-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Chait, David Poeppel, Jonathan Z. Simon, Neural Response Correlates of Detection of Monaurally and Binaurally Created Pitches in Humans, Cerebral Cortex, Volume 16, Issue 6, June 2006, Pages 835–848, https://doi.org/10.1093/cercor/bhj027
Close - Share Icon Share
Abstract
Recent magnetoencephalography (MEG) and functional magnetic resonance imaging studies of human auditory cortex are pointing to brain areas on lateral Heschl's gyrus as the ‘pitch-processing center’. Here we describe results of a combined MEG-psychophysical study designed to investigate the timing of the formation of the percept of pitch and the generality of the hypothesized ‘pitch-center’. We compared the cortical and behavioral responses to Huggins pitch (HP), a stimulus requiring binaural processing to elicit a pitch percept, with responses to tones embedded in noise (TN)—perceptually similar but physically very different signals. The stimuli were crafted to separate the electrophysiological responses to onset of the pitch percept from the onset of the initial stimulus. Our results demonstrate that responses to monaural pitch stimuli are affected by cross-correlational processes in the binaural pathway. Additionally, we show that MEG illuminates processes not simply observable in behavior. Crucially, the MEG data show that, although physically disparate, both HP and TN are mapped onto similar representations by 150 ms post-onset, and provide critical new evidence that the ‘pitch onset response’ reflects central pitch mechanisms, in agreement with models postulating a single, central pitch extractor.
Introduction
Pitch, one of the most salient features evoked by sound, is crucial to our ability to process voiced speech, segregate auditory streams, and enjoy music. Despite the importance of pitch, the mechanisms responsible for its extraction, as well as their location in the brain, are a matter of debate. The information necessary for the computation of pitch of some signals is available as early as the auditory nerve of either ear (Cariani and Delgutte, 1996; Moore, 1997). Others require combination of information from both ears within a central pitch processor. Houtsma and Goldstein (1971) demonstrated that the ‘missing fundamental’ effect (a harmonic complex tone has a pitch determined by its repetition rate even if a sinusoidal component at that frequency — the fundamental — is not physically present in the signal) can occur even when harmonics are played to different ears. This suggests that the ‘pitch extractor’ for such stimuli resides at or above the level of the superior olivary complex (SOC), where the information from the two ears is first combined. On the other hand, binaural interaction is not required for other stimuli and it has been suggested that both monaural and binaural pitch mechanisms might exist (e.g. Carlyon et al., 2001; see reviews in Moore, 1997; de Cheveigné, 2005).
Pitch processing in humans has primarily been studied via psychophysics (see Moore, 1997; Plack et al., 2005). These studies have been augmented by brain imaging research carried out with functional magnetic resonance imaging (fMRI)/positron emission tomography (PET) (Griffiths et al., 1998a; Patterson et al., 2002; Penagos et al., 2004) and electroencephalography (EEG)/magnetoencephalography (MEG) (Pantev et al., 1996; Fujioka et al., 2003; Krumbholz et al., 2003; Gutschalk et al., 2004; Ritter et al., 2005). Evidence from several studies (Patterson et al., 2002; Penagos et al., 2004; Ritter et al., 2005) points to an area immediately antero-lateral to primary auditory cortex (PAC) as the area where pitch extraction-related processes may operate. These have recently been complemented by similar findings in animal electrophysiology (Bendor and Wang, 2005).
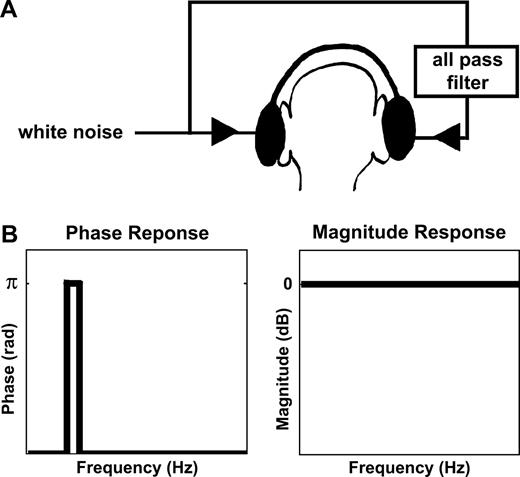
Whereas fMRI and PET are well suited to address questions related to where processing occurs, MEG excels in the investigation of the time course of processing. In the present study we combine a crafted auditory stimulus, Huggins pitch (HP), with the high temporal resolution of MEG recording, to investigate aspects of the processing of pitch information in cortex. Specifically, we investigate the timing of the response to tonal targets within background noise. Huggins pitch (Cramer and Huggins, 1958) is the auditory counterpart of the more famous ‘random dot stereogram’ (RDS) effect in vision (Julesz, 1971). An RDS is a binocularly presented pair of images with randomly distributed dots. Each image appears featureless when viewed individually but together they evoke a coherent 3D percept when displayed simultaneously, one to each eye. The illusion is created by presenting identical RDSs to the two eyes except that one image contains a group of dots that are slightly shifted relative to the other. The visual system fuses the shifted and non-shifted dots to create a 3D percept of an image (corresponding to the shifted dots) floating above the background (of the unshifted dots). Similarly in audition, if a random broadband noise signal is presented to one ear, and the same random noise — but with a phase shift of π over a narrow frequency band — is presented to the other ear, this results in the perception of a faint tonal object with a pure tone quality (with a pitch that matches the center frequency of the phase-shifted band), embedded in noise (Fig. 1). It is crucial that the input to either ear alone is just white noise, completely lacking any spectral or temporal cues to pitch. The fact that we are nevertheless able to perceive pitch when the two signals are presented dichotically implies that the HP percept is created by a central mechanism that receives the inputs from the two ears, collates them, and derives from the correspondence the percept of a tone. Here we compare the cortical auditory evoked responses to HP to a stimulus that is physically different but nonetheless elicits a very similar percept: a pure tone embedded in noise (TN).
Generation of the Huggins pitch stimuli. (A) The signals were created by introducing a constant phase shift of π in a narrow spectral region of the noise sample delivered to the right ear, while the original sample was delivered to the left ear (note that the particular ear that received the phase shifted noise is of no significance). (B) Schematic of the phase and magnitude responses of the all pass filter. The pitch of the perceived tonal object corresponds to the center of the phase-shifted band.
For our tonal stimuli, we chose four frequencies, ranging from 200 to 1000 Hz. The HP signals were generated by inverting a narrow spectral region of a noise sample in one ear, centered about the tonal frequency, while the original sample is delivered to the other. The corresponding TN signals were produced by adding a pure tone (with amplitude chosen to match the perceived tone loudness of the corresponding HP stimulus) to the original noise signal. Matched TN and HP stimuli result in a very similar perceptual experience.
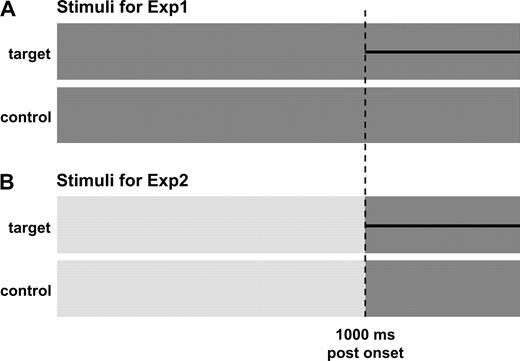
Experimentally it is important to isolate the processing that is specific to the detection of the onset of a tonal stimulus from that associated with generic stimulus onset. Typically this involves comparing responses to noise and tone-like stimuli. However, the large onset component common to both reduces the sensitivity to the tone-specific component. To attenuate this problem, noise was prepended to the TN and HP signals, so that the tonal response can be measured at the transition from noise to TN or HP, after the stimulus onset response has subsided. Conceptually, the transition response can be seen as evoked by the emergence of a tone-like target within a noise background. In order to investigate the degree to which binaural mechanisms affect the cortical response, the prepended noise was interaurally correlated in experiment 1 (Exp1) and interaurally uncorrelated in experiment 2 (Exp2; Fig. 2).
Schema of the stimuli used in the two experiments. (A) Stimuli for Exp1 consisted of 1500 ms of correlated wide-band noise (dark grey) with a 500 ms faint tonal object (HP/TN; black line) appearing at 1000 ms post-onset. Control stimuli were 1500 ms long correlated wide-band noise. (B) Stimuli for Exp2 consisted of 1000 ms long uncorrelated wide-band noise (light grey) followed by a 500 ms long correlated noise segment which either contained a tonal object (target condition) or did not (control condition). Crucially, the last 500 ms of the stimuli of Exp1 and Exp2 were identical.
