-
PDF
- Split View
-
Views
-
Cite
Cite
Mario Gaudino, John H Alexander, Faisal G Bakaeen, Karla Ballman, Fabio Barili, Antonio Maria Calafiore, Piroze Davierwala, Steven Goldman, Peter Kappetein, Roberto Lorusso, Darren Mylotte, Domenico Pagano, Marc Ruel, Thomas Schwann, Hisayoshi Suma, David P Taggart, Robert F Tranbaugh, Stephen Fremes, Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial—rationale and study protocol, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 6, December 2017, Pages 1031–1040, https://doi.org/10.1093/ejcts/ezx358
Close - Share Icon Share
The primary hypothesis of the ROMA trial is that in patients undergoing primary isolated non-emergent coronary artery bypass grafting, the use of 2 or more arterial grafts compared with a single arterial graft (SAG) is associated with a reduction in the composite outcome of death from any cause, any stroke, post-discharge myocardial infarction and/or repeat revascularization.
The secondary hypothesis is that in these patients, the use of 2 or more arterial grafts compared with a SAG is associated with improved survival. The ROMA trial is a prospective, unblinded, randomized event-driven multicentre trial comprising at least 4300 subjects. Patients younger than 70 years with left main and/or multivessel disease will be randomized to a SAG or multiple arterial grafts to the left coronary system in a 1:1 fashion. Permuted block randomization stratified by the centre and the type of second arterial graft will be used. The primary outcome will be a composite of death from any cause, any stroke, post-discharge myocardial infarction and/or repeat revascularization. The secondary outcome will be all-cause mortality. The primary safety outcome will be a composite of death from any cause, any stroke and any myocardial infarction. In all patients, 1 internal thoracic artery will be anastomosed to the left anterior descending coronary artery. For patients randomized to the SAG group, saphenous vein grafts will be used for all non-left anterior descending target vessels. For patients randomized to the multiple arterial graft group, the main target vessel of the lateral wall will be grafted with either a radial artery or a second internal thoracic artery. Additional grafts for the multiple arterial graft group can be saphenous veins or supplemental arterial conduits. To detect a 20% relative reduction in the primary outcome, with 90% power at 5% alpha and assuming a time-to-event analysis, the sample size must include 845 events (and 3650 patients). To detect a 20% relative reduction in the secondary outcome, with 80% power at 5% alpha, the sample size must include 631 events (and 3650 patients). To be conservative, the sample size will be set at 4300 patients. The primary outcome will be tested according to the intention-to-treat principle. The primary analysis will be a Cox proportional hazards regression model, with the treatment arm included as a covariate. If non-proportional hazards are observed, alternatives to Cox proportional hazards regression will be explored.
INTRODUCTION
In the 1980s, it was recognized that long-term survival was enhanced in patients undergoing coronary surgery when the left anterior descending coronary artery (LAD) was grafted with a left internal thoracic artery (ITA) rather than a saphenous vein [1]. This difference was predicated, at least in part, due to greater and more durable patency of the left ITA compared with an increased early occlusion rate and later progressive atherosclerosis of saphenous vein grafts (SVGs) [2].
For more than 20 years, it has generally been accepted that patients who receive multiple arterial grafts (MAGs) at the time of coronary artery bypass grafting (CABG) have increased postoperative survival compared with those who receive only 1 arterial graft (AG), especially over the long term [3–5]. The current US and European guidelines encourage the use of AGs in patients with a long life expectancy [6, 7]. Last year, a position paper from the Society of Thoracic Surgeons (STS) strongly recommended a wider use of AGs [8].
The putative mechanism underlying the AG hypothesis is greater patency. In line with the original findings of improved LAD graft patency with ITA versus SVG, data from randomized control trials (RCTs) as well as observational studies and a network meta-analysis [9] have demonstrated that the patency of the radial artery (RA), as well as the right ITA, exceed that of an SVG, providing mechanistic basis to support the AG hypothesis (Table 1).
Major randomized trials comparing the patency of arterial and venous conduits
| Trial . | Year . | Number of patients . | Conduits compared . | Mean follow-up (years) . | Main findings . |
|---|---|---|---|---|---|
| VA | 2011 | 733 | RA, SVG | 1 | No difference in patency |
| RSVP | 2008 | 142 | RA, SVG | 5 | Better patency for the RA (P = 0.004) |
| RAPS | 2012 | 561 | RA, SVG | 7.7 | Better patency for the RA (P = 0.002) |
| RAPCO | 2013 | 619 | RA, SVG, RITA | 10 | Better patency for the RA versus the SVG (P = 0.039), no difference in patency between RA and RITA (P = 0.19) |
| Trial . | Year . | Number of patients . | Conduits compared . | Mean follow-up (years) . | Main findings . |
|---|---|---|---|---|---|
| VA | 2011 | 733 | RA, SVG | 1 | No difference in patency |
| RSVP | 2008 | 142 | RA, SVG | 5 | Better patency for the RA (P = 0.004) |
| RAPS | 2012 | 561 | RA, SVG | 7.7 | Better patency for the RA (P = 0.002) |
| RAPCO | 2013 | 619 | RA, SVG, RITA | 10 | Better patency for the RA versus the SVG (P = 0.039), no difference in patency between RA and RITA (P = 0.19) |
RA: radial artery; RAPCO: Radial Artery Patency and Clinical Outcomes study [interim results and final results were presented at the 2016 AATS Annual Meeting (results unpublished to date), RA patency better than SVG (P = 0.03) and free RITA (P = 0.06)]; RAPS: Radial Artery Patency Study; RITA: right internal thoracic artery; RSVP: Radial Artery versus Saphenous Vein Patency trial; SVG: saphenous vein graft; VA: Veteran’s Administration.
