-
PDF
- Split View
-
Views
-
Cite
Cite
Madison H Fung, Brittany K Taylor, Michaela R Frenzel, Jacob A Eastman, Yu-Ping Wang, Vince D Calhoun, Julia M Stephen, Tony W Wilson, Pubertal Testosterone Tracks the Developmental Trajectory of Neural Oscillatory Activity Serving Visuospatial Processing, Cerebral Cortex, Volume 30, Issue 11, November 2020, Pages 5960–5971, https://doi.org/10.1093/cercor/bhaa169
Close - Share Icon Share
Abstract
Puberty is a period of substantial hormonal fluctuations that induce dramatic physical, neurological, and behavioral changes. Previous research has demonstrated that pubertal hormones modulate cortical development, as well as sex- and age-specific patterns of cognitive development during childhood and adolescence. However, the influence of pubertal hormones on the brain’s functional development, specifically neural oscillatory dynamics, has yet to be fully examined. Thus, in the current study, we used magnetoencephalography to investigate the oscillatory dynamics serving visuospatial perception and attention, and testosterone levels and chronological age as measures of development. Within a sample of typically developing youth, age was associated with changes in alpha, theta, and gamma oscillatory activity. Novel testosterone-by-sex interactions in the gamma range were identified in critical areas of the visual and attention networks. Females had increased gamma activity with increasing testosterone in the right temporal-parietal junction and occipital cortices, while males showed increased gamma activity in the right insula with increasing testosterone. These findings reveal robust developmental alterations in the oscillatory dynamics serving visuospatial processing during childhood and adolescence and provide novel insight into the hormonal basis of sexually dimorphic patterns of functional brain development during the pubertal transition that is at least partially mediated by endogenous testosterone.
Introduction
Puberty is a period of substantial hormonal fluctuations that induce dramatic physical, neurological, and behavioral changes. The onset of puberty triggers many physiological changes, specifically the production of the gonadal sex steroids testosterone and estradiol (Sisk and Foster 2004). These pubertal hormones facilitate sexually dimorphic physical development and are also known to stimulate sex-specific anatomical changes in the brain through synaptic pruning, programmed cell death, neurogenesis, and cell survival (Davis et al. 1996; Nuñez et al. 2002; Ahmed et al. 2008). In fact, gray matter develops within the frontal and parietal lobes 1–2 years earlier in females than in males (Giedd et al. 1999; Lenroot et al. 2007), which corresponds to sex differences in the timing of the onset of puberty (Dorn 2006). Other sex-related differences in frontal and parietal brain structures have also been linked to pubertal hormone levels (Neufang et al. 2009; Bramen et al. 2012; Nguyen et al. 2013; Koolschijn et al. 2014), and these appear to persist into adulthood (Witte et al. 2010), though the direction and extent of sex-specific gray matter development clearly vary by brain region. Thus, sex steroids are known to induce structural changes to critical brain areas that underlie both attention and salience networks. Although more broadly, sex-specific structural changes exist in overall linear white matter and non-linear gray matter development (Giedd et al. 1999; De Bellis et al. 2001; Gogtay et al. 2004; Shaw et al. 2008), in addition to negative associations between global gray matter and other measures of pubertal development (Peper et al. 2009; Paus et al. 2010; Vijayakumar et al. 2018).
Although there is mounting evidence that pubertal hormones impact cortical structure within fronto-parietal and other brain regions, there is a paucity of work examining whether developmental hormonal changes also affect the function of these cortical regions. Of note, several functional MRI (fMRI) studies have analyzed the behavioral and functional correlates of hormone levels during pubertal development on social, emotional, and reward processing (for review, see Vijayakumar et al. 2018), and a recent fMRI study examined the association between endogenous testosterone and the functional development of episodic memory (Selmeczy et al. 2019). The majority of these studies showed that neural responses are significantly associated with pubertal hormone levels, though the directionality of associations varied by brain region and process. Thus, despite the apparent impact of sex steroids on brain structure in fronto-parietal cortices, no developmental studies to date have examined whether puberty-related hormonal changes may also affect attention, visuospatial processing, and other brain functions known to be supported by these areas.
The mature visuospatial perception and attention networks, which allow the accurate detection and orientation towards behaviorally relevant stimuli, have been widely studied and consist of functional hubs localized across the frontal, parietal, and occipital cortices (Posner and Petersen 1990; Corbetta and Shulman 2002). These brain regions are also known to exhibit distinct spectro-temporal oscillatory dynamics during visuospatial processing, including rhythmic neural activity within the alpha, theta, and gamma frequency bands (Wiesman et al. 2017, 2018; Lew et al. 2019). These oscillatory responses are known to be modulated by age across the adult lifespan and to differ by sex (Wiesman and Wilson 2019); however, little is known about the development of these oscillatory dynamics serving visuospatial processing prior to adulthood.
