-
PDF
- Split View
-
Views
-
Cite
Cite
Hajime Kanamori, William A Rutala, David J Weber, The Role of Patient Care Items as a Fomite in Healthcare-Associated Outbreaks and Infection Prevention, Clinical Infectious Diseases, Volume 65, Issue 8, 15 October 2017, Pages 1412–1419, https://doi.org/10.1093/cid/cix462
Close - Share Icon Share
Abstract
Patient-care items can serve as a source or reservoir for healthcare-associated pathogens in hospitals. We reviewed healthcare- associated outbreaks from medical equipment and provide infection prevention recommendations. Multiple healthcare-associated outbreaks via a contaminated patient-care item were identified, including infections with multidrug-resistant organisms. The type of patient care items implicated as a fomite causing healthcare-associated infections (HAIs) has changed over time. Patient populations at risk were most commonly critically ill patients in adult and neonatal intensive care units. Most fomite related healthcare-associated outbreaks were due to inappropriate disinfection practices. Repeated healthcare-associated outbreaks via medical equipment highlight the need for infectious disease professionals to understand that fomites/medical devices may be a source of HAIs. The introduction of new and more complex medical devices will likely increase the risk that such devices serve as a source of HAIs. Assuring appropriate cleaning and disinfection or sterilization of medical equipment is necessary to prevent future fomite-associated outbreaks.
A fomite is defined as an inanimate object that can be the vehicle for transmission of an infectious agent [1]. In the hospital, fomites include patient care items and environmental surfaces [2–4]. It has been demonstrated that such items (eg, medical equipment, surfaces) are frequently contaminated and can serve as a reservoir or source for multidrug-resistant organisms (MDROs) such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Clostridium difficile [5, 6]. Transmission of MDROs from contaminated devices or surfaces to a patient may occur via direct contact, indirectly via the hands/gloves of healthcare personnel, or less commonly via aerosols, water, or food [7].
Our previous review in 1987 described that a variety of fomites present in the hospital environment were involved in healthcare-associated outbreaks, including carpets, mattresses, beds, stethoscopes, thermometers, intra-aortic balloon pumps, pressure transducers, enteral feeds, contaminated germicides, chutes, and others [8]. Almost 30 years have passed since this review and many additional outbreaks of fomite-associated infections have been published. Although there are also some previous reviews of environmental surfaces and medical equipment [5, 6, 9, 10], to our knowledge, there are no recent reviews focused on actual outbreaks via a specific patient care item. The purpose of this article was to review healthcare- associated outbreaks and infections via medical equipment, primarily by semicritical and noncritical patient care items commonly used in daily practice, and provide infection prevention recommendations for each healthcare fomite.
LITERATURE SEARCH AND SELECTION CRITERIA
We searched the published literature (January 1987–December 2016) via PubMed using patient care items and the following Medical Subject Headings (MeSH) as well as keywords: (hospitals OR hospital units OR nursing homes OR ambulatory care facilities OR ambulatory care OR dental facilities OR assisted living facilities OR healthcare settings) AND (healthcare- acquired infection OR nosocomial OR cross infection OR outbreak). We screened articles using titles and abstracts, then carefully reviewed selected articles. We included articles of outbreaks and infections via a patient care item when authors reported human cases with identification of same organism in clinical samples from patients and environmental samples from medical equipment, and excluded articles describing contamination only of a pathogen with each fomite, articles without abstracts, not written in English, and review articles. The following healthcare fomites were beyond the scope of this review, although some have been previously reviewed: environmental surfaces [5, 6, 10], laundry and bedding [11, 12], healthcare personnel’s attire and devices [13–15], endoscopes [16–20], water [21], air [22], invasive indwelling devices (eg, devices involved in ventilator-associated pneumonia, central line–associated bloodstream infection, or catheter-associated urinary tract infection), and sharps (eg, devices involved in percutaneous injuries).
In this review, we summarized characteristics of medical equipment and patient care items that have served as a fomite for a healthcare-associated outbreak (Tables 1 and 2). Clinical significance for healthcare-associated outbreaks and infections via a fomite was categorized as low (≤3 outbreaks), moderate (4–6 outbreaks), and high (≥7 outbreaks), based on the number of published reports during the 30-year review period. We also provided a trend of outbreaks related to selected fomites during January 1987 to December 2016 (Figure 1). The number of published articles describing outbreaks and infections relevant to a patient care item was counted. For selected medical equipment, we briefly discussed infection prevention issues. Details of cleaning, disinfection, and sterilization are available in the Centers for Disease Control and Prevention (CDC) guidelines [2–4], other reviews [23, 24], and the US Food and Drug Administration (FDA) and Environmental Protection Agency (EPA) websites (for FDA-cleared sterilants and high-level disinfectants (https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofReusableMedicalDevices/ucm437347.htm; for EPA-registered disinfectants, https://www.epa.gov/pesti cide-registration/selected-epa-registered-disinfectants).