Major randomized trials comparing the patency of arterial and venous conduits
| Trial . | Year . | Number of patients . | Conduits compared . | Mean follow-up (years) . | Main findings . |
|---|---|---|---|---|---|
| VA | 2011 | 733 | RA, SVG | 1 | No difference in patency |
| RSVP | 2008 | 142 | RA, SVG | 5 | Better patency for the RA (P = 0.004) |
| RAPS | 2012 | 561 | RA, SVG | 7.7 | Better patency for the RA (P = 0.002) |
| RAPCO | 2013 | 619 | RA, SVG, RITA | 10 | Better patency for the RA versus the SVG (P = 0.039), no difference in patency between RA and RITA (P = 0.19) |
| Trial . | Year . | Number of patients . | Conduits compared . | Mean follow-up (years) . | Main findings . |
|---|---|---|---|---|---|
| VA | 2011 | 733 | RA, SVG | 1 | No difference in patency |
| RSVP | 2008 | 142 | RA, SVG | 5 | Better patency for the RA (P = 0.004) |
| RAPS | 2012 | 561 | RA, SVG | 7.7 | Better patency for the RA (P = 0.002) |
| RAPCO | 2013 | 619 | RA, SVG, RITA | 10 | Better patency for the RA versus the SVG (P = 0.039), no difference in patency between RA and RITA (P = 0.19) |
RA: radial artery; RAPCO: Radial Artery Patency and Clinical Outcomes study [interim results and final results were presented at the 2016 AATS Annual Meeting (results unpublished to date), RA patency better than SVG (P = 0.03) and free RITA (P = 0.06)]; RAPS: Radial Artery Patency Study; RITA: right internal thoracic artery; RSVP: Radial Artery versus Saphenous Vein Patency trial; SVG: saphenous vein graft; VA: Veteran’s Administration.
The evidence on the survival benefits of AGs may appear compelling but is based almost exclusively on observational studies.
In the most recent meta-analysis of observational series comparing bilateral internal thoracic arteries (BITAs) and single ITA, Yi et al. [5] pooled data from 9 studies including over 15 000 patients. They reported a statistically significant 21% lower long-term mortality for patients receiving BITA [hazard ratio (HR) 0.79, 95% confidence interval (CI) 0.75–0.84] [5].
In contrast to this large amount of evidence, to date, only 2 large (>100 patients) RCTs comparing BITA and ITA have been published. A survival advantage with BITA grafting was not evident in either of these studies.
The Stand-in-Y Mammary study compared the early outcomes of a cohort of 850 patients randomized to 4 different surgical groups: BITA using 2 different configurations, ITA and RA or ITA and SVG [10]. At a mean follow-up of only 24.1 ± 9.8 months, no difference in survival was found between the groups (odds ratio 0.63, CI 0.27–1.47; P = 0.62).
The Arterial Revascularization Trial (ART) randomized 3102 patients to receive BITA (n = 1548) or a single ITA (n = 1554) [11]. The primary end-point was overall survival, and the study had adequate statistical power to demonstrate a 20% reduction in the primary end-point at 10 years. At the planned 5-year interim analysis, no difference in survival was found between groups [91.3% in the BITA group and 91.6% in the ITA group (HR 1.04, CI 0.81–1.32)].
There are several possible explanations for the negative results so far in ART. The sample size calculation for ART was mostly based on data from the 1980s and the 1990s, when the postoperative event rate was likely higher than in the more recent era. As ART is not an event-driven trial, the possibility of an underpowered sample size cannot be excluded. Also, it must be noted that in ART, a sizeable proportion (23%) of patients randomized to the single ITA arm also received an additional AG, the RA. Consequently, in almost a quarter of the patients, the ART compared BITA with ITA and RA instead of BITA with ITA and vein grafts.
Additional factors that might explain the lack of difference in this trial include the high rate of crossover (16.4% in the BITA series), the fact that the attrition rate of saphenous grafts increases almost exponentially after the 4th postoperative year and the uncommonly high compliance with optimal medical therapy (90% of patients on aspirin, beta-blockers and statins).
It is likely that the primary end-point will be reported in 2018; however, it seems highly improbable that differences will emerge with additional follow-up, as the survival (and event-free survival) curves seem superimposable so far.
There is also a discrepancy between the observational studies and RCTs in terms of late survival for the RA. The published matched observational studies comparing the clinical outcomes of CABG patients who received the RA or the SVG as the second conduit and including more than 1000 patients are summarized in Table 2. All of them are concordant in showing a moderate survival benefit for the RA.
Matched observational studies comparing the RA and the SVG as second conduit
| First author . | Year . | Number of patients . | Follow-up (years) . | Results . |
|---|---|---|---|---|
| Cohen | 2001 | 1434 | 3 | RA vs SVG late mortality: RR 0.60, CI 0.37–0.93; P = 0.02 |
| Zacharias | 2004 | 3161 | 6 | RA vs SVG mortality: RR 0.67, CI 0.984–0.462; P = 0.040 |
| Tranbaugh | 2010 | 4271 | 12 | RA vs SVG mortality: HR 0.71, CI 0.557–0.918; P = 0.0084 |
| Locker | 2013 | 8622 | 15 | RA vs SVG mortality: HR 0.56, CI 0.36–0.79; P < 0.001 |
| Schwann | 2015 | 11 261 | 15 | RA vs SVG mortality (for LVEF categories): EF > 50%: HR 0.51, CI 0.45–0.58; EF 50–36%: HR 0.48, CI 0.43–0.54 and EF < 35%: HR 0.56, CI 0.48–0.65; P < 0.001 for all |
| Shi | 2016 | 4006 | 15 | RA vs SVG mortality: HR 0.79, CI 0.71–0.89 |
| First author . | Year . | Number of patients . | Follow-up (years) . | Results . |
|---|---|---|---|---|
| Cohen | 2001 | 1434 | 3 | RA vs SVG late mortality: RR 0.60, CI 0.37–0.93; P = 0.02 |
| Zacharias | 2004 | 3161 | 6 | RA vs SVG mortality: RR 0.67, CI 0.984–0.462; P = 0.040 |
| Tranbaugh | 2010 | 4271 | 12 | RA vs SVG mortality: HR 0.71, CI 0.557–0.918; P = 0.0084 |
| Locker | 2013 | 8622 | 15 | RA vs SVG mortality: HR 0.56, CI 0.36–0.79; P < 0.001 |
| Schwann | 2015 | 11 261 | 15 | RA vs SVG mortality (for LVEF categories): EF > 50%: HR 0.51, CI 0.45–0.58; EF 50–36%: HR 0.48, CI 0.43–0.54 and EF < 35%: HR 0.56, CI 0.48–0.65; P < 0.001 for all |
| Shi | 2016 | 4006 | 15 | RA vs SVG mortality: HR 0.79, CI 0.71–0.89 |
CI: confidence interval; EF: ejection fraction; HR: hazard ratio; LVEF: left ventricular ejection fraction; RA: radial artery; SVG: saphenous vein graft.
