-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Wolbers, Eszter D. Schoell, Rolf Verleger, Stefanie Kraft, Adam McNamara, Piotr Jaśkowski, Christian Büchel, Changes in Connectivity Profiles as a Mechanism for Strategic Control over Interfering Subliminal Information, Cerebral Cortex, Volume 16, Issue 6, June 2006, Pages 857–864, https://doi.org/10.1093/cercor/bhj029
Close - Share Icon Share
Abstract
Human behavior can be influenced by information that is not consciously perceived. Recent behavioral and electrophysiological evidence suggests, however, that the processing of subliminal stimuli is not completely beyond an observer's conscious control. The present study aimed to characterize the cortical network that implements strategic control over interfering subliminal information at multiple stages. Fourteen participants underwent functional magnetic resonance imaging (fMRI) scanning while performing a metacontrast masking paradigm. We systematically varied the amount of conflicting versus non-conflicting trials across experimental blocks, and behavioral performance demonstrated strategic effects whenever a high proportion of subliminal prime stimuli induced response competition. A psychophysiological interaction analysis revealed the pre-supplementary motor area (pre-SMA) to exhibit context-dependent covariation with activation in the lateral occipital complex (LOC) and the putamen. The pre-SMA thereby appears to fulfill a superordinate function in the control of processing subliminal information by simultaneously modulating perceptual analysis and motor selection.
Introduction
Human behavior can be affected by stimuli that are not consciously perceived. During subliminal priming, stimuli that are rendered unidentifiable by backward masking can nevertheless prime a corresponding motor action (Eimer and Schlaghecken, 2003; Vorberg et al., 2003). If the latter is incompatible with a response required by a subsequently presented, consciously perceived target, prolonged reaction times and increased error rates can often be observed. These behavioral consequences presumably stem from competition between the different motor programs elicited by the masked priming stimulus and the target (Dehaene et al., 1998; Eimer and Schlaghecken, 1998; Leuthold and Kopp, 1998; Verleger et al., 2004).
How does the human brain resolve conflicts between different action plans? The anterior cingulate cortex (ACC) is crucial for coding the relationship between an action and its outcome (i.e. error detection; Garavan et al., 2003; Rushworth et al., 2004). Even though this structure may also be involved in the monitoring/resolution of conflicts that are consciously perceived, conflicts induced by subliminal primes are resolved without ACC contribution (Dehaene et al., 2003). Furthermore, a recent review indicates a central role of the superior frontal gyrus in encoding various kinds of response conflict in both humans and animals (Rushworth et al., 2004). For example, the pre-supplementary motor area (pre-SMA) has been shown to not only control the suppression of a voluntary action plan in favor of a subsequent one (Nachev et al., 2005), but also to be sensitive to conflict manipulations in a go/no-go task (Garavan et al., 2003). Whereas the supplementary eye field seems to implement the result of the conflict resolution process in the oculomotor domain (Nachev et al., 2005), the basal ganglia are involved in inhibiting unwanted manual responses activated by irrelevant, conscious or unconscious distracter stimuli (Aron et al., 2003; Seiss and Praamstra, 2004; Wylie et al., 2005).
Most studies on conflict resolution have focused on how the competition between conscious and/or unconscious action plans is resolved on a trial by trial basis. However, this process can be strongly influenced by context-dependent, strategic effects (Casey et al., 2000; Kunde, 2003; Stürmer and Leuthold, 2003). Electrophysiological evidence suggests that motor priming due to the irrelevant stimulus location in a Simon task (Simon, 1969) — as reflected by the ‘lateralized readiness potential’ (LRP) over motor and pre-motor cortex — can be reduced when an incompatible trial is preceded by a sequence of trials already inducing response conflict (Stürmer and Leuthold, 2003). A similar modulation, both of the LRP and of the posterior N2pc, a component of the event-related potential (ERP) presumably originating from lateral occipito-temporal regions (Hopf et al., 2000), has been demonstrated during subliminal priming (Jaśkowski et al., 2003). In that study, the proportion of conflicting trials in a given block affected participants' response criteria. When 80% of the trials induced competing motor responses, prime-related N2pc and LRP were reduced, as were the behavioral effects of the masked primes on responses to the visible targets. These findings indicate that strategic control might not only affect motor preparation, as indicated by LRP and behavioral effects, but also the identification of unconscious primes in subdivisions of the ventral visual pathway, as indicated by N2pc. Most interestingly, participants appeared to evaluate their general performance level to modulate the impact of primes in an unspecific way, as both compatible and incompatible trials were affected. Jaśkowski et al. (2003) speculated that the neurophysiological effects on LRP and N2pc reflect the operation of a gate-like mechanism that might originate from additional structures.
Based on the described electrophysiological and fMRI findings, it is intriguing to envisage a cortical network for strategic control that operates at multiple processing stages during subliminal priming. Specifically, motor cortex, the pre-SMA, the basal ganglia and subdivisions of the ventral visual pathway appear to be key structures with partially known anatomical connections (Mink, 1996; Inase et al., 1999; Nakano et al., 2000; Johansen-Berg et al., 2004; Lehericy et al., 2004). In the present study, we therefore employed functional magnetic resonance imaging (fMRI) and a modified version of our recently introduced metacontrast masking paradigm (Jaśkowski et al., 2003) (i) to identify the origin of the gate-like mechanism and (ii) to characterize precisely how perceptual analysis and motor selection are modulated by strategic control. A psychophysiological interaction (PPI) analysis allowed us to test how systematic variations of the ratio between compatible and incompatible trials modulate not only the respective hemodynamic responses but also the coupling between the network constituents.
Materials and Methods
Subjects
Fourteen right-handed healthy volunteers (11 men), aged 20–32 years, with normal or corrected-to-normal vision, gave written informed consent to participate. The study was approved by the local ethics committee. Three male participants were removed prior to image analysis due to corrupt behavioral data (see Results for details).
