-
PDF
- Split View
-
Views
-
Cite
Cite
Sabra L. Klein, Morgan A. Marks, Wei Li, Gregory E. Glass, Li-Qun Fang, Jia-Qi Ma, Wu-Chun Cao, Sex Differences in the Incidence and Case Fatality Rates From Hemorrhagic Fever With Renal Syndrome in China, 2004–2008, Clinical Infectious Diseases, Volume 52, Issue 12, 15 June 2011, Pages 1414–1421, https://doi.org/10.1093/cid/cir232
Close - Share Icon Share
Abstract
Background. Differences between male and female individuals in response to infectious diseases are an overlooked global health problem.
Methods. The relationship between sex and disease outcome was examined in populations of patients with hemorrhagic fever with renal syndrome (HFRS) in mainland China, where most cases of hantavirus exposure occur. HFRS in China is diagnosed on the basis of symptoms and is confirmed with serological testing. The geographical distribution, incidence, and case fatality rates (CFRs) of HFRS in China were estimated and compared by patient sex and age. In a subset of patients with HFRS, clinical manifestations of HFRS were assessed using latent class analysis and compared by sex.
Results. There were 80,671 HFRS cases reported during the period 2004–2008, with a majority of HFRS cases (39.2%) occurring among individuals 20–39 years of age. The incidence of HFRS was higher among male patients than among female patients for all individuals >10 years of age. There were 945 deaths (CFR, 1.17%) due to HFRS in China during the period 2004–2008. CFRs were higher among women than among men between the ages of 20–39 and ≥50 years of age. There were no sex differences in the geographical distribution of HFRS cases or deaths. Although the prevalence of each clinical marker did not differ by sex, 2 profiles of clinical markers were identified that were related to both severity of disease and sex.
Conclusions. These data illustrate a paradox in which the incidence of disease is greater for males, but the severity of disease outcome is worse for females. Several behavioral, societal, and biological factors are hypothesized to be involved.
Hemorrhagic fever with renal syndrome (HFRS) affects thousands of people each year in Asia and Europe, with over half of these cases occurring in China [1, 2]. In China, the causal agents of HFRS are Hantaan virus and Seoul virus, which are carried by striped field mice (Apodemus agrarius) and Norway rats (Rattus norvegicus), respectively. Hantaviruses (family Bunyaviridae) are shed in the feces, urine, and saliva of infectious rodents; therefore, exposure requires contact with infectious rodents or their secreta and excreta.
Disease in humans presents in 1 of 2 general forms: HFRS or hantavirus pulmonary syndrome (HPS). The mortality rate associated with HFRS varies from 0.1% to 15%, whereas HPS is associated with a higher mortality rate of 35%–50% [3]. Clinical symptoms of HPS and HFRS include an initial nonspecific prodrome of high fever, chills, malaise, and headache during the first few weeks of infection, followed by vomiting and gastrointestinal symptoms. The following 1–2 weeks are characterized by thrombocytopenia and acute kidney injury in HFRS or pulmonary edema in HPS; at this stage, patients have the highest risk for death [3].
Sex differences in hantaviral disease are reported in which the prevalence of disease is consistently higher in the male population than in the female population worldwide [4–11]. In Sweden, for example, although similar proportions of men and women are infected with Puumala virus (PUUV) [12], the incidence of nephropathia epidemica (NE; a mild form of HFRS) is higher among males ≥10 years of age [5]. Although NE is viewed as a mild disease and is associated with a mortality rate of <1%, during the acute phase of NE, standardized mortality rates are higher among female patients than they are among male patients [5]. Similar patterns of sex differences have been reported in response to human immunodeficiency virus, herpes simplex viruses, hepatitis B virus, influenza A viruses, coxsackievirus B-3, and West Nile virus [13]. There is no consensus about the mechanisms mediating differences between the sexes in the prevalence or outcome of viral diseases in humans; hormonal, behavioral, immunological, and genetic factors are hypothesized to be involved [13]. Whether this results in differential clinical presentation of viral diseases between the sexes has not been addressed.
Using well-characterized populations of patients with HFRS, we tested the hypothesis that the incidence and severity of hantaviral disease differ between the sexes and may reflect differences in the constellation of symptoms characteristic of this disease. We conducted retrospective analyses of HFRS cases in China using available datasets from the period 2004–2008. We hypothesized that, although incidence rates would be higher in the male population, the severity of disease might be worse in female patients. We further hypothesized that, if hormones mediate these differences, as they do in rodent reservoirs for hantaviruses [14–17], then the magnitude of the sex difference might be related to the age of the patients.
MATERIALS AND METHODS
Study Population
In China, HFRS is a notifiable disease, with all cases reported to the China Center for Disease Control. HFRS is diagnosed clinically by signs and symptoms and confirmed by standard serological tests (enzyme-linked immunosorbent assay–based methods; Lanzhou Institute of Biological Products). Patient samples were obtained 3–15 days after the onset of illness. Approval was received from the institutional review board of the Chinese National Center for Disease Prevention and Control for collection of all data that we retrospectively analyzed.
