-
PDF
- Split View
-
Views
-
Cite
Cite
Keith A. Crist, Zhongqiu Zhang, Ming You, William T. Gunning, Phillip B. Conran, Vernon E. Steele, Ronald A. Lubet, Characterization of rat ovarian adenocarcinomas developed in response to direct instillation of 7,12-dimethylbenz[ a ]anthracene (DMBA) coated suture , Carcinogenesis, Volume 26, Issue 5, May 2005, Pages 951–957, https://doi.org/10.1093/carcin/bgi039
Close - Share Icon Share
Abstract
Human ovarian cancer is predominantly of epithelial cell origin (>90% of malignant tumors) and most often presents at an advanced stage with poor prognosis. Most animal models of ovarian carcinoma yield thecal/granulosa cell tumors, rather than adenocarcinomas. Induction of adenocarcinoma in 10–45% of rats following an ovarian implantation of 7,12-dimethylbenz[ a ]anthracene (DMBA) coated silk suture has been reported. Here, DMBA of 99% purity was melted at 124°C to impregnate a 1 cm length of sterile suture for direct ovarian implantation in Wistar Furth rats at 7 weeks of age. DMBA-treated ovaries showed a nearly complete loss of primary follicles and degeneration of granulosa cells at 16 weeks, consistent with the known toxic response of the ovary to direct DMBA application. No tumors were present. Untreated right ovaries and sham dimethyl sulfoxide-treated ovaries were normal. Ovarian tumors in DMBA-treated rats were first noted at 26 weeks post implantation reaching a cumulative tumor incidence of 77% (23/30) at 52 weeks. Controls showed no evidence of tumor at 52 weeks (0/31). Tumor histology was distributed as well differentiated adenocarcinoma (1/23), poorly differentiated adenocarcinoma (8/23), thecal/granulosa cell tumor (8/23), undifferentiated sarcoma (5/23) and one undifferentiated carcinoma with no adeno character. Tumors occasionally seeded to peritoneal mesentery, spleen and abdominal wall. Adenocarcinomas appeared to originate from the ovarian surface epithelium, with focal papillary extension into cystic space. Epithelial derived tumor cells positively react with antibodies to cytokeratin (8/8), epithelial cell adhesion molecule (Ep-CAM 5/5) and prostaglandin synthetase-1 (COX-1 4/4). Vimentin positive epithelial cells when present in adenocarcinomas (4/7), showed perinuclear staining, quite distinct from the uniformly stained stromal cells in thecal/granulosa cell tumors (8/8). The thecal/granulosa cell tumors were Ep-CAM negative (0/5) and weakly COX-1 positive (4/4). Thus, the DMBA suture model in rats yields epithelial derived tumors histologically similar to humans and should prove suitable for the testing of preventive or therapeutic agents.
Introduction
Effective detection and treatment of ovarian cancer remains a significant clinical challenge. Approximately 90% of ovarian tumors derive from an epithelial origin, most often considered to be the ovarian surface epithelium; 80% of these develop an invasive component with evidence of clonality in metastatic lesions ( 1 , 2 ). When identified as stage I disease, 5 year disease-free survival approaches 95%, although 20% of these patients will eventually relapse to a treatment failure overall ( 3 ). However, early stage ovarian tumors lack specific clinical symptoms, hampering early detection and delaying diagnosis in two-thirds of all cases until the onset of abdominal distension associated with stage III or stage IV disease ( 4 ). At this point, 5 year survival is low, being <40% for stage III and 12% for stage IV, such that ovarian cancer accounts for more cancer-related deaths than all other gynecological tumors combined and ranks fifth among all sites ( 5 , 6 ). Recent use of lysophosphatidic acid and the α-folate receptor as biomarkers, in combination with CA125, have improved the specificity for detection of early stage malignant gynecologic tumors ( 7 ), and will probably be further advanced by the addition of proteomic screening of low molecular weight serum proteins using surface-enhanced laser desorption and ionization time-of-flight (SELDI–TOF) mass spectroscopy ( 8 ).
Chemoprevention is an alternative approach, which seeks to prevent or reverse the progression of initiated cells to malignant disease. Clinical trials of preventive agents have demonstrated an efficacy in patients at risk for both breast and colon cancer. The first prevention trials for women at high risk for the development of ovarian cancer and choosing prophylactic oophorectomy have now been initiated to evaluate biomarker modulation e.g. ovarian surface metaplasia, cellular atypia and cortical inclusion cysts following short term treatment with fenretinide, oral contraceptive therapy or placebo ( 9 ). These data will be essential to the expanded screening and identification of women at risk through application of novel technologies, which may have lower thresholds for detecting validated biomarkers of early stage disease ( 10 ).