Stimuli and Experimental Procedure
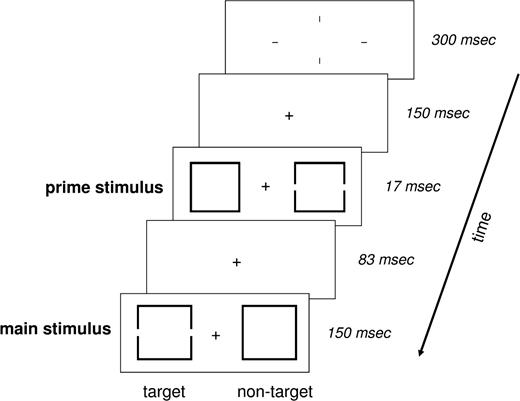
Stimuli consisted of a pair of square outlines whose centers were located 2.9° to the left/right of a centered fixation cross (Fig. 1). The square outline was either intact (the nontarget) or contained a hole in the center of each vertical side (the target). For each trial, two pairs were presented consecutively (83 ms apart), and each pair consisted of one target and one nontarget. The first pair, the prime, was slightly smaller than the second pair, the main stimulus (1.7 versus 1.9°). The gaps in the vertical sides were the exact same size throughout.
Experimental paradigm. Schematic representation of an incompatible trial consisting of a fixation cross, a prime stimulus and a main stimulus. A trial started with the convergence of four lines to a fixation cross. The fixation cross remained alone for 150 ms, followed by the prime stimulus for 17 ms. After a delay of 83 ms, the main stimulus was displayed for 150 ms. Participants were required to respond via button press to the target — the square with incomplete sides — in the main stimulus. The fixation cross remained until the response was given, at which point the screen went blank for 200 ms. The subsequent trial was initiated by the renewed converging of 4 lines to a fixation cross.
Before the beginning of each trial, four lines (5° above, below, right and left of center) converged to form the fixation cross (300 ms) and then remained during the trial, serving as both a warning signal and a fixation aid. The prime stimulus began 150 ms after formation of the fixation cross and was presented for 17 ms, followed 83 ms later by the slightly larger main stimulus displayed for 150 ms. The prime was thereby completely masked through metaconstrast.
Trials were categorized as compatible if the target square was on the same side of the fixation cross in both the prime and main stimuli; otherwise, the trial was incompatible. Each block consisted of 80 trials. Valid blocks consisted of 80% compatible trials and 20% incompatible trials, and invalid blocks of 80% incompatible trials and 20% compatible trials. The trials were pseudo-randomized within blocks; every 10 trials had a fixed ratio of 8:2. Six blocks, beginning with a valid block and then alternating between the two, comprised one session. At the beginning of each session, and between each block, a 20 s fixation period (the subjects were instructed to attend to the lone fixation cross in the center of the screen) served as a low-level baseline.
Participants held a response device in their right hand and were instructed to respond as quickly and accurately as possible via button press to the target in the main stimulus, using the index finger for left-sided targets and the ring finger for right-sided targets. They were not told that two pairs of stimuli were being consecutively presented. Participants had to respond for the presentation to continue and time from response to the next fixation aid was set at 200 ms. This self-paced design led to slightly varying block durations (valid blocks, 84.1 ± 3.1s; invalid blocks, 88.5 ± 2.5s).
To verify that the prime stimuli were not consciously perceived during the experiment, fMRI scanning was followed by a signal detection task. First, subjects were debriefed as to the real set-up of the stimuli by presenting 16 trials in slow motion. They then completed two blocks with equal numbers of compatible and incompatible trials, being instructed to respond to the target in the prime stimulus rather than in the main stimulus.
MRI Acquisition
A liquid crystal display video-projector (refresh rate: 60 Hz) back-projected the stimuli on a screen positioned on top of the head coil. Subjects lay on their backs within the bore of the magnet and viewed the stimuli comfortably via a 45° mirror that reflected the images displayed on the screen. To minimize head movement, all subjects were stabilized with tightly packed foam padding surrounding the head. Stimuli were displayed using Presentation (Neurobehavioral Systems, Albany, CA). Functional MRI was performed on a 3T system (Siemens Trio) using a standard head coil. Forty-eight axial slices (2 mm slice thickness, 0.5 mm gap) were acquired using a gradient echo echo-planar (EPI) T2*-sensitive sequence (TR = 2.75 s, TE = 25 ms, flip angle 80°, matrix 64 × 64, field of view 192 × 192 mm2). Due to the self-paced nature of the task we acquired slightly varying numbers of volumes per subject (session 1: 233.5 ± 4.5; session 2: 232.6 ± 6.6).
Image Processing and Statistical Analysis
Image processing and statistical analysis were carried out using SPM2 (www.fil.ion.ucl.ac.uk/spm/software/spm2). All volumes were realigned to the first volume, spatially normalized to a standard EPI template, smoothed using a 9 mm full-width at half-maximum isotropic Gaussian kernel and temporally filtered with a high-pass filter to remove baseline drifts. Data analysis was performed using the general linear model (GLM) and modeling the two block-types (valid, invalid) as delta functions convolved with a canonical hemodynamic response function (HRF) as implemented in SPM2. We used a block design since the environment of the block with its particular ratio of trial types was the interesting factor. In this context, it is important to note that due to the saturation of the hemodynamic response, any differences in activation between valid and invalid blocks could not be attributed to the slight variations in block duration.
Regression coefficients for all regressors were estimated using least squares within SPM2. Specific effects were tested with appropriate linear contrasts of the parameter estimates for the HRF regressors. Data was analyzed for each subject individually (first-level analysis) and for the group (second-level analysis). At the group level, a random effects approach was applied (Friston et al., 1999).