More broadly, multiple studies have shown that neural oscillations serving motor control are strongly modulated by development (Heinrichs-Graham et al. 2018; Trevarrow et al. 2019), and recent work in children and adolescents has extended these findings to working memory (Embury et al. 2019) and abstract reasoning (Taylor et al. 2020). These latter two studies of higher cognitive processing reported robust developmental sex differences in both alpha and theta oscillations, including within the fronto-parietal cortices, but notably both used chronological age as a proxy for development. Since age is often correlated with pubertal status, this approach is intuitive and common in the literature. However, there is also vast individual variability in the timing and tempo of pubertal maturation, which would act as noise in the overall analysis (Sisk and Foster 2004; Blakemore et al. 2010). Thus, there is a critical need for studies that disentangle chronological age and pubertal effects on cortical maturation. Currently, a vast knowledge of sex and age-specific patterns of cognitive development during adolescence exists, and abundant research demonstrates hormonally mediated developmental changes, yet the influence of pubertal hormones on the development of the oscillatory dynamics remains unknown.
In the present study, we used endogenous testosterone levels as a measure of pubertal maturation to assess developmental changes in the neural oscillatory dynamics serving visuospatial processing in a sample of typically developing children and adolescents. Testosterone levels rise sharply in both males and females after the onset of puberty and continue to rise throughout this transition period and into early adulthood (Root 1973; Blakemore et al. 2010; Handelsman et al. 2016). Thus, testosterone levels are a reliable metric of pubertal maturation. In addition to testosterone measures, all participants completed a visuospatial processing task during magnetoencephalography (MEG) and the resulting neural responses were transformed into the time-frequency domain and imaged using a beamforming approach. Whole-brain voxel-wise correlations between neural oscillatory responses and testosterone levels, while controlling for chronological age, were computed to assess the impact of pubertal hormones on visuospatial processing and attention. We also computed correlations using chronological age to identify changes that may occur independently of pubertal hormones, and examined sex differences in the context of both approaches. Based on the prior literature noted above indicating unique effects of pubertal hormones on structural brain development, we hypothesized that testosterone levels would be significantly correlated with the strength of neural oscillatory activity within critical brain regions serving visuospatial processing and attention, above-and-beyond the effects of chronological age. We also anticipated that these effects would differ between males and females due to the sexually dimorphic action of sex steroids during pubertal development. Importantly, no prior neuroimaging work has examined the impact of changing pubertal hormone levels, a biological proxy for pubertal development, on neural oscillatory activity and behavioral performance during visuospatial processing and attention. Thus, our findings help bridge critical gaps in the literature spanning specific cognitive processes (i.e., visuospatial processing and attention vs. emotional and reward processing), neurophysiological features (i.e., oscillations vs. fMRI activation), and biological and physiological measures of development (i.e., hormone levels vs. chronological age).
Materials and Methods
Participants
Thirty-nine typically developing children and adolescents ages 9–15 years completed a visuospatial discrimination task and provided saliva samples for hormonal analyses as part of the National Science Foundation-funded Developmental Chronnecto-Genomics (Dev-CoG) study. All participants were recruited from the University of Nebraska Medical Center (UNMC) site. Exclusionary criteria included neurological or psychiatric disorder, attention-deficit/hyperactivity disorder (ADHD) or other disorders affecting brain function, history of head trauma, and general MEG/MRI exclusionary criteria such as the presence of metal implants, dental braces or permanent retainers, or other metallic or otherwise magnetic non-removable devices. Other exclusionary criteria included major medical conditions such as cancer, history or diagnosis of alcohol or substance use disorder, or pregnancy. All procedures were approved by the UNMC Institutional Review Board, and informed consent from the child’s parent or legal guardian, as well as assent from the child, were obtained before proceeding with the study.
Procedure
Salivary Testosterone Collection and Measurement
At least 2.0 mL of whole unstimulated saliva was collected from each participant. Specifically, children were asked to passively drool into an Oragene DISCOVER (OGR-500; www.dnagenotek.com) collection tube until liquid saliva (not bubble) exceeded the fill line indicated on the tube. A single-channel pipette was then used to extract 0.5 mL from the collection tube (prior to the release of the protease inhibitors for long-term storage), and this 0.5 mL was immediately transferred into a labeled micro-centrifuge tube and placed in a −20 °C freezer for storage. Participants were instructed to refrain from consuming any food, liquids, or chewing gum for at least an hour before providing the saliva sample, and generally completed the study in the afternoon (15:45, SD = 3.23 h). All samples were assayed in duplicate using a commercially available assay kit for salivary testosterone (Salimetrics; www.salimetrics.com). The assay kit had a sensitivity of 1 pg/mL, with a range of 6.1–600 pg/mL. The intra- and inter-assay coefficients of variation were 5.28% and 8.93%, respectively. The average of the duplicate tests was used for further analyses in the present study.
Task Paradigm
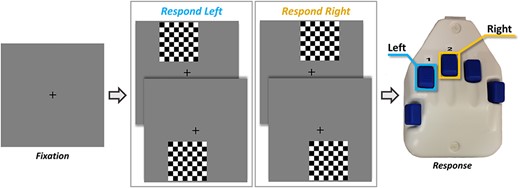
A visuospatial discrimination task, termed Vis-Attend, was used to engage the visuospatial processing circuitry (Wiesman et al. 2017). During this task, participants were told to fixate on a centrally presented crosshair. After a variable ISI (range: 1900–2100 ms), an 8 × 8 grid was presented for 800 ms at one of four positions relative to the fixation: above and to the right, below right, above left, or below left (Fig. 1). The left and right orientations were defined as a lateral offset of 75% of the grid from the center of fixation. Participants were instructed to respond via button press with their right hand as to whether the grid was positioned to the left (index finger) or right (middle finger) of the fixation point upon presentation of the grid. Each participant performed 240 trials (60 of each type) in a pseudo-randomized order concurrent with MEG recording. Responses with a reaction time 2.5 standard deviations (SDs) above or below the participant’s mean were excluded prior to averaging.