HFRS is categorized as (1) mild (temperature, <39°C; normal blood pressure; hemorrhagic spots in the skin or mucous membrane; mild acute kidney injury [AKI] with no oliguria; increased serum creatinine and blood urea nitrogen levels; and proteinuria [protein level, 0.1–2.9 g/L]); (2) moderate (at least 3 of the following: temperature, 39–40°C; systolic arterial pressure <90 mmHg and difference of pulse pressure <26 mmHg; hemorrhagic spots in the skin, mucous membrane, or viscera; AKI with oliguria present; increased serum creatinine and blood urea nitrogen levels; and proteinuria [protein level, 3.0–4.9 g/L]; (3) severe (at least 3 of the following: temperature, 40°C with vascular leakage; systolic arterial pressure <69.8 mmHg and difference of pulse pressure <20.3 mmHg; hemorrhage in the skin and viscera; AKI with oliguria ≤5 days in duration; increased serum creatinine and blood urea nitrogen levels; and proteinuria [protein level, 5.0–10.0 g/L]; or (4) extremely severe (the same clinical presentation as severe, but also includes shock, severe hemorrhage syndrome, AKI with oliguria ≥5 days in duration or anuresis ≥2 days in duration, blood urea nitrogen level >42.84 mmol/L, cardiac failure, pulmonary edema, respiratory distress, central nervous system involvement [defined as cerebral edema, encephalorrhagia, or cerebral hernia], secondary infection, or other severe complications) [18].
Statistical Analyses
Comparisons of the Incidence and Case Fatality Rates (CFRs) of HFRS by Age and Sex
Incidence rates were characterized using the 2008 Chinese population estimates reported by the National Bureau of Statistics of China and are reported as the number of cases per 100,000 persons. CFR is reported as the number of deaths per 1000 HFRS cases. We estimated the incidence rate and CFR of HFRS stratified by sex and age group (<10, 10–19, 20–39, 40–49, and ≥50 years of age). The age categorization was created to capture the different reproductive stages of male and female patients across the life-course: the <10-year-old and 10–19-year-old age groups represent prepubescent and postpubertal individuals, respectively; the 20–39-year-old age group represents individuals in the prime reproductive years; the 40–49-year-old age category encompasses women in the early stages of the menopausal transition; and the ≥50-year-old age category captures postmenopausal women. Differences in the incidence rate and CFR by sex within each age category were assessed using log-linear Poisson regression models. All models were stratified by age groups and adjusted for geographical location. A P value of <.05 was considered to be statistically significant. All analyses were performed using Stata software, version 11.0 (Stata).
Analysis of the Geographical Distribution of HFRS by Sex
The geographic distribution of cases and mortality patterns were mapped (ArcGIS software, version 9.3; ESRI) and were visually examined to generate a qualitative description of HFRS distribution and intensity. To examine geographic differences in the incidence and CFR for male and female patients, data were examined in 4 age classes for those >10 years of age, because few individuals ≤10 years of age were either confirmed to be sick or died as a result of infection. Using the geographic center of each province as the locations, Moran’s I was calculated for the sex ratio in HFRS incidence and the CFR for each of the 4 age groups to determine whether there was spatial clustering in the observed sex differences [19].
Assessment of Differences in the Presentation of HFRS by Sex
Detailed laboratory and clinical data were available for 221 patients with HFRS at 2 infectious diseases hospitals in Beijing and Shandong Province from 2005 through 2008. The clinical data were collected from all hospitalized patients with cases of HFRS and were acquired by the Chinese Center for Disease Control. The difference in the prevalence of signs and symptoms was compared by sex using Poisson regression, adjusted for age. Because HFRS is a condition diagnosed on the basis of a collection of markers that may differ by sex, latent class analysis (LCA) was performed to identify the presence of groups of individuals with shared manifestations of HFRS [20, 21]. Given the relatively small number of HFRS cases in this dataset, symptoms related to HFRS were selected for inclusion in LCA on the basis of the following a priori criteria: (1) laboratory markers were given preference over clinical signs and symptoms; (2) markers with a frequency of occurrence of >30% and <80%; and (3) lack of significant correlation with other markers. The factors selected for the LCA were thrombocytopenia, proteinuria, hematuria, and hypotension. Sample-size adjusted Bayesian Information Criteria (SSA BIC) were used to compare models and to identify the model with the best fit, with lower BIC scores denoting a better-fitting model. Log likelihood for each model with a given number of classes was estimated. Lo-Mendell-Rubin Adjusted Likelihood ratio test was used to assess the fit of the model with a given class number, compared with a similar model, with 1 less class with a low P value indicating a preferable model. An entropy measure was estimated to identify the quality of the classification of individuals included in the model, with a value approaching 1 indicating better classification. Latent class regression was used to determine whether class membership was related to severity of disease and sex, using Mplus V3 [22].