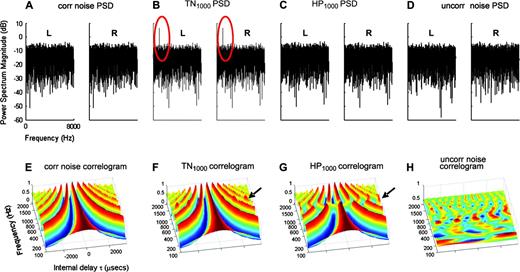
The physical differences between HP and TN are explained in Figure 3. Whereas the TN stimuli evoke patterns that can be detected monaurally as early as the auditory nerve (Fig. 3B), the HP stimuli are indistinguishable from white noise (Fig. 3C) up to the level of the medial superior olive (MSO), where phase and timing information from the two ears are first compared (Carr, 1993; Joris et al., 1998). Because HP stimuli are totally devoid of any spectral structure at each ear, they rule out the possibility that their pitch results from spectral processing at the level of the cochlea, auditory nerve or cochlear nucleus. Several studies (Griffiths et al., 1998a, 2001; Patterson et al., 2002; Krumbholz et al., 2003; Ritter et al., 2005) used a monaural stimulus with characteristics that resemble white noise, iterated rippled noise (IRN), to identify a hypothesized ‘pitch center’ in Heschl's gyrus (HG) whose activation increased with the degree of temporal regularity in the signal. Noise-like IRN stimuli are intended to reduce the likelihood that the response is related to changes of a tonotopic representation of the stimulus. However, this is true only if the iteration frequency is sufficiently low, or the stimulus is high-pass filtered to avoid resolution of spectral components within the cochlea. HP has no such constraint, and offers the advantage over IRN that spectral cues can be avoided over a range of parameters more typical of salient and musical pitch (Akeroyd et al., 2001).
Physical properties of HP and TN. Power spectral density (L = left ear; R = right ear) was computed for (A) 1000 ms correlated noise stimulus; (B) 500 ms 1000 Hz TN stimulus; (C) 500 ms 1000 Hz HP stimulus; and (D) 1000 ms uncorrelated noise stimulus. Pitch information for the TN (but not HP) stimuli is available monaurally at the input to the cochlea (see red circles in B). Physiological evidence indicates that MSO neurons may act as interaural cross-correlators (Joris et al., 1998). (E–H) Binaural cross-correlograms for the stimuli in (A–D), which model MSO activation as a neural array arranged by best frequency and best interaural delay (from −3500 to +3500 μs). The plots illustrate the long-term time average of the activity within such an array that would be evoked by our stimuli. The neuronal activation due to correlated noise is shown in (E): peaks at certain delays (main peak at zero ITD with side peaks spaced according to the neuronal best frequency) and troughs at others: some cells respond strongly to this stimulus (peaks) while others respond weakly (troughs). Activation due to TN1000 (F) is very similar to the correlated noise activation, except for mildly increased activation of already active neurons with best frequency of 1000 Hz (see arrow). In contrast, the HP1000 activation (G) differs sharply from the correlated noise activation — many neurons inactive under correlated noise become active under HP, due to the interaural phase shift in HP (see arrow). The uncorrelated noise stimulus (H) does not activate the MSO as strongly as correlated noise (cf. Polyakov and Pratt, 1998) and the activation is effectively random. The correlograms were generated using the ‘binaural toolbox’ (Akeroyd, 2001). The signal is fed through a filter-bank (100–2000 Hz with filter spacing 2/ERB) and half wave rectified. Left and right filter outputs are cross-multiplied and normalized by the average power in the two filter outputs.
The requirement for binaural processing before pitch extraction puts constraints on the available mechanisms, as processing can occur no earlier than the site of binaural convergence. By ruling out cues in the periphery, HP stimuli can be used to investigate the generality of the ‘pitch center’, as proposed by accumulating literature, as well as to refine our interpretation of auditory evoked responses. The M100 or N1 peak (for MEG and EEG, respectively) is the most prominent auditory evoked response. It occurs ∼100 ms after the onset of a stimulus and is thought to originate from the planum temporale (PT; Lütkenhöner and Steinsträter,1998). The latency and localization of the M100 have been shown to vary with stimulus parameters that determine pitch (Pantev et al., 1989, 1996; Ragot and Lepaul-Ercole, 1996; Roberts and Poeppel, 1996; Fujioka et al., 2003) and it has been hypothesized to reflect stimulus encoding (Salajegheh et al., 2004). Recently, Krumbholz et al. (2003) identified a magnetic deflection (the ‘pitch onset response’, POR) which shares some neural generators with the M100 (Seither-Priesler et al., 2004) and is evoked by a transition from noise to iterated rippled noise (IRN). The latency and amplitude of the POR were found to be dependent on the strength and pitch of the IRN stimulus, similarly to findings discussed extensively in the M100 literature. The latency of the M100 onset response to pure tones varies with the tone's frequency such that low frequencies evoke field responses ∼30 ms later than high frequencies (Roberts et al., 2000; Lütkenhöner et al., 2001). The period-dependency of latency has been attributed to differences in cochlear traveling wave delays (Greenberg et al., 1998; Borgmann et al., 2001) or latency differences between neural channels with different characteristic frequencies. By this account HP should not exhibit the same trends. Thus comparisons of auditory evoked responses to HP and TN stimuli enable us to test predictions about the architecture of the processing up to and including auditory cortex, and to determine at which point the perceptually similar but physically different stimuli converge on a single representation.
In our experimental paradigm subjects performed a pitch detection task while their brain activity was being recorded. The simultaneous recording of behavioral reaction times and MEG response latencies enables the investigation of the dynamics of the construction of perceptual representations and the degree of correspondence between behavioral and electrophysiological measures. The results reported here have important implications in several domains: in addition to posing new constraints for models of pitch and revealing neural processes associated with the extraction of tonal objects from noise, our data have specific and new implications for binaural processing mechanisms. Importantly, we also demonstrate that, even in humans, electrophysiological tools can measure processes not observable by behavioral, psychophysical means.
Materials and Methods
Subjects
Twenty subjects (mean age 24.6 years), took part in Exp1. Sixteen subjects (mean age 23.8 years) took part in Exp2. Twelve listeners participated in both experiments. Five subjects (from Exp1; mean age 26.6 years) took part in the control experiment for Exp1. All subjects were right handed (Oldfield, 1971), reported normal hearing and had no history of neurological disorder. The experimental procedures were approved by the University of Maryland institutional review board and written informed consent was obtained from each participant. Subjects were paid for their participation.
Stimuli
We chose four center frequencies (200, 400, 600, 1000 Hz) that span the frequency region for which HP is salient. A 1000 ms sample of ‘frozen’ noise was generated for each of these four conditions. The signals were created by choosing Gaussian distributed numbers (sampling frequency = 16 kHz, bandwidth = 8 kHz). The HP signals were generated by introducing a constant phase shift of π in a particular spectral region of the noise sample delivered to the right ear, while the original sample was delivered to the left ear (Yost et al., 1987). The width of the phase-shifted band was set to ±6% of its center frequency (Klein and Hartmann, 1981). The corresponding TN signals were produced by adding a pure tone (with one of the above frequencies) to the same noise samples used to create the HP stimuli. Listeners are able to match the HP signal to the pitch evoked by a pure tone (with a frequency that corresponds to the center of the phase-shifted band) with a standard error of ∼3% (Klein and Hartmann, 1981). Three versions of each TN stimulus were created: (i) TNcenter — perceived in the center of the head (same amplitude of pure tone to both ears); (ii) TNright — lateralized to the right (amplitude in the right ear higher than the left ear by ∼5 dB); and (iii) TNleft — lateralized to the left (amplitude in the left ear higher than the right ear by ∼5 dB). The amplitude of the pure tone signal was separately adjusted by two listeners to match the ‘perceived tone’ loudness of the corresponding HP stimulus, resulting in a signal to noise ratio (SNR) of ∼10 dB (see Fig. 3A–D). The match was verified for each subject in the beginning of the experiment.