The visuospatial task paradigm (Vis-attend). Each trial consisted of two periods: (1) fixation lasting 2000 ms on average (1900–2100 ms variable ISI) with a 400-ms baseline period, and (2) an 800 ms stimulus-presentation period with the grid appearing in one of four locations. Participants indicated the lateral location of the stimulus grid relative to the fixation with a button press (left or right).
Of note, we used this simple visuospatial processing task to maximize both compliance and performance in our youth population, and because of its known neural response profile. Prior work in clinical, normative adult, and aging populations has shown that the task is relatively easy and cleanly assesses visuospatial processing and attention without entangling higher-order cognitive functions or other sensory modalities (Wiesman et al. 2017, 2018; Lew et al. 2019; Wiesman and Wilson 2019). Additionally, these past studies have shown that the task elicits strong multispectral responses that are temporally distinct, which enables different neural populations, oscillatory responses, and cognitive and perceptual processes to be probed for developmental effects, thereby broadening the possible scope of the study. The developmental trajectory of neural oscillatory dynamics is broadly unstudied, therefore utilizing a task that involves basic sensory and attentional functions and elicits multispectral responses is beneficial, as it allows studies to provide more extensive foundational data which subsequent work can then build upon and target more complex cognitive functions.
MEG Data Acquisition
MEG recordings were conducted in a one-layer magnetically shielded room with active shielding engaged. Neuromagnetic responses were acquired with an Elekta/MEGIN MEG system with 306 magnetic sensors (204 planar gradiometers, 102 magnetometers; Elekta/MEGIN, Helsinki, Finland) using a bandwidth of 0.1–330 Hz, sampled continuously at 1 kHz. Each participant’s data were individually corrected for head motion, and noise reduction was applied using the signal-space separation method with a temporal extension (tSSS; Taulu et al. 2005; Taulu and Simola 2006).
MEG Coregistration and Structural MRI processing
Preceding MEG measurement, four coils were attached to the participant’s head and localized, together with the three fiducial points and scalp surface, using a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, each participant’s MEG data were coregistered with their individual structural T1-weighted MRI data prior to source space analyses using BESA MRI (Version 2.0). Structural T1-weighted MRI images were acquired using a Siemens Skyra 3T MRI scanner with a 32-channel head coil and a MP-RAGE sequence with the following parameters: TR = 2400 ms; TE = 1.94 ms; flip angle = 8°; FOV = 256 mm; slice thickness = 1 mm (no gap); voxel size = 1 × 1 × 1 mm. These data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. Following source reconstruction (i.e., beamforming), each participant’s 4.0 × 4.0 × 4.0 mm functional images were also transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled.
MEG Time-frequency Transformation and Statistics
Cardiac and ocular artifacts were removed from the data using signal-space projection (SSP), which was accounted for during source reconstruction (Uusitalo and Ilmoniemi 1997). The continuous magnetic time series was divided into epochs of 2700 ms duration, with the baseline extending from −400 to 0 ms before stimulus onset. Epochs containing artifacts (e.g., eye blinks, muscle artifacts, eye saccades, swallowing, coughing) were rejected based on a fixed-threshold method, supplemented with visual inspection. Briefly, the distribution of amplitude and gradient values per participant were computed using all trials, and the highest amplitude/gradient trials relative to the total distribution were excluded by selecting a threshold that rejected extreme values. Notably, thresholds were set independently for each participant due to differences among individuals in head size and sensor proximity, which strongly affect MEG signal amplitude. After artifact rejection, an average of 195.5 (SD = 15.8) trials per participant were used for further analysis, and this number was not significantly correlated with chronological age (P = 0.73) or testosterone levels (P = 0.78).
Artifact-free epochs were transformed into the time-frequency domain using complex demodulation (resolution: 2.0 Hz, 25 ms; Papp and Ktonas 1977; Kovach and Gander 2016), and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized using the respective bin’s baseline power, which was calculated as the mean power during the −400 to 0 ms time period. The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. To reduce the risk of false-positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, two-tailed paired-sample t-tests against baseline were conducted on each data point, and the output spectrograms of t-values were thresholded at P < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, the time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also below the P < 0.05 threshold, and a cluster value was derived by summing all the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values and the significance level of the observed clusters (from stage one) was tested directly using this distribution (Ernst 2004; Maris and Oostenveld 2007). For each comparison, 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time-frequency windows that contained significant oscillatory events across all participants were subjected to a beamforming analysis (see Results). Of note, time-frequency clusters that became significant after the mean reaction time across all participants were not considered in further analyses, as the aims of the study were to focus on visuospatial processing rather than other processes involved in task completion (e.g., motor initiation, response/error-checking, etc.).