Matched observational studies comparing the RA and the SVG as second conduit
| First author . | Year . | Number of patients . | Follow-up (years) . | Results . |
|---|---|---|---|---|
| Cohen | 2001 | 1434 | 3 | RA vs SVG late mortality: RR 0.60, CI 0.37–0.93; P = 0.02 |
| Zacharias | 2004 | 3161 | 6 | RA vs SVG mortality: RR 0.67, CI 0.984–0.462; P = 0.040 |
| Tranbaugh | 2010 | 4271 | 12 | RA vs SVG mortality: HR 0.71, CI 0.557–0.918; P = 0.0084 |
| Locker | 2013 | 8622 | 15 | RA vs SVG mortality: HR 0.56, CI 0.36–0.79; P < 0.001 |
| Schwann | 2015 | 11 261 | 15 | RA vs SVG mortality (for LVEF categories): EF > 50%: HR 0.51, CI 0.45–0.58; EF 50–36%: HR 0.48, CI 0.43–0.54 and EF < 35%: HR 0.56, CI 0.48–0.65; P < 0.001 for all |
| Shi | 2016 | 4006 | 15 | RA vs SVG mortality: HR 0.79, CI 0.71–0.89 |
| First author . | Year . | Number of patients . | Follow-up (years) . | Results . |
|---|---|---|---|---|
| Cohen | 2001 | 1434 | 3 | RA vs SVG late mortality: RR 0.60, CI 0.37–0.93; P = 0.02 |
| Zacharias | 2004 | 3161 | 6 | RA vs SVG mortality: RR 0.67, CI 0.984–0.462; P = 0.040 |
| Tranbaugh | 2010 | 4271 | 12 | RA vs SVG mortality: HR 0.71, CI 0.557–0.918; P = 0.0084 |
| Locker | 2013 | 8622 | 15 | RA vs SVG mortality: HR 0.56, CI 0.36–0.79; P < 0.001 |
| Schwann | 2015 | 11 261 | 15 | RA vs SVG mortality (for LVEF categories): EF > 50%: HR 0.51, CI 0.45–0.58; EF 50–36%: HR 0.48, CI 0.43–0.54 and EF < 35%: HR 0.56, CI 0.48–0.65; P < 0.001 for all |
| Shi | 2016 | 4006 | 15 | RA vs SVG mortality: HR 0.79, CI 0.71–0.89 |
CI: confidence interval; EF: ejection fraction; HR: hazard ratio; LVEF: left ventricular ejection fraction; RA: radial artery; SVG: saphenous vein graft.
The 7 RCTs, which to date have compared the RA with the SVG, had primary angiographic outcomes and were individually underpowered to detect moderate differences in mortality. A meta-analysis of 6 of them including 1860 patients, however, showed no difference in survival between the RA and the SVG [12].
A possible explanation for the differential results between the RCTs and the observational series is that the observational studies suffer from intrinsic selection biases in favour of the AGs that no matching system can eliminate.
The contradiction between the observational and randomized evidence questions the clinical effect of the use of MAGs and the current guidelines of various societies.
There are 2 other important considerations:
Overall mortality may be the wrong primary outcome for studies investigating the clinical effect of the second conduit in CABG patients. Survival following CABG is primarily determined by the status of the LAD and that grafts to non-LAD vessels are more likely to affect other non-fatal cardiac end-points (myocardial infarction, angina recurrence and need for revascularization) but not overall survival [13–15]. Of note, in the Stand-in-Y trial, cardiac event-free survival (including cardiac death, myocardial infarction, recurrent angina, graft failure, redo CABG or percutaneous coronary intervention) was significantly better in patients receiving 2 AGs [10].
Recent evidence suggests that the use of a third AG is associated with an additional survival benefit compared with the use of 2 AGs. Last year, 2 independent meta-analyses evaluated the effect of the addition of a third arterial graft on outcome in CABG patients. In a meta-analysis of 10 287 propensity-matched patients receiving 2 vs 3 arterial conduits, the use of a third arterial graft was not associated with an increase in the operative risk; in fact, a 24% relative survival benefit was evident at a mean follow-up period of 77.9 months [16]. In a meta-analysis of the observational studies comparing total arterial revascularization with CABG with 1 or 2 AGs (130 305 patients), Yanagawa et al. [17] found that total arterial grafting was associated with a 15% relative increase in survival at a mean follow-up period ranging from 1 to 14 years. In the great majority, the MAGs used were the 2 ITAs and the RA. The right gastroepiploic artery (RGEA) was used in a minority of the studies.
Finally, it is worth noting that the use of MAGs has the potential to increase postoperative morbidity and, in particular, surgical site complications. The use of bilateral ITAs is a known risk factor for postoperative sternal complications, especially in some categories of patients (diabetics and the obese). In the ART, the incidence of sternal wound reconstruction was 0.6% in the single ITA group and 1.9% in the BITA group (P = 0.002) [11]. A meta-analysis of observational studies, including 173 000 patients, reported that deep sternal wound infections were 38% higher when a second ITA was used (1.6% vs 2.05%; RR 1.38, CI 1.29–1.45) [21]. The risk of sternal complications can be significantly reduced using a skeletonized ITA harvesting technique [22].
