-
PDF
- Split View
-
Views
-
Cite
Cite
Naoki Mita, Xiaojuan He, Kodai Sasamoto, Tomohide Mishiba, Toshio Ohshima, Cyclin-Dependent Kinase 5 Regulates Dendritic Spine Formation and Maintenance of Cortical Neuron in the Mouse Brain, Cerebral Cortex, Volume 26, Issue 3, March 2016, Pages 967–976, https://doi.org/10.1093/cercor/bhu264
Close - Share Icon Share
Abstract
Cyclin-dependent kinase 5 (Cdk5) activity is dependent on its association with 1 of 2 neuron-specific activators, p35 or p39. Cdk5 and its activators play an important role in brain development as well as higher functions like synaptic plasticity, learning, and memory. Reduction in p35 was reported in postmortem schizophrenia brain, in which reduced dendritic spine density was observed. Previous in vitro experiments have shown that Cdk5 is involved in dendritic spine formation, although in vivo evidence is limited. We examined dendritic spine formation in inducible-p35 conditional knockout (p35 cKO); p39 KO mice. When we deleted the p35 gene either during early postnatal days or at adult stage, we observed reduced spine densities of layer V neurons in the cerebral cortex and CA1 pyramidal neurons in the hippocampus. We further generated CA1-specific p35 conditional knockout (CA1-p35 cKO) mice and also CA1-p35 cKO; p39 KO mice in which have specific deletion of p35 in the CA1 region of hippocampus. We found a greater reduction in spine densities in CA1 pyramidal neurons in CA1-p35 cKO; p39 KO mice than in CA1-p35 cKO mice. These results indicate that dendritic spine formation and neuronal maintenance are dependent on Cdk5 activity.
Introduction
Postsynaptic excitatory neurons contain dendritic spines. Their proper density and morphology are important for higher brain functions like learning and memory. Dendritic spines are important for neurotransmission efficiency because neuronal activity is dependent on plastic changes in their density and morphology. Theta burst stimulation induces long-term potentiation (LTP) and leads to the formation of new spines and enlargement of existing ones (Nägerl et al. 2004; Okamoto et al. 2004), whereas low-frequency stimulation induces long-term depression and causes spine retraction or shrinkage (Nägerl et al. 2004; Okamoto et al. 2004; Zhou et al. 2004). Spine formation begins when a dendrite comes in contact with an axon. Spines mature with the accumulation of synaptic proteins including dreblin and PSD95 and the dynamic reconstruction of the actin cytoskeleton (Takahashi et al. 2003). In mature spines, the regulation of the actin cytoskeleton affects spine morphology and plastic changes in neurotransmission efficiency (Fischer et al. 1998). In the rodent brain, spine density peaks at puberty and decreases at adulthood (Koss et al. 2014). Reduced dendritic spine density has been reported in multiple brain regions in schizophrenia (Glausier and Lewis 2013).
The activity of cyclin-dependent kinase 5 (Cdk5) is dependent on its association with 1 of 2 neuron-specific activators, p35 or p39. Mutant mouse studies have shown involvement of Cdk5 and its activators in brain development in addition to higher brain functions like synaptic plasticity and learning and memory (Ohshima et al. 2005; Hawasli et al. 2007; Guan et al. 2011). Although genetic mutation of CDK5 gene in human patients is yet to be reported, reduction in p35 has been reported in postmortem brain samples from patients with schizophrenia (Engmann, Hortobágyi, Pidsley, et al. 2011). Recent studies have identified Cdk5 substrates and showed that Cdk5 is involved in regulation of spine formation. Synaptic proteins, phosphorylated by Cdk5, ephexin 1 (Fu et al. 2007), WAVE1 (Kim et al. 2006), CRMP1 (Yamashita et al. 2007), TrkB (Cheung et al. 2007), and PSD95 (Morabito et al. 2004) have been shown to play a role in spine formation (Morabito et al. 2004; Kim et al. 2006; Yamashita et al. 2007) and maintenance (Fu et al. 2007; Lai and Ip 2009). However, their functions differ such that some of them are crucial for spine formation (Yamashita et al. 2007) and some for spine retraction (Fu et al. 2007). Thus, the overall impact of Cdk5 on dendritic spine formation and maintenance remains unclear.