Pathogens Associated With a Fomite, Transmission Mechanism, and Infection Prevention Recommendations in Moderate to High Significance Categories of Healthcare-Associated Outbreaks
| Fomite | Pathogen | Transmission | Significancea | Prevention/Control |
| Hand soap/ sanitizer dispenser | Enterobacter, Pseudomonas, Serratia | Contact | Moderate | Use disposable dispenser, antiseptic with greater bactericidal activity (eg, chlorhexidine) and/or alcohol-based handrub, use antimicrobial at recommended concentration |
| Humidifier | Acinetobacter, Acremonium, Burkholderia, Klebsiella, Legionella, Mycobacterium, Pseudomonas, Stenotrophomonas | Inhalation, airborne | High | Avoid use of humidifier when possible, use sterile water, disinfect between use |
| Nebulizer | Burkholderia, Legionella, Pseudomonas, Staphylococci | Inhalation, contact, airborne | High | Avoid sharing multidose medications between patients, use sterile water, disinfect device between use |
| Pressure transducer | Pseudomonas, Serratia | Contact | Moderate | Disinfect pressure transducer between patients, use disposable dome, adhere to aseptic technique |
| Stethoscope | Acinetobacter, Klebsiella, Pseudomonas | Contact | Moderate | Prudent to disinfect between patients |
| Suction apparatus | Acinetobacter, Enterobacter, Klebsiella, Pseudomonas, Salmonella, Serratia, Staphylococcus, Stenotrophomonas | Contact, droplet | High | Avoid backflow, avoid aerosolization, disinfect suction apparatus properly |
| Thermometer | Clostridium, Enterobacter, Enterococcus, Klebsiella | Contact | High | Proper disinfection between uses of thermometer, use single-use disposable thermometer when available |
| Ultrasound probe | Burkholderia, Enterobacter, Mycobacterium, Pseudomonas, Salmonella, Serratia, Staphylococcus | Contact | High | Low-level disinfect for surface probes and high-level disinfect for endocavitary probes between patients; label gel bottles and use sterile gels if available |
| Fomite | Pathogen | Transmission | Significancea | Prevention/Control |
| Hand soap/ sanitizer dispenser | Enterobacter, Pseudomonas, Serratia | Contact | Moderate | Use disposable dispenser, antiseptic with greater bactericidal activity (eg, chlorhexidine) and/or alcohol-based handrub, use antimicrobial at recommended concentration |
| Humidifier | Acinetobacter, Acremonium, Burkholderia, Klebsiella, Legionella, Mycobacterium, Pseudomonas, Stenotrophomonas | Inhalation, airborne | High | Avoid use of humidifier when possible, use sterile water, disinfect between use |
| Nebulizer | Burkholderia, Legionella, Pseudomonas, Staphylococci | Inhalation, contact, airborne | High | Avoid sharing multidose medications between patients, use sterile water, disinfect device between use |
| Pressure transducer | Pseudomonas, Serratia | Contact | Moderate | Disinfect pressure transducer between patients, use disposable dome, adhere to aseptic technique |
| Stethoscope | Acinetobacter, Klebsiella, Pseudomonas | Contact | Moderate | Prudent to disinfect between patients |
| Suction apparatus | Acinetobacter, Enterobacter, Klebsiella, Pseudomonas, Salmonella, Serratia, Staphylococcus, Stenotrophomonas | Contact, droplet | High | Avoid backflow, avoid aerosolization, disinfect suction apparatus properly |
| Thermometer | Clostridium, Enterobacter, Enterococcus, Klebsiella | Contact | High | Proper disinfection between uses of thermometer, use single-use disposable thermometer when available |
| Ultrasound probe | Burkholderia, Enterobacter, Mycobacterium, Pseudomonas, Salmonella, Serratia, Staphylococcus | Contact | High | Low-level disinfect for surface probes and high-level disinfect for endocavitary probes between patients; label gel bottles and use sterile gels if available |
aClinical significance for outbreaks via a fomite was categorized as low (≤3 outbreaks), moderate (4–6 outbreaks), and high (≥7 outbreaks).