MEG Source Imaging and Statistics
Cortical responses were imaged through an extension of the linearly constrained minimum variance vector beamformer (Van Veen et al. 1997; Gross et al. 2001; Hillebrand et al. 2005), which employs spatial filters in the time-frequency domain to calculate source power for the entire brain volume. The single images are derived from the cross-spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, we computed noise-normalized, source power per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth (Hillebrand et al. 2005). Such images are typically referred to as pseudo-t maps, with units (i.e., pseudo-t) that reflect noise-normalized power differences (i.e., active vs. passive) per voxel. MEG preprocessing and imaging used the BESA (V 6.1) software. Further details about our MEG data processing pipeline are available in a recent publication (Wiesman and Wilson 2020).
Normalized differential source power was computed for the statistically selected time-frequency bands (see below) over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. The resulting 3D maps of brain activity were then averaged across participants to assess the neuroanatomical basis of the significant oscillatory responses identified through the sensor-level analysis. Next, whole brain correlations were computed between participant-level maps and two measures of pubertal maturation, endogenous testosterone levels and chronological age, to examine overall developmental changes in the neural responses. When using hormone levels as a proxy for development, age was used as a covariate of no-interest in order to detect the unique effects of testosterone on neural oscillatory activity above and beyond chronological age. To identify testosterone-by-sex and age-by-sex interactions, whole-brain correlation maps were computed separately between males and females. Fisher’s r to Z transformation was then applied to these maps, which provided a voxel-wise map of Z-scores representing the normalized sex differences in the oscillatory coding of visuospatial processing. All maps were thresholded at P < 0.005 and corrected for multiple comparisons using a spatial extent threshold of 500 contiguous voxels based on the theory of Gaussian random fields (Worsley et al. 1996; Woo et al. 2014).
Results
Demographic and Behavioral Results
Of the 39 participants that completed the task, three were excluded due to excessively noisy MEG data or abnormally high hormone levels. Thus, the final sample consisted of 36 children and adolescents (Mage = 12.76 years, SD = 1.52; 20 females).
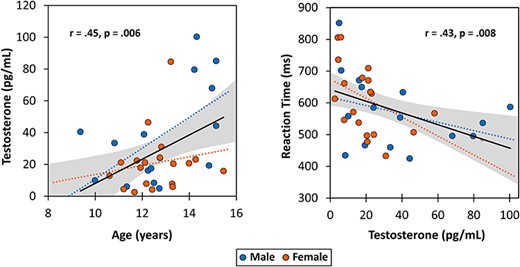
Participants performed well on the visuospatial processing and attention task. The average accuracy on the task was 94.19 ± 6.34% and the average reaction time was 588.0 ± 110.87 ms. There was a significant negative correlation between testosterone levels and reaction time (P = 0.008; Fig. 2) and between age and reaction time (P = 0.04), such that reaction time decreased with both increasing age and increasing testosterone. Accuracy in the task was not significantly correlated with age, testosterone, or reaction time (i.e., no speed/accuracy tradeoff was apparent). Finally, there were no sex differences in performance, as neither reaction time nor accuracy significantly differed between males and females.
Correlations between age, endogenous testosterone, and reaction time for the whole sample. (Left) A significant positive correlation between age and testosterone. (Right) A significant negative association between testosterone and average reaction time for the visuospatial processing task. Solid black lines indicate correlations for the whole sample. Gray bands represent 95% confidence intervals for all participants. Male (blue) and female (red) data plotted separately, although these correlations did not significantly differ between the sexes (age and testosterone: P = 0.412; testosterone and reaction time: P = 0.542).
Testosterone Results
As expected, chronological age was positively correlated with testosterone levels in the whole sample (r = 0.447, P = 0.006), such that older children tended to have higher levels of testosterone (Fig. 2). No significant sex differences existed between age and testosterone levels.
Neural Oscillatory Responses to the Task
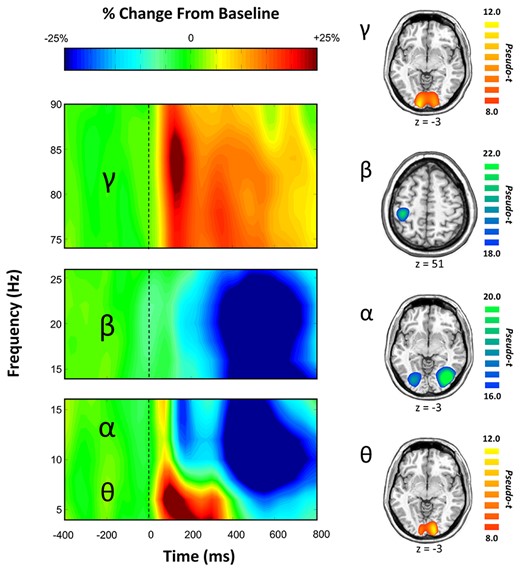
Statistical analysis of the time-frequency spectrograms showed significant oscillatory responses in four distinct time-frequency windows (Fig. 3 and Supplementary Fig. 1). These included a significant increase from baseline (i.e., synchronization) in the theta range (4–8 Hz) from stimulus onset to 300 ms post-stimulus (P < 0.001, corrected), and a significant increase in the high gamma range (78–84 Hz) from 100–150 ms (P < 0.001, corrected) across a cluster of occipital sensors. Additionally, a significant decrease from baseline (i.e., desynchronization) in the alpha frequency (8–14 Hz) range occurred from 350–500 ms over occipital sensors, and a significant desynchronization in the beta range (18–24 Hz) from 350–650 ms was identified in sensors near sensorimotor cortices (both P < 0.001, corrected). These four oscillatory responses were imaged using a beamformer and the resulting maps were grand-averaged per oscillatory response. As shown in Figure 3, the theta and gamma responses were strongest in the medial occipital cortices, while the alpha oscillations were centered in more lateral occipital cortices bilaterally, and beta activity was tightly clustered on the contralateral primary motor cortex. Since the beta activity was clearly indicative of the motor response (i.e., button press) and not related to perceptual or attention processing, we did not further examine this oscillatory component.