The stimuli in Exp1 were 1500 ms long, consisting of 1000 ms interaurally correlated white noise followed by either HP or TN, as described above, or interaurally correlated noise (control). The stimuli of Exp2 were identical to those of Exp1 except that the first 1000 ms of all stimuli were replaced by interaurally uncorrelated noise (Fig. 2). The stimuli of the control experiment consisted of 1000 ms interaurally correlated white noise followed by the same noise used to generate the HP stimuli but with one (narrow) band amplified (noise band stimuli, BN). The amplified bands have the same bandwidth as the phase shifted region in the corresponding HP stimulus, but no interaural phase difference. The loudness of the pitch in the BN stimuli was separately adjusted by two listeners to match the perceived loudness of the TN stimuli.
When HP is perceived, the background noise is always lateralized to the center of the head but the tonal object may be reported as being at a lateral position away from the midline. It is lateralized to the left or to the right by some listeners but mostly evokes an inconsistent (ambiguous) lateralization, especially by inexperienced listeners (Yost et al., 1987; Zhang and Hartmann, 2004). For the purpose of making the TN and HP stimuli as perceptually similar as possible, prior to the beginning of the MEG experiment proper, each listener's lateralization of HP was assessed. HP stimuli as well as TNcenter, TNleft, and TNright stimuli of the different frequencies were presented in a random order (all stimuli were preceded by correlated or uncorrelated noise, in Exp1 and 2 respectively). Each condition was presented five times (giving a total of 80 trials). For each stimulus, the subjects were asked to indicate the perceived location of the tonal object. In cases when subjects were consistent at lateralizing the HP stimuli to the left (three subjects in Exp1, 5 in Exp2) or right (one in Exp1, three in Exp2), the corresponding TNleft or TNright stimuli were chosen for the MEG experiment. If the subjects were inconsistent or indicated that HP was heard in the center, TNcenter was chosen. Lateralization could also have been obtained by introducing an interaural time difference (ITD), but this would have engaged binaural masking level difference (BMLD) mechanisms similar to those that occur for HP (see discussion). We decided to introduce an interaural level difference (ILD) instead so as to simplify the interpretation of HP/TN differences.
The stimuli were created offline, gated on and off using 15 ms cosine-squared ramps (with no gating in the transition at 1000 ms post-onset), and saved in 16-bit stereo WAV format at a sampling rate of 16 kHz. The signals were delivered to the subjects' ears with a tubephone (E-A-RTONE 3A 50 Ω, Etymotic Research, Inc), attached to E-A-RLINK foam plugs inserted into the ear-canal and presented at ∼75 dB SPL, to ensure a salient pitch. HP saliency increases with increasing noise level (Durlach, 1962).
In total each subject heard 100 presentations of each of the eight pitch conditions (HP 200, 400, 600, 1000 Hz; TN 200, 400, 600, 1000 Hz) and 800 (50% of all) presentations of the control stimulus. The order of presentations was randomized, with the inter-stimulus interval (ISI) semi-randomized between 500 and 2000 ms (depending on the subject's RT).
Procedure
Subjects lay supine inside a magnetically shielded room. Before the recording began, each subject's HP lateralization was assessed as described above and the appropriate stimuli were selected. The recording (∼1.5 h) consisted of two parts. First (pre-experiment) subjects listened to 200 repetitions of a 1 kHz 50 ms sinusoidal tone (ISI randomized between 750 and 1550 ms). These responses were used to verify that signals from auditory cortex had a satisfactory SNR. In the second part of the experiment, subjects, who were not informed about the existence of different types (HP versus TN) of tonal stimuli, performed a pitch detection task (50% of the trials) by pressing a button, held in the right hand, as soon as they heard a tone popping out of the noise. Reaction times (RT) and accuracy scores were stored and analyzed. Exit interviews showed that subjects were unaware of the existence of different (HP versus TN) tonal stimuli.
For the purposes of relative (i.e. no MR overlay) source localization, five electromagnetic coils were attached to the head of 14 participants in Exp1 prior to the MEG measurement. The locations of the coils were calculated with respect to anatomical landmarks on the scalp using 3D digitizer software (Source Signal Imaging, Inc.) and digitizing hardware (Polhemus, Inc.). In order to transform the MEG measurements into each participant's individual head coordinate system, the coils were also localized with respect to the MEG sensors. A 3D head-shape, used to estimate a spherical head model for each participant, was also acquired during digitization.
Neuromagnetic Recording and Data Analysis
The magnetic signals were recorded using a 160-channel, whole-head axial gradiometer system (KIT, Kanazawa, Japan). The data for the pre-experiment were acquired with a sampling rate of 1000 Hz, filtered online between 1 and 58.8 Hz, baseline corrected to the 100 ms pre-onset interval and stored in 500 ms (100 ms pre-onset) stimulus-related epochs. The data for Exp1, Exp2 and the control experiment were acquired continuously with a sampling rate of 1 kHz, filtered online between 1 and 200 Hz, with a notch at 60 Hz, and stored for later analysis. Effects of environmental magnetic fields were reduced based on several sensors distant from the head using the CALM algorithm (Adachi et al., 2001), and responses were then smoothed by low pass filtering with cutoff at 55 Hz.
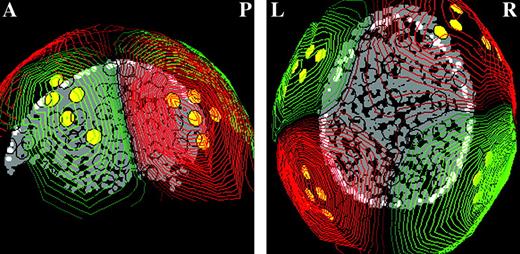
In the pre-experiment, auditory evoked responses to the onset of the pure tones were examined and the M100 response was identified for each subject as a dipole-like pattern (i.e. a source/sink pair) in the magnetic field contour plots distributed over the temporal region of each hemisphere. For each subject, the 20 strongest channels at the peak of the M100 (five in each sink and source, yielding 10 in each hemisphere) were considered to best reflect activity in the auditory cortex and thus chosen for the analysis of the experimental data (Fig. 4).
Channel selection from the pre-experiment. Different channels were chosen for each individual subject depending on their M100 response; The figure shows a sagittal view (A = anterior, P = posterior) of the LH and axial view (L = left, R = right) of the digitized head-shape of a representative subject, the dipole-like pattern in the iso-field maps distributed over the temporal region (red: sink; green: source), and the locations of the 20 chosen channels (yellow circles).
In Exp1 and 2, 1500 ms long epochs (50 ms pre onset) were created for each of the nine stimulus conditions. Epochs with amplitudes >3 pT (∼10%) were considered artifactual and discarded. The rest were averaged, low-pass filtered at 20 Hz and baseline corrected to the full range of the epoch. In each hemisphere, the root mean square (RMS) of the field strength across the 10 channels, selected in the pre-experiment, was calculated for each sample point. Eighteen RMS time series, one for each condition and each hemisphere, were thus created for each subject. To evaluate congruity across subjects, the individual RMS time series were combined into 18 group-RMS (RMS of individual RMSs) time series. Consistency of peaks in each group-RMS was automatically assessed with the Bootstrap method (1000 iterations; balanced; Efron and Tibshirani, 1993), a computationally intensive resampling method that allows the treatment of situations in which the exact sampling distribution of the statistic of interest is unknown. Source locations were estimated at the RMS peak latency using the model of an equivalent current dipole with the best-fit sphere for each subject's head. A single dipole model was applied for each hemisphere and all channels over that hemisphere were used for the computation.
Since response latencies, which are the major experimental parameter in this study, are naturally characterized by positive skew and the prevalence of outliers, assuming a normal distribution may be misleading. For that reason, for each statistical test presented here, we performed the applicable standard parametric test as well as a form of bootstrapped hypothesis testing (see Efron and Tibshirani, 1993). The two methods yielded very similar results so only the standard parametric test results are reported here. The α level was set a priori to 0.05. The lower-bound correction was applied where applicable.
Results
The stimuli of Exp1 (Fig. 2A) sound like a 1500 ms continuous noise located in the center of the head with a faint tonal object appearing at 1000 ms post-onset. The initial portion of the stimuli of Exp2 sounds like a diffused noise (the binaural stimuli are not fused to a unitary auditory object). At 1000 ms the noise changes from diffused to centered, and at the same time a faint tonal object appears (Fig. 2B). This description applies only to binaurally presented stimuli. When listening with only one ear, the stimuli of Exp1 and the corresponding stimuli of Exp2 sound the same (1500 ms of noise in the case of the control stimulus or the HP stimuli and 1500 ms of noise with a tonal object appearing at 1000 ms post-onset in the case of TN stimuli).