The use of the RA does not seem to increase the risk of surgical site complications. In a subanalysis of an RCT comparing the RA and the saphenous vein as the second conduit, the incidence of surgical site infections and the incision-related pain at 1 year of follow-up was similar for the 2 conduits [23]. Observational studies have reported high patient satisfaction and less scar discomfort using the RA instead of the saphenous vein for CABG [24].
It is worth noting that the use of AGs remains limited all around the world. Currently, in the USA, less than 6% of CABG patients receive more than 1 AG [25]. In a 2009 report from the Australasian Society of Cardiac Surgery, the rate of bilateral mammary artery use is slightly above 12% [26], and similar data are quoted in Europe. A possible explanation for the slow diffusion of the use of AGs is the lack of solid evidence on its clinical benefits.
The immense clinical, social and economic implications of CABG are clear. There remain important unanswered questions: (i) whether 2 or more AGs are associated with a reduction in major adverse cardiac and cerebral events and improved overall survival compared with a single arterial graft (SAG) and (ii) whether the right ITA or RA is the preferred second arterial conduit. The ROMA trial is an international randomized event-driven clinical trial comprising 4300 patients, with sufficient power and duration, to address these important clinical questions.
STUDY DESIGN
The ROMA trial is a 2-arm event-driven, international multicentre randomized clinical trial aimed at evaluating the impact of the use of 1 ITA versus 2 or more AGs for CABG on a composite outcome of death from any cause, any stroke, postdischarge myocardial infarction and/or repeat revascularization. Patients will be randomized to a SAG or MAGs. Investigators and patients will not be blinded but end-point assessors will be blinded to treatment allocation (PROBE). The trial is powered to detect a 20% relative reduction in the primary outcome with 90% power at 5% alpha.
The sample size is also sufficient to detect a 20% relative difference with 80% power at 5% alpha in overall survival, which addresses the secondary hypothesis.
PILOT PHASE
In the initial pilot phase, the study will enrol 10% of the sample size (430 patients) in 25 core centres.
Hypothesis for the pilot phase
The pilot phase is aimed at establishing the feasibility of the project, the adherence to the protocol and the enrolment rate.
Objective of the pilot phase
The main objective of the pilot phase is to enrol 430 patients in 5 months at the 25 core centres, with a predicted enrolment rate of approximately 1 patient/centre/week. This pilot phase will be carried out without external funding and using the research infrastructure of the participating centres. The results of the pilot phase will be used to apply for grants from health care agencies in the USA, Canada and Europe asking for support to expand the network of participating centres and to complete the follow-up. A consent rate of >50% of eligible patients, an enrolment rate 80% of the predicted, a rate of compliance with the protocol >90% and a loss of follow-up of less than 1% will be considered as a demonstration of feasibility.
The patients enrolled in the pilot phase will be included in the main trial analysis. If substantive changes to the protocol are required based on the pilot, inclusion of these pilot patients may be reconsidered by the Steering Committee.
MAIN TRIAL
Hypotheses
The primary hypothesis of the ROMA trial is that in patients undergoing primary isolated non-emergent CABG, the use of 2 or more arterial grafts compared with a SAG is associated with a reduction in the composite outcome of death from any cause, any stroke, post-discharge myocardial infarction and/or repeat revascularization.
The secondary hypothesis is that in patients undergoing primary isolated non-emergent CABG, the use of 2 or more arterial grafts compared with a SAG is associated with improved survival.
Objectives
The primary aim is to conduct a multicentre international RCT to test the hypothesis that the use of 2 or more AGs compared with a SAG is associated with a reduction in the composite outcome of death from any cause, any stroke, postdischarge myocardial infarction and/or repeat revascularization.
The secondary aim is to conduct a multicentre international RCT to test the hypothesis that the use of 2 or more AGs compared with a SAG is associated with improved survival.
Eligibility
Inclusion criteria
Primary isolated CABG patients with disease of the left main coronary artery or of the left anterior descending and the circumflex coronary system with or without disease of the right coronary artery. Coronary artery disease will be defined as a stenosis ≥70% based on coronary angiography, an FFR value ≤0.80 or iFr value ≤0.89, a left main diameter stenosis ≥50%, left main IVUS MLA value ≤4.5 mm2 or equivalent OCT measurements will also be considered.
Exclusion criteria
Age >70 years
Planned single graft
Emergency operation
Preoperative myocardial infarction within 48 h
Ejection fraction <35%
Any concomitant cardiac or non-cardiac procedure
Previous cardiac operation
Preoperative severe end-organ dysfunction (dialysis, liver failure and respiratory failure), cancer or any comorbidity that reduces life expectancy to less than 5 years.
Inability to use either the saphenous vein or both the right ITA and the RA as grafts
Anticipated need for coronary thromboendarterectomy.
Planned hybrid revascularization
Special considerations regarding age and ejection fraction limits
These conservative limits were adopted to select a patient population, where the effect of the intervention could be maximized. In the case of a slow recruitment rate, these limits will be reviewed by the Steering Committee.
Randomization and enrolment
All patients scheduled for CABG at the study centres will be screened for inclusion. Eligible patients who provide informed consent can be enrolled. Each centre will keep a log of all screened patients with details on inclusion or reasons for exclusion.
Randomization will be performed through a web-based randomization system. A confirmation email with the details of the randomization will be sent to the contact investigators of the single centres, the lead principal investigators and the Data Monitoring Office at randomization.
Patients will be randomized in an 1:1 fashion between the 2 groups. Permuted block randomization with randomly defined blocks stratified by the centre and the type of planned second arterial graft will be used to provide treatment distribution in equal proportion.
Surgery should take place within 2 weeks from randomization to reduce the possibility of events occurring after randomization and before surgery.