RESULTS
The Incidence of HFRS Is Higher Among Males of All Ages
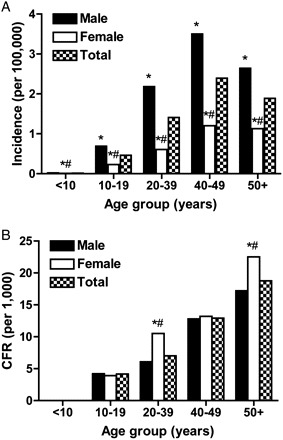
During 2004–2008, there were 80,671 confirmed cases of HFRS in China. Of the confirmed HFRS cases, the greatest number of cases occurred among individuals 20–39 and ≥50 years of age (Table 1). The total number and incidence rate of HFRS were significantly higher among male individuals than among female individuals for all ages (P < .05 for all; Table 1 and Figure 1A).
Total Hemorrhagic Fever With Renal Syndrome Cases and Deaths in China, 2004–2008
| Age, years | Cases (n = 80,671) | Deaths (n = 945) | ||||
| Male | Female | Total | Male | Female | Total | |
| <10 | 69 | 31 | 100 | 0 | 0 | 0 |
| 10–19 | 4051 | 1251 | 5302 | 17 | 5 | 22 |
| 20–39 | 25,019 | 6637 | 31,656 | 152 | 70 | 222 |
| 40–49 | 15,096 | 4841 | 19,937 | 193 | 64 | 257 |
| ≥50 | 16,700 | 6976 | 23,676 | 287 | 157 | 444 |
| Age, years | Cases (n = 80,671) | Deaths (n = 945) | ||||
| Male | Female | Total | Male | Female | Total | |
| <10 | 69 | 31 | 100 | 0 | 0 | 0 |
| 10–19 | 4051 | 1251 | 5302 | 17 | 5 | 22 |
| 20–39 | 25,019 | 6637 | 31,656 | 152 | 70 | 222 |
| 40–49 | 15,096 | 4841 | 19,937 | 193 | 64 | 257 |
| ≥50 | 16,700 | 6976 | 23,676 | 287 | 157 | 444 |
Total Hemorrhagic Fever With Renal Syndrome Cases and Deaths in China, 2004–2008
| Age, years | Cases (n = 80,671) | Deaths (n = 945) | ||||
| Male | Female | Total | Male | Female | Total | |
| <10 | 69 | 31 | 100 | 0 | 0 | 0 |
| 10–19 | 4051 | 1251 | 5302 | 17 | 5 | 22 |
| 20–39 | 25,019 | 6637 | 31,656 | 152 | 70 | 222 |
| 40–49 | 15,096 | 4841 | 19,937 | 193 | 64 | 257 |
| ≥50 | 16,700 | 6976 | 23,676 | 287 | 157 | 444 |
| Age, years | Cases (n = 80,671) | Deaths (n = 945) | ||||
| Male | Female | Total | Male | Female | Total | |
| <10 | 69 | 31 | 100 | 0 | 0 | 0 |
| 10–19 | 4051 | 1251 | 5302 | 17 | 5 | 22 |
| 20–39 | 25,019 | 6637 | 31,656 | 152 | 70 | 222 |
| 40–49 | 15,096 | 4841 | 19,937 | 193 | 64 | 257 |
| ≥50 | 16,700 | 6976 | 23,676 | 287 | 157 | 444 |
Incidence rate (per 100,000 people) (A) and case fatality rate (CFR; per 1000 people) (B) for hemorrhagic fever with renal syndrome (HFRS) in China, 2004–2008. Total incidence and CFR represent the incidence or CFR of HFRS collapsed across both male and female patients. A number (#) symbol designates statistically significant differences between male and female patients, and an asterisk (*) denotes statistically significant differences between the incidence or CFR for either male or female patients and the total incidence or CFR.
CFRs Are Higher Among the Female Population and Are Age Dependent
There were 945 deaths associated with HFRS, resulting in a per year fatality rate of 1.17%, which is within the range reported previously [2, 3]. The greatest number of deaths from HFRS occurred among individuals ≥20 years of age (Table 1). The total number of deaths from HFRS did not differ significantly between the sexes (Table 1). CFRs, however, were significantly higher among female patients than among male patients 20–39 and ≥50 years of age (P < .05 for all; Figure 1B). Among individuals 20–39 years of age, the CFR was significantly higher for female patients within each 5-year age group (data not shown).