Experiment 1
Behavioral Data
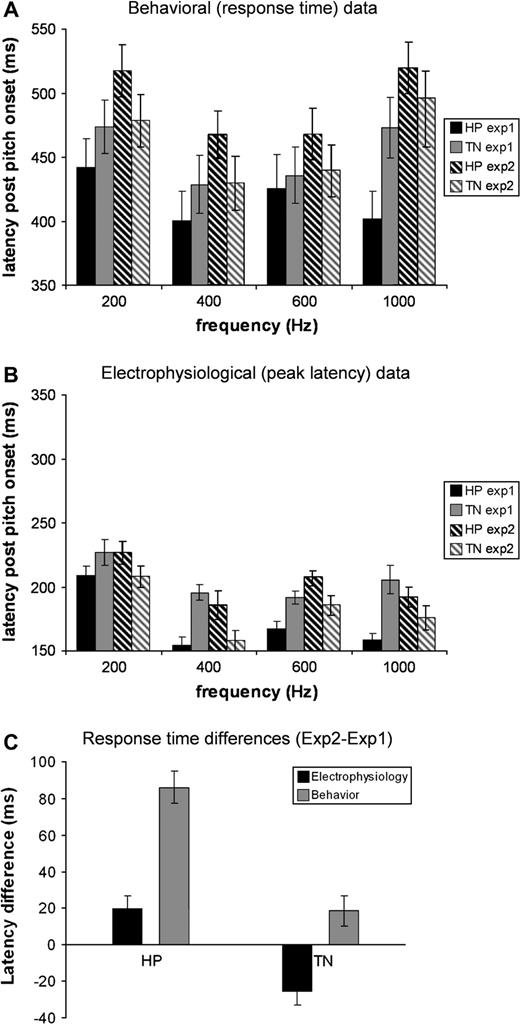
Subjects were generally accurate at detecting the auditory targets. In Exp1, the mean miss rate was 2% of the pitch trials and the mean false positive count was 2% of the control (noise) trials resulting in d′ = 4.12. The response time (RT) data are summarized in Figure 5A. An analysis of variance (ANOVA) with type (HP,TN) and frequency as factors showed significant main effects for both factors [F(1,19) = 107.456, P < 0.001; F(1,19) = 33.167, P < 0.001] as well as a significant interaction [F(1,19) = 30.788, P < 0.001]. In Exp1, subjects responded faster to HP stimuli than to TN stimuli, regardless of frequency tested. This effect was significant for all but the 600 Hz stimuli (paired t tests, df = 19: 200 Hz, t = −7.35, P < 0.01; 400 Hz, t = −8.26, P < 0.01; 1000 Hz, t = −11.83, P < 0.01).
Behavioral versus electrophysiological responses. (A) Average behavioral RT for the different conditions in Exp1 (solid bars) and Exp2 (striped bars). (B) Electrophysiological peak latency of responses in the LH for the different conditions in Exp1 (solid bars) and Exp2 (striped bars). The time scales are different in the two plots but both show a 200 ms interval to facilitate the visual comparison. (C) Average response time differences (collapsed over frequencies) between Exp2 and Exp1 for electrophysiology and behavior for the 12 subjects common to both experiments. Positive values indicate responses in Exp2 that were delayed relative to Exp1. Electrophysiological responses to TN were earlier in Exp2 than Exp1, opposite to the behavioral pattern and both types of responses to HP. All error bars (in A, B, C) represent 1 SE.
Electrophysiological Data
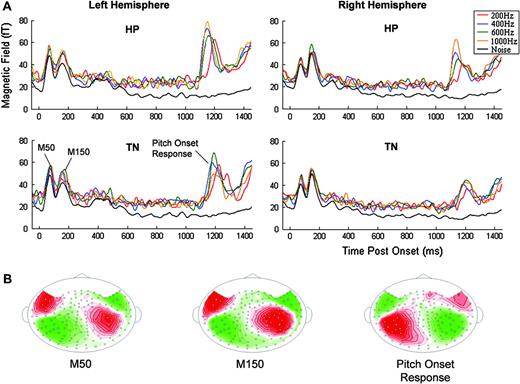
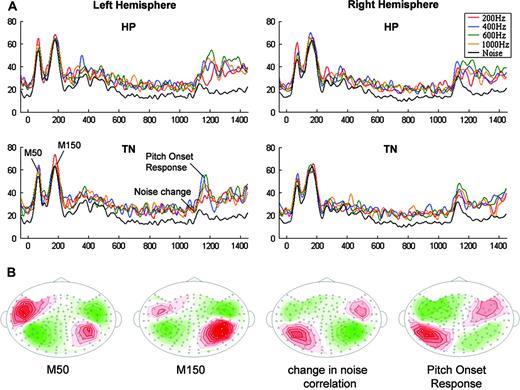
Waveform analysis reveals that all participants had comparable response trajectories. Figure 6A shows the group-RMS (RMS of the individual-subject RMSs) of the auditory evoked response for each of the conditions in the left and right hemispheres (LH and RH, respectively). The responses contained a two-peaked ‘noise onset response’ at ∼70 ms and ∼150 ms post-stimulus onset (both with a characteristic M50 spatial distribution) and a POR (with a characteristic M100 spatial distribution) at ∼1160 ms, i.e. ∼160 ms after onset of TN/HP. The POR (nomenclature introduced by Krumbholz et al., 2003) was modulated in latency by perceived pitch. The dipolar patterns observed in the iso-contour maps for a representative subject are displayed in Figure 6B. Interestingly, the initial ‘noise onset response’ lacks the usual M100 evoked by stimulus onset. It is likely that the lack of an M100 peak is a consequence of the task performed by the subjects that led them to attend to the later part of the stimulus and regard the former part as a noise background. This is discussed in detail elsewhere (Chait et al., 2004).
Summary of the electrophysiological data from Exp1. (A) The group-RMS in the LH and RH for all tested conditions. The control condition (noise) is lower because it is computed by averaging over many more (800 vs. 100) repetitions. The response is characterized by a two-peaked noise onset response, and a pitch onset response at ∼160 ms post-HP/TN onset, modulated by frequency. (B) Contour maps from a representative subject at the critical time periods (10 fT/iso-contour). Source = red; sink = green.
By adding a stretch of noise before the onset of the HP/TN portions of the stimulus, we were able to isolate the brain response to the onset of the stimulus from the response to the onset of the tonal signal. Figure 6A shows that the transient response due to the onset of the noise has faded by ∼600 ms. The onset of the pitch corresponds to a prominent increase of activity at ∼160 ms post-pitch onset (∼1160 post-stimulus onset) and shares important characteristics with the standard M100 response, including its spatial distribution (reflected in the contour plot) and its dependence on perceived pitch (for a review, see Roberts et al. 2000). The existence of such a vigorous response is surprising, as it contrasts with the relatively weak perceived loudness of the tonal signals. Repeated measurers ANOVA with hemisphere, type (HP, TN) and frequency (200, 400, 600, 1000 Hz) as factors showed main effects of type [F(1,19) = 65.445, P < 0.001] and frequency [F(1,19) = 15.194 P < 0.001]. The latency of the POR is affected both by the frequency and the type (HP versus TN) of the signal. HP stimuli elicit a response with a peak latency that is ∼30 ms earlier than the corresponding TN condition. The observed POR in this study is similar to the POR reported by Krumbholz et al. (2003) in both its spatial distribution and dependency of latency on pitch.
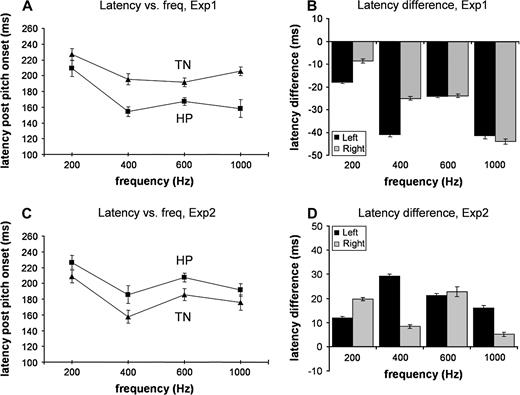
Figure 7A shows the average peak latency in the LH as a function of frequency. The latency of the peak of the POR for each of the eight conditions was determined automatically for each listener by choosing the maximum value of the RMS in the interval 1100–1300 ms post-pitch onset. Note that the peak latency for the lowest frequency is longer, i.e. the 200 Hz signals are associated with significant latency delays, a finding that has been reported and discussed extensively in the context of the M100 literature (Roberts et al., 2000; Lütkenhöner et al., 2001). To estimate the latency differences between TN and HP, for each listener and each frequency, the latency of the peak of TN was subtracted from that of HP (Fig. 7B). The response to HP stimuli is consistently earlier than the response to TN stimuli, 30 ms on average. The amplitude difference between HP and TN peaks was not statistically significant.