Psychophysiological Interaction (PPI)
Our primary interest in the present study was focused on assessing the interactions among the network constituents responsible for exerting strategic control over interfering subliminal information. Based on the results of previous studies (Garavan et al., 2003; Rushworth et al., 2004; Nachev et al., 2005), we hypothesized that the pre-SMA might fulfill a superordinate function by modulating the activity of regions involved in perceptual processing and/or motor control. We therefore performed a psychophysiological interaction analysis (Friston et al., 1997) to identify regions whose activation would exhibit a substantial covariation with the pre-SMA during strong strategic control (i.e. invalid blocks) and only a weak covariation during weak control (i.e. valid blocks). More specifically, we checked for areas whose activation profile could be explained by the interaction between experimental context (valid versus invalid block) and activation in the pre-SMA.
As outlined in the Results section, the overall comparison between block types revealed the pre-SMA to display greater activity during invalid than during valid blocks. We used the peak voxel from this analysis (x = 6; y = 16; z = 50) to serve as a landmark for the individual seed voxels. For each participant, we searched within a 4 mm sphere around this peak voxel from the group analysis to determine his or her individual peak voxel. Next, the time series of the individual peak voxels were extracted, and PPI regressors were computed as the element-by-element product of the mean-corrected pre-SMA activity and a vector coding for the differential effect of strong versus weak strategic control. The individual contrast images reflecting the interaction between the psychological variable (block type) and the activation time course in the pre-SMA were subsequently entered into a one-sample t-test.
For all group analyses (categorical comparisons and PPI analyses), we employed the false discovery rate (FDR; Genovese et al., 2002) at P < 0.05 to correct the resulting statistical parametric maps for multiple comparisons. Instead of controlling the chance of any false positives (as Bonferroni or random field methods do), FDR controls the expected proportion of false positives among suprathreshold voxels. Based on the results from previous studies on subliminal priming and conflict resolution (Aron et al., 2003; Dehaene et al., 2003; Garavan et al., 2003), we used the WFU Pickatlas (Maldjian et al., 2003) to define anatomical regions of interest (ROI) for the following structures: precentral gyrus, anterior cingulate, caudate nucleus and putamen. In addition, we defined the lateral occipital complex (LOC) and the pre-SMA as a spherical volumes with a 10 mm radius surrounding previously reported coordinates (LOC: x = ±43, y = −73, z = −18, Malach et al., 1995; pre-SMA: x = ±5, y = 18, z = 57, Casey et al., 2000). For all regions of interest, correction for multiple comparisons was based on these regions; elsewhere in the brain, correction was based on the entire search volume.
Results
Behavioral Data
Three participants were excluded from the final data set after detailed inspection of their behavioral performance. One subject inserted excessively long breaks between trials, strongly indicating that he did not continuously pay attention to the task. Two other participants had very long reaction times irrespective of trial and block types, exceeding the group means by >2 SD.
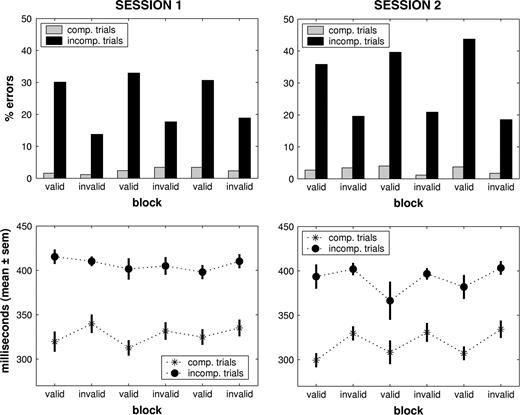
We analyzed the behavioral results (Fig. 2) of the remaining 11 participants with a 2 × 2 × 3 × 2 repeated-measures analysis of variance [factors: trial type (compatible versus incompatible), block type (valid versus invalid), block repetition within sessions (1–3) and session (1st versus 2nd)]. Using error rate as the dependent variable, we observed significant main effects of session (F = 7.170, P = 0.023), trial type (F = 48.068, P < 0.001) and block type (F = 39.541, P < 0.001), and a trend towards significance for block repetition (F = 3.449, P = 0.054). Most importantly, a strong interaction between trial and block type (F = 30.655, P < 0.001) indicated the emergence of strategic control during invalid blocks. As the error rate for compatible trials was very low throughout the experiment (valid blocks: 2.96 ± 0.91%; invalid blocks: 2.18 ± 1.04%), strategic control predominantly affected incompatible trials by reducing the error rate during invalid as compared with valid blocks (Fig. 2). The magnitude of this modulation increased over time as evidenced by significant interactions between trial type and session (F = 5.747, P = 0.037) and between all four factors (F = 4.405, P = 0.029). In sum, whereas error rates for compatible trials were very low for both block types, performance for incompatible trials was significantly affected by experimental context. The strong reduction during blocks with a high proportion of incompatible trials (invalid blocks) indicates the emergence of strategic control.
Behavioral results. Upper panels: behavioral performance across sessions. Whereas error rates for compatible trials were very low for both block types, performance for incompatible trials was significantly affected by experimental context. The strong reduction during blocks with a high proportion of incompatible trials (invalid blocks) indicates the emergence of strategic control. Note that this reduction was stronger in session 2 as compared with session 1. Lower panels: reaction times across sessions. The consistent difference between compatible and incompatible trials demonstrates an overall priming effect. In addition, reaction times for both trial types were slower during invalid blocks, particularly in session 2. This suggests that participants set a more conservative response criterion whenever prime stimuli predominantly induced conflicting motor responses.
The analysis of the reaction time data also revealed significant main effects of trial type and session (F = 10.281, P = 0.009; F = 30.655 P < 0.001), in contrast to block repetition and block type (P > 0.05). The consistent difference between compatible and incompatible trials demonstrates an overall priming effect. Moreover, significant interactions between block repetition and session (F = 4.685, P = 0.028) and between block repetition and block type (F = 3.804 P = 0.049) indicated reaction time changes over time that were different between sessions and for valid versus invalid blocks. Reaction times for both trial types were slower during invalid blocks, particularly in session 2. This suggests that participants set a more conservative response criterion whenever prime stimuli predominantly induced conflicting motor responses.