There is clearly a need for a validated animal model possessing histologic characteristics appropriate to the presentation of human ovarian tumors to evaluate the efficacy of newly developed chemopreventive compounds. Spontaneous rodent ovarian tumors of epithelial origin have been reported but these do not appear to occur in experimentally significant numbers ( 11 ). A spontaneous tumor incidence of 10–25% can be achieved in (SWR X SWXJ-9) F1 mice but these are entirely of granulosa cell origin. The only model system for spontaneously occurring ovarian adenocarcinomas has been described in the laying hen ( 12 ). Birds were followed for 3.5 years to achieve 24% incidence of ovarian adenocarcinomas with an overall tumor incidence rate of 46%. However, constraints associated with animal housing, as well as difficulties of extrapolation from the avian species to humans, make this model problematic. Most reported rodent models utilizing ovarian transplantation to the spleen ( 13 ), irradiation ( 14 ), transgenic expression of SV40 T-antigen ( 15 ) or estrogen stimulation ( 16 ) produce thecal/granulosa cell tumors. An exception to this experience has been the use of DMBA coated suture for direct implantation into the rat ovary, first ascribed to Kato et al . ( 17 ). Incidence of ovarian adenocarcinoma has varied between 10 and 45%, perhaps, owing to the strain of rat employed or the chemical form of the DMBA utilized and the fact that different studies have observed a wide variance in the incidence of tumors of stromal origin ( 18 , 19 ). No immunohistochemical characterization of these rodent ovarian tumors has been carried out for a comparison to known human ovarian markers. Direct implantation of carcinogen appears to be critical as a single intragastric instillation or intravenous injection of DMBA in mice yields a comparable total tumor incidence but virtually every tumor exhibits a stromal tumor histology ( 20 ). We report here that the use of high purity DMBA absorbed onto silk suture and implanted into Wistar Furth rat ovary resulted in 77% tumor incidence at the end of 1 year with 39% (9/23) of all tumors diagnosed as adenocarcinoma. All of these tumors were positively stained by monoclonal antibodies for epithelial cell adhesion molecule and cyclooxygenase 1 protein, as well as a polyclonal cytokeratin wide spectrum screening antibody.
Materials and methods
Chemicals
DMBA (obtained from from Acros Organics, Hanover Park, IL) was heated to 124°C. Approximately 2 cm of sterile 3-0 silk suture (Owens and Minor, Plymouth, MI) proximal to the needle was first knotted and then the distal 1 cm immersed in melted DMBA for 10 s and air dried. The suture retained on average 200 µg of carcinogen, calibrated by weight from replicate control suture samples on a micro analytical balance. Coated suture was retained at −20°C until transportation to the surgical site and used within 1 week. Control suture was coated in DMSO (Sigma Chemical Co., St Louis, MO).
Animals and surgical procedures
Five-week-old female Wistar Furth rats (Harlan Sprague Dawley, Indianapolis, IN) were housed two per cage and acclimated to the animal room for 1 week before surgery. A Harlan Teklad control diet (Indianapolis, IN) and water was available ad libitum . The room was controlled for constant temperature (22 ± 2°C) and relative humidity (50 ± 20%) with a 12 h light/dark cycle. Surgical anesthesia was induced by an intraperitoneal injection of ketamine hydrochloride (90 mg/kg)/acepromazine (2 mg/kg) and buprenorphine (0.01 mg/kg sq) was administered following anesthesia for post-surgical analgesia. The lower back, left of the midline, was shaved and swabbed with Nolvasan (Fort Dodge Laboratories, Inc., Fort Dodge, IA) followed by 70% ethanol. The retroperitoneal cavity was entered and the left ovary was exteriorized by grasping the ovarian fat pad. The bursa was divided using microsurgical scissors to expose the ovary. Suture impregnated with either DMBA (60 rats) or DMSO as a sham treatment (60 rats) was embedded by three passes at right angles through the ovarian parenchyma. The abdominal cavity was closed with 3-0 silk and the skin with 3-0 nylon.