Geographical Clustering of HFRS Cases and Deaths Do Not Differ by Sex
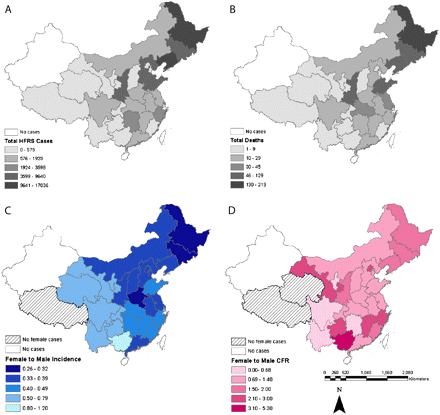
Confirmed HFRS cases were widespread throughout the country, with both the number of cases and the total mortality being lower in the west than in the northeast (Figures 2A and B). Confirmed cases were reported in 29 of 31 provinces in mainland China, with numbers ranging from 1 to 17,036 cases per province, similar to previous reports [2]. The only provinces without HFRS cases in mainland China were Hainan Province and Xinjiang Autonomous Region. There were more male patients with HFRS than female patients with HFRS in each province, with this trend being strongest in those provinces that had the most cases (Figure 2C).
Geographic distribution of hemorrhagic fever with renal syndrome (HFRS) cases (A) and deaths (B) for all individuals ≥10 years of age, by province. Total HFRS cases and deaths were lower in the western provinces than in the northeastern provinces, with darker shading indicating more cases. Female-to-male ratio in the incidence (C) and case fatality rate (CFR); (D) for all individuals ≥10 years of age, by province. Moran’s I was calculated for the sex ratio in HFRS incidence and CFR collapsed across age, with a value <1 indicating that the incidence/CFR was lower and a value >1 indicating that the incidence/CFR was higher for females than for males in an individual province, which is shown by the color of the shading.
Fatalities ranged from 0 deaths per 1000 cases (in provinces with <20 total cases; n = 3 provinces) to a maximum of 54.5 deaths per 1000 cases (mean crude CFR, 18.1 deaths per 1000 cases). Overall, there was no spatial clustering in the sex ratio for CFR among any of the age groups (Figure 2D). The lack of geographical clustering of the sex ratio in HFRS deaths was confirmed with Poisson regression analyses, which revealed that women 20–39 years of age and ≥50 years of age with HFRS still had a significantly higher relative risk of death than that for male patients after adjustment for geographical location (data not shown).
Clinical Symptoms of HFRS May Differ Between the Sexes
Differences in the presentation of classic markers of HFRS were evaluated between male and female patients in a limited number of patients with HFRS, with 174 male (78.3%) and 47 female (21.3%) patients and a sex and age distribution that was similar to that for the total population of patients with HFRS (Supplemental Table 1). The mean age (± standard deviation [SD]) of the sample was 46.4 ± 16.2 years, which did not differ significantly between male patients (45.3 ± 15.6 years) and female patients (50.3 ± 17.8 years). The median time between onset of symptoms and clinical diagnosis was 5 days (interquartile range [IQR], 3–7 days), which also did not differ significantly between the sexes (median time to diagnosis for both sexes, 5 days; IQR, 3–7 days). Based on the standard diagnostic criteria for HFRS severity, 89 (40.3%), 107 (48.4%), 15 (6.8%), and 10 (4.5%) of the patients presented with mild, moderate, severe, and extremely severe disease, respectively; the classification of disease severity did not differ with respect to sex or age.
In the clinical subsample, male patients had higher prevalences of back pain, neck ecchymosis, palpebral edema, and petechiae, compared with prevalences among female patients in univariate analyses (P≤ .05 for all); these differences, however, were not statistically significant after adjustment for age (Table 2).