Pathogens Associated With a Fomite, Transmission Mechanism, and Infection Prevention Recommendations in Moderate to High Significance Categories of Healthcare-Associated Outbreaks
| Fomite | Pathogen | Transmission | Significancea | Prevention/Control |
| Hand soap/ sanitizer dispenser | Enterobacter, Pseudomonas, Serratia | Contact | Moderate | Use disposable dispenser, antiseptic with greater bactericidal activity (eg, chlorhexidine) and/or alcohol-based handrub, use antimicrobial at recommended concentration |
| Humidifier | Acinetobacter, Acremonium, Burkholderia, Klebsiella, Legionella, Mycobacterium, Pseudomonas, Stenotrophomonas | Inhalation, airborne | High | Avoid use of humidifier when possible, use sterile water, disinfect between use |
| Nebulizer | Burkholderia, Legionella, Pseudomonas, Staphylococci | Inhalation, contact, airborne | High | Avoid sharing multidose medications between patients, use sterile water, disinfect device between use |
| Pressure transducer | Pseudomonas, Serratia | Contact | Moderate | Disinfect pressure transducer between patients, use disposable dome, adhere to aseptic technique |
| Stethoscope | Acinetobacter, Klebsiella, Pseudomonas | Contact | Moderate | Prudent to disinfect between patients |
| Suction apparatus | Acinetobacter, Enterobacter, Klebsiella, Pseudomonas, Salmonella, Serratia, Staphylococcus, Stenotrophomonas | Contact, droplet | High | Avoid backflow, avoid aerosolization, disinfect suction apparatus properly |
| Thermometer | Clostridium, Enterobacter, Enterococcus, Klebsiella | Contact | High | Proper disinfection between uses of thermometer, use single-use disposable thermometer when available |
| Ultrasound probe | Burkholderia, Enterobacter, Mycobacterium, Pseudomonas, Salmonella, Serratia, Staphylococcus | Contact | High | Low-level disinfect for surface probes and high-level disinfect for endocavitary probes between patients; label gel bottles and use sterile gels if available |
| Fomite | Pathogen | Transmission | Significancea | Prevention/Control |
| Hand soap/ sanitizer dispenser | Enterobacter, Pseudomonas, Serratia | Contact | Moderate | Use disposable dispenser, antiseptic with greater bactericidal activity (eg, chlorhexidine) and/or alcohol-based handrub, use antimicrobial at recommended concentration |
| Humidifier | Acinetobacter, Acremonium, Burkholderia, Klebsiella, Legionella, Mycobacterium, Pseudomonas, Stenotrophomonas | Inhalation, airborne | High | Avoid use of humidifier when possible, use sterile water, disinfect between use |
| Nebulizer | Burkholderia, Legionella, Pseudomonas, Staphylococci | Inhalation, contact, airborne | High | Avoid sharing multidose medications between patients, use sterile water, disinfect device between use |
| Pressure transducer | Pseudomonas, Serratia | Contact | Moderate | Disinfect pressure transducer between patients, use disposable dome, adhere to aseptic technique |
| Stethoscope | Acinetobacter, Klebsiella, Pseudomonas | Contact | Moderate | Prudent to disinfect between patients |
| Suction apparatus | Acinetobacter, Enterobacter, Klebsiella, Pseudomonas, Salmonella, Serratia, Staphylococcus, Stenotrophomonas | Contact, droplet | High | Avoid backflow, avoid aerosolization, disinfect suction apparatus properly |
| Thermometer | Clostridium, Enterobacter, Enterococcus, Klebsiella | Contact | High | Proper disinfection between uses of thermometer, use single-use disposable thermometer when available |
| Ultrasound probe | Burkholderia, Enterobacter, Mycobacterium, Pseudomonas, Salmonella, Serratia, Staphylococcus | Contact | High | Low-level disinfect for surface probes and high-level disinfect for endocavitary probes between patients; label gel bottles and use sterile gels if available |
aClinical significance for outbreaks via a fomite was categorized as low (≤3 outbreaks), moderate (4–6 outbreaks), and high (≥7 outbreaks).