Study design
After recovery from surgery, the rats were housed, fed and watered as described above. Rats were palpated once every month over the first 16 weeks at which time half of the rats in each group were terminated to evaluate the ovaries for early signs of neoplasia. The remaining animals were palpated weekly throughout the observation period, which terminated 1 year after surgery. Rats with palpable tumors were terminated before any observable morbidity and the tumors were sampled to provide both frozen and formalin-fixed tissue. Right ovaries of tumor bearing rats and left DMSO-treated ovaries of control rats were fixed to provide histologic controls. All tissue for histologic evaluation was fixed overnight in 10% neutral buffered formalin, sectioned at 5 µm and stained with hematoxylin and eosin or processed for immunohistochemistry.
Immunohistochemistry
Paraffin-embedded sections for immunohistochemistry were dewaxed, rehydrated and processed for antigen unmasking by heating to near boiling in citrate buffer (0.01 M, pH 6.0, 3 × 5 min) in a microwave oven, then allowed to cool for 20 min. Following this and all subsequent incubations except for the blocking serum, the slides were washed in phosphate-buffered saline (PBS, 3 × 5 min). Endogenous peroxidase activity was blocked by hydrogen peroxide incubation (0.3% in methanol) for 20 min. Frozen sections were mounted onto slides, fixed in cold acetone (−20°C) for 10 min and air dried before peroxidase block incubation. After incubation for 30 min in blocking serum (diluted 1:50 in PBS) appropriate to each secondary antibody, the slides were incubated with primary antibody at 4°C overnight (cytokeratin, wide spectrum screening polyclonal, 85 µg/ml, DAKO Corporation, Carpenteria, CA; vimentin, COX-1 and CD45, 1 µg/ml, Santa Cruz Biotechnology, Santa Cruz, CA; Ep-CAM clone 2X63, 1 µg/ml, U.S. Biologicals, Swampscott, MA). All reagents except for the chromagen substrate were diluted with PBS containing 2% bovine serum albumin. Detection of primary antibody proceeded with the binding of biotinylated secondary antibody (diluted 1:200 for 30 min) followed by avidin–biotin peroxidase complex (Vectastain Elite, Vector Laboratories, Burlingame, CA) and diaminobenzidine (DAB) chromagen substrate.
Results and discussion
Measurement of DMBA-treated left ovaries from rats terminated 16 weeks after suture implantation, processed and embedded as one batch along with DMSO-treated ovaries, showed a small but significant increase in size (3.8 ± 1.2 mm versus 2.4 ± 0.5 mm, mean ± SD, P < 0.001, n = 19 and 28, respectively). Untreated right ovaries from both groups, also processed at the same time, were not different in size (2.7 ± 0.5 versus 2.2 ± 0.5 mm) and did not differ in size from the DMSO-treated left ovary. The characteristic nodular appearance was replaced in DMBA-treated ovary by a more regular contour consistent with the loss of nearly all primary and most secondary follicles observed on histologic evaluation. Disappearance of follicles has been routinely observed to precede neoplastic change following either intrasplenic transplantation ( 21 ) or painting of the ovarian surface with DMBA ( 22 ). Polycyclic aromatic hydrocarbons (PAH) require metabolic transformation to genotoxic compounds by microsomal P-450 dependent monooxygenase (AHH) activity ( 23 ). Previous studies with intraovarian injection of benzopyrene have demonstrated that the destruction of primary follicles occurs only in the injected ovary without induction of AHH activity in liver or contralateral ovary and is inhibited by i.p. administration of the competitive substrate α-naphthoflavone ( 24 ). In the absence of liver AHH activation, generation of the 7-hydroxymethyl-12-methylbenz[ a ]thracene metabolite responsible for adrenal toxicity known to occur after an i.p. administration of DMBA is not expected ( 25 ), and no abnormalities in adrenal histology have been noted in the present work in tissue collected from tumor-bearing rats. Ovarian parenchyma at 16 weeks was largely replaced by dense fibroplasia, areas of necrosis and inflammatory infiltrate. Ovarian architecture was unaffected by the implantation of DMSO-coated suture.