Frequency of Clinical and Laboratory Markers Among Male and Female Patients With a Diagnosis of Hemorrhagic Fever With Renal Syndrome
| Dependent measure | No. (%) of patients | PRa (95% CI) | ||
| Total (n = 221) | Male (n = 174) | Female (n = 47) | ||
| Self-reported symptoms | ||||
| Headache | 190 (85.9) | 149 (85.6) | 41 (87.2) | 1.02 (0.89–1.15) |
| Back pain | 174 (78.7) | 142 (81.6) | 32 (68.1) | 0.83 (0.67–1.03) |
| Nausea | 131 (59.3) | 108 (62.1) | 23 (48.9) | 0.78 (0.67–1.07) |
| Vomiting | 128 (57.9) | 100 (57.5) | 28 (59.6) | 1.02 (0.78–1.33) |
| Joint pain | 107 (48.4) | 90 (51.7) | 17 (36.2) | 0.69 (0.46–1.03) |
| Abdominal pain | 87 (39.4) | 72 (41.4) | 15 (31.9) | 0.75 (0.48–1.18) |
| Diarrhea | 29 (13.1) | 22 (12.6) | 7 (14.9) | 1.15 (0.52–2.53) |
| Constipation | 11 (4.9) | 9 (5.2) | 2 (4.3) | 0.85 (0.19–3.89) |
| Clinical signs | ||||
| Fever | 218 (98.6) | 171 (98.3) | 47 (100) | 1.02 (0.99–1.03) |
| Neck ecchymosis | 118 (53.4) | 99 (56.9) | 19 (40.4) | 0.71 (0.49–1.03) |
| Conjunctival congestion | 106 (47.9) | 89 (51.2) | 17 (36.2) | 0.70 (0.47–1.05) |
| Petechiae | 97 (44.1) | 78 (45.1) | 19 (40.4) | 0.91 (0.62–1.34) |
| Palpebral edema | 92 (41.6) | 79 (45.4) | 13 (27.7) | 0.59 (0.36–0.97) |
| Mucosal hemorrhage | 68 (31.3) | 56 (32.9) | 12 (25.5) | 0.76 (0.45–1.28) |
| Jaundice | 13 (5.9) | 10 (5.8) | 3 (6.4) | 1.36 (0.39–4.68) |
| Laboratory measures | ||||
| Fever | 218 (98.6) | 171 (98.3) | 47 (100) | 1.02 (0.99–1.03) |
| Proteinuriab | 190 (85.9) | 150 (86.2) | 40 (85.1) | 0.96 (0.88–1.05) |
| Thrombocytopenia | 145 (65.6) | 114 (65.5) | 31 (65.9) | 1.03 (0.83–1.27) |
| Oliguriac | 97 (43.9) | 79 (45.4) | 18 (38.3) | 0.79 (0.53–1.18) |
| Hematuria | 63 (28.5) | 51 (29.3) | 12 (25.5) | 0.82 (0.48–1.40) |
| Hypotensiond | 35 (15.8) | 27 (15.5) | 8 (17.0) | 1.01 (0.49–2.08) |
| Shock | 16 (7.2) | 13 (7.5) | 3 (6.4) | 0.88 (0.26–2.98) |
| Dependent measure | No. (%) of patients | PRa (95% CI) | ||
| Total (n = 221) | Male (n = 174) | Female (n = 47) | ||
| Self-reported symptoms | ||||
| Headache | 190 (85.9) | 149 (85.6) | 41 (87.2) | 1.02 (0.89–1.15) |
| Back pain | 174 (78.7) | 142 (81.6) | 32 (68.1) | 0.83 (0.67–1.03) |
| Nausea | 131 (59.3) | 108 (62.1) | 23 (48.9) | 0.78 (0.67–1.07) |
| Vomiting | 128 (57.9) | 100 (57.5) | 28 (59.6) | 1.02 (0.78–1.33) |
| Joint pain | 107 (48.4) | 90 (51.7) | 17 (36.2) | 0.69 (0.46–1.03) |
| Abdominal pain | 87 (39.4) | 72 (41.4) | 15 (31.9) | 0.75 (0.48–1.18) |
| Diarrhea | 29 (13.1) | 22 (12.6) | 7 (14.9) | 1.15 (0.52–2.53) |
| Constipation | 11 (4.9) | 9 (5.2) | 2 (4.3) | 0.85 (0.19–3.89) |
| Clinical signs | ||||
| Fever | 218 (98.6) | 171 (98.3) | 47 (100) | 1.02 (0.99–1.03) |
| Neck ecchymosis | 118 (53.4) | 99 (56.9) | 19 (40.4) | 0.71 (0.49–1.03) |
| Conjunctival congestion | 106 (47.9) | 89 (51.2) | 17 (36.2) | 0.70 (0.47–1.05) |
| Petechiae | 97 (44.1) | 78 (45.1) | 19 (40.4) | 0.91 (0.62–1.34) |
| Palpebral edema | 92 (41.6) | 79 (45.4) | 13 (27.7) | 0.59 (0.36–0.97) |
| Mucosal hemorrhage | 68 (31.3) | 56 (32.9) | 12 (25.5) | 0.76 (0.45–1.28) |
| Jaundice | 13 (5.9) | 10 (5.8) | 3 (6.4) | 1.36 (0.39–4.68) |
| Laboratory measures | ||||
| Fever | 218 (98.6) | 171 (98.3) | 47 (100) | 1.02 (0.99–1.03) |
| Proteinuriab | 190 (85.9) | 150 (86.2) | 40 (85.1) | 0.96 (0.88–1.05) |
| Thrombocytopenia | 145 (65.6) | 114 (65.5) | 31 (65.9) | 1.03 (0.83–1.27) |
| Oliguriac | 97 (43.9) | 79 (45.4) | 18 (38.3) | 0.79 (0.53–1.18) |
| Hematuria | 63 (28.5) | 51 (29.3) | 12 (25.5) | 0.82 (0.48–1.40) |
| Hypotensiond | 35 (15.8) | 27 (15.5) | 8 (17.0) | 1.01 (0.49–2.08) |
| Shock | 16 (7.2) | 13 (7.5) | 3 (6.4) | 0.88 (0.26–2.98) |
NOTE. CI, confidence interval; PR, prevalence ratio.