Given that we aimed at investigating how strategic control affects the processing of subliminal primes and ensuing response conflicts, we used a signal detection task (see Methods) to verify as far as possible that strategic control was independent of prime perceptibility. We correlated individual discrimination accuracy (d′; mean: 0.73, range: −0.04 to +1.67) in the signal detection task with the magnitude of strategic control — expressed as the difference in error rate for incompatible trials between valid and invalid blocks — during the two experimental sessions. Because neither correlation approached significance (session 1: r = 0.21, P = 0.27; session 2: r = 0.09, P = 0.40), the effects reported in the following sections are not influenced by a potential supraliminarity of the primes.
Imaging Data
Main Effects of Task
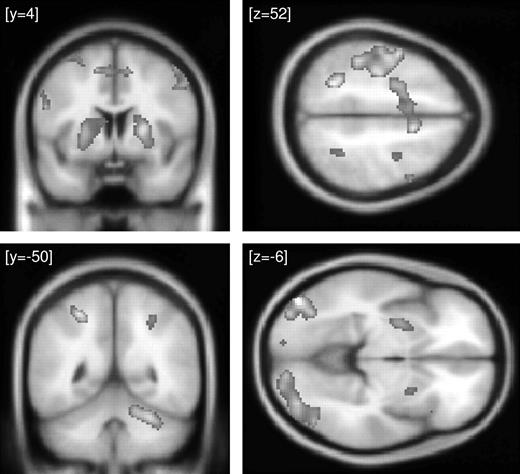
Contrasting each type of block with the low-level, fixation baseline revealed extensive activation within the occipital cortex including the lateral occipital complex, inferior parietal cortex, the basal ganglia, motor and premotor cortex, the supplementary motor area and the cerebellum. Because we observed similar effects for both valid and invalid blocks, Table 1 and Figure 3 present the overall activation averaged across blocks and sessions. A direct comparison between both block types revealed the pre-SMA and the left precentral gyrus to show greater BOLD responses during invalid as compared with valid blocks (Table 1). As we had expected activation in the pre-SMA to emerge during periods of strong strategic control (i.e. invalid blocks), we used the peak voxel in this region [x = 6, y = 16, z = 50] as a landmark for defining individual seed voxels for the psychophysiological interaction analysis (see Materials and Methods for details).
Main effect of task. Activation of the task compared with the low-level fixation baseline. Effects are averaged across sessions and block types, thereby indicating the mean response to the task. Results of the random effects analysis are displayed with a threshold of P < 0.001 (uncorrected) on the averaged MNI template brain.
Spatial coordinates of the local maxima in the group analysis (P < 0.05 corrected)
| Region . | Coordinate (x, y, z, in mm) . | . | Voxel–level (z–score) . | |||
|---|---|---|---|---|---|---|
. | LH . | RH . | . | |||
| Local activation maxima for the contrast valid / invalid blocks minus fixation | ||||||
| Motor cortex | −42, −14, 60 | 5.16 | ||||
| −26, 0, 62 | 4.89 | |||||
| −42, −34, 50 | 4.31 | |||||
| Pre–supplementary motor area | −6, −2, 54 | 3.78 | ||||
| 10, 8, 52 | 4.38 | |||||
| Basal ganglia | −22, −8, 8 | 4.52 | ||||
| 22, 2, 8 | 4.38 | |||||
| 22, 10, 2 | 4.28 | |||||
| Inferior parietal cortex | −24, −54, 52 | 4.60 | ||||
| −42, −34, 50 | 4.31 | |||||
| 44, −34, 44 | 3.85 | |||||
| 30, −48, 50 | 3.57 | |||||
| Occipital cortex | −44, −82, −6 | 4.93 | ||||
| −20, −92, −16 | 3.97 | |||||
| 50, −70, −2 | 4.95 | |||||
| 18, −92, −10 | 4.18 | |||||
| Cerebellum | −36, −56, −30 | 3.44 | ||||
| 28, −52, −26 | 4.41 | |||||
| Local activation maxima for the contrast invalid minus valid blocks | ||||||
| Pre–supplementary motor area | 6, 16, 50 | 2.90 | ||||
| Precentral gyrus | −28, −12, 50 | 3.61 | ||||
| Local activation maximum for the PPI analysis in session 1 | ||||||
| Lateral occipital complex | −46, −74, −12 | 2.18 (n.s.) | ||||
| 42, −74, −20 | 3.25 | |||||
| 52, −78, 4 | 3.04 (n.s.) | |||||
| Local activation maximum for the PPI analysis in session 2 | ||||||
| Putamen | −18, 12, 4 | 5.02 | ||||
| −24, 20, 0 | 4.51 | |||||
| 20, 14, −6 | 4.27 | |||||
| Lateral occipital complex | −44, −78, −10 | 3.74 | ||||
| −50, −74, −14 | 3.70 | |||||
| 40, −72, −24 | 3.59 | |||||
| 46, −68, −10 | 3.17 | |||||
| Region . | Coordinate (x, y, z, in mm) . | . | Voxel–level (z–score) . | |||
|---|---|---|---|---|---|---|
. | LH . | RH . | . | |||
| Local activation maxima for the contrast valid / invalid blocks minus fixation | ||||||
| Motor cortex | −42, −14, 60 | 5.16 | ||||
| −26, 0, 62 | 4.89 | |||||
| −42, −34, 50 | 4.31 | |||||
| Pre–supplementary motor area | −6, −2, 54 | 3.78 | ||||
| 10, 8, 52 | 4.38 | |||||
| Basal ganglia | −22, −8, 8 | 4.52 | ||||
| 22, 2, 8 | 4.38 | |||||
| 22, 10, 2 | 4.28 | |||||
| Inferior parietal cortex | −24, −54, 52 | 4.60 | ||||
| −42, −34, 50 | 4.31 | |||||
| 44, −34, 44 | 3.85 | |||||
| 30, −48, 50 | 3.57 | |||||
| Occipital cortex | −44, −82, −6 | 4.93 | ||||