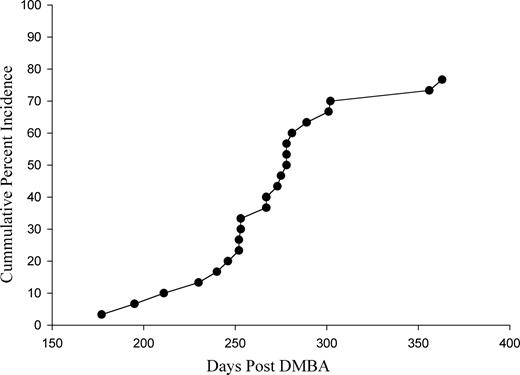
Ovarian tumors became palpable 175 days after DMBA suture implantation with a rapid onset beginning at 230 days ( Figure 1 ). Cumulative incidence reached 77% at the end of 1 year when the remaining animals were killed. Tumors were attached to the left uterine horn and adherent to kidney, omentum, pancreas and the abdominal incision marked by the suture used for closure. Tumors were dissected to locate the implanted suture on which the DMBA coating was clearly evident and the immediately surrounding tissue was sampled for histologic diagnosis. In some cases, tumors were observed to seed to the spleen, diaphragm and intestinal mesentery or invade through the abdominal musculature. No mammary gland or uterine tumors were noted.
Cumulative incidence of ovarian tumors from the date of suture implantation to the time of palpation or discovery at termination of the experiment. DMBA-coated suture was implanted at 7 weeks of age.
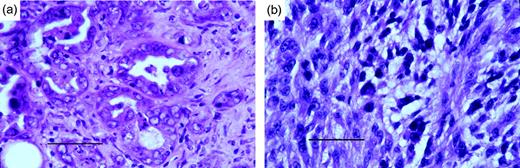
The majority of tumors (74%) were of two histologic types. Adenocarcinomas, most (8/23) poorly differentiated, were characterized by nests of glandular structures, often back to back, lined by a single layer of cells that defined a lumina and have a high nuclear/cytoplasmic ratio, nuclear pleomorphism with granular chromatin and prominent nucleoli ( Figure 2A ). In contrast to other reports using DMBA-coated suture, only one papillary serous cystadenocarcinoma was noted among these ( 19 ). One additional tumor was scored as well differentiated, displaying apparently productive ductal structures. Thecal/granulosa cell tumors (8/23) retained some normal follicular structure with cuboidal granulosa cells having a large central nucleolus, but were predominantly characterized by interwoven palisades and whorls of plump spindle shaped thecal cells. Grossly, these were lobulated, densely packed fibrous tumors ( Figure 2B ). The mean time to first palpation for the adenocarcinomas was not different from that of thecal/granulosa cell tumors (275 versus 258 days, pooled SD = 37 days).
Histologic differentiation between ovarian tumors having epithelial or mesenchymal character (H&E, ×63, scale bar = 50 μm). Poorly differentiated adenocarcinomas were characterized by nests of glandular structures, often back to back, lined by a single layer of cells that defined a lumina ( a ). Mesenchymal tumors contained interwoven palisades and whorls of plumb spindle shaped thecal cells ( b ).
To confirm histologic observations, we employed a number of immunohistochemical reagents for epithelial or mesenchymal cell types ( Table I ). Antibodies to intermediate filament (IF) proteins are often useful for this purpose, differentiating cytokeratin positive cells of epithelial origin from vimentin positive cells of mesenchymal origin. The rat ovarian mesothelium is reported to express cytokeratin and desmoplakin IF proteins, while only vimentin is weakly expressed in granulosa cells and strongly expressed in stromal cells ( 26 ). IF expression is somewhat more complex in human ovary where mesothelial and granulosa cells co-express cytokeratin, desmoplakin and vimentin proteins, a rare event in situ , while stromal cells express only vimentin ( 26 – 28 ). Antibodies with specificity for high molecular weight cytokeratins are not reactive in granulosa cell tumors ( 29 ). This cell of origin expression pattern is maintained not only in most primary tumors but in most solid metastatic tumors as well ( 30 ).
Comparison of selected immunohistochemical markers in rat and human epithelial ovarian tumors
| Marker . | Human epithelial ovarian cancer (Ref.) . | Rat epithelial ovarian cancer . | Rat granulosa cell cancer . |
|---|---|---|---|
| Cytokeratin | 26 | 1+, 5++, 2+++ | +/− |
| Vimentin | 26 | 1+, 6++ | 5+++, 3++ |
| Ep-CAM | 37 | 2++, 3+++ | — |
| Cox-1 | 47 | 2++, 3+++ | 2+, 1++, 1+++ |
| Marker . | Human epithelial ovarian cancer (Ref.) . | Rat epithelial ovarian cancer . | Rat granulosa cell cancer . |
|---|---|---|---|
| Cytokeratin | 26 | 1+, 5++, 2+++ | +/− |
| Vimentin | 26 | 1+, 6++ | 5+++, 3++ |
| Ep-CAM | 37 | 2++, 3+++ | — |
| Cox-1 | 47 | 2++, 3+++ | 2+, 1++, 1+++ |
The number of tumors at each observed level of staining is presented when one staining level did not characterize all tumor specimens stained within the tumor type.