Pathogens Associated With a Fomite in Low Significance Category of Healthcare-Associated Outbreaks
| Fomite | Pathogen |
| Atomizer | Alcaligenes, Achromobacter |
| Breast pump | Acinetobacter, Serratia |
| Computer keyboard/ mobile phone/tablet | Acinetobacter, Chryseobacterium, Clostridium, Enterococcus, Pseudomonas, Staphylococcus |
| Electrocardiography/ lead wire | Enterococcus, Serratia |
| Enteral feed | Salmonella, Serratia |
| Medical chart | Acinetobacter, Escherichia, Klebsiella, Staphylococcus, Streptococcus |
| Shaving razor | Klebsiella, Microsporum, Serratia |
| Tourniquet/ exsanguinator | Acinetobacter, Enterococcus, Candida, Staphylococcus, Proteus |
| Toys | Bacillus, Micrococcus, Pseudomonas, Staphylococcus, Stenotrophomonas, Streptococcus |
| Measuring cup/automated urine analyzer | Pseudomonas, Shewanella |
| Wheelchairs | Acinetobacter, Pseudomonas, Staphylococcus |
| Fomite | Pathogen |
| Atomizer | Alcaligenes, Achromobacter |
| Breast pump | Acinetobacter, Serratia |
| Computer keyboard/ mobile phone/tablet | Acinetobacter, Chryseobacterium, Clostridium, Enterococcus, Pseudomonas, Staphylococcus |
| Electrocardiography/ lead wire | Enterococcus, Serratia |
| Enteral feed | Salmonella, Serratia |
| Medical chart | Acinetobacter, Escherichia, Klebsiella, Staphylococcus, Streptococcus |
| Shaving razor | Klebsiella, Microsporum, Serratia |
| Tourniquet/ exsanguinator | Acinetobacter, Enterococcus, Candida, Staphylococcus, Proteus |
| Toys | Bacillus, Micrococcus, Pseudomonas, Staphylococcus, Stenotrophomonas, Streptococcus |
| Measuring cup/automated urine analyzer | Pseudomonas, Shewanella |
| Wheelchairs | Acinetobacter, Pseudomonas, Staphylococcus |
Pathogens Associated With a Fomite in Low Significance Category of Healthcare-Associated Outbreaks
| Fomite | Pathogen |
| Atomizer | Alcaligenes, Achromobacter |
| Breast pump | Acinetobacter, Serratia |
| Computer keyboard/ mobile phone/tablet | Acinetobacter, Chryseobacterium, Clostridium, Enterococcus, Pseudomonas, Staphylococcus |
| Electrocardiography/ lead wire | Enterococcus, Serratia |
| Enteral feed | Salmonella, Serratia |
| Medical chart | Acinetobacter, Escherichia, Klebsiella, Staphylococcus, Streptococcus |
| Shaving razor | Klebsiella, Microsporum, Serratia |
| Tourniquet/ exsanguinator | Acinetobacter, Enterococcus, Candida, Staphylococcus, Proteus |
| Toys | Bacillus, Micrococcus, Pseudomonas, Staphylococcus, Stenotrophomonas, Streptococcus |
| Measuring cup/automated urine analyzer | Pseudomonas, Shewanella |
| Wheelchairs | Acinetobacter, Pseudomonas, Staphylococcus |
| Fomite | Pathogen |
| Atomizer | Alcaligenes, Achromobacter |
| Breast pump | Acinetobacter, Serratia |
| Computer keyboard/ mobile phone/tablet | Acinetobacter, Chryseobacterium, Clostridium, Enterococcus, Pseudomonas, Staphylococcus |
| Electrocardiography/ lead wire | Enterococcus, Serratia |
| Enteral feed | Salmonella, Serratia |
| Medical chart | Acinetobacter, Escherichia, Klebsiella, Staphylococcus, Streptococcus |
| Shaving razor | Klebsiella, Microsporum, Serratia |
| Tourniquet/ exsanguinator | Acinetobacter, Enterococcus, Candida, Staphylococcus, Proteus |
| Toys | Bacillus, Micrococcus, Pseudomonas, Staphylococcus, Stenotrophomonas, Streptococcus |
| Measuring cup/automated urine analyzer | Pseudomonas, Shewanella |
| Wheelchairs | Acinetobacter, Pseudomonas, Staphylococcus |
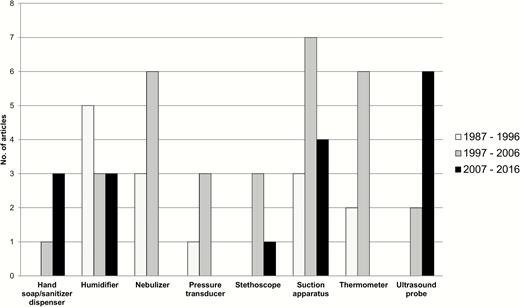
Trend in patient care items as a fomite causing healthcare-associated outbreaks during a 30-year period. During January 1987–December 2016, the number of published articles describing outbreaks relevant to each patient care item is shown.