| −20, −92, −16 | 3.97 | |||||
| 50, −70, −2 | 4.95 | |||||
| 18, −92, −10 | 4.18 | |||||
| Cerebellum | −36, −56, −30 | 3.44 | ||||
| 28, −52, −26 | 4.41 | |||||
| Local activation maxima for the contrast invalid minus valid blocks | ||||||
| Pre–supplementary motor area | 6, 16, 50 | 2.90 | ||||
| Precentral gyrus | −28, −12, 50 | 3.61 | ||||
| Local activation maximum for the PPI analysis in session 1 | ||||||
| Lateral occipital complex | −46, −74, −12 | 2.18 (n.s.) | ||||
| 42, −74, −20 | 3.25 | |||||
| 52, −78, 4 | 3.04 (n.s.) | |||||
| Local activation maximum for the PPI analysis in session 2 | ||||||
| Putamen | −18, 12, 4 | 5.02 | ||||
| −24, 20, 0 | 4.51 | |||||
| 20, 14, −6 | 4.27 | |||||
| Lateral occipital complex | −44, −78, −10 | 3.74 | ||||
| −50, −74, −14 | 3.70 | |||||
| 40, −72, −24 | 3.59 | |||||
| 46, −68, −10 | 3.17 | |||||
RH — right hemisphere, LH — left hemisphere, n.s. — non significant
Spatial coordinates of the local maxima in the group analysis (P < 0.05 corrected)
| Region . | Coordinate (x, y, z, in mm) . | . | Voxel–level (z–score) . | |||
|---|---|---|---|---|---|---|
. | LH . | RH . | . | |||
| Local activation maxima for the contrast valid / invalid blocks minus fixation | ||||||
| Motor cortex | −42, −14, 60 | 5.16 | ||||
| −26, 0, 62 | 4.89 | |||||
| −42, −34, 50 | 4.31 | |||||
| Pre–supplementary motor area | −6, −2, 54 | 3.78 | ||||
| 10, 8, 52 | 4.38 | |||||
| Basal ganglia | −22, −8, 8 | 4.52 | ||||
| 22, 2, 8 | 4.38 | |||||
| 22, 10, 2 | 4.28 | |||||
| Inferior parietal cortex | −24, −54, 52 | 4.60 | ||||
| −42, −34, 50 | 4.31 | |||||
| 44, −34, 44 | 3.85 | |||||
| 30, −48, 50 | 3.57 | |||||
| Occipital cortex | −44, −82, −6 | 4.93 | ||||
| −20, −92, −16 | 3.97 | |||||
| 50, −70, −2 | 4.95 | |||||
| 18, −92, −10 | 4.18 | |||||
| Cerebellum | −36, −56, −30 | 3.44 | ||||
| 28, −52, −26 | 4.41 | |||||
| Local activation maxima for the contrast invalid minus valid blocks | ||||||
| Pre–supplementary motor area | 6, 16, 50 | 2.90 | ||||
| Precentral gyrus | −28, −12, 50 | 3.61 | ||||
| Local activation maximum for the PPI analysis in session 1 | ||||||
| Lateral occipital complex | −46, −74, −12 | 2.18 (n.s.) | ||||
| 42, −74, −20 | 3.25 | |||||
| 52, −78, 4 | 3.04 (n.s.) | |||||
| Local activation maximum for the PPI analysis in session 2 | ||||||
| Putamen | −18, 12, 4 | 5.02 | ||||
| −24, 20, 0 | 4.51 | |||||
| 20, 14, −6 | 4.27 | |||||
| Lateral occipital complex | −44, −78, −10 | 3.74 | ||||
| −50, −74, −14 | 3.70 | |||||
| 40, −72, −24 | 3.59 | |||||
| 46, −68, −10 | 3.17 | |||||
| Region . | Coordinate (x, y, z, in mm) . | . | Voxel–level (z–score) . | |||
|---|---|---|---|---|---|---|
. | LH . | RH . | . | |||
| Local activation maxima for the contrast valid / invalid blocks minus fixation | ||||||
| Motor cortex | −42, −14, 60 | 5.16 | ||||
| −26, 0, 62 | 4.89 | |||||
| −42, −34, 50 | 4.31 | |||||
| Pre–supplementary motor area | −6, −2, 54 | 3.78 | ||||
| 10, 8, 52 | 4.38 | |||||
| Basal ganglia | −22, −8, 8 | 4.52 | ||||
| 22, 2, 8 | 4.38 | |||||
| 22, 10, 2 | 4.28 | |||||
| Inferior parietal cortex | −24, −54, 52 | 4.60 | ||||
| −42, −34, 50 | 4.31 | |||||
| 44, −34, 44 | 3.85 | |||||
| 30, −48, 50 | 3.57 | |||||
| Occipital cortex | −44, −82, −6 | 4.93 | ||||
| −20, −92, −16 | 3.97 | |||||
| 50, −70, −2 | 4.95 | |||||
| 18, −92, −10 | 4.18 | |||||
| Cerebellum | −36, −56, −30 | 3.44 | ||||
| 28, −52, −26 | 4.41 | |||||
| Local activation maxima for the contrast invalid minus valid blocks | ||||||
| Pre–supplementary motor area | 6, 16, 50 | 2.90 | ||||
| Precentral gyrus | −28, −12, 50 | 3.61 | ||||
| Local activation maximum for the PPI analysis in session 1 | ||||||
| Lateral occipital complex | −46, −74, −12 | 2.18 (n.s.) | ||||
| 42, −74, −20 | 3.25 | |||||
| 52, −78, 4 | 3.04 (n.s.) | |||||
| Local activation maximum for the PPI analysis in session 2 | ||||||
| Putamen | −18, 12, 4 | 5.02 | ||||
| −24, 20, 0 | 4.51 | |||||
| 20, 14, −6 | 4.27 | |||||
| Lateral occipital complex | −44, −78, −10 | 3.74 | ||||
| −50, −74, −14 | 3.70 | |||||
| 40, −72, −24 | 3.59 | |||||
| 46, −68, −10 | 3.17 | |||||
RH — right hemisphere, LH — left hemisphere, n.s. — non significant
Psychophysiological Interaction
Given that both behavioral measures indicated significant performance changes across sessions, we conducted separate PPI analyses for each session. On average, the coordinates of the individual seed voxels in the pre-SMA were as follows: x = 7.45 ± 3.11, y = 16 ± 5.06, z = 53.27 ± 5.24.