Comparison of selected immunohistochemical markers in rat and human epithelial ovarian tumors
| Marker . | Human epithelial ovarian cancer (Ref.) . | Rat epithelial ovarian cancer . | Rat granulosa cell cancer . |
|---|---|---|---|
| Cytokeratin | 26 | 1+, 5++, 2+++ | +/− |
| Vimentin | 26 | 1+, 6++ | 5+++, 3++ |
| Ep-CAM | 37 | 2++, 3+++ | — |
| Cox-1 | 47 | 2++, 3+++ | 2+, 1++, 1+++ |
| Marker . | Human epithelial ovarian cancer (Ref.) . | Rat epithelial ovarian cancer . | Rat granulosa cell cancer . |
|---|---|---|---|
| Cytokeratin | 26 | 1+, 5++, 2+++ | +/− |
| Vimentin | 26 | 1+, 6++ | 5+++, 3++ |
| Ep-CAM | 37 | 2++, 3+++ | — |
| Cox-1 | 47 | 2++, 3+++ | 2+, 1++, 1+++ |
The number of tumors at each observed level of staining is presented when one staining level did not characterize all tumor specimens stained within the tumor type.
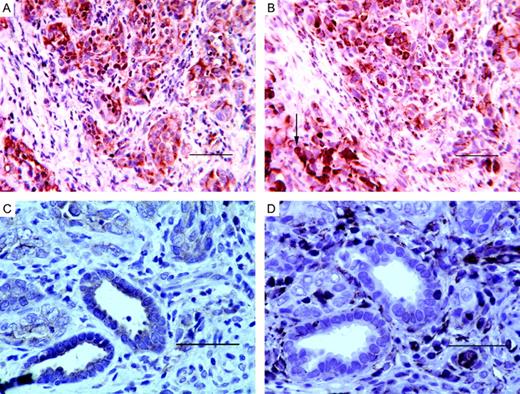
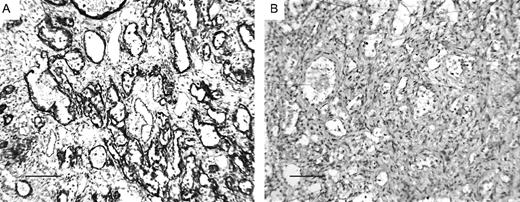
In DMBA-induced rat ovarian adenocarcinomas, neoplastic epithelial cells demonstrated a positive staining for cytokeratin distributed diffusely throughout the cytoplasm ( Figure 3A ). Staining for vimentin was positive in the corresponding cells from serial sections but the distribution pattern was either strongly perinuclear, polar or weakly diffuse ( Figure 3B ). In other adenocarcinomas, neoplastic cytokeratin positive epithelial cells forming luminal structures were clearly vimentin negative while the surrounding stromal cells were positive ( Figure 3C and D ). Since mesotheliomas are also known to express the class of keratins detected by the polyclonal antibody used, initially frozen tumor demonstrating the histologic appearance of adenocarcinoma was post-fixed in glutaraldehyde solution and processed for electron microscopy. Although resolution was compromised by the initial freezing, relatively short microvilli were readily discernible, excluding mesothelioma as a potential diagnosis for these tumors.
Variation in staining for cytokeratin and vimentin in adenocarcinomas( A and B 40×; C and D 63×, scale bar = 50 μm). Cytokeratin staining of epithelial cells was most often diffuse (A) but also appeared as localized to a luminal boundary (C). Epithelial cell staining of vimentin appeared as perinuclear (B, black arrow), polar (B, white arrow), diffuse in undifferentiated areas, or was absent from epithelial cells in more differentiated ductal structures and present only in the surrounding stroma (D).