OVERALL TREND IN PATIENT CARE ITEMS AS FOMITE IN HEALTHCARE SETTINGS
Fomites recognized in the 1987 previous review [8] (eg, humidifier, nebulizer, urine-measuring device, stethoscope, thermometer, suction apparatus, pressure transducer) have continued to be implicated in healthcare-associated outbreaks (Table 1). There were also various contaminated fomites implicated without having clear evidence of healthcare-associated outbreaks and infections (Table 2). During the 3 decades since our last review, additional healthcare fomites (eg, hand soap/sanitizer dispenser, ultrasound probe/gel, computer keyboards) have been identified. The type of patient care items as a fomite has changed over time (Figure 1), and some of them were likely to be reduced (eg, nebulizer, pressure transducer, thermometer), but others were not. The number of healthcare-associated outbreaks via a patient care item may be affected by publication bias, depending on authors’ interest (eg, rare organism or fomite) and findings [25]. Furthermore, there likely would be more unpublished healthcare-associated outbreaks than published ones, given concern about reduced reputation of healthcare facility.
MAJOR PATIENT CARE ITEMS AS A HEALTHCARE FOMITE
Respiratory Care Equipment (Humidifier, Nebulizer, and Suction Apparatus)
Contamination with Acinetobacter baumannii of the temperature probe of a humidifier caused a hospital outbreak of pneumonia among patients in an intensive care unit (ICU), even though the probe was disinfected with 70% ethanol as recommended by the manufacturer. The outbreak was ended when low-temperature plasma sterilization was utilized for decontamination [26]. Acinetobacter calcoaceticus sepsis outbreak in a neonatal ICU and an A. baumannii ventilator-associated pneumonia outbreak were caused by contamination of warm air humidifiers [27] and the oxygen humidifying chambers [28], respectively. An outbreak of Burkholderia cepacia respiratory tract infection in a pediatric ICU was caused by patient-to-patient transmission via respiratory devices and heated humidifier water. Control measures could include use of disposable, sterilizable, or easy-to-clean/disinfect materials [29]. A Klebsiella oxytoca (K1 β-lactamase overproduction) outbreak among neonates in a neonatal ICU was associated with water reservoirs of humidifiers [30]. An outbreak of Pseudomonas cepacia respiratory infection in an ICU was due to contamination of reusable electronic temperature probes used with servo-controlled ventilator humidifiers, and highlighted the need to facilitate adequate disinfection practices for reusable patient care items [31]. A Stenotrophomonas maltophilia outbreak in a surgical ICU was also caused by electronic temperature probes used with servo-controlled humidifiers that were wiped with a quaternary ammonium compound disinfectant [32].
Legionellosis in patients with nonrespiratory diseases was related to use of contaminated oxygen bubble humidifiers filled with tap water, suggesting sterile water should be used in humidifiers [33]. An outbreak of Legionella pneumophila infection in neonates was also probably due to aerosol generated by a cold mist ultrasonic humidifier filled with contaminated water, indicating that use of such humidifiers should be avoided in a neonatal ICU [34]. The CDC guideline recommends that large-volume room air humidifiers producing aerosols should be avoided unless they are subjected to high-level disinfection and filled with sterile water [2].
An outbreak of Mycobacterium chelonae eye infection in an outpatient clinic of laser-assisted in situ keratomileusis (LASIK) was likely led by water reservoir of the misting humidifier that was utilized to maintain the high level of humidity recommended by the manufacturers of lasers for LASIK. This underscored the importance for careful adherence to current guidelines even in outpatient settings [35]. A fungal endophthalmitis outbreak due to Acremonium kiliense among patients after cataract surgery in an outpatient setting was associated with a contaminated high-efficiency particulate air (HEPA) filter, followed by the humidifier water contamination in the ventilation system [36]. Appropriate engineering humidity control of the heating, ventilation, and air conditioning system is essential for preventing spread of airborne pathogens [2].