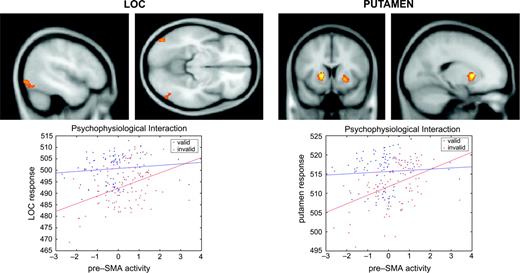
In session 1, significant context-dependent effects were restricted to the posterior part of the lateral occipital complex (LOC) in both hemispheres (Table 1). In other words, the covariation between activation in the pre-SMA and in LOC was stronger during invalid than during valid blocks, suggesting the emergence of strategic control in blocks with a high proportion of incompatible trials. In accordance with the behavioral results, which indicated stronger strategic control in session 2, psychophysiological interaction effects in LOC were larger in the second half of the experiment (Fig. 4 and Table 1). Moreover, we also obtained robust indicia for context-dependent effects in the putamen bilaterally (Fig. 4 and Table 1), which was not observed in session 1.
Psychophysiological interaction in session 2. Upper left panels: regions in the lateral occipital complex showing a significant context-dependent covariation with the pre-SMA. This coupling was stronger during invalid as compared with valid blocks, suggesting a modulation of perceptual analysis in blocks with a high proportion of incompatible trials. Results of the random effects analysis are displayed with a threshold of P < 0.001 (uncorrected) on the averaged MNI template brain. Lower left panel: regression of LOC activation (at x = −46, y = −80, z = −8) on pre-SMA activation (at x = 8, y = 14, z = 52) for a representative participant during valid and invalid blocks. Observed data are adjusted for the effects of interest. Strategic control can be seen to augment the contribution of pre-SMA to LOC activation. Upper right panels: regions in the putamen showing a significant context-dependent covariation with the pre-SMA. This coupling was stronger during invalid as compared with valid blocks, suggesting a modulation of motor selection in blocks with a high proportion of incompatible trials. Results of the random effects analysis are displayed with a threshold of P < 0.001 (uncorrected) on the averaged MNI template brain. Lower right panel: Regression of putamen activation (at x = −32, y = 10, z = −4) on pre-SMA activation (at x = 8, y = 14, z = 52) during valid and invalid blocks for the same participant. Observed data are adjusted for the effects of interest. Strategic control can be seen to augment the contribution of pre-SMA to putamen activation.
To further illustrate the relationship between strategic control and the covariation between the pre-SMA and the posterior part of LOC/the putamen, the lower panels of Figure 4 display activation profiles for one participant during valid and invalid blocks in session 2. Both for LOC and the basal ganglia, the regression slopes were steeper during invalid blocks, suggesting a tighter coupling with the pre-SMA whenever this participant suppressed the influence of the subliminal prime stimuli. As a consequence, strategic control appeared to affect prime-related processing both at the level of perceptual analysis and of motor preparation.
Finally, to verify as far as possible that the described context-dependent interactions were independent of prime perceptibility, we correlated d′ scores with the beta values from the individual PPI analyses in the peak voxels from LOC (sessions 1 + 2) and the putamen (session 2). Because none of the correlations was significant (all P > 0.05), we conclude that the results from the psychophysiological interaction analyses do not relate to variations in prime perceptibility, but rather indicate the emergence of strategic control processes.
In addition to the pre-SMA, the precentral gyrus had exhibited stronger activation during invalid as compared with valid blocks. Therefore, we tested whether the context-dependent covariations were specific to the pre-SMA-LOC/putamen circuits by conducting separate PPI analyses with the precentral gyrus as a seed region (mean seed voxel coordinates: x = −28 ± 5.66, y = −9.09 ± 3.51, z = 50.72 ± 3.38). In session 1, these analyses did not reveal any significant effects either in LOC or in the putamen. In the second half of the experiment, we also did not observe significant covariations in the putamen; however, in LOC these effects reached statistical significance [left hemisphere: z = 3.74 (at x = −38, y = −70, z = −14); right hemisphere: z = 3.44 (at x = 52, y = −72, z = −8)].
Discussion
The present study aimed to characterize the cortical network mediating strategic control over interfering subliminal information. By varying the proportion of compatible versus incompatible trials, we obtained clear evidence for the emergence of strategic effects in those blocks where 80% of the trials induced response competition. These effects consisted of increased reaction times and decreased error rates, indicating that participants changed their overall response criteria. Neuroimaging results revealed the pre-SMA and the left precentral gyrus to exhibit stronger activation during invalid than during valid blocks. Most importantly, activation in LOC and the putamen showed a significant context-dependent covariation with the pre-SMA. Psychophysiological interaction analyses revealed a tight coupling specifically during blocks with 80% incompatible trials. These results suggest that the pre-SMA might fulfill a superordinate function in the control of processing subliminal primes by modulating perceptual analysis and response selection.