(Left) Time Frequency Spectrogram. Data are from two occipital sensors (top: M2032, bottom: M2112) and one left fronto-parietal sensor (middle: M1813) averaged across all correct trials and all participants. Warm colors reflect power increases relative to the baseline, and cool colors represent decreases relative to baseline. Time frequency windows for source imaging (beamforming) were derived from statistical analyses of these sensor-level spectrograms, which indicated significant bins in theta, alpha, beta, and gamma activity. (Right) Group-averaged beamformer images of each time-frequency oscillatory bin of interest across all participants. Theta, alpha, and gamma oscillatory responses were strongest in bilateral occipital cortices, whereas beta was centered on the motor cortex and thus not further examined. Color scale bars indicate the strength of responses (pseudo-t). Warm colors indicate synchronizations; cool colors indicate desynchronizations.
Chronological Age and Sex Effects on Neural Oscillatory Activity
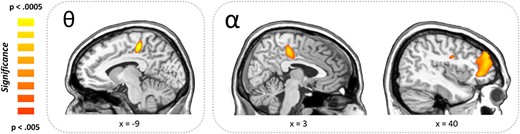
Developmental effects on neural oscillatory responses were examined by correlating each participant’s functional map, per oscillatory component, with their chronological age. These analyses indicated five significant clusters across the alpha, theta, and gamma responses. Specifically, alpha activity was positively correlated with chronological age within right frontal areas, including the dorsolateral prefrontal cortex (r = 0.635, P < 0.001), cingulate cortex (r = 0.574, P = 0.002), and precentral gyrus (r = 0.579, P = 0.002; Fig. 4). Thus, older children exhibited a weaker alpha desynchronization (i.e., weaker neural response) with increasing age in these regions. In contrast, theta activity was positively correlated with age within the left cingulate cortex (r = 0.667, P = 0.003; Fig. 4) and the same was true for gamma activity in the right cerebellum (r = 0.566, P < 0.001). These positive correlations among theta and gamma bands indicate stronger neural responses with increasing chronological age.
Whole-brain correlations between chronological age and oscillatory activity. Greek letters indicate frequency band. (Right) Positive associations between chronological age and alpha oscillatory activity were detected in the right cingulate cortex, right prefrontal cortex, and the right precentral gyrus indicating that alpha oscillations in these areas decreased (i.e., weaker desynchronization) with increasing age. (Left) Positive associations were also found in the left cingulate cortex in the theta frequency range, which in this case indicates stronger responses (i.e., greater synchronizations) with increasing age.
Next, the influence of biological sex on age-specific oscillatory alterations was examined by performing Fisher’s r to Z transformations on age-wise correlation maps between males and females. Sex-specific developmental effects occurred in the left intraparietal sulcus, where males exhibited stronger theta synchronizations with increasing age compared to females (Z = 3.12, P = 0.002). There were no other significant sex differences in the chronological age correlations with theta, alpha, or gamma activity. For completeness, we also examined sex differences irrespective of age. This showed a significant difference in the right cingulate gyrus in the theta band, where males showed stronger synchronizations than females (P = 0.002). No other sex differences were significant for the alpha or gamma activity.
Testosterone Effects on Neural Oscillatory Activity
To examine puberty-specific effects on neural oscillatory activity, whole-brain correlation maps with testosterone levels were computed for each oscillatory component. These analyses used testosterone levels as the covariate of interest and age as the covariate of no-interest in order to separate pubertal effects from the previously identified chronological age effects. When correcting for age, there were no significant regions exhibiting correlations between testosterone levels and oscillatory activity in theta, alpha, or beta that met our significance thresholds (P < 0.005).