Surgical procedures
In all patients, 1 ITA will be anastomosed to the LAD. For patients randomized to the SAG group, SVG grafts will be used for all non-LAD target vessels. For patients randomized to the MAG group, the second ITA or the RA will be used to graft the main target vessel of the lateral wall. Identification of the second ITA or RA target will be based on coronary angiography and will be left to the judgement of the operating surgeon. The choice between the second ITA and RA will be decided by the individual surgeon. The use of RAs previously submitted to catheterization for diagnostic or interventional procedures is strongly discouraged. For the use of RA grafts, a high-grade stenosis of the coronary target is highly recommended. A moderate stenosis is sufficient for the right ITA. The use of supplementary AGs will be allowed in the MAG group. For the right coronary targets, a high-grade target vessel stenosis is recommended for both the RA and the RITA. The use of the RGEA will be allowed only if the operating surgeon has a personal experience of at least 250 cases using the RGEA. It is recommended that the RGEA be used to graft vessels of the inferior wall with >90% stenosis and is harvested in a skeletonized fashion.
Surgical revascularization will be performed with the current standard technique in use at every single centre. The choice of anaesthetic technique, harvesting technique, vasodilatory protocol, graft configuration (insitu or Y/T), on or off pump, sequential grafting and myocardial protection will be left to the individual centres. For all SVG grafts proximal aortic anastomosis and conventional harvesting technique will be required.
The intraoperative assessment of graft patency using transit time flowmeter is a Class IIA recommendation, level of evidence C of the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) [7]. The assessment of graft patency is not mandated as part of the protocol, but it is recommended.
Postoperative assessment of graft patency will be performed in those centres where this is the standard of care, using the method routinely used in those centres. The criteria used for the definition of graft status are summarized in the Appendix. Results of the patency studies when available will be entered in the case report form.
Details of secondary prevention will be left at the discretion of the individual centre. However, the use of evidence-based medication, including aspirin, statins, beta-blockers and angiotensin-converting enzyme inhibitors, is strongly recommended. The use of dual antiplatelet therapy is recommended for 3 months after off-pump procedures, coronary endarterectomy or angioplasty and for 1 year in acute coronary syndromes.
All data will be recorded in the case report form and prospectively entered in the dedicated online electronic database.
Outcome measures
Primary outcome
The primary outcome will be a composite of death from any cause, any stroke, postdischarge myocardial infarction and/or repeat revascularization.
Secondary outcome
The secondary outcome will be all-cause mortality.
Additional secondary outcomes will be as follows:
Thirty-day mortality
Major postoperative complications (revision for bleeding, perioperative myocardial infarction, any stroke, need for dialysis, need for tracheostomy and surgical site complications)
Sternal wound complications
A composite of death from any cause, postdischarge myocardial infarction and/or repeat revascularization (i.e. primary outcome without stroke)
A composite of death from any cause, any stroke, any myocardial infarction and/or repeat revascularization (i.e. primary outcome with perioperative myocardial infarction)
Cause-specific death (cardiac versus non-cardiac)
Hospital readmission for congestive heart failure
Safety
The primary safety outcome will be a composite of death from any cause, any stroke and any myocardial infarction. Secondary safety outcomes will be major postoperative complications (revision for bleeding, perioperative myocardial infarction, any stroke, need for dialysis, need for tracheostomy and sternal wound complications).
Definitions of outcomes are given in the Appendix.
All primary and secondary outcome measures will be assessed and recorded at designated time intervals by research personnel at each individual centre. Data will be prospectively entered in the dedicated online electronic database.
Data regarding every event will be reviewed and adjudicated centrally by a blinded Event Review Committee.
Follow-up
Patients will be seen in clinic 6–12 weeks postoperatively as per individual institutional routine. Subsequent follow-up will be performed at 6 months postoperatively and every 6 months thereafter by telephone. At the end of the enrolment phase, the possibility of establishing centralized national centres for follow-up will be considered.
Details on current medications, clinical status, clinical events, rehospitalization and revascularization will be recorded. For patients who have been or are hospitalized, hospital records and/or a death certificate will be acquired if possible. Follow-up case report forms will be completed; additional specific study forms will be completed for patients who experience one or more study events.
Crossover/protocol violation and loss to follow-up
A 5% crossover/protocol violation and a 3% loss of follow-up are anticipated for the primary outcome.
Sample size calculation
The ROMA trial is an event-driven trial.
Sample size calculations have been performed for the primary outcome, a composite of death from any cause, any stroke, postdischarge myocardial infarction and/or repeat revascularization, and for the principal secondary outcome, all-cause mortality.
The sample size calculation for the primary outcome has been performed on the basis of the data derived from the surgical arms of the FREEDOM, EXCEL, NOBLE and SYNTAX trials and the ART, CORONARY and ROOBY trials (see Table 3).
Incidence of outcomes in the major contemporary RCTs including CABG patients
| Study . | Follow-up (years) . | Primary composite outcome . | Perioperative MI . | Incidence of primary outcome . | Death (%) . | MI (%) . | Stroke (%) . | Repeat revascularization (%) . | 1-Year mortality (%) . |
|---|---|---|---|---|---|---|---|---|---|
| ART | 5 | D + MI + S | Yes | 12.5 | 8.6 | 3.5 | 2.9 | 2.4 | |
| Coronary | 5 | D + MI + S + RR + renal failurea | Yes | 23.4 | 14.1 | 7.9 | 2.6 | 2.6 | 5.1 |
| Excel | 3 | D + MI + S | Yes | 14.7 | 5.9 | 8.3 | 2.9 | 1.1 | |
| Freedom | 5 | D + MI + S | Yes | 18.7 | 10.9 | 6 | 5.2 | 4.2 | |
| Noble | 5 | D + MI + S + RR | No | 19 | 9 | 2 | 2 | 10 | 3.0 |
| Rooby | 1 | D + MI + RR | No | 7.4 (ONCAB) | 3.5 | 2.1 | 4 | 3.5 | |
| PREVENT IV | 5 | D + MI + RR | Yes | 25.5 | 10.7 | 3.2 | 13.9 | 2.9 | |
| SYNTAX | 5 | D + MI + S + RR | Yes | 26.9 | 11.4 | 3.8 | 3.7 | 13.7 | 3.5 |
| Study . | Follow-up (years) . | Primary composite outcome . | Perioperative MI . | Incidence of primary outcome . | Death (%) . | MI (%) . | Stroke (%) . | Repeat revascularization (%) . | 1-Year mortality (%) . |
|---|---|---|---|---|---|---|---|---|---|
| ART | 5 | D + MI + S | Yes | 12.5 | 8.6 | 3.5 | 2.9 | 2.4 | |
| Coronary | 5 | D + MI + S + RR + renal failurea | Yes | 23.4 | 14.1 | 7.9 | 2.6 | 2.6 | 5.1 |
| Excel | 3 | D + MI + S | Yes | 14.7 | 5.9 | 8.3 | 2.9 | 1.1 | |
| Freedom | 5 | D + MI + S | Yes | 18.7 | 10.9 | 6 | 5.2 | 4.2 | |
| Noble | 5 | D + MI + S + RR | No | 19 | 9 | 2 | 2 | 10 | 3.0 |
| Rooby | 1 | D + MI + RR | No | 7.4 (ONCAB) | 3.5 | 2.1 | 4 | 3.5 | |
| PREVENT IV | 5 | D + MI + RR | Yes | 25.5 | 10.7 | 3.2 | 13.9 | 2.9 | |
| SYNTAX | 5 | D + MI + S + RR | Yes | 26.9 | 11.4 | 3.8 | 3.7 | 13.7 | 3.5 |
Renal failure: 1.8%.
