.
The closure technique is another topic of debate. Currently, direct suturing and patching are the 2 main repair strategies. In previous studies, the rate of patching closure varied, but most rates were greater than 50% [7, 20, 21]. Some researchers believe that small defects can be closed with pledgeted interrupted sutures [22]. However, others worry that direct suturing may lead to a higher recurrence rate and recommend patching for all ruptured SVAs [7, 14, 23]. In our study, the proportion of patching (90.9%) was higher than that of direct suturing (9.1%). Currently, there is no clear, unified consensus on this issue. We look forward to further studies.
Most postoperative complications of SVA repair are not fatal. However, the most notable complication is newly emerging or worsening AR, which may increase the risk of secondary surgery and reduce the survival rate [18]. Some studies have noted that AR at discharge is a risk factor for postoperative AR [18, 24]. Moreover, the results of a study performed by Liu et al. [24] showed that aortic valvuloplasty was a risk factor correlated with the late postoperative worsening of AR. A study by Jung et al. [19] also found that aortic valvuloplasty during the initial operation was a risk factor for postoperative AR, which emphasized the importance of the aortic valve management strategy. In our study, more patients underwent AVR than aortic valvuloplasty (84.8% vs 15.2%). This result may not only be related to the surgeon’s experience in valve repair but also reflect economic considerations. The poor economic condition of patients in our country makes AVR a solid choice for most surgeons to avoid the inherent risk of reoperation in the future.
Limitations
There are some limitations to our study. The sample size of 286 cases is still not sufficient to draw a very reliable conclusion. The follow-up period was also not long, indicating that the results of the survival analysis are not fully generalizable. Additionally, the nature of retrospective studies may add inevitable biases to the results of our study. We look forwards to more large-scale and rigorously designed studies on ruptured SVAs.
CONCLUSION
The surgical repair of ruptured SVAs is effective and safe and has satisfactory early clinical results and long-term outcomes. The surgical approach could be transaortic or through the cardiac chamber involved, but the selected option is based on the involved chamber and accompanying deformities. The vast majority of patients who undergo surgical repair can survive for a long time.
Footnotes
Presented at the 32nd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 18–20 October 2018.
ACKNOWLEDGEMENTS
The authors express their gratitude to all the surgeons, cardiologists and technicians involved in providing the necessary data.
Conflict of interest: none declared.