Latency results. (A) Exp1: peak latency of the average POR in the LH as a function of frequency. Squares: HP; triangles: TN. (B) Exp1: residual latency in the two hemispheres on an individual subject basis. (C) Exp2: peak latency of the average POR in the LH as a function of frequency. Squares: HP; triangles: TN. (D) Exp2: residual latency in the two hemispheres on an individual subject basis. All error bars are 1 SE derived from bootstrap. The values on the x-axis in all figures are category names and are not presented as being on scale.
The observed latency differences might conceivably be attributed to the difference in bandwidth of the tonal parts of TN and HP stimuli. The EC model of Durlach (1962, 1963), suggests that the internal representation of the HP stimulus resembles that of a narrow band of noise, whereas the TN stimulus is a pure tone. In order to investigate this possibility, a control experiment was run with five subjects to compare the TN stimuli used in Exp1 with noise band stimuli (BN; see Materials and Methods). The experimental parameters and procedure were as in Exp1. The data (not shown) demonstrate no significant latency difference between BN and TN for any frequency. In contrast, the same five subjects showed a significant effect in Exp1. Thus, the different activation patterns observed in Exp1 cannot be attributed to a bandwidth difference between the HP and TN stimuli. In the Discussion we argue that they may instead reflect the mechanisms that process binaural stimuli.
A striking finding is that the POR had significantly larger amplitudes in the LH compared with the RH. This effect is found both for HP and TN stimuli (paired t-tests at the peak of the PORs, df = 19: TN200, t = 3.41, P < 0.01; TN400, t = 4.16, P < 0.01; TN600, t = 6.02, P < 0.01; TN1000, t = 2.66, P = 0.015; HP200, t = 3.81, P < 0.01; HP400, t = 3.52, P < 0.01; HP600, t = 5.18, P < 0.01; HP1000, t = 5.18, P < 0.01). This observation is interesting insofar as no such hemispheric differences were found for the M100 response for pure tones in the pre-experiment (Fig. 8D). Similar findings have been reported (Hertrich et al., 2005; Hautus and Johnson, 2004; Johnson et al., 2003), but are harder to interpret in the latter case because no leading noise or tone controls were used. Krumbholz et al. (2003) measured MEG signals only over the left hemisphere.
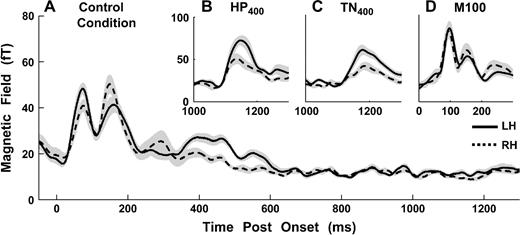
Comparison of hemispheric activation: LH, solid lines; RH, dashed lines. Grey areas are 1 SE derived by bootstrap. (A) Control condition in Exp1. M50 peak has stronger activation in the LH; M150 peak shows stronger activation in the RH. (B, C) POR for all HP and TN conditions (400 Hz shown here as an example) exhibited stronger left hemispheric activation. (D) The M100 response in the pre-experiment (1 kHz tones) showed no significant hemispheric differences.
The stimulus onset responses also showed significant hemispheric differences (discussed in Chait et al., 2004). Hemispheric lateralization thus switched (at least) three times during the full course of the 1500 ms stimulus (Fig. 8). The first peak, M50, is stronger in the LH, the second one, M150, is stronger on the RH, and the third peak, POR (the 400 Hz stimuli are shown as an example), is again significantly larger in the LH.
As discussed in the methods section, we compensated for listener-specific subjective lateralization of HP by using TN stimuli with similar perceived lateralization as HP (see methods). In all of the findings discussed above, there was no difference between the subjects who perceived HP on the left or right (and thus were presented with TNleft or TNright in the experiment) and the subjects who heard HP at the center of the head or at an ambiguous location (and thus were presented with TNcenter). Therefore, the hemispheric lateralization and the latency differences of the MEG response do not seem to be related to the perceived location of the stimuli. Additionally, in experiments specifically designed to investigate spatial (ITD) effects on auditory evoked potentials the observed latency effects were on the scale of a few milliseconds (McEvoy et al., 1993), an order of magnitude smaller than those observed here.
The Source of the Pitch Onset Response
For each of the 14 subjects for whom a digitized head shape was obtained, the M50 peak (mean latency = 69.8 ms) in the control condition in the LH was fitted to a single equivalent current dipole. One listener with a goodness of fit (GOF) of <80% was discarded from the analysis. The mean GOF for the 13 remaining listeners was 88.77%. The M50 component is believed to originate in or near PAC. In a recent study, Yvert et al. (2001) found it to activate the antero-lateral portion of HG and Heschl's sulcus. This might reflect activity in the human counterpart of the anterior areas in the core line region or in the antero-lateral belt region described in monkey (Kaas et al., 1999).
In order to compute the proportion of the POR field explained by the current dipole obtained for the M50 components, we estimated the GOF of that M50 dipole (maintaining a fixed location and orientation but allowing for a 180° flip in polarity) for the pitch onset component. The 400 Hz stimuli, HP400 (mean latency = 1135 ms) and TN400 (mean latency = 1186 ms), were characterized by the clearest and most prominent POR peaks and thus were chosen for this analysis (for the same reason only the LH response is fit). The resulting mean GOF was 77.3% for HP400 and 76% for TN400, with no significant difference between conditions. These findings indicate that the M50 dipole produces a good fit to the field of the POR. The M50 and the POR have opposite polarities, so the two processes cannot originate from identical neuronal populations. The good negative fit suggests at least one possible explanation: that the sources of the activity lie in close proximity in auditory cortex, though perhaps on opposite sides of a cortical fold. When looking at the proportion of the field explained by the M50 dipole in the time range 0–1400 ms, the time windows with the highest GOF are around the onset of the stimulus (M50 time window) and the onset of the pitch. The average GOF of the remainder, including, crucially, the time window around 100 and 150 ms post-noise onset, is below 40%, suggesting a different source and further affirming the specificity of the relation between the M50 component and the POR. These findings (that the POR originates in an area not in PAC but very close to it) are consistent with recent fMRI studies: Penagos et al. (2004) report that a region immediately anterolateral to PAC exhibits sensitivity to pitch salience. In Patterson et al. (2002), the contrast between noise and pitch eliciting iterated rippled noise activated a relatively small, bilateral region, lateral to PAC on HG, which the authors suggest might correspond to the R or RT region of core auditory cortex.
The small number of repetitions per condition, in combination with the spatial resolution of the MEG system and inter-subject variability prevent us from making any further spatial conclusions with a reasonable degree of certainty.
Experiment 2
The only respect in which Exp2 differed from Exp1 was that the initial 1000 ms of all stimuli (including controls) were uncorrelated instead of correlated noise. Crucially, the last 500 ms (HP, TN, or noise for the control stimuli) remained exactly the same as in Exp1. In particular, the noise in these segments was correlated, and thus switched from uncorrelated to correlated at 1000 ms post-onset (see Fig. 2).
Behavioral Data
Performance in Exp2 was slightly worse than in Exp1. Those subjects that participated in both experiments (n = 12) observed that Exp2 was more difficult. They reported that in addition to the change in the noise that occurred simultaneously with the appearance of pitch and hindered the detection, the quality of the noise (as two unfused objects at each ear) in the first 1000 ms of the stimulus made it harder to detect the tonal object. This was reflected in the moderately increased error rate in Exp2: the mean miss rate was 7% of the tonal trials and the mean false positives count was 4% of the control (noise) trials resulting in d′ = 3.23.
A repeated-measures ANOVA with type (HP, TN) and frequency as factors revealed main effects of both [F(1,15) = 29.044, P < 0.001; F(1,15) = 52.77, P < 0.001]. In contrast to Exp1, in Exp2 subjects responded faster to TN stimuli than to HP stimuli (paired t-tests, df = 15: 200 Hz, t = 5.45, P < 0.01; 400 Hz, t = 6.13, P < 0.01; 600 Hz, t = 4.11, P = 0.01; 1000 Hz, t = 2.12, P = 0.051; Fig. 5A).