Although several studies have indicated that Cdk5 is involved in spine formation and maintenance, most are based on findings in cultured neurons. Thus, in vivo evidence is limited. Here, we generated inducible-p35 cKO; p39 KO mice, and induced p35 deletion at postnatal day (P) 3–4 to examine spine formation at P18. We also generated CA1-specific p35 conditional knockout (CA1-p35 cKO) mice and CA1-p35 cKO; p39 KO mice with p35 deletion in CA1 region of the hippocampus after P17. We found a reduction in spine densities in cerebral layer V neurons and hippocampal CA1 pyramidal neurons in inducible-p35 cKO; p39 KO mice, indicating that Cdk5 is required for spine formation. We also observed a reduction in spine densities in CA1 pyramidal neurons of CA1-p35 cKO mice and an even further reduction in CA1-p35 cKO; p39 KO mice at 4 weeks of age. Furthermore, we found reduced spine densities in cerebral layer V neurons and hippocampal CA1 pyramidal neurons in inducible-p35 cKO; p39 KO mice when we deleted p35 gene at 4 months of age. These results indicate that spine formation and maintenance are dependent on Cdk5/p35 and Cdk5/p39 kinase activity in the mouse brain.
Materials and Methods
Mice
Inducible-p35 cKO; p39 KO Mice
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Waseda University. Throughout the experiment, all efforts were made to minimize the number of animals used and their suffering. Mice were fed ad libitum with standard laboratory food and water in animal cages under a 12-h light/dark cycle. p35-flox mice were generated in a C57BL/6 background (He et al. 2014). CAGGCre-ER mice (Hayashi and McMahon 2002) were obtained from the Jackson laboratory (stock number 004682). By intercrossing p35 flox/+; p39−/−; CAGGCre-ER, p35 flox/flox; p39−/−; CAGGCre-ER mice were obtained. Cre activity was induced by the intraperitoneal injection of 4-OH-tamoxifen (2 mg/30 g body weight in corn oil, Sigma) daily at P3–4. p35 flox/flox; p39−/− mice and wild-type (WT) mice treated with 4-OH-tamoxifen were used as control mice. In other experiments, cre activity was induced at 4 months old by oral administration of tamoxifen (3 mg/40 g body weight in corn oil) daily for 3 days.
CA1-p35 cKO and CA1-p35 cKO; p39 KO Mice
CaMKII-Cre mice (Tsien et al. 1996) were obtained from the Jackson Laboratory (stock number 005359). By crossing p35 flox/+; CaMKII-Cre and p35 flox/flox mice, CaMKII-Cre (CA1-p35 cKO) mice were obtained. Cre activity was expressed in the hippocampal CA1 region after P17 (Tsien et al. 1996). p39 mutant mice (Ko et al. 2001) were crossed with p35 flox/+; CaMKII-Cre mice. Offspring were further intercrossed to obtain p35 flox/flox; p39−/−; CaMKII-Cre (CA1-p35 cKO; p39 KO) mice.
Biochemical Analysis
Western Blotting
Hippocampi were collected from 5-week-old CA1-p35 cKO, CA1-p35 cKO; p39 KO mice and control mice. Western blot analysis was conducted as previously described (Ohshima et al. 2007). The primary antibodies used were polyclonal antibody against p35 (C-19, 1 : 300, Santa Cruz), anti-actin (1 : 1000, Sigma), and anti-β-tubulin (1 : 1000, Sigma). Statistical analyses were conducted using the Student's t-test. Mean ± standard error of mean (SEM) were reported in graphs, and a value of P < 0.05 was considered to be statistically significant.
Histological Analysis
Immunohistochemistry
Mice were anesthetized using diethyl ether and then perfused transcardially with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). Brain samples were fixed in 4% PFA in PBS overnight at 4 °C. After dehydration in 20% sucrose in PBS, samples were embedded in OCT compound (Sakura Finetek, Japan). Cryosections were cut at 14 µm and stored at −20 °C until immunostaining. For immunostaining, cryosections were incubated with the primary antibodies at 4 °C overnight. After washing with PBS, the secondary antibody, AlexaFluor, was applied, and sections were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA). Anti-pCRMP2S522, which recognizes phospho-CRMP2 at Ser522 (1 : 100, rabbit, EMC Biosciences), anti-glial fibrillary acidic protein (GFAP; 1 : 500, rabbit, Dako), and anti-Iba1 (1 : 500, mouse, Millipore) were used as primary antibodies. All immunostaining images were captured by a BX50 microscope (Olympus, Japan).