Multiple outbreaks of B. cepacia respiratory infection and bacteremia, mostly among patients, occurred in association with nebulizers (eg, solution, medication, tube), and the use of multidose vials for multiple patients should be avoided [37–42]. An outbreak of P. cepacia pneumonia among immunocompromised patients was associated with contamination of nebulizers that were utilized for prophylactic inhalations of polymyxin B and amphotericin B [43]. Ultrasonic nebulizers used to humidify tracheostomies were involved in an MRSA outbreak in a head and neck surgical ward [44]. Contaminated tap water to rinse medication nebulizers resulted in an outbreak of legionellosis among patients with chronic obstructive pulmonary disease; thus, tap water should be avoided in filling or rinsing nebulizers [45]. Importantly, fluid-containing respiratory equipment such as humidifiers and nebulizers can be heavily contaminated by bacteria capable of proliferating in water; these pathogens may be transferred to patients by healthcare personnel, aerosolization into room air, direct airway inoculation through connected ventilation system, or contaminated medications [8].
Pathogens from contaminated suction collection units can be transmitted to patients through healthcare personnel’s hands or retrograde spread during their use or can be dispersed by aerosols [8]. Contamination of suction apparatus (eg, catheter, tube, rubber pipe, quiver, portable suction device) was involved in the following outbreaks: MDR A. baumannii, MDR Pseudomonas aeruginosa, S. maltophilia, and Serratia marcescens postoperative empyema in ICU [46–49]; MDR A. baumannii, extended- spectrum β-lactamase (ESBL)–producing Klebsiella pneumoniae, Enterobacter aerogenes, Salmonella worthington, and MRSA in neonatal or pediatric ICU [50–54]; MDR P. aeruginosa in a long-term-care facility [55]; and A. baumannii meningitis in neurosurgical patients [56]. The cause of these outbreaks was mainly improper disinfection and reuse, as well as external contamination of suction catheters. Adherence to a disinfection practice as determined by the intended use of the patient care item and the tissue(s) the item is expected to contact; use of sterile, single-use catheters when the open-system suction is employed; and use of sterile fluid in removing secretions from the suction catheter are recommended [4, 24, 57].
THERMOMETER
Rectal thermometers served as a fomite for outbreaks of Enterobacter cloacae in neonatal ICU, VRE in an ICU, or C. difficile infection among older patients partially because of improper disinfection, and replacement to single-use disposable or tympanic thermometers resulted in the reduction in VRE and C. difficile infection [58–63]. Transmission of VRE in a community hospital occurred via an electric ear probe thermometer, likely due to the disposable probe sheath contaminated from the handle of the thermometer [64]. ESBL-producing K. pneumoniae in a neonatal unit was caused in part by incorrect use of a personal electric thermometer and contamination of an oxygen saturation probe with a fabric-covered transducer [65]. Thermometers should be low-level disinfected using EPA-registered disinfectants between patient uses or be replaced by single-use disposable thermometers [4].
ULTRASOUND PROBE
Contaminated ultrasound gels led to B. cepacia infection and bacteremia, S. aureus pyoderma, or Mycobacterium massiliense surgical site infection in neonates, children, or ICU patients [66–69]. Contamination of transesophageal echocardiography (TEE) probes was involved in outbreaks of E. cloacae, S. marcescens, or MDR P. aeruginosa in cardiac surgical patients [70–72] and an outbreak of ESBL-producing Salmonella enterica serotype Isangi among surgical patients for transplant [73]. Legionella pneumophila pneumonia cases were also associated with contaminated water to rinse TEE probes [74]. Although surface probes used on intact skin are considered as noncritical items that are subjected to at least low-level disinfection between patients, endocavitary probes such as TEE, transvaginal, or transrectal probes contact directly with mucous membranes and are considered semicritical items [75]. Therefore, high-level disinfection of the endocavitary probes is recommended even if probe covers have been used to reduce their contamination since probe covers and low-level disinfection could fail [4].
HAND SOAP/SANITIZER DISPENSER
Outbreaks of S. marcescens, New Delhi metallo-β-lactamase (NDM)–producing E. cloacae in neonatal ICUs, or P. aeruginosa in patients with hematologic malignancy were associated with contamination of refillable liquid soap or triclosan soap dispensers, facilitating transmission of the pathogen via hands of healthcare personnel [76–79]. The use of disposable antiseptic with greater bactericidal activity (eg, chlorhexidine) and/or alcohol-based handrub is recommended, and refilling soap dispensers should be avoided [78, 80]. Small-volume dispensers refilled from large-volume stock containers should be consumed until they are entirely empty, rinsed with tap water, and then air dried before refilling [81].