Adjusted for age.
Acetic acid was used to measure protein in the urine, which can range from 0.1–10.0 g/L.
Defined as <500 mL urine within 24 h.
Systolic arterial pressure <90 mmHg and the difference of pulse pressure <26 mmHg.
Frequency of Clinical and Laboratory Markers Among Male and Female Patients With a Diagnosis of Hemorrhagic Fever With Renal Syndrome
| Dependent measure | No. (%) of patients | PRa (95% CI) | ||
| Total (n = 221) | Male (n = 174) | Female (n = 47) | ||
| Self-reported symptoms | ||||
| Headache | 190 (85.9) | 149 (85.6) | 41 (87.2) | 1.02 (0.89–1.15) |
| Back pain | 174 (78.7) | 142 (81.6) | 32 (68.1) | 0.83 (0.67–1.03) |
| Nausea | 131 (59.3) | 108 (62.1) | 23 (48.9) | 0.78 (0.67–1.07) |
| Vomiting | 128 (57.9) | 100 (57.5) | 28 (59.6) | 1.02 (0.78–1.33) |
| Joint pain | 107 (48.4) | 90 (51.7) | 17 (36.2) | 0.69 (0.46–1.03) |
| Abdominal pain | 87 (39.4) | 72 (41.4) | 15 (31.9) | 0.75 (0.48–1.18) |
| Diarrhea | 29 (13.1) | 22 (12.6) | 7 (14.9) | 1.15 (0.52–2.53) |
| Constipation | 11 (4.9) | 9 (5.2) | 2 (4.3) | 0.85 (0.19–3.89) |
| Clinical signs | ||||
| Fever | 218 (98.6) | 171 (98.3) | 47 (100) | 1.02 (0.99–1.03) |
| Neck ecchymosis | 118 (53.4) | 99 (56.9) | 19 (40.4) | 0.71 (0.49–1.03) |
| Conjunctival congestion | 106 (47.9) | 89 (51.2) | 17 (36.2) | 0.70 (0.47–1.05) |
| Petechiae | 97 (44.1) | 78 (45.1) | 19 (40.4) | 0.91 (0.62–1.34) |
| Palpebral edema | 92 (41.6) | 79 (45.4) | 13 (27.7) | 0.59 (0.36–0.97) |
| Mucosal hemorrhage | 68 (31.3) | 56 (32.9) | 12 (25.5) | 0.76 (0.45–1.28) |
| Jaundice | 13 (5.9) | 10 (5.8) | 3 (6.4) | 1.36 (0.39–4.68) |
| Laboratory measures | ||||
| Fever | 218 (98.6) | 171 (98.3) | 47 (100) | 1.02 (0.99–1.03) |
| Proteinuriab | 190 (85.9) | 150 (86.2) | 40 (85.1) | 0.96 (0.88–1.05) |
| Thrombocytopenia | 145 (65.6) | 114 (65.5) | 31 (65.9) | 1.03 (0.83–1.27) |
| Oliguriac | 97 (43.9) | 79 (45.4) | 18 (38.3) | 0.79 (0.53–1.18) |
| Hematuria | 63 (28.5) | 51 (29.3) | 12 (25.5) | 0.82 (0.48–1.40) |
| Hypotensiond | 35 (15.8) | 27 (15.5) | 8 (17.0) | 1.01 (0.49–2.08) |
| Shock | 16 (7.2) | 13 (7.5) | 3 (6.4) | 0.88 (0.26–2.98) |
| Dependent measure | No. (%) of patients | PRa (95% CI) | ||
| Total (n = 221) | Male (n = 174) | Female (n = 47) | ||
| Self-reported symptoms | ||||
| Headache | 190 (85.9) | 149 (85.6) | 41 (87.2) | 1.02 (0.89–1.15) |
| Back pain | 174 (78.7) | 142 (81.6) | 32 (68.1) | 0.83 (0.67–1.03) |
| Nausea | 131 (59.3) | 108 (62.1) | 23 (48.9) | 0.78 (0.67–1.07) |
| Vomiting | 128 (57.9) | 100 (57.5) | 28 (59.6) | 1.02 (0.78–1.33) |
| Joint pain | 107 (48.4) | 90 (51.7) | 17 (36.2) | 0.69 (0.46–1.03) |
| Abdominal pain | 87 (39.4) | 72 (41.4) | 15 (31.9) | 0.75 (0.48–1.18) |
| Diarrhea | 29 (13.1) | 22 (12.6) | 7 (14.9) | 1.15 (0.52–2.53) |
| Constipation | 11 (4.9) | 9 (5.2) | 2 (4.3) | 0.85 (0.19–3.89) |
| Clinical signs | ||||
| Fever | 218 (98.6) | 171 (98.3) | 47 (100) | 1.02 (0.99–1.03) |
| Neck ecchymosis | 118 (53.4) | 99 (56.9) | 19 (40.4) | 0.71 (0.49–1.03) |
| Conjunctival congestion | 106 (47.9) | 89 (51.2) | 17 (36.2) | 0.70 (0.47–1.05) |
| Petechiae | 97 (44.1) | 78 (45.1) | 19 (40.4) | 0.91 (0.62–1.34) |
| Palpebral edema | 92 (41.6) | 79 (45.4) | 13 (27.7) | 0.59 (0.36–0.97) |
| Mucosal hemorrhage | 68 (31.3) | 56 (32.9) | 12 (25.5) | 0.76 (0.45–1.28) |
| Jaundice | 13 (5.9) | 10 (5.8) | 3 (6.4) | 1.36 (0.39–4.68) |
| Laboratory measures | ||||
| Fever | 218 (98.6) | 171 (98.3) | 47 (100) | 1.02 (0.99–1.03) |