Rapid Golgi Staining
For modified Golgi-Cox staining, an FD Rapid GolgiStain kit was used (FD NeuroTechnologies, MD, USA). Stained slices were sectioned at 200 μm thickness. Pyramidal hippocampal CA1 neurons and thick tufted pyramidal layer V neurons in the somatosensory cortex of cerebrum in each mouse were selected for the analysis as described in our previous work (Yamashita et al. 2007). The numbers of dendritic spines in apical dendrites of CA1 pyramidal neurons or those in basal dendrites of cerebral layer V neurons were counted under a BX50 microscope (Olympus, Japan) with a UPlanSApo ×40 (NA = 0.95) objective. Spines were counted in 50 µm segments of proximal dendrites. In a typical experiment, more than 2000 spines were counted on more than 50 dendritic segments in 25 neurons. Average spine densities per 50 µm dendritic segments were then calculated for each treatment group, along with the SEM. Groups of spines were compared using the Student's t-test.
Results
Reduction of Dendritic Spine Density in the Cortical Neurons of Inducible-p35 cKO; p39 KO Mice
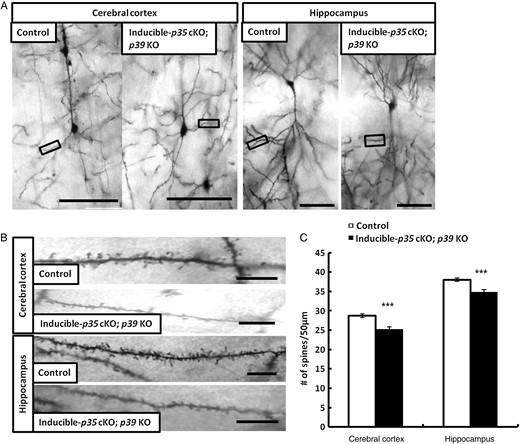
We generated inducible-p35 cKO; p39 KO mice with p35 deletion by mating p35-flox; p39 KO mice with CreER-mice (Hayashi and McMahon 2002). We induced Cre activity via intraperitoneal administration of 4-OH tamoxifen at P3–4 and analyzed spine density after 2 weeks at P18. Reductions in p35 protein were confirmed by western blotting (data not shown). To evaluate the impact of p35 and p39 expression on dendritic spines in inducible-p35 cKO; p39 KO mice, we conducted rapid Golgi staining. Golgi staining showed a reduction in the numbers of dendritic spines in layer V neurons of the somatosensory cortex and hippocampal CA1 pyramidal neurons of inducible-p35 cKO; p39 KO mice, compared with those in control mice at P18 (Fig. 1A–C). These results indicate that Cdk5 is required for dendritic spine formation in vivo.
Reduction of dendritic spine density in layer V pyramidal neurons of the cerebral cortex and hippocampal CA1 pyramidal neurons in Inducible-p35 cKO; p39 KO mice. (A) Representative photographs of dendritic segments of layer V pyramidal neurons of the cerebral cortex and hippocampal CA1 pyramidal neurons in p35 flox/flox (control) and Inducible-p35 cKO; p39 KO mice. Scale bar, 50 µm. (B) Magnified images of areas indicated in A. Scale bar, 10 µm. (C) Reduced numbers of spines were observed in layer V pyramidal neurons in cerebral cortex and hippocampal CA1 pyramidal neurons of Inducible-p35 cKO; p39 KO mice compared to those in control mice. *** p < 0.001.