Recognizing that adenocarcinomas induced by DMBA in the rat ovary are most often less well differentiated than routinely observed in human ovarian tumors, we next evaluated immunohistochemical markers with reported positive expression in human tumors and cross reactivity in rodents, to assess similarities in marker expression. Potential markers were identified by a comparison of the gene expression profiles between adenocarcinoma (three poorly differentiated, one serous cystadenocarcinoma) or mesenchymal tumors (three thecal/granulosa cell, one sarcoma) and untreated normal ovary of similarly aged rats. Affymetrix RG34A chips were used to query gene expression; model-based expression results of normalized perfect match data were analyzed using dChip software (Harvard University). No attempt was made to microdissect epithelial cells from adenocarcinomas; however, while both tumor types have stromal cell components, only adenocarcinomas contain epithelial cells when the tissue is sampled from the central tumor mass. Candidate genes were selected under stringent conditions ( P < 0.00025) for overexpression in three adenocarcinomas versus three stromal tumors. Epithelial cell adhesion molecule (Ep-CAM) and a cyclooxygenase gene ( COX-1 ) were chosen from that pool ( Table I ).
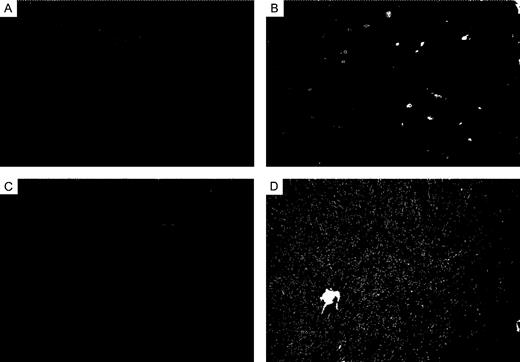
Ep-CAM is a 40 kDa transmembrane epithelial glycoprotein product of the GA733-2 gene that shares 80–81% sequence identity with the rodent homologues ( 31 , 32 ). Antibodies to various epitopes have been independently raised (17-1A, MOC31, KS1/4, Ber-EP4, etc.) that are now recognized to target the same protein ( 33 – 36 ). In adult humans, normal tissue expression is entirely epithelial and localized to the basolateral surface of glandular, pseudo-stratified and transitional epithelia, but absent in normal squamous stratified epithelia ( 31 ). Ep-CAM expression is increased in association with malignant transformation of most carcinomas, including ovary, breast, lung, prostate and colon but not with squamous carcinoma of the skin ( 37 , 38 ). It can also serve as a useful marker to differentiate ovarian adenocarcinomas from non-epithelioid mesotheliomas, particularly ovarian serous neoplasms, which are immunophenotypically identical to mesotheliomas with respect to CK7, CK20, CA125 and CEA expression ( 39 – 41 ). The role of Ep-CAM in malignant progression is not currently defined. A lack of expression was found to correlate with nodal metastasis from primary laryngeal carcinomas ( 42 ), while an increased expression was found to correlate with the nodal involvement in primary breast tumors ( 43 ). All rat ovarian adenocarcinomas yielding frozen tissue for analysis in this study (three poorly differentiated, 2++, 1+++; one well differentiated, +++; one poorly differentiated cystadenocarcinoma, +++) showed a strong positive staining of epithelial cells, while thecal/granulosa cell tumor frozen sections (0/4) were uniformly negative ( Figure 4A and B , positive and negative controls).
Staining with Ep-CAM was limited to malignant epithelial cells in adenocarcinoma frozen sections (16×, scale bar = 100 μm). The majority of epithelial cells were positively stained ( A , +++) compared with an isotype matched negative control antibody ( B , CD24).
Prostaglandin synthetases 1 and 2 (COX-1 and COX-2) are involved in the catalysis of prostaglandins from arachidonic acid. COX-2 is the form of the enzyme that is expressed in inflammatory cells and is highly induced by various stimuli (growth factors, UV, etc.) in a wide variety of cells. In contrast, it is minimally expressed in most normal epithelial cells. In the past 5–10 years, it has been shown that COX-2 is expressed in a wide variety of epithelial cancers, which has made it a primary target for various chemoprevention studies employing specific COX-2 inhibitors or NSAIDS that inhibit both COX-1 and COX-2 ( 44 ). In contrast, COX-1 is regarded as a constitutively active regulator of prostaglandin generated from the metabolism of arachidonic acid and it is detectable by immunohistochemical techniques in normal human bladder, breast, colon and lung, but not in normal ovary ( 45 – 47 ). Upregulation of this isoform has been identified during ovarian-induced differentiation by phorbol ester ( 48 ) and in ovarian adenocarcinomas from patients selected for the absence of prior cytoreductive chemotherapy, since prior treatment is known to induce COX-2 expression ( 47 ). Elevated levels of prostaglandin is associated with the stimulation of vascular endothelial growth factor (VEGF) and consequent angiogenic activity required for the healing of normal tissue and also for the expansion of tumor cell mass. Upregulation of COX-1 in ovarian carcinomas is therefore a potential marker of aggressive phenotypes, as is similarly the case for VEGF expression ( 49 ). Increased expression of COX-1 was similarly observed here in adenocarcinomas generated by DMBA. COX-1 expression was confined to neoplastic ductal epithelium in the 5/5 adenocarcinoma frozen sections examined ( Figure 5A and B ). Thecal/granulosa cell tumor staining, when present, was focal in three-quarters of the cases ( Figure 5C ), with one-quarter of the tumors showing +++ positively stained spindle shaped cells ( Figure 5D ).