PRESSURE TRANSDUCER
An outbreak of P. aeruginosa urinary tract infection was reported after cystometry; a contaminated pressure dome that covered a pressure transducer of urodynamic system was implicated as the source [82]. Several Serratia outbreaks also occurred as follows: S. marcescens bacteremia in a cardiac care unit via a pressure transducer of intra-aortic balloon pump, S. marcescens endophthalmitis after cataract surgery via a pressure transducer of the virectomy apparatus, and Serratia liquefaciens infection in an ICU via a pressure monitoring system [83–85]. Wiping reusable transducer heads with 70% sterile isopropyl alcohol between patients or use of disposable domes, and careful adherence to aseptic technique, should be used to prevent such outbreaks [8, 86, 87].
STETHOSCOPE
Healthcare-associated outbreaks via stethoscope occurred in combination with other reservoirs (eg, artificial nails, computer mouse, ointment, sink, other environment) and were caused by A. baumannii in an ICU, ESBL-producing K. pneumoniae, K. pneumoniae bacteremia in neonatal ICUs, or Verona integron-mediated metallo-β-lactamase (VIM)–producing carbapenem-resistant P. aeruginosa in various ICUs, suggesting dissemination of an outbreak strain via healthcare personnel [88–91]. Stethoscope and other noncritical items should be disinfected with an EPA-registered low-level disinfectant between patient uses, given the nature and degree of contamination [2, 4].
MISCELLANEOUS
For articles falling into the low clinical significance category and contamination, each pathogen and fomite are described in Table 2. Contamination of disinfectant atomizers (eg, chlorhexidine diluted, didecyl diammonium chloride) was involved in outbreaks of Alcaligenes xylosoxidans wound infection in burn patients [92] and Achromobacter bacteremia in pediatric patients with hematologic malignancy [93], respectively, which highlighted the need to use disinfectants appropriately [4, 24]. To prevent microbial contamination of disinfectants and antiseptics, the CDC guideline recommends as follows: (1) following the manufacturers’ instruction regarding dilution (if needed); (2) avoiding contamination of disinfectants with water used for dilution, containers, and hospital preparation areas; and (3) storing stock solutions of disinfections as indicated on the product label [4].
A pseudo-outbreak of extremely drug-resistant P. aeruginosa urinary tract infections was caused by contamination of an automated urine cytometric analyzer following nonobservances of the standard routine sample process [94]. Reused and shared measuring cup for catheter drainage also caused a Shewanella outbreak in a surgical ward, which highlighted the need of strict adherence to standard precautions [95]. For enteric feeding, outbreaks of Salmonella enteritidis and S. marcescens were associated with contamination of lyophilized enteral nutrition [96] and a bottle of enteral feed additive [97], respectively. It is essential to use sterile commercial feeds, minimize manipulation, and use a closed administration set [8]. In addition to outbreaks of A. baumannii and S. marcescens infection in neonatal ICUs via contaminated breast milk pumps [98, 99], there are additional outbreaks associated with contamination of breast milk (eg, expressed, pooled, or shared breast milk) by a pathogen (eg, Bacillus cereus, ESBL-producing Escherichia coli, MRSA, or S. marcescens) [100–103].
A VRE outbreak in a burn ICU involved contaminated electrocardiogram leads, necessitating strict barrier precautions in the hydrotherapy area [104], and a S. marcescens outbreak in cardiac surgical patients associated with electrocardiogram rubber Welsh bulbs required replacement of the reusable electrocardiogram bulbs with disposable leads [105]. Toys have been reported to be contaminated with various pathogens [106, 107], and an MDR P. aeruginosa outbreak occurred via water-retaining bath toys in a pediatric oncology ward, suggesting that use of water-retaining toys and other toys that are difficult to clean and dry should be limited to a single child [108]. Outbreaks of C. difficile infection in non–isolation rooms or Chryseobacterium meningosepticum infection in neonates and pediatric patients implicated computer keyboards and other medical equipment. Contamination with multiple pathogens had been identified on computer keyboards, mobile phones, and tablets [109–115]. Razors used for preoperative shaving by barbers were implicated in outbreaks of S. marcescens or carbapenemase-producing K. pneumoniae postoperative infection in neurosurgical patients [116, 117], and shared razors have also been associated with tinea corporis due to Microsporum canis [118]. Thus, only single-use disposable razors should be used, or the razor heads should be disinfected between patient uses. Ideally, if hair removal is necessary, an electric shaver with a disposable head should be used as it is less damaging to the skin (razors should not be used for surgical site preparation). Other patient care items as a potential fomite in articles describing mainly contamination of pathogens included tourniquet and exsanguinator [119, 120], wheelchair [121], and medical record [122].