| Proteinuriab | 190 (85.9) | 150 (86.2) | 40 (85.1) | 0.96 (0.88–1.05) |
| Thrombocytopenia | 145 (65.6) | 114 (65.5) | 31 (65.9) | 1.03 (0.83–1.27) |
| Oliguriac | 97 (43.9) | 79 (45.4) | 18 (38.3) | 0.79 (0.53–1.18) |
| Hematuria | 63 (28.5) | 51 (29.3) | 12 (25.5) | 0.82 (0.48–1.40) |
| Hypotensiond | 35 (15.8) | 27 (15.5) | 8 (17.0) | 1.01 (0.49–2.08) |
| Shock | 16 (7.2) | 13 (7.5) | 3 (6.4) | 0.88 (0.26–2.98) |
NOTE. CI, confidence interval; PR, prevalence ratio.
Adjusted for age.
Acetic acid was used to measure protein in the urine, which can range from 0.1–10.0 g/L.
Defined as <500 mL urine within 24 h.
Systolic arterial pressure <90 mmHg and the difference of pulse pressure <26 mmHg.
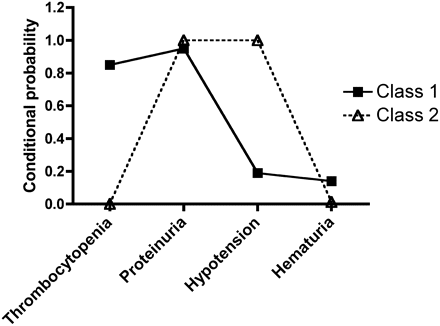
LCA of thrombocytopenia, proteinuria, hypotension, and hematuria revealed 2 groups of individuals with different profiles of clinical presentation (Supplemental Table 2; Figure 3). Class 1 comprised the majority of patients with HFRS (83.7%) and was defined as having a high probability of thrombocytopenia and a low probability of hypotension. Class 2 comprised a smaller proportion of patients with HFRS (16.3%) and was differentiated from Class 1 by having a low probability of thrombocytopenia but a high probability of hypotension (Figure 3). Class 2 membership was associated with a diagnosis of extremely severe disease (odds ratio [OR], 3.42; 95% confidence interval [CI], 1.31–8.92), and female patients were less likely to belong to Class 2 than were male patients (9.5% vs 20.4%); this sex difference did not achieve statistical significance (OR, 0.47; 95% CI, 0.17–1.25).
The conditional probability of class assignment for individuals with symptoms of hemorrhagic fever with renal syndrome (HFRS). Individuals assigned to class 1 were more likely to present with thrombocytopenia and hematuria and were less likely to have hypotension than were individuals assigned to class 2. All individuals assigned to class 2 had proteinuria and hypotension. Female patients were less likely to be assigned to class 2 than were male patients.
DISCUSSION
Sex differences are reported in the outcome of many viral infections, but the significance of these differences for the diagnosis and treatment of disease is often overlooked. Although the incidence of HFRS was higher in the male population, CFRs associated with HFRS were higher in the female population. These outcomes were age dependent; differences in HFRS incidence were only observed at or after the time of puberty and into the later stages of adulthood. Sex differences in CFR were only apparent among individuals 20–39 and ≥50 years of age. Age-dependent sex differences in mortality rates also have been observed for NE in Sweden, and death from NE has only been reported in individuals ≥50 years of age [5]. Whether age-dependent CFRs reflect the prevalence of other risk factors associated with worse outcome needs to be evaluated.
Differences between the male and female populations with respect to the incidence of HFRS may reflect sex differences in the likelihood of contact with infectious rodents or their excrement [23]. The male-biased incidence of disease has been reported for both HFRS and HPS in several countries, in addition to China [4–11]. Data from Sweden illustrate that the prevalence of people with antibody against PUUV does not differ between the sexes, which suggests that differences in exposure may not be involved [12].