( A and C 63×, scale bar = 50 μm, B and D 16×, scale bar = 100 μm) Specific staining with COX-1 was primarily limited to malignant epithelial cells in adenocarcinomas. Strongly positive staining of ductal structures was observed (A, B) compared with the isotype matched control antibody. Some diffuse staining was seen in stromal tumors (C) identified by the arrow, with one exception where staining of stromal cells occupied a greater percentage of the section (D) but always clearly less than in the adenocarcinomas.
Ovarian adenocarcinomas in the rat have been induced by exposure of the ovary to embedded suture coated with DMBA by us and several other independent groups ( 17 – 19 ). The limited amount of ovarian surface epithelium and the difficulty in palpation of early lesions have hindered the detection of a developmental course comparable with the early metaplasia of surface epithelium in the inclusion cysts of human ovaries. The variety of histologies seen, including aggressive undifferentiated sarcomas, suggests that DMBA induces tumors in various cell types. Thirty-nine percent (9/23) of tumors present as adenocarcinoma and express epithelial and metabolic markers similar to human ovarian epithelial tumors. Altering the progression of chemically initiated cells to the formation of an established tumor is a useful paradigm in chemoprevention research. Chemopreventive studies using this model system are now in progress to evaluate compounds having potential activity to inhibit ovarian tumorigenesis.
While this manuscript was in final review, a paper employing the DMBA suture technique was published ( 50 ). These investigators employed a much lower dose of DMBA, which did not cause obvious damage to the ovary but yielded only one tumor (1% incidence) after 12 months, in the absence of exogenous human chorionic gonadotropin. Interestingly, these authors found that all tumors which did arise had a p53 mutation, which is common in human ovarian cancer. These findings combined with our own results support the potential relevance of this model.
We thank Dr Robert D.Cardiff for assistance with the histologic diagnosis and helpful discussion in completion of this manuscript. This project has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. N01-CN-05103.
References
Scully,R., Young,R. and Clement,P. (eds) (
Tsao,S., Mok,C., Knapp,R., Oike,K., Muto,M., Welch,W., Goodman,H., Sheets,E., Berkowitz,R. and Lau,C. (
Dent,S.F., Klaassen,D., Pater,J.L., Zee,B. and Whitehead,M. (
Benedet,J.L., Bender,H., Jones,H.,3rd, Ngan,H. and Pecorelli,S. (
Nguyen,H., Averette,H., Hoskins,W., Sevin,B., Penalver,M. and Steren,A. (
Greenlee,R., Hill-Harmon,M.B., Murray,T. and Thun,M. (
Xu,Y., Shen,Z., Wiper,D.W., Wu,M., Morton,R.E., Elson,P., Kennedy,A.W., Belinson,J., Markman,M. and Casey,G. (
Petricoin,E.F., Ardekani,A.M., Hitt,B.A., Levine,P.J., Fusaro,V.A., Steinberg,S.M., Mills,G.B., Simone,C., Fishman,D.A., Kohn,E.C. and Liotta,L.A. (
Ozols,R.F., Daly,M.B., Klein-Szanto,A., Hamilton,T.C., Bast,R.C.,Jr and Brewer,M.A. (
Ramanujam,N., Mitchell,M.F., Mahadevan-Jansen,A., Thomsen,S.L., Staerkel,G., Malpica,A., Wright,T., Atkinson,N. and Richards-Kortum,R. (
Prejean,J., Peckham,J., Casey,A., Griswold,D., Weisburger,E. and Weisburger,J. (