Testosterone by Sex Interactions
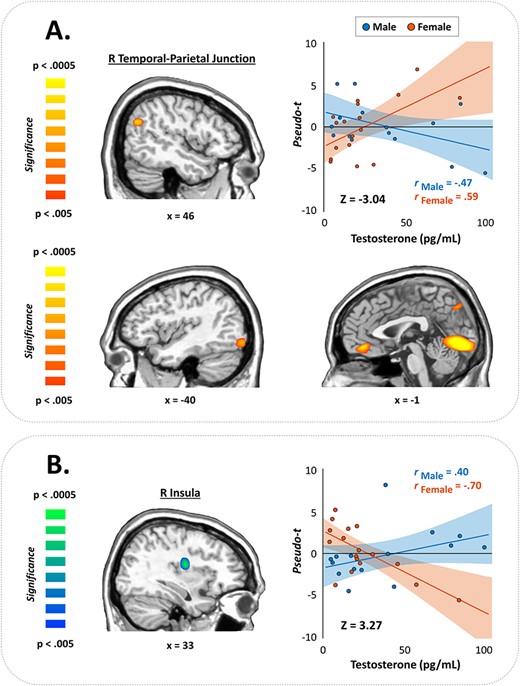
Finally, we examined sex differences in the correlations among testosterone levels and oscillatory neural responses, controlling for chronological age. These Fisher’s r to Z transformations revealed significant sex differences within the gamma band, but the direction varied by brain region. Specifically, females showed stronger gamma activity with increasing testosterone across five brain regions, including the left occipital cortex (Z = −3.52, P < 0.0014), left middle frontal gyrus (Z = −3.12, P = 0.002), right temporal-parietal junction (TPJ; Z = −3.04, P = 0.002), left inferior occipital cortex (Z = −2.94, P = 0.003), and the right cuneus (Z = −2.84, P = 0.005), whereas males exhibited decreases in gamma activity with increases in testosterone across these same regions (Fig. 5). Conversely, within the right insula, males exhibited stronger gamma synchronizations with increasing testosterone (controlling for age), while females showed reduced gamma activity in this region with increasing testosterone levels (Z = 3.27, P = 0.001; Fig. 5). No significant sex-by-testosterone interactions were observed in the theta or alpha range.
Testosterone-by-sex interactions in the gamma band. (A) Fisher’s r to Z maps showed significant sex differences in gamma oscillatory activity across several key nodes for visuospatial processing and attention. Warm colors indicate regions where females exhibited stronger positive associations between testosterone and gamma activity than males, evident in the right TPJ (top) and other brain regions shown in (A). Conversely, cool colors indicate where males showed more positive correlations than females, which occurred in the right insula (B). Color scale bars indicate the significance of the interaction effects. Scatterplots show correlations between testosterone levels and gamma activity in the peak voxels extracted from the corresponding map to the left of each. Bands represent 95% confidence intervals.
Discussion
The present study examined the effects of age, testosterone, and sex on the oscillatory dynamics of visuospatial processing in a sample of typically developing youth. Behaviorally, reaction time was negatively correlated with both age and testosterone levels, while accuracy was high and showed no relationship to either variable. Importantly, the data indicated that both males and females improved similarly in performance with increasing chronological age and testosterone level. In regard to the neural dynamics serving task performance, we observed robust increases (i.e., neural synchronizations) in the theta (4–8 Hz) and high gamma (78–84 Hz) ranges during early sensory processing, and a desynchronization (i.e., decrease in power) in the alpha range (8–14 Hz) slightly later from 350–500 ms. All of these responses localized to the occipital cortices. Importantly, we found novel developmental shifts in these neural oscillations in terms of chronological age and pubertal-specific effects, as indexed by testosterone levels, which interacted with biological sex to alter the trajectory of how these variables affect oscillatory dynamics in both sensory regions and association cortices. Below, we discuss the implications of these findings for understanding the developmental trajectory of neural oscillations during the pubertal transition and the impact of biological sex.
First, it is important to acknowledge that these oscillatory responses have been widely characterized in previous studies, and are known to be involved in initial sensory (theta) and stimulus feature (gamma) encoding, and later processing of visual stimuli in lateral occipital cortices (alpha; Hoogenboom et al. 2006; Doesburg et al. 2008; Klimesch 2012; Muthukumaraswamy and Singh 2013). Further, these findings are consistent with prior studies utilizing the same visuospatial processing and attention task in adult populations (Wiesman et al. 2017, 2018; Lew et al. 2019; Wiesman and Wilson 2019). However, far less is known about the neural dynamics of visuospatial processing during development, and that is especially true for the pubertal period (i.e., late childhood and early adolescence), when the levels of testosterone and other sex hormones are undergoing major shifts.
One of our most interesting findings was the significant testosterone-by-sex interaction observed in the high gamma frequency range during task performance. These interactions were clustered in critical brain areas for visuospatial processing and attention, including the right temporal-parietal junction (TPJ) where females showed strong positive correlations between testosterone levels and gamma oscillatory power, while males exhibited the opposite pattern. The right TPJ has been linked with detecting and directing attention towards behaviorally relevant stimuli (i.e., bottom-up processing) and comprises part of the ventral attention network (VAN; Corbetta and Shulman 2002). Conversely, males exhibited stronger gamma responses with increasing endogenous testosterone in the right insula, while females exhibited less insular activity with increasing testosterone. The insula has a diverse range of functions, including serving as a critical node within the salience network, which detects salient stimuli and then facilitates further bottom-up processing (Menon and Uddin 2010). Thus, these sex differences may suggest differential network development or processing strategies for detecting behaviorally relevant visual stimuli during puberty.