CABG: coronary artery bypass grafting; D: death; MI: myocardial infarction; RCT: randomized controlled trial; RR: repeat revascularization; S: stroke.
Incidence of outcomes in the major contemporary RCTs including CABG patients
| Study . | Follow-up (years) . | Primary composite outcome . | Perioperative MI . | Incidence of primary outcome . | Death (%) . | MI (%) . | Stroke (%) . | Repeat revascularization (%) . | 1-Year mortality (%) . |
|---|---|---|---|---|---|---|---|---|---|
| ART | 5 | D + MI + S | Yes | 12.5 | 8.6 | 3.5 | 2.9 | 2.4 | |
| Coronary | 5 | D + MI + S + RR + renal failurea | Yes | 23.4 | 14.1 | 7.9 | 2.6 | 2.6 | 5.1 |
| Excel | 3 | D + MI + S | Yes | 14.7 | 5.9 | 8.3 | 2.9 | 1.1 | |
| Freedom | 5 | D + MI + S | Yes | 18.7 | 10.9 | 6 | 5.2 | 4.2 | |
| Noble | 5 | D + MI + S + RR | No | 19 | 9 | 2 | 2 | 10 | 3.0 |
| Rooby | 1 | D + MI + RR | No | 7.4 (ONCAB) | 3.5 | 2.1 | 4 | 3.5 | |
| PREVENT IV | 5 | D + MI + RR | Yes | 25.5 | 10.7 | 3.2 | 13.9 | 2.9 | |
| SYNTAX | 5 | D + MI + S + RR | Yes | 26.9 | 11.4 | 3.8 | 3.7 | 13.7 | 3.5 |
| Study . | Follow-up (years) . | Primary composite outcome . | Perioperative MI . | Incidence of primary outcome . | Death (%) . | MI (%) . | Stroke (%) . | Repeat revascularization (%) . | 1-Year mortality (%) . |
|---|---|---|---|---|---|---|---|---|---|
| ART | 5 | D + MI + S | Yes | 12.5 | 8.6 | 3.5 | 2.9 | 2.4 | |
| Coronary | 5 | D + MI + S + RR + renal failurea | Yes | 23.4 | 14.1 | 7.9 | 2.6 | 2.6 | 5.1 |
| Excel | 3 | D + MI + S | Yes | 14.7 | 5.9 | 8.3 | 2.9 | 1.1 | |
| Freedom | 5 | D + MI + S | Yes | 18.7 | 10.9 | 6 | 5.2 | 4.2 | |
| Noble | 5 | D + MI + S + RR | No | 19 | 9 | 2 | 2 | 10 | 3.0 |
| Rooby | 1 | D + MI + RR | No | 7.4 (ONCAB) | 3.5 | 2.1 | 4 | 3.5 | |
| PREVENT IV | 5 | D + MI + RR | Yes | 25.5 | 10.7 | 3.2 | 13.9 | 2.9 | |
| SYNTAX | 5 | D + MI + S + RR | Yes | 26.9 | 11.4 | 3.8 | 3.7 | 13.7 | 3.5 |
Renal failure: 1.8%.
CABG: coronary artery bypass grafting; D: death; MI: myocardial infarction; RCT: randomized controlled trial; RR: repeat revascularization; S: stroke.
We consider a 20% relative risk reduction in the composite primary outcome variable to be the minimal clinically important difference that would influence a change in surgeons’ behaviour and in line with the observed results in observational studies. A 17% composite event rate at 5 years for the control arm is consistent with the event rates in Table 3. To detect a 20% relative reduction (from 17% to 14.2%) in the primary outcome, with 90% power at 5% 2-sided alpha and assuming a time-to-event analysis, the sample size must include 3650 patients or 845 events.
There are no contemporary RCT data on the long-term mortality of similar CABG patients, so no direct inferences are possible. However, based on the 1- and 5-year data of the published RCTs, a linearized rate of death of 1.5–2% between Year 1 and 5 was observed, and we estimate that the 5-year mortality in the ROMA trial will be 8–10%. Because of the known increase in graft attrition rate after the 5th postoperative year, we expect to see a linearized rate of death of 2% between 5 and 10 years postoperatively and estimate that the overall 10-year mortality will be 18–20% [27].
We consider a 20% relative risk reduction in mortality to be clinically meaningful and sufficient to change practice. To detect a 20% relative reduction (from 18% to 14.4%) in 10-year mortality, with 80% power at 5% alpha, the sample size must include 3650 patients or 631 events.
Considering 5% crossover/protocol violation and up to 10% loss to follow-up, 4198 patients are required. To be conservative, the sample size can be set at 4300 patients.
The aim of the trial is to enrol at least 4300 patients in at least 25 centres in the USA, Canada, Europe and Asia. Assuming an enrolment rate of 1 patient/centre/week, 3.5 years will be necessary to complete the enrolment phase. In case of funding, we assume to have about 50 centres for the main trial, so that the duration of the enrolment phase will be shorter or unchanged even in case of a lower enrolment rate.
Planned subgroup analysis
RITA versus RA
2 versus >2 arterial grafts
Diabetic versus non-diabetic
Male versus female
Obese versus not obese
Stratification by race
On pump versus off pump
Composite versus in situ grafts
Stratification by ejection fraction
Open versus occluded grafts
Complete versus incomplete revascularization
Region
Operator experience
Left main disease versus multiple vessel disease
Anaortic CABG
Statistical analysis
Representativeness of study sample and flow of patients
A CONSORT flow diagram will be presented showing the numbers of patients screened, randomized, received surgery (with details) and followed up.
Baseline comparability of randomized groups
Demographic and clinical characteristics of patients at baseline will be presented by randomized group.
Numbers (with percentages) for binary and categorical variables and means (and standard deviations) or medians (with lower and upper quartiles) for continuous variables will be presented; there will be neither tests of statistical significance nor CIs for differences between randomized groups on any baseline variable.
We will also present data on operative details such as number of grafts, % off pump, sequentials and Y grafts.
Comparison of losses to follow-up
The numbers (with percentages) of losses to follow-up (defaulters and withdrawals) over the period of follow-up will be reported and compared between the intervention groups using the absolute risk difference with 95% CI.
Description of available data
Completeness of outcomes will be recorded at each assessment.
Description of interventions received
We will summarize the interventions performed in each treatment group and describe the characteristics of any patients that crossed over or did not receive surgery.
Analysis of outcomes
The primary outcome will be tested according to the intention-to-treat principle. Modified intention-to-treat analyses will also be conducted. As treated analyses will be reported but considered hypothesis generating or exploratory.
Safety (harms)
In the analysis of safety/harms, patients will be analysed both by intention-to-treat principle and according to the operation received (i.e. per-protocol). The primary safety outcome will be a composite of death from any cause, any stroke and any myocardial infarction. Secondary safety outcomes will be major postoperative complications (revision for bleeding, perioperative myocardial infarction, any stroke, need for dialysis, need for tracheostomy and sternal wound complications). Only patients who received the surgical procedure to which they were randomly allocated will be included in the per-protocol analysis of safety data. All patients who underwent CABG will be included in the safety analysis. Patients randomized to 1 surgical procedure but who received the alternative (for whatever reason), those who received no surgery (e.g. death, patient withdrawal and ineligibility at the time of surgery) and those who received another surgical procedure will be excluded. If the difference in the safety outcomes exceeds 10%, the trial accrual will be suspended so the study team can evaluate safety. The study team will report to and consult with the trial Steering Committee and the Data and Safety Monitoring Board (DSMB) prior to making a final decision, which would be one of the following [1]: reopen the trial with no changes [2], reopen the trial with a modified protocol or [3] permanently stop accrual to the trial.
Analysis of primary aims
After assessing normality, intraoperative and early postoperative variables will be analysed using the χ2 and the Student’s t-test as appropriate. In the case of non-normal distribution, non-parametric tests will be used.
Primary outcome
The primary analysis for the primary outcome, a composite of death from any cause, any stroke, postdischarge myocardial infarction and/or repeat revascularization will be completed after 845 events. The analysis for the principal secondary outcome, all-cause mortality, will be completed after 631 events.
The analysis time, in the case of patients who have any of the events included in the primary outcome, will be the time from randomization to the occurrence of the first event. For patients with whom a status is not known, the time from randomization until they were last recorded will be calculated.
Data will be graphically displayed using the Kaplan–Meier plots, comparing the curves using a log-rank test. The primary analysis will be a Cox proportional hazards regression model, with treatment arm included as a covariate. The estimated HR for the 2 groups will be reported, with a 95% CI and the associated P-value.
If non-proportional hazards are observed, alternatives to Cox proportional hazards regression will be explored. These would include ‘restricted mean survival time’ analysis [28].
This analysis will make no adjustment to account for clustering of patients within surgeons.
Analysis of the primary outcome will include estimates of effect and 95% CI. A P-value of 0.05 will be deemed to indicate ‘statistical significance’. In the analysis of secondary outcomes, 95% CIs will be constructed around point estimates.
As a sensitivity analysis, the same analysis will be carried out but will include a number of potentially prognostic baseline covariates in the Cox model. These will be as follows: age, gender, diabetes status, ejection fraction, extent of coronary disease (2 vessels, 3 vessels and left main), on- or off-pump procedure, surgical priority, completeness of revascularization and participating centre, which will be included as a frailty term (gamma distribution).
Each item of the composite will also be analysed individually and results reported.
These analyses will account for the competing risk of death using cumulative incidence statistics.
Subgroup analysis
To test for a differential effect of randomized treatment across subgroups, an interaction term (treatment group by subgroup) will be fitted in the Cox proportional hazards model. If non-proportional hazards are observed, alternatives to Cox proportional hazards regression will be explored. These would include ‘restricted mean survival time’ analysis.
Subgroup analyses will be conducted only with respect to the primary outcome. Treatment effect in each subgroup shall be presented along with the P-value for the interaction term in the model.
TRIAL ORGANIZATION
The study will be co-ordinated by the principal investigators and the Weill Cornell Medicine (WCM) Joint Clinical Trial Office.
TRIAL STEERING COMMITTEE
The Committee will design the trial as well as monitor and supervise its progress. The members of the committee are listed in Supplementary Material, Table S1. The Committee will include 3 independent members whose institutions do not actively participate in the trials: Fabio Barili, cardiac surgeon and statistician; Professor Hisayoshi Suma, cardiac surgeon and Professor Steve Goldman, cardiologist.
DATA MONITORING AND SAFETY COMMITTEE
The WCM Data Monitoring and Safety Committee (DMSC) will monitor the trial. The WCM DMSB is an independent multidisciplinary committee based at WCM and aimed at providing an independent means of data and safety monitoring for clinical trials that involve significant risk to research subjects. The WCM DSMB reviews interim data on a schedule commensurate with the needs of a given protocol to evaluate research subject safety, rates of accrual and efficacy of experimental intervention. After each evaluation, the Board provides the principal investigator with recommendations for protocol modification, continuation or termination.
The DMSC will perform 3 interim analysis reports to specifically address any potential safety issue and report them to the Steering Committee. Three interim efficacy analyses will occur when 25%, 50% and 75% of the enrolment data are available. These analyses will be based on the primary outcome and blinded to the randomization status. The DMSC will employ the modified Haybittle–Peto rule of 4 SDs for analyses in the first half of the study and 3 SDs for all analyses in the second half [29, 30]. To be considered significant, these predefined boundaries will have to be exceeded in at least 2 consecutive analyses, 3 or more months apart. The corresponding critical χ2 values are 16.0 (i.e. α = 0.0001) for the first 2 planned analyses and 12.25 (α = 0.00047) for the third analysis. The α level for the final analysis will remain the conventional α = 0.05, given the infrequent interim analyses, their extremely low α levels and the requirement for confirmation with a subsequent analysis. If 1 or both interventions should surpass the modified Haybittle–Peto rule, then the DMSC will advise the Steering Committee of such a finding and recommend stopping the study. The DMSC in making such a recommendation will also consider the consistency of the secondary end-points and any relevant external data. Given the anticipated timing of enrolment and events, it is highly unlikely that the study will be stopped for efficacy early after 25%, 50% or 75% enrolment, although clearly would be advantageous from a scientific and economic analysis.
It is conceivable that the short-term results will favour the SAG arm—the use of BITA is more invasive. Monitoring this trial, where the short-term results are likely to be evident well before clear long-term results emerge will pose special challenges during the interim monitoring. Specifically, we anticipate that a reduction in early outcomes in the SAG group may occur first and prior to a long-term excess of clinical events that will only be evident with longer follow-up. Therefore, if at any stage, the results clearly favour 1 group in terms of the early data and cross the prespecified statistical monitoring boundaries, the DMSC will also examine the available long-term follow-up data to assess its reliability, its direction and overall clinical implications before making any recommendations to prematurely discontinue recruitment of subjects.
The statistical monitoring boundaries that we have described should not be viewed as absolute rules but rather a guide that should be considered along with both the short- and long-term outcomes, the patterns of data observed, the types of events impacted and the potential impact on both patient safety and clinical practice.
CENTRAL EVENT REVIEW COMMITTEE
The Committee will be located at WCM and formed by a cardiologist, an intensivist and a cardiac surgeon not actively participating in the trial. Adverse events will be independently adjudicated by committee members. Consensus will require agreement between 2 members. Disagreement will be resolved by adhoc independent external review.
ETHICS
This trial will conform to the Medical Research Council (MRC) Guidelines for Good Clinical Practice in Clinical Trials and the Declaration of Helsinki. The study protocol will be approved by the local ethics committee in each centre participating in the trial before the study commences.
FUNDING
This work will be supported by the Department of Cardiothoracic Surgery of WCM, which will fund the core clinical trial unit and the research support for it.
The Core and Vanguard centres (see Supplementary Material, Table S2) will start the trial with limited funding. In an initial pilot phase, the study will enrol 10% of the sample size (430 patients). This pilot phase will have the main aims of establishing the feasibility of the project, the adherence to the protocol and the enrolment rate. The results of the pilot phase will be used to apply for national grants in the USA, Canada and Europe. In case of funding, more financial support will be given to the participating centres, and other centres will be enrolled. Private funding will also be explored.
PROFESSIONAL SOCIETIES’ SUPPORT
Support from the STS and European Association for Cardio-Thoracic Surgery (EACTS) will be requested.
REGISTRATION
The trial has been registered on ClinicalTrials.gov (no. 1703018094).
PUBLICATIONS
Publications of trial data will take place at the following time points:
Study protocol
In-hospital results (descriptive analysis)
Harvest site complications at 6 months
One-year outcomes and analysis of sternal complications
Primary composite outcome (major adverse cardiac and cerebral events)
Secondary outcome (all-cause mortality)
Other secondary outcomes and subgroups
TIMELINE
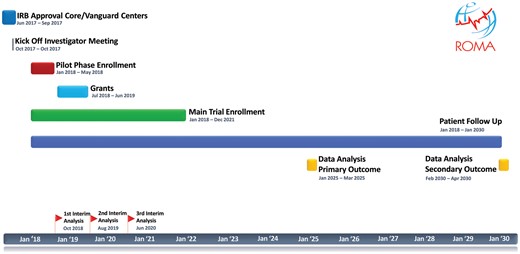
The Gantt chart of the trial is shown in Fig. 1.
CONCLUSION
Despite more than 25 years of clinical research, several unanswered questions concerning the use of MAGs for CABG remain. The effect of a second AG on postoperative outcomes, the effect of using 3 or more AGs and the relative role of the right ITA and the RA as second or third AG are all open interrogatives that have not been tested in an adequate randomized trial.
The ROMA trial was conceived to provide answers to those open questions.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: Pieter Kappetein is currently an employee of Medtronic. All other authors declared no conflict of interest.
REFERENCES
Author notes
Presented at the 31st Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 7–11 October 2017.
- myocardial infarction
- coronary artery bypass surgery
- survival analysis
- internal thoracic artery
- anterior descending branch of left coronary artery
- saphenous vein graft
- conduit implant
- arterial graft
- saphenous vein
- cerebrovascular accident
- ischemic stroke
- follow-up
- radial artery
- safety
- surgical procedures, operative
- tissue transplants
- arm
- mortality
- treatment outcome
- revascularization
- intention to treat
- multi vessel coronary artery disease
- primary outcome measure
- composite outcomes