Chamson-Reig,A., Bianchi,M., Rey-Roldan,E., Sorianello,E., Libertun,C. and Lux-Lantos,V. (
van der Houven van Oordt,C.W., Smits,R., Schouten,T.G., Houwing-Duistermaat,J.J., Williamson,S.L., Luz,A., Meera Khan,P., van der Eb,A.J., Breuer,M.L. and Fodde,R. (
Rahman,N., Kananen Rilianawati,K., Paukku,T., Mikola,M., Markkula,M., Hamalainen,T. and Huhtaniemi,I. (
Silva,E., Tornos,C., Deavers,M., Kaisman,K., Gray,K. and Gershenson,D. (
Kato,T., Yakushiji,M., Tsunawaki,A. and Ide,K. (
Nishida,T., Sugiyama,T., Kataoka,A., Ushijima,K. and Yakushiji,M. (
Tunca,J., Erturk,E., Erturk,E. and Bryan,G. (
Kuwahara,I. (
Guthrie,M. (
Krarup,T. (
Conney,A.H. (
Shiromizu,K. and Mattison,D.R. (
Bengtsson,M., Montelius,J., Mankowitz,L. and Rydstrom,J. (
Czernobilsky,B., Moll,R., Levy,R. and Franke,W.W. (
Santini,D., Ceccarelli,C., Mazzoleni,G., Pasquinelli,G., Jasonni,V.M. and Martinelli,G.N. (
Costa,M.J., DeRose,P.B., Roth,L.M., Brescia,R.J., Zaloudek,C.J. and Cohen,C. (
Otis,C.N., Powell,J.L., Barbuto,D. and Carcangiu,M.L. (
Osborn,M. and Weber,K. (
Balzar,M., Winter,M.J., de Boer,C.J. and Litvinov,S.V. (
Kubuschok,B., Passlick,B., Izbicki,J.R., Thetter,O. and Pantel,K. (
Herlyn,M., Steplewski,Z., Herlyn,D. and Koprowski,H. (
Durbin,H., Rodrigues,H. and Bodmer,W. (
Edwards,D., Grzyb,K., Dressler,L., Mansel,R., Zava,D., Sledge,G. and McGuire,W. (
Latza,U., Niedobitek,G., Schwarting,R., Nekarda,H. and Stein,H. (
Wimberger,P., Xiang,W., Mayr,D., Diebold,J., Dreier,T., Baeuerle,P.A. and Kimmig,R. (
Tellechea,O., Reis,J.P., Domingues,J.C. and Baptista,A.P. (
Sheibani,K., Shin,S., Kezirian,J. and Weiss,L. (
Ryan,P., Oates,J. and Stableforth,D. (
McCluggage,W. (
Takes,R.P., De Jong,R.J.B., Schuuring,E., Hermans,J., Vis,A.A., Litvinov,S.V. and Van Krieken,J.H.J.M. (
Tandon,A.K., Clark,G.M., Chamness,G.C. and McGuire,W.L. (
Dannenberg,A.J., Altorki,N.K., Boyle,J.O., Dang,C., Howe,L.R., Weksler,B.B. and Subbaramaiah,K. (
Komhoff,M., Guan,Y., Shappell,H.W., Davis,L., Jack,G., Shyr,Y., Koch,M.O., Shappell,S.B. and Breyer,M.D. (
Soslow,R.A., Dannenberg,A.J., Rush,D., Woerner,B.M., Khan,K.N., Masferrer,J. and Koki,A.T. (
Gupta,R.A., Tejada,L.V., Tong,B.J., Das,S.K., Morrow,J.D., Dey,S.K. and DuBois,R.N. (
Smith,C.J., Morrow,J.D., Roberts,L.J.,II and Marnett,L.J. (
Yamamoto,S., Konishi,I., Mandai,M., Kuroda,H., Komatsu,T., Nanbu,K., Sakahara,H. and Mori,T. (
Author notes
1Department of Surgery and 2Department of Pathology, Medical College of Ohio, Toledo, OH 43614, USA, 3Department of Surgery and The Alvin J. Siteman Cancer Center, Washington University School of Medicine, St Louis, MO, USA and 4Chemoprevention Agent Development Group, National Cancer Institute, Rockville, MD, USA
- prostaglandins
- cancer
- adenocarcinoma
- epithelium
- 9,10-dimethyl-1,2-benzanthracene
- anthracenes
- granulosa cell tumor
- granulosa cells
- hair follicle
- keratins
- ligase
- mesentery
- animal model
- ovarian follicle
- ovarian neoplasms
- rats, inbred wf
- sarcoma
- stromal cells
- sutures
- vimentin
- antibodies
- histology
- neoplasms
- ovary
- rats
- spleen
- ovarian cancer
- tumor cells
- epithelial cells
- cyclooxygenase-1
- germinal epithelium of ovary
- prevention
- epcam gene