Electrophysiological Data
Figure 9A shows the group-RMS of the auditory evoked response for each of the conditions in the LH and RH. The dipolar pattern in the iso-contour maps for a representative subject is displayed in Figure 9B. The onset of the stimulus is characterized by two peaks, at 70 and 180 ms in the LH, and at 70 and 170 ms in the RH. As in Exp1, the classical M100 response is absent at the onset of the noise (Chait et al, 2004).
Summary of the electrophysiological data from Exp2. (A) The group-RMS of in the LH and RH for all tested conditions. The control condition (noise) is lower because it is computed by averaging over many more (800 vs. 100) repetitions. The responses contain a two peaked noise onset response, a peak that corresponds to the change in noise at ∼1130 ms post-onset, and a pitch onset response at ∼160 ms post-HP/TN onset, modulated by perceived frequency. (B) Contour maps from a representative subject at the critical time periods (10 fT/iso-contour). Source = red; sink = green.
In contrast to Exp1, in Exp2 the separation of the response to the onset of the tonal target from the response to the noise is not as complete because there are two simultaneous changes occurring in the stimuli at 1000 ms post-onset: (i) the noise changes from uncorrelated to correlated and (ii) onset of HP/TN (see Fig. 2). However, comparison of responses to the control stimulus and pitch-evoking stimuli indicates that these two changes are processed at different times: The cortical activity due to the change in the noise is reflected in a peak in the control condition at 1134 ms in the LH and 1130 ms in the RH. The latency of this peak was not modulated by the perceived pitch of the tonal stimulus (when present). The POR is evident in the pitch conditions (especially in the LH) as a separate, second peak at ∼1160 ms post-onset or later, so the two responses can be distinguished. A repeated-measures ANOVA with hemisphere, type (HP, TN) and frequency as factors showed main effects of type [F(1,14) = 11.8 P = 0.004] and frequency [F(1,14) = 2.88, P = 0.047]. As in Exp1, the latency of this peak covaries with perceived pitch and type: HP stimuli elicit a response with a peak latency that is ∼20 ms later than the corresponding TN condition. The difference between the amplitude of the peaks of HP and corresponding TN stimuli is not significant.
Figure 7C shows the average peak latency in the LH as a function of the frequency of the perceived tonal object. The latency of the peak of the POR for each of the eight conditions was automatically determined for each listener by choosing the maximum value of the RMS in the interval 1100–1300 ms post-pitch onset. The data in the 200 Hz condition were noisy and peaks were not found in the data of two subjects. For five additional participants, the maximum value of the RMS was at the peak associated with the change in noise, in those cases the POR was defined as the second highest peak in the above specified interval. In order to estimate the latency differences, for each listener and each frequency the latency of the peak of TN was subtracted from that of HP (Fig. 7D). Positive values reveal that the response to HP stimuli is consistently later than the response to TN stimuli by ∼20 ms.
The physiological data from Exp2 were noisier than the data from Exp1. However, the main difference with Exp1, the switch in latency between TN and HP stimuli, is robust across frequencies and listeners (see Fig. 7) and cannot be attributed solely to a decrease in SNR.
Hemispheric comparisons do not yield significant differences in Exp2, possibly as a consequence of the noisier nature of the data, but the trend is in the same direction as Exp1. Interestingly, the peak at ∼1130 ms, associated with the change in the perception of the noise, had higher amplitude in the RH (not statistically significant, but approaching significance). This might be related to fMRI reports of stronger activation in the RH when subjects were listening to changes in binaural timing (Griffiths et al., 1998b). The higher amplitude in the RH is probably masking more of the pitch onset signal, which in turn might explain the noisier results in the RH (see Fig. 9).
As in Exp1, the hemispheric lateralization or the latency differences between the stimuli were not related to the perceived location of the stimuli.
Comparison between Experiment 1 and Experiment 2
Behavioral Data
When comparing the results of the two experiments (three-way repeated-measures ANOVA for the 12 subjects who participated in both experiments; corrected) there is a main effect of experiment [F(1,11) = 13.33 P < 0.01] and frequency [F(1,11) = 45.61 P < 0.01], as well the interactions experiment × frequency [F(1,11) = 12.12 P < 0.01], stimulus type × frequency [F(1,11) = 11.91 P < 0.01], experiment × stimulus type [HP/TN, F(1,11) = 48.15 P < 0.01] and experiment × stimulus type × frequency [F(1,11) = 9.96 P < 0.01], indicating that RTs for both HP and TN were longer in Exp2 than in Exp1. Despite the three-way interaction, effects at all frequencies are similar. When collapsed across frequencies (Fig. 5C) a paired sample t-test showed that RTs for both HP and TN stimuli were greater in Exp2 relative to Exp1 (df = 47; HP: t = −9.837, P < 0.01; TN: t = −2.261, P < 0.028), corresponding to subjects' reports that Exp2 was harder; individual frequency results are similar but noisier.
Electrophysiological Data
We compared POR peak latencies of the two experiments using a three-way repeated-measures ANOVA (with experiment, hemisphere, type and frequency as factors) for the 12 subjects that participated in both experiments. Unlike for the behavioral data, there were no main effects of experiment or stimulus type (due to the latency reversal in the two experiments) but there is a main effect of frequency [F(1,10) = 5.82, P = 0.037], as well as an interaction between experiment × stimulus type [HP/TN, F(1,10) = 34.732 P < 0.01].
Figure 5B summarizes the latency data of the PORs from the two experiments (we report results for the LH; RH results are similar but noisier). When collapsed across frequencies (Fig. 5C) a paired sample t-test showed a significantly greater latency in Exp2 relative to Exp1 for HP stimuli, but smaller latency to TN stimuli (df = 47; HP: t = −2.789, P < 0.01; TN: t = 3.17, P < 0.01); the amplitude of the POR in the LH did not show a significant change between the stimuli of Exp1 and those of Exp2. We argue in the discussion that the different peak latencies of responses to HP and TN in the two experiments may be attributable to the different activation that they produce in the binaural system.
When performing the pitch detection task, subjects pressed a button held in their right hand. It could be argued that this might have influenced the amplitude and left lateralization of the pitch onset peak. Such an effect can be dismissed by comparing the data from the two experiments: the experimental procedure was identical in both experiments, accuracy of pressing the button was comparable (see above), but the latency, amplitude and lateralization of the peaks differed significantly. This suggests that these characteristics of the responses reflect the processing of the acoustic stimuli and not the motor event.
Discussion
The primary objective of this whole-head auditory MEG study was to investigate the timing of the formation of the percept of tone in two physically very different signals that elicit a very similar pitch percept — Huggins pitch (HP) and a pure tone in noise (TN). Several aspects of the data, as well as new unresolved issues, are discussed in turn below.
Implications for Pitch
In this study we observed a prominent cortical response to the onset of both HP and TN stimuli, occurring at ∼150–200 ms post-tonal onset. By prepending noise to the HP/TN stimuli we are able to separate the response to the tonal onset from other processing associated with the onset of an acoustic stimulus. Unlike other studies that used the M100 response at the onset of a tonal stimulus to probe brain processes that handle pitch, we measured the response to the transition from a noise to a pitch-like stimulus embedded in noise (as in Krumbholz et al, 2003). The latency of the M100 response at the onset of a tonal stimulus is known to covary with pitch, but this response confounds stimulus-onset-related computation with pitch computation. In contrast, the response to the transition from noise to a pitch-like stimulus (POR) allows pitch computation to be isolated. Additional evidence for the fact that the POR truly reflects tonal processing and not just general change in the input come from Exp2 where the deflection corresponding to the change in noise from uncorrelated to correlated occurs ∼30 ms earlier than the response to the onset of the tonal targets, even though both changes occurred simultaneously in the signal (see Fig. 2). If the POR were just a detector of change in the ongoing stimulation (e.g. Jones et al., 1991), we would expect to see a single peak rather than the two peaks observed in Exp2, each with distinct temporal and spatial properties: POR stronger on the LH, response to change in noise stronger on the RH, indicating distinct neural generators.
The POR peaked at 160 ms post-tonal onset, ∼60 ms later than a classic M100, although M100 latencies to near-threshold tonal signals are comparably delayed (Stufflebeam et al., 1998). The early sensory processing of HP differs from TN and the other diotic stimuli that were investigated in previous experiments (regular interval noise in Patterson et al., 2002, Krumholtz et al., 2003; Ritter et al., 2005; stimuli with and without resolved harmonic in Penagos et al., 2004). The comparison of the activation evoked by HP and TN stimuli therefore affords an opportunity to distinguish neural processing common to these pitch-like stimuli from those specific to early sensory processing. The responses to both (TN and HP) were localized to the same cortical area and exhibited similar frequency dependence. For both, low frequency stimuli elicited longer latencies than high frequency ones, similar to the ‘classic’ M100 response (Roberts et al., 2000). Since HP showed this effect, an inevitable conclusion is that the dependence on frequency originates from central mechanisms (beyond the stage where binaural information is combined); explanations based solely on the cochlear traveling wave delay (Greenberg et al., 1998; see also Borgmann et al., 2001) must therefore be reassessed. Crucially, these data indicate that by ∼150 ms post-onset both types of tonal objects are mapped to very similar representations in cortex. The similarity of the MEG responses is consistent with behavioral data indicating that HP generates pitch and timbre percepts that are like those of monaural tones (Bilsen, 1977) and suggests that these signals are processed similarly, despite their physical differences.
Our data provide new evidence that the processes that give rise to the POR reflect central pitch mechanisms. An fMRI study (Patterson et al., 2002) and a MEG study (Krumbholz et al., 2003), using monaural IRN stimuli, identified a hypothesized ‘pitch center’ in HG, whose activation increased with the degree of temporal regularity in the signal. Since the pitch-evoking structure of IRN is present in the stimulus at both ears, its processing could begin as early as the cochlear nucleus (as suggested by Griffiths et al., 2001). Such is not the case for HP. According to generic models of auditory processing (McFadden, 1975; Colburn and Durlach, 1978; Stern and Trahiotis, 1995), auditory information is processed via monaural and binaural analyzers. Huggins pitch requires binaural presentation, and thus cannot be extracted by low-level processors within a monaural pathway that precedes binaural interaction. The pitch of the TN stimulus (like IRN) can, in theory, be detected monaurally, and consequently could be extracted peripherally or at some low-level stage (cf. Cariani and Delgutte, 1996). Parsimony, and the fact that TN varies with frequency similarly to HP suggests that both stimuli are processed beyond the stage of binaural convergence, and argues against the hypothesis of separate pathways for monaural and binaural pitch phenomena. Different latencies for TN and HP responses might be taken as evidence for separate processing levels, but the fact that HP MEG responses preceded TN responses in Exp1 would imply processing of the binaural HP stimulus occurs at an earlier stage than that of the monaural TN stimulus, which seems unlikely. Instead, we propose the latency differences arise as a result of binaural processing that both signals undergo (see below).
Interestingly, Carlyon et al. (2001), using dichotic pulse trains with no place information, were led to conclude that temporal pitch mechanisms operate on the input to each ear alone rather than on the output of the binaural system (combination of the information from the two ears). That conclusion was obtained using unresolved stimuli. Our study suggests the opposite conclusion (not only for HP, but also for TN), but as we used resolved stimuli (in the sense that the tonal components are isolated sinusoids) we do not know whether our conclusion applies to spectral (place) or temporal pitch mechanisms or both.
Our results are consistent with a pitch processor that is driven by a central spectrum, computed from all available information irrespective of it being monaural or binaural in nature. The EC model of Durlach (1962, 1963), for example, suggests that the central representation of HP has a similar time and place profile to that of TN. In this study we have only used pure tones (TN) and ‘pure tone’-like stimuli (HP). Nevertheless, the data reported here are a significant first step and constitute a generalizable paradigm for use with richer pitch stimuli. An intriguing next step is comparing brain responses to complex tones with a missing fundamental with response to missing fundamental HP complexes (Bilsen, 1977). The ‘Bilsen multiple phase shift pitch’ has phase shifts at several harmonics, and therefore is the counterpart of the pitch of harmonic complex tones. HP and its generalizations are ideal tools to study ‘missing fundamental’ effects because combination-tones resulting from cochlear non-linearities (Pantev et al., 1989) do not exist.
The LH advantage at the peak of the POR is unexpected. No hemispheric difference is reported in studies involving detection or discrimination of pure tones (Papanicolaou et al., 1999; Shtyrov et al., 2000; Johnsrude et al., 2000; but see Devlin et al., 2003), or in studies with IRN stimuli (Patterson et al., 2002; though see Griffiths et al., 1998a). In fact, pitch-related tasks are usually reported to produce stronger responses from the RH (Zatorre, 2001). One possible explanation is that the pitch onset peak does not reflect the extraction of pitch per se, but the segregation of the tonal object within the auditory scene and its separation from the noise background, which might incur more significant involvement of LH mechanisms. The onset of the pitch-evoking stimulus activates pitch sensitive mechanisms that produce a pitch-like percept, but also segregation (or pop-out-of-background) mechanisms that produce the perception of a pitched object. The POR may be tapping into the latter stage. Alain et al. (2002; see also Dyson and Alain 2004), in EEG studies of concurrent sound segregation, reported that the perception of a mistuned harmonic as a separate sound is associated with a negative wave peaking at ∼150 ms after sound onset. In their stimuli the onset of mistuning coincides with that of the stimulus, so the response components of the two cannot be isolated, but the properties of the wave (referred to as object-related negativity) are very similar to those of the POR (see also Hautus and Johnson, 2004). This alternative interpretation, which could equally be applied to the results of the other fMRI/MEG studies cited here (e.g. Pantev et al., 1996; Patterson et al., 2002; Fujioka et al., 2003; Krumbholz et al., 2003; Penagos et al., 2004), still allows the responses to be interpreted in terms of pitch processing. Extraction of the pitch ‘feature’ must precede object emergence, and indeed the cortical response latency showed a dependence on the pitch of the tonal object. This study cannot disambiguate between an interpretation in terms of a pitch-specific response and this alternative. A more systematic and detailed examination of this question is warranted.
Binaural Processing
Models of auditory processing are often discussed in terms of ‘monaural’ and ‘binaural’ pathways (McFadden, 1975; Colburn and Durlach, 1978). The former are invoked to account for phenomena involving monaural (or diotic) stimulation, the latter for phenomena that arise only from dichotic stimulation. The pitch evoked by HP must arise in the latter, but that evoked by TN might conceivably arise in a monaural pathway. We do not see evidence of such a division in the present study. As discussed above, when presented monaurally the stimuli of Exp1 and Exp2 are indistinguishable; consequently any differences between the two experiments must be due to binaural mechanisms. In particular, the latency differences observed between Exp1 and Exp2 for TN (Fig. 5C) must result from the binaural processing of correlated versus uncorrelated noise. The change in the interaural configuration of the leading noise caused an earlier brain response to TN in Exp2 relative to Exp1. Note that a difference of the opposite sign would have been compatible with the hypothesis of monaural processing of the pitch of TN with subsequent interference from the result of binaural processing at a later stage. The fact that the responses in Exp2 were earlier indicates that binaural processing aided the detection of TN, and thus that detection occurred within a binaural rather than monaural pathway. HP is commonly hypothesized to be mediated by the mechanism of binaural unmasking (Raatgever and Bilsen 1986; Culling et al., 1998): a target that is just masked by binaurally correlated noise can be made easier to detect by inverting the noise or the target in one ear. Unmasking depends on mechanisms that are sensitive to the similarity of the signals at the two ears. Cells exhibiting these properties are found in the MSO, and in animal studies, the inferior colliculus (IC), the projection target of the MSO, exhibits correlates of binaural unmasking (Jiang et al., 1997a,b; Palmer et al., 2000).