Behavioral performance was characterized by an overall priming effect, since reaction times were shorter and error rates were lower for compatible than for incompatible trials. This indicates a substantial influence of the subliminal primes, presumably consisting of a fast activation of the corresponding motor response (Eimer and Schlaghecken, 2003) as well as a cueing of attention to the side of the target shape in the prime (Jaśkowski et al., 2002; Mattler, 2003; Scharlau and Ansorge, 2003). In accordance with the results from the previous ERP study (Jaśkowski et al., 2003), the proportion of compatible versus incompatible trials in a given block had a significant impact on participants' behavior. Valid blocks were characterized by shorter reaction times and higher error rates for incompatible trials in particular. In contrast, participants set a more conservative response criterion in blocks with a high probability of incompatible trials, trading speed for accuracy (Fitts, 1966; Rinkenauer et al., 2004): reaction times increased and error rates were reduced, suggesting a weakening of the impact of the prime stimuli. Interestingly, these performance differences between both block types increased over time, which parallels the stronger neuroimaging effects in session 2 as obtained from the PPI analysis. Of note, when debriefed after the experiment about the fact that alternating blocks differed by some feature, none of the participants stated that he or she had noticed this. Thus, strategic control reflected by the speed-accuracy trade-off apparently did not become conscious. Neither did participants in the four experiments reported in Jaśkowski et al. (2003) mention any differences between the blocks. Therefore, it appears that participants attributed their errors to subjective lapses rather than to the objective ambiguities of visual input caused by the masked primes of which they were not aware.
It is reasonable to assume that participants generally evaluate their performance, trying to keep error rate on a low level. The critical assumption is that on the basis of this evaluation, participants can modulate the impact of masked primes. Earlier investigations have shown that top-down modulation is indeed possible for the effects of masked primes. Such modulations may be caused, as in the present study, by varying the frequency of identical prime-target sequences (Bodner and Masson, 2001; Ansorge et al., 2002; Jaśkowski et al., 2003), by changing the potential task relevance of the primes (Kunde et al., 2003), by varying the frequency of associated prime-target sequences (Bodner and Masson, 2003) or by varying the temporal window of attention (Naccache et al., 2002). Similar effects have been reported for modulation by attention on the effects of stimuli in the blind visual field (Kentridge et al., 2004) and on learning to distinguish systematic background movements (Seitz and Watanabe, 2003). The question emerging from all these effects is how the modulations of subliminal input can be performed. Possibly, there is a ‘gate’ through which the input of the masked stimuli passes, along with a ‘gatekeeper’ modulating the gate. Based on these considerations, the PPI analysis investigated whether the pre-SMA might have such a gate-keeping function in the present paradigm.
The lateral occipital complex, a region in the lateral occipital cortex extending anteriorly in the temporal cortex, is involved in shape processing and may thereby mediate object recognition (Malach et al., 1995; Grill-Spector et al., 2000; Bar et al., 2001). Recent evidence suggests a functional dissociation within LOC, with the anterior part representing the perceived 3-D shape of objects and their position in visual scenes, and the posterior subdivision processing 2-D features independent of image transformations (Kourtzi et al., 2003). The location of the context-dependent covariation observed in LOC in the present study is consistent with the posterior part as described by Kourtzi et al. (2003). We therefore suggest that it is the identification of target shapes based on 2-D features that may undergo a modulation by strategic control. Originating from the pre-SMA, this control could amplify perceptual processing of the main stimuli and inhibit the subliminal primes, which would be in accord with the reduction of the prime-related N2pc component observed in our previous ERP-study (Jaśkowski et al., 2003). As a consequence, the competition between automatically activated motor responses is biased towards the response to the main stimulus, which ultimately entails a reduction in error rate.
The basal ganglia receive widespread input from various cortical areas including motor, premotor and supplementary motor regions. The output structures — globus pallidus and the substantia nigra pars reticulata — are inhibitory and project to motor areas in the brainstem and thalamus (Mink, 1996, 2003). The basal ganglia–thalamocortical loops can therefore mediate selection between competing motor programs (Mink, 2003); and basal ganglia dysfunction entails substantial problems with inhibiting primed response tendencies (Aron et al., 2003; Seiss and Praamstra, 2004; Wylie et al., 2005). In the present study, only 20% of the trials during valid blocks induced a need to resolve competition between alternative motor responses. In contrast, 80% of the trials during invalid blocks required a focused selection and partial inhibition of competing motor programs. The significant covariation between activation in the putamen and the pre-SMA during invalid blocks indicates how these processes might be implemented in the human brain. First of all, strategic control did not result in a higher net activation in the putamen, because the direct comparison did not reveal significant differences between valid and invalid blocks. We therefore conclude that it is the local distribution of inhibitory signals in the basal ganglia that is coordinated by strategic control processes. This, in turn, changes the relative weighting of wanted versus unwanted motor actions. On the one hand, prime-related responses are suppressed by increasing tonic inhibitory signals sent via the thalamus to the corresponding motor pattern generators in the cerebral cortex. Conversely, responses related to the main stimulus are strengthened by removing tonic inhibition from the specific target neurons in the thalamus. Consistent with this view, Casey et al. (2000) also observed strong activation for trials embedded in a sequence of incompatible trials in the superior frontal gyrus, but not in the basal ganglia.
The differences between sessions, observed on both the behavioral and the neuroimaging level, support the idea that the strategic control processes operate simultaneously on perceptual analyses and motor selection. In session 1, the PPI analysis revealed significant context-dependent covariation only in LOC, which already led to an error rate reduction and a reaction time increase during invalid blocks. These behavioral effects became significantly stronger in the second half of the experiment, presumably due to the additional, intensified coupling between the pre-SMA and the putamen. Hence, strategic control is obviously able to simultaneously affect multiple processing stages, which seems to translate into additive effects on behavioral responses.