Specifically, the increased gamma activity in the right TPJ of females may reflect more mature cortical activation patterns, which more strongly recruit the VAN nodes as a function of increasing testosterone. Prior research has shown that children have increased functional connectivity between the VAN and salience network, while adults demonstrate more intrinsic connectivity between VAN nodes, suggesting a reduced segregation of the ventral attention and salience networks in children (Farrant and Uddin 2015). Therefore, the decreasing activity in the insula in females further demonstrates more precise and mature VAN activity in females, where recruitment of the salience network reduces with increasing testosterone levels. The stronger pattern of insular activity observed in males relative to females then may represent a more immature neural response profile, involving both the salience and ventral attention networks, indicating that the segregation of these systems during visuospatial processing has not yet fully occurred in males. Alternatively, the insular activity could reflect an immature pattern of diffuse cortical activity in males. Children exhibit more diffuse recruitment of task-relevant brain areas relative to adults in order to perform the same cognitive attention tasks, due to both enhanced activation of key functional nodes and decreased recruitment of other regions uncorrelated with task performance (Konrad et al. 2005; Durston et al. 2006; Rubia et al. 2010). The anterior insula is anatomically adjacent to the ventral frontal cortex (VFC), another critical node of the VAN (Corbetta and Shulman 2002; Farrant and Uddin 2015). Thus, the increased pattern of insular activation in males may represent diffuse recruitment of the VFC node, indicative of an immature pattern of activity, while females demonstrated more focal and mature responses restricted to the VAN nodes serving performance in the visuospatial attention task. Either way, these sexually dimorphic patterns of oscillatory activity would generally support an earlier onset of pubertal development in females relative to males (Dorn 2006).
Additional significant sex-by-testosterone interactions were also identified within key visual areas, including the occipital cortices and right cuneus, where females showed stronger gamma responses with increasing testosterone levels compared to males. These findings are in accordance with prior literature, which have shown that the coordination of neuronal synchronizations continues to mature into adulthood; specifically, gamma activity during visual processing increases in synchrony throughout development, at least as a function of chronological age (Werkle-Bergner et al. 2009; Uhlhaas et al. 2010). When considering all of the sex-by-testosterone interactions in visual gamma represented increasing activity in females, it is likely that these reflect accelerated development of cortical areas underlying visuospatial processing in females, while the developmental increases in gamma activity with increasing testosterone levels were not captured in the sample of young males.
The fact that all of the significant sex-by-testosterone interactions were within the gamma frequency range is also worthy of discussion, as it may suggest a key role of pubertal hormones in the development of these high-frequency oscillatory responses. Specifically, GABA-mediated local inhibitory networks have been extensively associated with gamma oscillatory activity in the frontal, parietal, and occipital cortices (Bartos et al. 2007; Fries et al. 2007; Edden et al. 2009; Muthukumaraswamy et al. 2009; Buzsáki and Wang 2012). The maturation of this GABAergic system continues into adolescence and adulthood (Hashimoto et al. 2009; Kilb 2012), and sex steroids can influence GABA subunit expression and modulate GABAergic activity (Zhang et al. 1999; Friess et al. 2000; Belelli et al. 2006). Therefore, the pubertal rises in androgenic hormones and other sex steroids likely contribute to the development of gamma oscillatory behavior through interactions with the GABAergic neurotransmitter system, which appears to occur in a sex-specific manner. Future studies should directly test this hypothesis by quantifying GABA levels in the occipital cortices, or other regions, by combining magnetic resonance spectroscopy with MEG/EEG in a developmental sample.
In addition to the noted effects of testosterone on oscillatory activity, unique effects of chronological age were identified. Specifically, alpha activity was significantly modulated by age in the full sample, with positive correlations observed between alpha oscillatory activity and age in the right medial prefrontal, precentral, and cingulate cortices. Though it is important to note, the alpha response itself was a desynchronization, or decrease from baseline. Therefore, the observed correlations indicate that alpha responses become weaker with increasing age in these regions. It is possible that younger children have to engage in more effortful processing to complete the task compared to their older peers, who likely perform this simple visuospatial task more automatically. Future studies could probe this more directly using parametric task designs in the context of working memory or other functions.
More broadly, a previous MEG study examined the oscillatory dynamics of visuospatial processing across the adult lifespan (Wiesman and Wilson 2019) and found spectrally specific changes with chronological age, sex differences, and age-by-sex interactions. Our findings of age-related changes within the theta, alpha, and gamma frequency ranges during the pubertal transition further contribute by extending our understanding of the overall developmental trajectory. However, most importantly, the present findings also provide insight into the pubertal component of these neurophysiological changes, since this study is the first to examine the influence of testosterone on the development of oscillatory brain activity above and beyond the effects of chronological age. Other MEG studies have mapped the developmental trajectory of oscillatory activity serving working memory and abstract reasoning, which also changed with age and differed by sex (Embury et al. 2019; Taylor et al. 2020). Though the influence of pubertal hormones on these cognitive domains remains largely unknown, the sex differences observed in these studies may suggest that higher-order cognitive processes and their underlying neural architecture may be differentially sensitive to endogenous sex steroid levels during pubertal development. Interestingly, another recent MEG study did not find sex differences or age-by-sex interactions in motor oscillatory developmental patterns (Trevarrow et al. 2019), though again, this study only utilized chronological age as a measure of maturation. The significant interactions that we observed in visual areas in the present study provides evidence that sex-specific functional development during puberty may still extend to early sensory cortices, which are thought to mature relatively early. Therefore, it is possible the motor network and other cortical regions that also mature earlier in development may still be selectively sensitive to hormonal influence during the pubertal period between sexes, but are not related to the effects of sex or chronological age independently. However, further work is needed to identify the extent to which specific cortical networks or oscillatory rhythms mature later and the net effect of pubertal hormone changes.