The auditory-evoked magnetic fields, measured outside the head by MEG, are generated by neuronal currents flowing in tens of thousands of cortical pyramidal cells on the supratemporal gyrus (Hämäläinen et al., 1993), but the observed response latency differences might originate as early as the SOC. One possibility is that the latency disparity reflects constraints of processing within the MSO itself. The MSO has been likened to an array of cross-correlators fed from both ears (Jeffress, 1948; Joris et al., 1998). Figure 3E–H illustrates the long-term time average of the activity within such an array that would be evoked by our stimuli. Correlated noise (Fig. 3E) evokes an orderly arrangement of ‘valleys’ and ‘peaks’ (resulting from each cell's sensitivity to a particular relative phase between the two ears at its best frequency; cf. Yin and Chan, 1990). TN stimuli (Fig. 3F) evoke the same pattern with slightly higher amplitude at the frequency of the tone, whereas HP stimuli (Fig. 3G) produce a more complex pattern, with a crossover between ridges at the frequency of the phase transition. Uncorrelated noise (Fig. 3H) evokes an irregular pattern with low amplitude. The frequency-local features of target stimuli (TN or HP) distinguish them from non-target correlated noise. The influence of the preceding context (correlated or uncorrelated noise) on their detection might explain the latency differences observed between Exp1 and Exp2. In Exp1, the onset from correlated noise to HP activates neurons, within the frequency region of the phase transition, that were previously inactive (compare Figs 3E and 3G). In Exp2 the onset from uncorrelated noise to HP also increments activity, but in this case it is distributed across the frequency axis. Easier detection of tonal HP targets in the former case might explain the smaller latencies of brain responses observed in Exp1. For TN, in Exp1 the onset of the tonal target causes a local increment in the activity of neurons that were already strongly activated by the correlated noise (compare Figs 3E and 3F). In Exp2 the same neurons were less strongly activated by the uncorrelated noise (compare Figs 3F and 3G). Easier detection of tonal TN targets in the latter case might explain the smaller latencies observed in Exp2 for brain (but not behavioral) responses. The patterns illustrated in Figure 3 reflect the generic cross-correlation model of Jeffress, but a similar account could be applied to the recent model of McAlpine and colleagues (McAlpine and Grothe, 2003). Thus, latency differences may result from constraints of binaural processing as early as the MSO, but it is not clear how they would result in the relatively large latency differences observed. It is also not clear whether they arise at the MSO itself, or in subsequent stages that interpret its output. To the best of our knowledge, no physiological studies have tried to measure the latency of responses to such stimulus events at the level of MSO or IC. Several different models of unmasking (and HP) have been suggested to account for the available psychophysical, physiological and electrophysiological data (Durlach, 1963; Raatgever and Bilsen, 1986). Physiological measurement of the latency of responses to signals such as those used here might greatly clarify our understanding of the processing of these events.
Behavior versus Electrophysiology
Although behavioral and electrophysiological responses mostly follow similar directions, there is also a striking difference between them (Fig. 5C). In the behavioral data, the average RT for HP was significantly greater in Exp2 than in Exp1. Similarly RTs for TN were either equal or greater in Exp2 relative to Exp1. In contrast, in the electrophysiological data responses to TN stimuli are faster in Exp2 than Exp1 (negative values in Fig. 5C), while the responses to HP stimuli are slower in Exp2 than Exp1 (positive values in Fig. 5C). Thus, although HP responses follow the same pattern as behavior, brain responses to TN in Exp2 (where changing binaural cues are present) are earlier than those to TN in Exp1 (no changing binaural cues). The difference between behavior and brain response patterns can be understood by supposing that the transition from correlated to uncorrelated background in Exp2 introduced an additional difficulty (reported by the subjects) that caused RTs to be overall longer in Exp2 than in Exp1. The fact that it was not apparent in the POR brain responses suggests that it affected a different pathway (or a stage subsequent) to that which produced those responses.
Behavior and electrophysiology, studied separately, might lead to different conclusions about the nature of the processing involved. The simultaneous acquisition of both MEG and behavioral data puts stronger constraints on the interpretation, revealing a multi staged process where early (∼150 ms post-onset) cortical responses (POR) reflect the operation of low-level mechanisms but behavior is affected by additional mechanisms. For example, these mechanisms might incorporate the outputs from the POR generating system as well as the outputs from a separate system sensitive to the change in the background noise into some decision variable, in this manner making the conscious detection of the tone onsets in Exp2 slower than in Exp1.
The incongruence between behavior and electrophysiology observed here demonstrates that there is a limitation on what can be learned from behavioral or electrophysiological measure alone. In the study of the processes that underlie the construction of perceptual experiences, electrophysiological measures can usefully supplement the wealth of data that have accumulated over the (relatively) long history of behavioral research.
We are grateful to Alain de Cheveigné, Catherine Carr, Shihab Shamma and Barbara Shinn-Cunningham for insightful comments and discussion, and to Jeff Walker for excellent technical support. M.C. and D.P. are supported by NIH R01DC05660. During the preparation of this manuscript M.C. was visiting at the ‘Audition’ lab, DEC-ENS, France and DP was a fellow at the Wissenschaftskolleg zu Berlin and the American Academy Berlin.
References
Adachi Y, Shimogawara M, Higuchi M, Haruta Y, Ochiai M (
Akeroyd MA (
Akeroyd MA, Moore BC, Moore GA (
Alain C, Schuler BM, McDonald KL (
Bendor D, Wang X (
Bilsen F (
Borgmann C, Roß B, Draganova R, Pantev C (
Cariani PA, Delgutte B (
Carlyon RP, Demany L, Deeks J (
Chait M, Simon JZ, Poeppel D (
Colburn HS, Durlach NI (
Cramer E, Huggins WH (
Culling J, Marshall D, Summerfield Q (
de Cheveigné A (
Devlin JT, Raley J, Tunbridge E, Lanary K, Floyer-Lea A, Narain C, Cohen I, Behrens T, Jezzard P, Matthews PM, Moore DR (
Durlach NI (
Durlach NI (
Dyson BJ, Alain C (
Fujioka T, Ross B, Okamoto H, Takeshima Y, Kakigi R, Pantev C (
Greenberg S, Poeppel D, Roberts T (
Griffiths TD, Büchel C, Frackowiak RSJ, Patterson RD (
Griffiths TD, Rees G, Rees D, Green GGR, Witton C, Rowe D, Büchel C, Turner R, Frackowiak RSJ (
Griffiths TD, Uppenkamp S, Johnsrude I, Josephs O, Patterson RD (
Gutschalk A, Patterson RD, Scherg M, Uppenkamp S, Rupp A (
Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounamaa OV (
Hautus MJ, Johnson BW (
Hertrich I, Mathiak K, Menning H, Lutzenberger W, Ackermann H (
Houtsma AJM, Goldstein JL (
Jiang D, McAlpine D, Palmer AR (
Jiang D, McAlpine D Palmer AR (
Johnson BW, Hautus M, Clapp WC (
Johnsrude IS, Penhune VB, Zatorre RJ (
Jones SJ, Pitman JR, Halliday AM (
Joris PX, Smith PH, Yin TC (
Kaas JH, Hackett TA, Tramo MJ (
Krumbholz K, Patterson RD, Seither-Preisler A, Lammertmann C, Lütkenhöner B (
Lütkenhöner B, Steinsträter O (
Lütkenhöner B, Lammertmann C, Knecht S (
McAlpine D, Grothe B (
McEvoy L, Hari R, Imada T, Sams M (
McFadden D (
Oldfield RC (
Palmer AR, Jiang D, McAlpine D (
Pantev C, Hoke M, Lütkenhöner B, Lehnertz K (
Pantev C, Elbert T, Ross B, Eulitz C, Terhardt E (
Papanicolaou AC, Panagiotis SG, Breier JI, Zouridakis G, Willmore J, Wheless JW, Constantinou JEC, Maggio W, Gormley WB (
Patterson RD, Uppenkamp S, Johnsrude IS, Griffiths TD (
Penagos H, Melcher JR, Oxenham AJ (
Plack CJ, Oxenham A, Fay RR, Popper AN (
Polyakov A, Pratt H (
Raatgever J, Bilsen F (
Ragot R, Lepaul-Ercole R (
Ritter S, Gunter Dosch H, Specht HJ, Rupp A (
Roberts T, Poeppel D (
Roberts T, Ferrari P, Stufflebeam S, Poeppel D (
Salajegheh A, Link A, Elster C, Burghoff M, Sander T, Trahms L, Poeppel D (
Seither-Preisler A, Krumbholz K, Patterson R, Seither S, Lutkenhoner B (
Shtyrov Y, Kujala T, Lyytinen H, Ilmoniemi RJ, Näätänen R (
Stern RM, Trahiotis C (
Stufflebeam SM, Poeppel D, Rowley HA, Roberts TP (
Yin TC, Chan JC (
Yost WA, Harder PJ, Dye RH (
Yvert B, Crouzeix A, Bertrand O, Seither-Preisler A, Pantev C (
Author notes
1Neuroscience and Cognitive Science Program, University of Maryland, College Park, MD, USA, 2Department of Linguistics, University of Maryland, College Park, MD, USA, 3Department of Biology, University of Maryland, College Park, MD, USA and 4Department of Electrical and Computer Engineering, University of Maryland, College Park, MD, USA