Reduction of Dendritic Spine Density in the Hippocampal CA1 Pyramidal Neurons of CA1-p35 cKO Mice
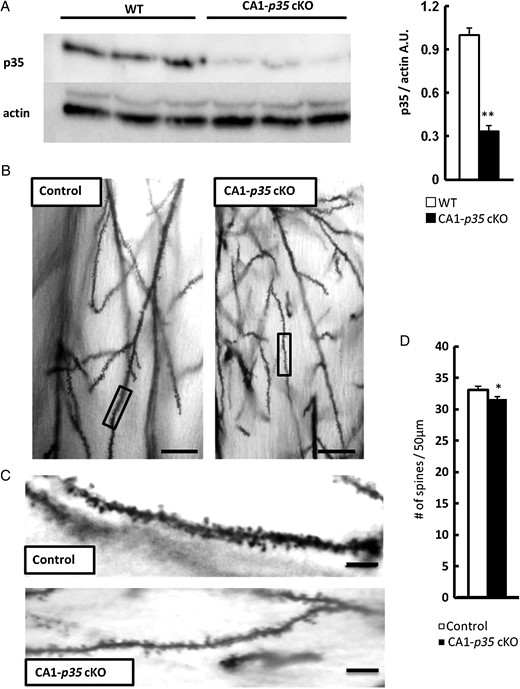
We generated CA1-specific p35 conditional KO (CA1-p35 cKO) mice by crossing p35-flox and CaMKII-cre mice in which cre recombinase is expressed in the pyramidal neurons of the hippocampal CA1 region after P17 (Tsien et al. 1996). To confirm the reduction in p35 protein and evaluate the impact of loss of p35 expression on dendritic spines in CA1-p35 cKO mice, we conducted a western blot analysis. Results showed a remarkable reduction in p35 protein level in the hippocampus of CA1-p35 cKO mice, compared with that in WT mice at 4 weeks (Fig. 2A). Next, we examined dendritic spine density by Golgi staining in 5-week-old CA1-p35 cKO mice. Golgi staining of the forebrain slice showed a reduction in the number of spines in hippocampal CA1 pyramidal neurons in CA1-p35 cKO mice compared with that in control mice (Fig. 2B–D), but not in layer V pyramidal neurons in the cerebral cortex of CA1-p35 cKO mice (data not shown). These results indicate that Cdk5 is required for maintaining dendritic spine density in the mouse brain.
Reduction of dendritic spine density in hippocampal CA1 pyramidal neurons of CA1-p35 cKO mice. (A) Western blot analysis of hippocampal tissue from WT and CA1-p35 cKO mice for protein levels of p35 and actin. Reduced levels of p35 protein were observed in the hippocampus of CA1-p35 cKO compared with those in 4-week-old WT mice (n = 6, **P < 0.01). (B) Representative photographs of dendritic segments of hippocampal CA1 pyramidal neurons of p35 flox/flox (control) and CA1-p35 cKO mice. Scale bar, 50 μm. (C) Magnified images of areas indicated in B. Scale bar, 10 μm. (D) Reduced numbers of spines were observed in the CA1 pyramidal neurons of CA1-p35 cKO mice compared with those in control mice. *P < 0.05.
CA1-p35 cKO; p39 KO Mice Exhibit Extended Reduced Dendritic Spine Density in Hippocampal CA1 Pyramidal Neurons, Reduced Body Weight, and Earlier Lethality
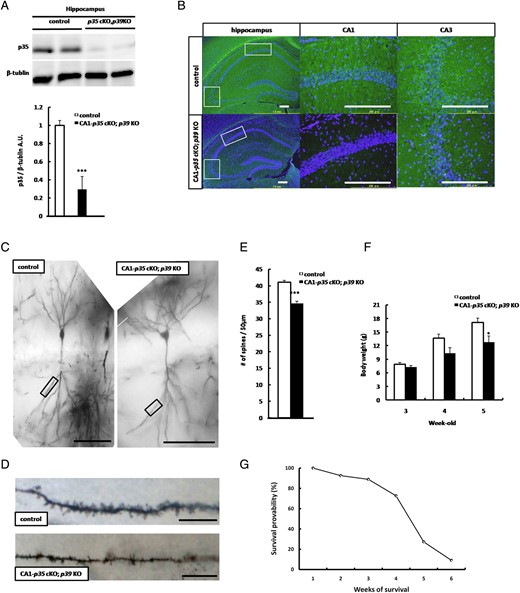
Since p39, another isoform of the Cdk5 activating subunit may compensate for the phenotype of CA1-p35 cKO mice, as observed in p35 KO mice (Chae et al. 1997; Ko et al. 2001; Ohshima et al. 2001), we generated CA1-p35 cKO; p39 KO mice. To confirm the reduction of p35 protein and reduced Cdk5 activity in the hippocampal CA1 region of CA1-p35 cKO; p39 KO mice, we conducted western blotting. The western blot analysis showed reduced levels of p35 protein in the hippocampus of CA1-p35 cKO; p39 KO mice compared with that in p35-flox/flox mice at age 4 weeks (Fig. 3A). To evaluate Cdk5 kinase function loss in hippocampal CA1 pyramidal neurons of CA1-p35 cKO; p39 KO mice, we conducted immunostaining by using a phospho-specific antibody of CRMP2 at Ser522 pCRMP2 (S522), which can detect Cdk5 phosphorylation of CRMP2 (Uchida et al. 2005). Immunostaining for pCRMP2(S522) in a hippocampal section showed a reduction in the immunoreactivity of pCRMP2(S522) in the CA1 area of CA1-p35 cKO; p39 KO mice compared with that in control mice, and no reduction in the CA3 area (Fig. 3B). To evaluate the impact of p35 and p39 loss on spine density in CA1-p35 cKO; p39 KO mice, we conducted rapid Golgi staining. Golgi staining showed a reduction in the number of dendritic spines in hippocampal CA1 pyramidal neurons in CA1-p35 cKO; p39 KO mice compared with control mice at 5 weeks old (Fig. 3C–E) and no reduction in the number of spines in layer V pyramidal neurons of the cerebral cortex (Supplementary Fig. 1). While CA1-p35 cKO mice did not show phenotypic changes, CA1-p35 cKO; p39 KO mice exhibited reduced body weight (Fig. 3F) and early lethality at 4 weeks of age (Fig. 3G). Approximately 80% of CA1-p35 cKO; p39 KO mice died by 5 weeks of age (Fig. 3G).
CA1-p35 cKO; p39 KO mice exhibit reduced dendritic spine density in hippocampal CA1 pyramidal neurons, reduced body weight, and early lethality. (A) Western blot analysis of hippocampal tissue from p35 flox/flox (control) and CA1-p35 cKO; p39 KO mice for protein levels of p35 and β-tublin, respectively, ***P < 0.001. (B) Immunostaining for pCRMP2 (S522) in coronal sections of hippocampal areas from p35 flox/flox (control) and CA1-p35 cKO; p39 KO mice at age 5 weeks. Scale bar 200 µm. (C) Representative photographs of dendritic segments of hippocampal CA1 pyramidal neurons at age 5 weeks. Scale bar, 100 μm. (D) Magnified images of the areas indicated in A. Scale bar, 10 μm. (E) Reduced dendritic spine density in hippocampal CA1 pyramidal neurons spine density was observed in hippocampal CA1 pyramidal neurons of CA1-p35 cKO; p39 KO mice compared with those of control mice, ***P < 0.001. (F) Loss of body weight in CA1-p35 cKO; p39 KO mice was observed at age 4 weeks. *P < 0.05. (G) Early lethality was observed at 4 weeks of age. Approximately 80% CA1-p35 cKO; p39 KO mice were dead at age 5 weeks.
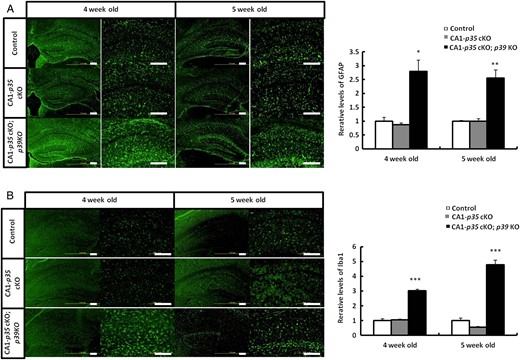
Increasing Reactive Astrocytes and Activated Microglia in the Hippocampus of CA1-p35 cKO; p39 KO Mice
To investigate the cause of early lethality of CA1-p35 cKO; p39 KO mice, we analyzed neuroinflammation that has been observed in Cdk5 cKO mice previously (Takahashi et al. 2010). Coronal sections of the hippocampus of p35 flox/flox (control), CA1-p35 cKO, and CA1-p35 cKO; p39 KO mice were stained with anti-GFAP antibody for astrocytes and anti-Iba1 for microglia. Immunostaining with these antibodies showed that reactive astrocytes and activated microglia increased in CA1-p35 cKO; p39 KO mice than in control mice at age 4 and 5 weeks, while no differences could be seen between CA1-p35 cKO mice and control mice (Fig. 4A,B). These results indicate that a severe reduction in Cdk5 activity leads to an increase in reactive astrocytes and activated microglia in the mouse brain at this age.
Increase in reactive astrocytes and activated microglia in the hippocampus of CA1-p35 cKO; p39 KO mice. Immunostaining for astrocyte (GFAP) (A) and microglia (Iba1) (B) in hippocampal areas in coronal sections from p35 flox/flox (control), CA1-p35 cKO, and CA1-p35 cKO; p39 KO mice. Scale bar, 200 µm. Reactive astrocytes (A) and activated microglia (B) were increased in CA1-p35 cKO; p39 KO mice than in control mice, while no differences were shown between CA1-p35 cKO mice and control mice. *P < 0.05, **P < 0.01, ***P < 0.001.
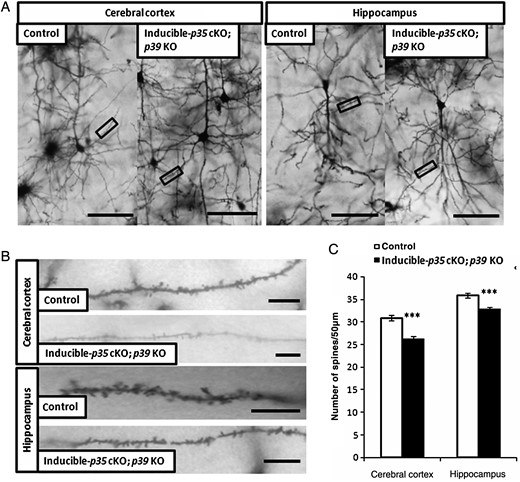
Reduced Dendritic Spine Densities in Adult Inducible-p35 cKO; p39 KO Mice
We analyzed the impact of induced deletion of p35 gene on dendritic spine density in 4-month-old mice. Reductions in p35 protein level in the cerebral cortex and hippocampus were confirmed by western blotting at 1 week post-deletion (data not shown). At the end of 2 weeks, we analyzed the numbers of dendritic spines in layer V neurons of the somatosensory cortex and hippocampal CA1 pyramidal neurons in the inducible-p35 cKO; p39 KO mice and compared it with those in the control mice. As shown in Figure 5, dendritic spine densities decreased in the neurons of both the brain regions in the inducible-p35 cKO; p39 KO mice, suggesting that Cdk5 activity is required for the maintenance of proper dendritic spine density in cortical and hippocampal neurons in the adult mouse brain.
Reduction of dendritic spine density in layer V pyramidal neurons of the cerebral cortex and hippocampal CA1 pyramidal neurons in adult inducible-p35 cKO; p39 KO mice. (A) Representative photographs of dendritic segments of layer V pyramidal neurons of the cerebral cortex and hippocampal CA1 pyramidal neurons in p35 flox/flox (control) and inducible-p35 cKO; p39 KO mice. Scale bar, 50 µm. (B) Magnified images of areas indicated in A. Scale bar, 10 µm. (C) Reduced numbers of spines were observed in layer V pyramidal neurons in the cerebral cortex and hippocampal CA1 pyramidal neurons of adult inducible-p35 cKO; p39 KO mice as compared to those in the control mice. ***p < 0.001.
Discussion
In vitro studies suggest that Cdk5 plays an important role in dendritic spine formation and maintenance (Lai and Ip 2009). However, up until now, in vivo studies of Cdk5 functioning has been limited. Previous studies examined if dendritic spine formation was dependent on Cdk5 activity through the phosphorylation of synaptic proteins (Morabito et al. 2004; Kim et al. 2006; Yamashita et al. 2007). These results indicated that the phosphorylation of WAVE1 and PSD95 by Cdk5 contributes to the inhibition of dendritic spine formation (Kim et al. 2006) and PSD95 regulation (Morabito et al. 2004), respectively. On the other hand, phosphorylation of CRMP1 by Cdk5 has been shown to contribute to increasing dendritic spine density in vitro (Yamashita et al. 2007). Because these results contradict one another, the overall effect of Cdk5 activity on dendritic spine formation remains unclear. In the present study, we generated inducible-p35 cKO; p39 KO mice with p35 deletion at P3–4 and examined spine formation at P18. We found a reduction in dendritic spine densities of layer V neurons of cerebral cortex and CA1 pyramidal neurons in inducible-p35 cKO; p39 KO mice (Fig. 1), indicating that Cdk5 is required for dendritic spine formation in vivo. Because we analyzed dendritic spine density at only this time point, we cannot exclude the possibility that the development of spines is delayed or the rate of spine elimination is altered in inducible-p35 cKO; p39 KO mice. In addition, we could not analyze morphological distributions of dendritic spines because of the technical limitations. In present study, we quantified spine densities of cortical neurons in the mutant mice by Golgi staining. Further studies are required for detailed analysis of spine formation in loss of Cdk5 activity in vivo. We plan to characterize the changes of their spine shapes by using GFP-M mice to visualize spine morphology of cortical neurons in the mouse brain.
Other studies examined the relationship between Cdk5 activity and dendritic spine maintenance (Fu et al. 2007; Lai et al. 2012). Previous results indicated that phosphorylation of ephexin1 by Cdk5 contributes to the inhibition of ephrinA1-triggered dendritic spine retraction (Fu et al. 2007). Lai et al. (2012) showed that phosphorylation of TrkB by Cdk5 is required for brain-derived neurotrophic factor-induced dendritic spine formation and enlargement of hippocampal neurons. In the present study, we generated CA1-p35 cKO mice and CA1-p35 cKO; p39 KO mice with a p35 deletion in the CA1 region of the hippocampus after initial spine formation. We observed a reduction in dendritic spine densities in CA1 pyramidal neurons of CA1-p35 cKO mice and an even further reduction in CA1-p35 cKO; p39 KO mice, indicating that dendritic spine maintenance is dependent on Cdk5/p35 or Cdk5/p39 kinase activity in vivo.
Koss et al. (2014) studied ontogenic changes in the dendritic spine density in pyramidal neurons of layer V of the medial prefrontal cortex (mPFC) in P20 (juvenile), P35 (puberty), and P90 (adulthood) rat brains. The density of dendritic spines in the mPFC neurons increases between P20 and P35, while it significantly decreases between P35 and P90. In the cerebral cortex of macaque and rhesus monkeys and humans, the number of dendritic spines rapidly increases after birth and peaks in the early infantile period (Huttenlocher 1990; Bourgeois et al. 1994; Petanjek et al. 2011). Thereafter, spine density decreases during the later infantile period and adolescence period to reach the low adult levels (Missler et al. 1993). A decrease in dendritic spine density during the transition from puberty to adulthood has also been reported in mouse hippocampus (Meyer et al. 1978). These studies indicate ontogenic similarity between rodents, primates, and humans in spine formation and pruning. The mechanism of spine formation and pruning is a research hotspot because of its importance in several developmental disorders and psychiatric diseases (Penzes et al. 2011). In schizophrenia, the over-pruning of spines during late childhood or adolescence has been implicated as the cause for a decreased number of spines (Feinberg 1982; Glantz and Lewis 2000; Faludi and Mirnics 2011). Most pronounced decreased dendritic spine density was found in the prefrontal cortex in schizophrenia patients (Hill et al. 2006). Interestingly, reduction in p35 was reported in postmortem schizophrenic brain including prefrontal cortex and hippocampus, and p35+/− mice showed cognitive endophenotypes of schizophrenia (Engmann, Hortobágyi, Pidsley, et al. 2011). Therefore, we induced deletion of the p35 gene at 4 months of age in mice and analyzed spine densities in layer V neurons of the cerebral cortex and in CA1 pyramidal neurons 2 weeks later. We found reduction in spine densities in both brain regions (Fig. 5), suggesting involvement of Cdk5/p35 in spine pruning. We also analyzed spine densities of apical dendrites in layer V pyramidal neurons of the cerebral cortex and basal dendrites in hippocampal CA1 pyramidal neurons in adult inducible-p35 cKO; p39 KO mice. Our preliminary results indicate that reductions of spine densities occur in apical and basal dendrites of these cortical neurons in adult inducible-p35 cKO; p39 KO mice (preliminary observation by T.O. and K.S.).
p25 overexpression has been shown to enhance Cdk5 activity in adult mice and increase the number of dendritic spines and synapses (Fischer et al. 2005), and enhanced memory formation (Angelo et al. 2003; Ris et al. 2005; ; Engmann, Hortobágyi, Thompson, et al. 2011). This result is consistent with our finding that a reduction in Cdk5 activity leads to decreased spine density. Enhanced hippocampal LTP and improved learning and memory were also shown in mice overexpressing p25 (Fischer et al. 2005). Therefore, in a situation in which there is reduced Cdk5 activity, impaired LTP and learning and memory could be expected. However, some reports make this expectation less warranted. Hawasli et al. (2007) demonstrated that Cdk5 cKO mice exhibited improved performance in spatial learning tasks and enhanced hippocampal LTP. In contrast, Guan et al. (2011) suggested that Cdk5 ablation impaired spatial memories and LTP. These results cannot exclude the possibility of secondary phenotypes caused by an increase in NR2B (Hawasli et al. 2007) or the protein kinase C signal transduction system (Guan et al. 2011) due to Cdk5 loss. CA1-p35 cKO mice, which can be easily analyzed except secondary changes mentioned above, are ideal for studying Cdk5/p35 function on learning and memory by electrophysiological and behavioral analyses. Furthermore, the hippocampal neuroinflammation observed in CA1-p35 cKO; p39 KO mice may affect dendritic spine density in these mutant mice. We previously showed that reactive astrocytes, activated microglia, and neuronal loss lead to early lethality in Cdk5 cKO mice (Takahashi et al. 2010). CA1- p35 cKO; p39 KO mice also showed early lethality (Fig. 3G). In CaMKII-Cre mice, we used in this study (Tsien et al. 1996), Cre-mediated p35 deletion is relatively specific to CA1 area in 3–4 weeks of age. However, deletion of p35 was spreading to cerebral cortex in 5 weeks of age, as we noted reduced immunoreactivity of pCRMP2(S522) in the cerebral cortex of CA1-p35 cKO; p39 KO mice at age 5 weeks (Fig. 3B). These changes of Cre activity in this CaMKII-Cre mouse line were also reported by other groups (Rampon et al. 2000; Fukaya et al. 2003; Guan et al. 2011). Neuroinflammation in other Cdk5 cKO mice have not been studied yet (Hawasli et al. 2007; Guan et al. 2011). Since there is no inflammatory reaction in CA1-p35 cKO mice, decreasing Cdk5 activity must lead to a reduction in dendritic spine density of cortical neurons in the brain.
It remains unclear which substrate of Cdk5 is involved in the reduction of dendritic spine density. There is no change of constitutive spine density in TrkB knock-in (TrkB-KI) mice lacking serine phosphorylation by Cdk5 (Lai et al. 2012). Because Cdk5 phosphorylation is necessary for activity-dependent regulation of dendritic spine density by TrkB (Lai et al. 2012), constitutive regulation of dendritic spine density differs from activity-dependent regulation of dendritic spine density. To identify the Cdk5 substrate(s) that regulate(s) dendritic spine density, the spine density should be analyzed in mice lacking Cdk5 phosphorylation sites like CRMP2-KI mice (Yamashita et al. 2012). Our preliminary analysis on dendritic spine densities of hippocampal CA1 and cerebral layer V neurons in CRMP2-KI mice at P18 showed reduced spine densities in these neurons compared with controls (preliminary observation by T.O. and K.S.). Since we previously reported reduced spine densities in layer V neurons of the cerebral cortex in CRMP1KO mice (Yamashita et al. 2007), these results indicate that collapsin response mediator proteins are one of the important Cdk5 substrates for spine formation. The results obtained from the present study will provide a new insight into the regulatory mechanisms underlying the effect of Cdk5 on dendritic spine density. Studying the involvement of Cdk5 in neurodevelopmental disorders associated with a reduction in dendritic spine density will contribute to developing therapeutic strategies that enhance learning and memory by controlling Cdk5 activity.
Funding
This work was partly supported by a grant-in-aid fund of the Ministry of Education, Science, Technology, Sports and Culture of Japan and by Waseda University Grant for Special Research Projects.
Notes
The authors thank Dr Tsai for p39 mutant mice and Dr Ichinohe for critical reading of the manuscript.Conflict of Interest: None declared.
References