DISCUSSION
Healthcare-associated outbreaks via patient care items commonly used in daily practice still occur, but the number of articles describing actual infections caused by these healthcare fomites has been limited except for some items. Despite the dramatically lower documented risk of transmitting pathogens to patients through noncritical items compared to critical and semicritical items noted in a previous review [123], there is a potentially substantial burden of medical equipment on healthcare-associated outbreaks as identified in the current review. We also found more articles that reported only contamination of medical devices with a pathogen without evidence of patient-associated infections, suggesting potential fomites in healthcare settings. However, in assessing microbial contamination with a fomite in different studies, meaningful comparison of contamination levels is difficult or impossible since a standard environmental sampling method of medical equipment is not well-established, and permissible levels of microbial contamination are undefined [2, 3, 9]. Moreover, in many articles of outbreaks associated with a healthcare fomite, levels of hand hygiene and environmental cleaning as well as disinfection were not evaluated, complicating the ability to subscribe the infections directly to the fomite [5].
Our review demonstrated a variety of healthcare-associated outbreaks via a patient care item due to bacterial pathogens, including gram-negative rods, as well as increasing reports of fomite-associated outbreaks due to MDROs (eg, carbapenem-resistant Enterobacteriaceae). A previous review showed that the majority of bacterial species isolated from contaminated equipment were flora (eg, coagulase-negative staphylococci) commonly identified from normal skin and environment, but the minority were gram-negative rods [9]. These pathogens in association with healthcare fomites were transmitted primarily by direct contact with contaminated items and other environment, via healthcare personnel, or by inhalation of aerosols generated from the contaminated water for respiratory equipment. Patient populations at risk for outbreaks and infections via a patient care item included critically ill patients in adult and neonatal ICUs. In our review, the main cause of healthcare-associated outbreaks was inappropriate disinfection practice for sharing items. Other reviews also noted that medical equipment used in noncritical settings rarely had cleaning protocol and may be involved in frequent transfer of pathogens compared to critical settings, suggesting the need for appropriate cleaning and disinfection protocols for patient care items commonly used in daily practice [9]. Cleaning must precede high-level disinfection or sterilization of any reused patient care items [4, 24]. Thus, assuring disinfectants for noncritical medical equipment in addition to improving thoroughness of cleaning and disinfection practice is imperative in terms of infection prevention. As currently available disinfectants have both advantages and disadvantages, 5 components to help select optimal disinfectants in healthcare facilities—including relevant kill claims, appropriate wet-contact and kill times, safety, ease of use, and other factors (eg, user training and support by the manufacturer, costs, and standardization)—have been discussed [124]. Hospitals should not reprocess single-use devices; they may use third-party reprocessors who comply with the same regulatory requirements of the device as when the device was originally manufactured as well as all applicable FDA labeling requirements when single-use devices are reused [4]. In addition, hospitals should follow the latest FDA guidance documents on reprocessing and reuse of single-use devices as their reuse remains controversial due to economic, medical, ethical, regulatory, and legal issues.
Any patient care items used in healthcare settings can be contaminated with a healthcare-associated pathogen and are a potential fomite, but outbreaks via these fomites can be prevented or minimized by adhering to current recommendations (ie, manufacturers and CDC) for cleaning and disinfection/sterilization of devices and surfaces [4, 24]. Although the trend in healthcare-associated outbreaks via a fomite may be affected by reporting bias, sharing lessons learned from outbreaks and accumulation of practical evidence would help improve infection prevention for each fomite. It is important for healthcare personnel to recognize the increasing role of patient care items as a fomite and adhere to prevention strategies for fomite-associated outbreaks based on current guidelines and the literature. Further investigations for healthcare fomites, including causation between contamination of a pathogen with a fomite and actual healthcare-associated outbreaks, elucidation of direct and indirect transmission mechanisms via a fomite using advanced molecular typing, establishment of standard environmental sampling of medical equipment, and improvement of adherence to cleaning and disinfection practice against a fomite, are warranted.
Notes
Acknowledgments. We thank Lara Handler, a librarian at Health Sciences Library, University of North Carolina, for her assistance with literature search.
Financial support. H. K. received financial support necessary for studying abroad from the Japan Society for the Promotion of Science Overseas Research Fellowships.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References





Comments