How can we characterize the function of the supplementary motor area in mediating strategic control over interfering subliminal information? The two subdivisions of the SMA, the caudal SMA proper and the rostral pre-SMA, exhibit different functional responses and have distinct anatomical connectivity (Inase et al., 1999; Johansen-Berg et al., 2004; Lehericy et al., 2004). Given that the pre-SMA has been shown to support conflict resolution between competing motor responses and switching between action selection rules (Rushworth et al., 2004; Nachev et al., 2005), this structure may be of special importance for exerting task control. Moreover, its sensitivity to changes in experimental context indicates that it may have access to at least a limited history of presence or absence of response conflict (Casey et al., 2000). Our results demonstrate that the pre-SMA does not only increase its activation in situations with a high probability of motor competition induced by visual stimulation, but that it selectively intensifies the coupling with LOC and the putamen. These changes in coupling strengths could indicate a parallel modulation of prime-related perceptual processing in LOC and of the response selection process in the basal ganglia. As a consequence, the subliminal primes lose some of their influence, as reflected by the decreased error rate.
With regard to the underlying neuroanatomical foundations, the pre-SMA shares dense connections with rostral parts of the striatum and the thalamus, medial parietal cortex and various frontal regions (Johansen-Berg et al., 2004; Lehericy et al., 2004). Whereas direct projections between the pre-SMA and the basal ganglia are well-established (Künzle, 1978; Inase et al., 1999), we are not aware of any evidence for monosynaptic connections between the pre-SMA and LOC. However, both anatomical studies in monkeys (Luppino et al., 1993) and diffusion tensor imaging in humans (Johansen-Berg et al., 2004) have demonstrated cortico-cortical connectivity between the pre-SMA and parietal regions. In addition, ventral visual areas also share projections with subdivisions of parietal cortex (Cavada and Goldman-Rakic, 1989), which are thought to constitute a neuroanatomical foundation of top-down attentional control (Friedman-Hill et al., 2003). Therefore, we propose that the strategic control processes, originating from the pre-SMA, may affect motor selection via direct connections with the putamen. In contrast, the modulation of perceptual processing in LOC is mediated by parietal regions that are generally involved in attentional selection during visual processing.
Given that a psychophysiological interaction analysis is a correlative approach, it should be noted that the observed results cannot only be explained by a top-down modulation of perceptual analysis and motor selection. Rather, the enhanced covariation between SMA and LOC/putamen during blocks with a high proportion of conflicting trials could also be driven by bottom-up signals. These may convey the increased presence of response conflict, which subsequently necessitates top-down control. The interpretation of the present results should therefore be treated with appropriate caution. With regard to anatomical specificity, we did not observe similar context-dependent modulations when the precentral gyrus was chosen as a seed region for the PPI analyses, except for an effect in LOC in session 2. We therefore believe that the emergence of strategic control is primarily mediated by an intensified coupling between the pre-SMA and cortical structures involved in object identification (LOC) and motor selection (putamen).
In closing, changes in experimental context during visual subliminal priming affect not only behavioral performance and neuronal activation but also the connectivity between the crucial brain structures. These results raise several questions to be addressed in future studies. For example, strategic control processes might differentially affect the processing of sub- versus supraliminal interfering information and/or varying response modalities (i.e. manual, verbal, oculomotor). In addition, replicating the present experiment with an event-related design would provide further insights into possible interactions between strategic control and interfering versus non-interfering subliminal information.
We thank the Physics and Methods group at NeuroImage Nord in Hamburg. This work was supported by grants from the German Research Foundation (DFG) to C.B. (Bu1323/4-1) and R.V. (Ve110/13-1).
References
Ansorge U, Heumann M, Scharlau I (
Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, Sahakian BJ, Robbins TW (
Bar M, Tootell RB, Schacter DL, Greve DN, Fischl B, Mendola JD, Rosen BR, Dale AM (
Bodner GE, Masson ME (
Bodner GE, Masson ME (
Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA (
Cavada C, Goldman-Rakic PS (
Dehaene S, Naccache L, Le Clec HG, Koechlin E, Mueller M, Dehaene-Lambertz G, van de Moortele PF, Le Bihan D (
Dehaene S, Artiges E, Naccache L, Martelli C, Viard A, Schurhoff F, Recasens C, Martinot ML, Leboyer M, Martinot JL (
Eimer M, Schlaghecken F (
Eimer M, Schlaghecken F (
Fitts PM (
Friedman-Hill SR, Robertson LC, Desimone R, Ungerleider LG (
Friston KJ, Büchel C, Fink GR, Morris J, Rolls E, Dolan RJ (
Garavan H, Ross TJ, Kaufman J, Stein EA (
Genovese CR, Lazar NA, Nichols T (
Grill-Spector K, Kushnir T, Hendler T, Malach R (
Hopf JM, Luck SJ, Girelli M, Hagner T, Mangun GR, Scheich H, Heinze HJ (
Inase M, Tokuno H, Nambu A, Akazawa T, Takada M (
Jaśkowski P, van der Lubbe RH, Schlotterbeck E, Verleger R (
Jaśkowski P, Skalska B, Verleger R (
Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM (
Kentridge RW, Heywood CA, Weiskrantz L (
Kourtzi Z, Erb M, Grodd W, Bülthoff HH (
Kunde W (
Kunde W, Kiesel A, Hoffmann J (
Künzle H (
Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS (
Leuthold H, Kopp B (
Luppino G, Matelli M, Camarda R, Rizzolatti G (
Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB (
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (
Mink JW (
Mink JW (
Naccache L, Blandin E, Dehaene S (
Nachev P, Rees G, Parton A, Kennard C, Husain M (
Nakano K, Kayahara T, Tsutsumi T, Ushiro H (
Rinkenauer G, Osman A, Ulrich R, Muller-Gethmann H, Mattes S (
Rushworth MF, Walton ME, Kennerley SW, Bannerman DM (
Scharlau I, Ansorge U (
Seiss E, Praamstra P (
Stürmer B, Leuthold H (
Verleger R, Jaśkowski P, Aydemir A, van der Lubbe RH, Groen M (
Vorberg D, Mattler U, Heinecke A, Schmidt T, Schwarzbach J (
Author notes
1NeuroImage Nord, Department of Systems Neuroscience, University Medical Center Hamburg-Eppendorf, Germany, 2Department of Neurology, University of Lübeck, Germany and 3Department of Cognitive Psychology, University of Finance and Management, Warsaw, Poland