The data from this study illustrate a paradox; the incidence of HFRS was higher in the male population, but mortality rates were higher in the female population and were age dependent. Similarly, in Sweden, the incidence of NE is greater in the male population, but fatality rates during the acute phase of NE are higher in the female population [5]. There are sex differences in health-seeking behaviors and access to health care in several regions of the world [24]. Because female patients experience a worse disease outcome both in Sweden [5] and in China, differences in access to health care may not explain why female patients experience a worse outcome from hantaviral disease. Sex differences in the diagnosis of HFRS by physicians cannot be discounted, because gender biases have been shown for the diagnosis of other diseases [25, 26].
To test the hypothesis that differences between the male and female populations with respect to the incidence of HFRS and its associated CFR reflect the differential distribution of male and female cases across provinces, we evaluated differences in the geographical distribution and clustering of HFRS cases and deaths. Overall, there was geographic heterogeneity in the distribution of HFRS cases, with the number of cases generally being lower in the western provinces than in the northeastern provinces. The male-biased incidence of HFRS was most prevalent in those provinces that had the greatest number of cases (ie, the northeastern provinces). The difference in mortality rates between female patients and male patients, however, showed no significant spatial clustering at a countrywide level or in Poisson regression models, indicating that the absolute case numbers did not drive differential mortality rates.
We considered whether the constellations of HFRS markers present differently in male and female individuals and whether sex differences in clinical presentation of disease could explain the higher CFR observed in female patients. Although standard regression analyses revealed no differences in the prevalence of symptoms by sex, after adjusting for age, LCA using a subset of 4 HFRS-related markers identified 2 distinct profiles. A majority of patients with HFRS had mild disease with a high probability of thrombocytopenia but a low probability of hypotension. Conversely, a second, smaller group of patients presented with extremely severe HFRS and had an elevated probability of hypotension but a low probability of thrombocytopenia. Notably, there was a dissociation between thrombocytopenia and hypotension, which has been observed in other patients with HFRS [27]. The probability of proteinuria did not differ between LCA classes or by sex, which suggests that this remains a reliable diagnostic criterion of HFRS in both sexes, but that it is not an effective marker for differentiating subgroups of patients. Female patients with HFRS tended to be in the symptom class defined by an elevated probability of thrombocytopenia and a low probability of hypotension, which may indicate that they had a less severe presentation of HFRS illness. This observation conflicts with the higher CFR observed among female patients and suggests that the symptoms traditionally used as clinical diagnostic markers to assess the severity of HFRS may differ by sex.
This study has several limitations. The relatively small size of the clinical subsample, particularly of women, and the nonrandom sampling of the subsample make it difficult to apply these findings to the broader population of individuals with HFRS. There was, however, no significant difference in the age and sex distribution of this clinical subsample when compared with the larger population of individuals with HFRS for the period 2004–2008. This study used data from >80,000 patients with HFRS that were collected from a highly effective surveillance system in mainland China. Although the total number of cases documented is very large, differential ascertainment of HFRS cases by age or sex may potentially bias estimates of the true burden of HFRS in specific groups. The higher incidence of HFRS among the male population and the higher CFR among female patients across all provinces in China suggests a negligible influence of systemic differences in reporting.
The biological mechanisms for these sex differences in the outcome of disease must be explored. During acute PUUV infection, circulating concentrations of CXCL8 and CXCL10 are higher, whereas concentrations of interleukin 9 and granulocyte macrophage colony-stimulating factor are lower in men than in women [28]. Whether the inflammatory response to hantavirus infection [29–31] results in more-severe disease in female individuals because of a stronger, more sustained antiviral response should be evaluated [32]. Sexually dimorphic immune responses are mediated, in part, by sex hormone activity [13, 15]. Concentrations of androgens, estrogens, and progesterone should be characterized, especially because sex differences in the outcome of HFRS are age dependent and correspond with puberty and the menopausal transition. The current study raises awareness about the significance of sex-specific differences in response to hantaviruses by providing one of the largest patient samples systematically evaluated for sex differences in response to an emerging infectious disease.
The authors thank the medical personnel for investigation and reporting of HFRS cases, the Chinese Center for Disease Control and Prevention for providing the HFRS surveillance data, and Dr. Karen Bandeen-Roche for advice about latent class analyses.
Financial support. The Special Program for Prevention and Control of Infectious Diseases (No. 2008ZX10004-012), the Natural Science Foundation of China (No. 30810103903 and 30972521), the W. Harry Feinstone endowment, and an institutional post-doctoral training fellowship in Cancer Prevention, Etiology and Control, National Cancer Institute (5T32CA009314-28).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgements section.





Comments