Though the present study focused on systems-level physiological maturation, it is important to integrate these findings into the broader context of neural circuit development. Prior cellular and molecular studies have revealed that neurons undergo complex patterns of structural development with unique magnitudes and kinetics, which vary by brain region and cortical layer (Anderson et al. 1995; Huttenlocher and Dabholkar 1997). For example, human prefrontal pyramidal neurons demonstrate an early overproduction of dendritic spines, followed by a plateau in dendritic spine density and then gradual elimination until reaching adult-levels, although the rate of this process appears to vary between cortical layers (Petanjek et al. 2011). This overall pattern of initial growth, plateau, and refinement appears to be present throughout the developing brain, including the visual cortex (Rakic et al. 1994), though the specific trajectories may be heterochronous across the cortex. However, despite similarities in the overall pattern, neural circuit development is an exceptionally complex process that is influenced by age, experience, and environmental factors, including sex steroids that reorder and activate neural circuits during adolescent development (Sisk and Foster 2004). In fact, sex-specific hormones have been shown to alter the architecture of brain circuitry differentially between the sexes. For example, during the period of synaptic elimination, gonadectomized female rats may have less neuronal loss in the primary visual cortex than their intact female counterparts (i.e., gonadectomized females did not display female-typical patterns of cell loss), while neuronal loss during this period was similar in gonadectomized and intact males (Nuñez et al. 2002). Though few studies have directly probed the impact of pubertal hormonal levels on neural circuit development in humans, our current results may provide evidence for distinct functional outcomes during the structural maturation process. Such changes in cortical anatomy and neurotransmitter systems (e.g., GABA) are consistent with the changes observed in local- and long-range oscillatory synchronizations during development (Uhlhaas et al. 2010). Therefore, the present findings may reflect important correlates of the region-, layer-, and temporally specific developmental patterns of complex brain circuit construction observed in animal models.
Before closing, it is important to note some limitations of the current study. First, while salivary assays are less invasive than blood samples, making them a much more attractive option for developmental studies, saliva samples are also less sensitive at capturing low levels of biologically active hormones (Herting and Sowell 2017). Thus, future studies could improve precision by implementing blood draws. Endogenous testosterone, like that studied here, is also cyclic in nature and has some inherent inter-individual baseline differences. Circulating levels of hormones are subject to fluctuations throughout the menstrual cycle in females and the circadian cycle in both sexes, with pubertal testosterone generally peaking early in the morning and decreasing throughout the day (Matchock et al. 2007; Herting and Sowell 2017). These temporal hormonal fluctuations likely had at least some effect on our data, although our sample collection times were generally similar and did not differ by sex or chronological age. Nonetheless, further standardizing collection times would enhance future work and reduce inter-individual differences. Future studies should also investigate the influence of other pubertal hormones, such as DHEA, progesterone, estradiol, and other sex steroids that dramatically change throughout adolescent development. Similarly, future work should also utilize alternative tasks during MEG to probe how other cognitive functions and networks are affected. Given our findings, it would not be surprising if these other hormones also modulate neurophysiological maturation during puberty, which would likely also vary by cognitive domain. Finally, the current study had a somewhat narrow age range (i.e., 9–15 years) and a moderate sample size. Thus, additional studies should include a larger sample size with both younger and older participants in order to capture a more comprehensive understanding of the developmental trajectory throughout the entire pubertal transition.
In conclusion, the current study was the first to investigate the influence of both chronological age and pubertal testosterone levels on neural oscillatory activity in typically developing children and adolescents. Using a visuospatial attention task, spectrally specific developmental changes as a function of chronological age were identified, which reflect continuous development of visuospatial functions within this developmental period. Further, unique testosterone-by-sex interactions within the gamma frequency range were observed, and these were concentrated in critical visual attention and perceptual networks. These findings emphasize the dramatic neurophysiological changes that occur in childhood and adolescence, and provide novel insight into the influence of sex-specific hormones on functional neural development during the pubertal transition.
Funding
National Institutes of Health (grants R01-MH121101, R01-MH103220, R01-MH116782, R01-MH118013, P20-GM130447 to (T.W.W.), R01-EB020407 to V.D.C.); National Science Foundation (grant #1539067 to T.W.W., V.C.D., Y.-P.W., and J.M.S.); At Ease USA (T.W.W.).
Notes
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All salivary assays and analyses were performed at the University of Nebraska—Lincoln Salivary Bioscience Laboratory. We thank Dr Jessica L. Calvi for her insight and assistance with this aspect of the study. Conflict of Interest: None declared.
References
Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR.