-
PDF
- Split View
-
Views
-
Cite
Cite
S. F. Witelson, H. Beresh, D. L. Kigar, Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors, Brain, Volume 129, Issue 2, February 2006, Pages 386–398, https://doi.org/10.1093/brain/awh696
Close - Share Icon Share
Abstract
The neural basis of variation in human intelligence is not well delineated. Numerous studies relating measures of brain size such as brain weight, head circumference, CT or MRI brain volume to different intelligence test measures, with variously defined samples of subjects have yielded inconsistent findings with correlations from ∼0 to 0.6, with most correlations ∼0.3 or 0.4. The study of intelligence in relation to postmortem cerebral volume is not available to date. We report the results of such a study on 100 cases (58 women and 42 men) having prospectively obtained Full Scale Wechsler Adult Intelligence Scale scores. Ability correlated with cerebral volume, but the relationship depended on the realm of intelligence studied, as well as the sex and hemispheric functional lateralization of the subject. General verbal ability was positively correlated with cerebral volume and each hemisphere's volume in women and in right-handed men accounting for 36% of the variation in verbal intelligence. There was no evidence of such a relationship in non-right-handed men, indicating that at least for verbal intelligence, functional asymmetry may be a relevant factor in structure–function relationships in men, but not in women. In women, general visuospatial ability was also positively correlated with cerebral volume, but less strongly, accounting for ∼10% of the variance. In men, there was a non-significant trend of a negative correlation between visuospatial ability and cerebral volume, suggesting that the neural substrate of visuospatial ability may differ between the sexes. Analyses of additional research subjects used as test cases provided support for our regression models. In men, visuospatial ability and cerebral volume were strongly linked via the factor of chronological age, suggesting that the well-documented decline in visuospatial intelligence with age is related, at least in right-handed men, to the decrease in cerebral volume with age. We found that cerebral volume decreased only minimally with age in women. This leaves unknown the neural substrate underlying the visuospatial decline with age in women. Body height was found to account for 1–4% of the variation in cerebral volume within each sex, leaving the basis of the well-documented sex difference in cerebral volume unaccounted for. With finer testing instruments of specific cognitive abilities and measures of their associated brain regions, it is likely that stronger structure–function relationships will be observed. Our results point to the need for responsibility in the consideration of the possible use of brain images as intelligence tests.
Introduction
Brain weight and intelligence: postmortem studies
The neurobiological correlates of the variation in human intelligence are unknown. Regardless of the combination of genetic and environmental factors which contribute to intelligence, some variation in the neural substrate—whether at a gross structural, histological, physiological or neurochemical level—must underlie variation in intelligence. This issue received considerable attention in 19th century neuroanatomical studies of postmortem brain weight in relation to inferred ability in groups and individual cases of exceptional and notable people. More than 100 case reports of eminent individuals were reviewed by Spitzka (1907) who concluded that greater ability was associated with greater brain weight. These early conclusions were challenged on the basis of limitations in methodology: intelligence was based on subjective judgements of occupation or degree of eminence; quantitative analyses of the data were not available; and numerous exceptions existed (e.g. Gould, 1981). In the last few decades, about a dozen studies assessed groups of people using the rough measure of external head size in relation to quantitative intelligence test scores and the average correlation was found to be 0.15 (reviewed in Rushton and Ankney, 1996). However, to date no study has directly assessed brain weight obtained for neurologically normal individuals in relation to prospectively designed quantitative measurements of intelligence.
Brain volume and intelligence: imaging studies
With the advent of brain imaging the issue of the relationship between brain size and intelligence has been revived. Studies using various brain-volume imaging and intelligence measures have reported predominantly positive correlations ranging from 0 to 0.6 with the majority of MRI studies yielding correlations ∼0.4 (e.g. Willerman et al., 1991; Andreasen et al., 1993; Gur et al., 1999; Pennington et al., 2000). At least some of this inconsistency may be due to whether the factors of chronological age, sex or body size were controlled, the cognitive measure studied and the method of imaging analyses. Moreover, brain volume may be more precisely determined by the water displacement method in postmortem studies than by MRI measurement which depends on differentiation between brain tissue and other structures from computer images.
Factors relevant to the relationship between brain size and intelligence
Age
It has been well documented that brain weight decreases with age starting in early adulthood. For example, Dekaban and Sadowksy (1978) reviewed six major studies and reported their own work based on >3000 adults: each study showed a decrease in brain weight within the age interval of 30–90 years of age. Correlations were not reported but mean scores revealed a 4–10% change over the age span studied. A more recent large-sample study (n = 1261) reported a similar change of 12% from 25 to 80 years, with correlations of r = −0.27 for men and r = −0.15 for women (P < 0.01 in each case) (Ho et al., 1980). More recently, numerous MRI studies have assessed changes in intracranial or cerebral volume in relation to chronological age. In a review of three dozen reports on volume of different brain regions in relation to age, all but one showed a negative correlation between brain volume and age in the age span studied (Coffey, 2000).
Numerous studies have documented that visuospatial skills, as reflected in the Performance Scale of the Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1955), decrease with advancing age. In contrast, measures of verbal ability such as the WAIS Verbal Scale showed no decrease (in fact a slight rise) over the same age range (Kaufman et al., 1991).
Sex
It is well documented that mean brain size (weight or volume) is 9–12% larger in men than in women (postmortem: Dekaban and Sadowsky, 1978; MRI: Gur et al., 2002). It is thus necessary to consider the sexes separately or to control statistically for the difference in brain size with some method when assessing intelligence in relation to brain size. The issue of sex appears further complicated in that there may be an interaction of the effects of sex and age on brain size. Some studies indicated that brain size decreased with age to a greater extent in men than women (postmortem: Ho et al., 1980; MRI: Cowell et al., 1994; Murphy et al., 1996; Gur et al., 1999), although other studies with apparently adequate power found no sex difference in the effect of age on brain size (postmortem: Pakkenberg and Voigt, 1964; MRI: Raz et al., 1997).
Hemispheric functional lateralization
Recent MRI studies of monozygotic versus dizygotic twin pairs have shown that brain size is significantly tied to genetic factors: the volume of each cerebral hemisphere showed 65% heritability (Geschwind et al., 2002); the temporal and frontal cortical regions showed 90% heritability (Thompson et al., 2001); the total grey or white matter volume showed 85% heritability (Baaré et al., 2001); and the midsagittal area of the corpus callosum showed 94% heritability (Scamvougeras et al., 2003). The factor of hand preference was considered in the Geschwind et al. (2002) report of male twins. Among monozygotic twin pairs, the genetic contribution was greater in twin pairs concordant for strong right-hand preference than in discordant twin pairs. Geschwind et al. suggested that the greater within-pair similarity of brain volume observed in the right-hand concordant monozygotic twin pairs is indicative of greater genetic control over cerebral volume in this group. In other words there may be less genetic control of brain size in non-right-handers.
The large majority of right handers constitute a relatively homogeneous population with respect to cortical localization of verbal and visuospatial functions to the left and right hemispheres, respectively. In contrast, the pattern of lateralization of cognitive functions is more variable among left handers (Bryden, 1982). The expression of the genetic factors that influence brain size and possibly brain functional organization may be modified by non-genetic factors, such as chance environmental events (Annett, 2002) or prenatal environment or birth stress (Coren and Halpern, 1991), to a greater extent in left than in right handers. These findings suggest that functional asymmetry may be a relevant factor in the relationship between brain size and intelligence.
The aim of this study was to examine the quantitative relationship between postmortem cerebral volume and prospectively obtained general measures of verbal and visuospatial intelligence in a sample of 100 brain specimens from cognitively normal men and women. The possibility of different relationships depending on sex and the pattern of functional asymmetry was examined. In addition, we assessed the effect of the decrease in brain volume with age on the relationship between brain volume and intelligence.
Methods
Sample
The sample consisted of 106 consecutive cases from the Witelson Normal Brain Collection (Witelson and McCulloch, 1991) which consists of brains from non-neurological cancer patients recruited to participate in a study that required taking a battery of neuropsychological tests and agreeing to a postmortem examination. All subjects were Caucasian and documented to have no cognitive or neuropsychiatric dysfunction on the basis of prospectively designed neuropsychological testing and detailed medical, educational and social histories. Subjects and their next-of-kin provided written informed consent and the study was approved by the Research Ethics Board of McMaster University, Hamilton, Canada. Of the 106 cases, 6 were excluded due to brain pathology that could affect measurements of brain size.
Table 1 presents the descriptive data of the sample of 100 cases divided into four subgroups (hand/sex subgroups) classified by sex and category of hand preference (consistent-right-handed, CRH; non-consistent-right-handed, non-CRH, described in detail in a subsequent section). Mean scores are presented for chronological age, body height (crown–heel length), postmortem interval (PMI, time between death and brain removal), Full Scale IQ on the Wechsler Adult Intelligence Scale and a handedness integer score. Mean handedness scores and standard deviations were similar for each hand-preference group between the sexes. The integer scores for the CRH groups reveal almost no variation and for the non-CRH groups ranged from −10 to +10 for women and −12 to +10 for men. Mean age for the total sample of 100 cases was 56 years with min/max = 25/83 years. Two-way ANOVAs were done for each variable. Only height differed, with men being taller. WAIS scores indicated that the subgroups were in the ‘bright normal’ range of intelligence (min/max was 99/133 for women and 93/131 for men).
Descriptive data for the four hand/sex subgroups
| . | Women . | . | Men . | . | ||
|---|---|---|---|---|---|---|
. | CRH (n = 38) Mean (SD) . | Non-CRH (n = 20) Mean (SD) . | CRH (n = 20) Mean (SD) . | Non-CRH (n = 22) Mean (SD) . | ||
| Age (years) | 55.2 (10.8) | 54.0 (8.9) | 58.6 (13.3) | 58.6 (9.4) | ||
| Height (inches)* | 64.0 (3.2) | 64.8 (2.8) | 69.3 (2.8) | 70.5 (2.5) | ||
| PMI (h) | 9.2 (5.6) | 10.5 (7.5) | 7.4 (5.9) | 7.8 (7.1) | ||
| WAIS:FSIQa | 116.4 (8.8) | 110.7 (5.8) | 116.4 (9.6) | 117.1 (9.3) | ||
| Handedness score | 11.5 (1.2) | 5.6 (6.0) | 11.4 (1.0) | 4.0 (7.6) | ||
| . | Women . | . | Men . | . | ||
|---|---|---|---|---|---|---|
. | CRH (n = 38) Mean (SD) . | Non-CRH (n = 20) Mean (SD) . | CRH (n = 20) Mean (SD) . | Non-CRH (n = 22) Mean (SD) . | ||
| Age (years) | 55.2 (10.8) | 54.0 (8.9) | 58.6 (13.3) | 58.6 (9.4) | ||
| Height (inches)* | 64.0 (3.2) | 64.8 (2.8) | 69.3 (2.8) | 70.5 (2.5) | ||
| PMI (h) | 9.2 (5.6) | 10.5 (7.5) | 7.4 (5.9) | 7.8 (7.1) | ||
| WAIS:FSIQa | 116.4 (8.8) | 110.7 (5.8) | 116.4 (9.6) | 117.1 (9.3) | ||
| Handedness score | 11.5 (1.2) | 5.6 (6.0) | 11.4 (1.0) | 4.0 (7.6) | ||
PMI = time interval between death and brain removal at postmortem; CRH = consistent-right-handed; Non-CRH = non-consistent- right-handed; WAIS: Wechsler Adult Intelligence Scale; FSIQ = Full Scale IQ;
t-test indicated men were taller than women (P < 0.0001);
n's vary, see Table 2.
Descriptive data for the four hand/sex subgroups
| . | Women . | . | Men . | . | ||
|---|---|---|---|---|---|---|
. | CRH (n = 38) Mean (SD) . | Non-CRH (n = 20) Mean (SD) . | CRH (n = 20) Mean (SD) . | Non-CRH (n = 22) Mean (SD) . | ||
| Age (years) | 55.2 (10.8) | 54.0 (8.9) | 58.6 (13.3) | 58.6 (9.4) | ||
| Height (inches)* | 64.0 (3.2) | 64.8 (2.8) | 69.3 (2.8) | 70.5 (2.5) | ||
| PMI (h) | 9.2 (5.6) | 10.5 (7.5) | 7.4 (5.9) | 7.8 (7.1) | ||
| WAIS:FSIQa | 116.4 (8.8) | 110.7 (5.8) | 116.4 (9.6) | 117.1 (9.3) | ||
| Handedness score | 11.5 (1.2) | 5.6 (6.0) | 11.4 (1.0) | 4.0 (7.6) | ||
| . | Women . | . | Men . | . | ||
|---|---|---|---|---|---|---|
. | CRH (n = 38) Mean (SD) . | Non-CRH (n = 20) Mean (SD) . | CRH (n = 20) Mean (SD) . | Non-CRH (n = 22) Mean (SD) . | ||
| Age (years) | 55.2 (10.8) | 54.0 (8.9) | 58.6 (13.3) | 58.6 (9.4) | ||
| Height (inches)* | 64.0 (3.2) | 64.8 (2.8) | 69.3 (2.8) | 70.5 (2.5) | ||
| PMI (h) | 9.2 (5.6) | 10.5 (7.5) | 7.4 (5.9) | 7.8 (7.1) | ||
| WAIS:FSIQa | 116.4 (8.8) | 110.7 (5.8) | 116.4 (9.6) | 117.1 (9.3) | ||
| Handedness score | 11.5 (1.2) | 5.6 (6.0) | 11.4 (1.0) | 4.0 (7.6) | ||
PMI = time interval between death and brain removal at postmortem; CRH = consistent-right-handed; Non-CRH = non-consistent- right-handed; WAIS: Wechsler Adult Intelligence Scale; FSIQ = Full Scale IQ;
t-test indicated men were taller than women (P < 0.0001);
n's vary, see Table 2.
Brain measures
Mean scores for the brain measures in each of the four hand/sex subgroups are given in Table 2. Fresh whole brain weight (brain severed at the dorsal end of the medulla) was obtained at autopsy immediately following removal of the brain from the skull. The brain was then fixed by suspension in 10% buffered formalin for three weeks. PMI was on average 8.8 h with min/max = 1/22 h (see Table 1). For the total sample, the intraclass correlation between fresh brain weight and formalin-fixed brain weight revealed that r = 0.88 (P < 0.0001) and that fixed brain weight tended to be 1.5% greater than fresh brain weight throughout the size interval of the sample.
Brain size and WAIS measures for the four hand/sex subgroups
| . | Women . | . | . | . | Men . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CRH . | . | Non-CRH . | . | CRH . | . | Non-CRH . | . | ||||||||
. | n . | Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | ||||||||
| Brain size | ||||||||||||||||
| Fresh whole brain weight (g)a,* | 38 | 1278 (103) | 20 | 1218 (80) | 20 | 1373 (102) | 22 | 1383 (114) | ||||||||
| Cerebral weight (g)* | 38 | 1046 (77) | 20 | 1033 (77) | 20 | 1173 (88) | 22 | 1166 (98) | ||||||||
| Cerebral volume (ml)b,* | 38 | 985 (81) | 20 | 962 (79) | 20 | 1103 (92) | 22 | 1097 (97) | ||||||||
| WAIS | ||||||||||||||||
| VSS | 27 | 75 (10.4) | 13 | 67 (7.3) | 17 | 71 (10.8) | 20 | 74 (8.5) | ||||||||
| PSS | 23 | 50 (10.0) | 10 | 45 (7.5) | 13 | 51 (11.1) | 18 | 49 (8.2) | ||||||||
| . | Women . | . | . | . | Men . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CRH . | . | Non-CRH . | . | CRH . | . | Non-CRH . | . | ||||||||
. | n . | Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | ||||||||
| Brain size | ||||||||||||||||
| Fresh whole brain weight (g)a,* | 38 | 1278 (103) | 20 | 1218 (80) | 20 | 1373 (102) | 22 | 1383 (114) | ||||||||
| Cerebral weight (g)* | 38 | 1046 (77) | 20 | 1033 (77) | 20 | 1173 (88) | 22 | 1166 (98) | ||||||||
| Cerebral volume (ml)b,* | 38 | 985 (81) | 20 | 962 (79) | 20 | 1103 (92) | 22 | 1097 (97) | ||||||||
| WAIS | ||||||||||||||||
| VSS | 27 | 75 (10.4) | 13 | 67 (7.3) | 17 | 71 (10.8) | 20 | 74 (8.5) | ||||||||
| PSS | 23 | 50 (10.0) | 10 | 45 (7.5) | 13 | 51 (11.1) | 18 | 49 (8.2) | ||||||||
CRH = consistent-right-handed; Non-CRH = non-consistent-right-handed; WAIS = Wechsler Adult Intelligence Scale; VSS = Verbal Scaled Score; PSS = Performance Scaled Score;
Two-way ANOVAs indicated that sex was a significant factor (P < 0.0001);
Fresh whole brain weight in this study is very close to that for comparably aged samples from other postmortem studies [e.g. Pakkenberg and Voigt (1964): (n = 1090), 1280 and 1436 g for women and men, respectively; Dekaban and Sadowsky (1978): (n = 972), 1255 and 1392 g for women and men, respectively];
Cerebral volume has not been reported in other postmortem studies. Values from MRI studies vary considerably [e.g. Gur et al. (1991): (n = 69), 1280 ml for women and 1370 ml for men; Mayhew and Olsen (1991): (n = 14), 622–851 ml for women and 697–1094 ml for men].
Brain size and WAIS measures for the four hand/sex subgroups
| . | Women . | . | . | . | Men . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CRH . | . | Non-CRH . | . | CRH . | . | Non-CRH . | . | ||||||||
. | n . | Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | ||||||||
| Brain size | ||||||||||||||||
| Fresh whole brain weight (g)a,* | 38 | 1278 (103) | 20 | 1218 (80) | 20 | 1373 (102) | 22 | 1383 (114) | ||||||||
| Cerebral weight (g)* | 38 | 1046 (77) | 20 | 1033 (77) | 20 | 1173 (88) | 22 | 1166 (98) | ||||||||
| Cerebral volume (ml)b,* | 38 | 985 (81) | 20 | 962 (79) | 20 | 1103 (92) | 22 | 1097 (97) | ||||||||
| WAIS | ||||||||||||||||
| VSS | 27 | 75 (10.4) | 13 | 67 (7.3) | 17 | 71 (10.8) | 20 | 74 (8.5) | ||||||||
| PSS | 23 | 50 (10.0) | 10 | 45 (7.5) | 13 | 51 (11.1) | 18 | 49 (8.2) | ||||||||
| . | Women . | . | . | . | Men . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CRH . | . | Non-CRH . | . | CRH . | . | Non-CRH . | . | ||||||||
. | n . | Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | ||||||||
| Brain size | ||||||||||||||||
| Fresh whole brain weight (g)a,* | 38 | 1278 (103) | 20 | 1218 (80) | 20 | 1373 (102) | 22 | 1383 (114) | ||||||||
| Cerebral weight (g)* | 38 | 1046 (77) | 20 | 1033 (77) | 20 | 1173 (88) | 22 | 1166 (98) | ||||||||
| Cerebral volume (ml)b,* | 38 | 985 (81) | 20 | 962 (79) | 20 | 1103 (92) | 22 | 1097 (97) | ||||||||
| WAIS | ||||||||||||||||
| VSS | 27 | 75 (10.4) | 13 | 67 (7.3) | 17 | 71 (10.8) | 20 | 74 (8.5) | ||||||||
| PSS | 23 | 50 (10.0) | 10 | 45 (7.5) | 13 | 51 (11.1) | 18 | 49 (8.2) | ||||||||
CRH = consistent-right-handed; Non-CRH = non-consistent-right-handed; WAIS = Wechsler Adult Intelligence Scale; VSS = Verbal Scaled Score; PSS = Performance Scaled Score;
Two-way ANOVAs indicated that sex was a significant factor (P < 0.0001);
Fresh whole brain weight in this study is very close to that for comparably aged samples from other postmortem studies [e.g. Pakkenberg and Voigt (1964): (n = 1090), 1280 and 1436 g for women and men, respectively; Dekaban and Sadowsky (1978): (n = 972), 1255 and 1392 g for women and men, respectively];
Cerebral volume has not been reported in other postmortem studies. Values from MRI studies vary considerably [e.g. Gur et al. (1991): (n = 69), 1280 ml for women and 1370 ml for men; Mayhew and Olsen (1991): (n = 14), 622–851 ml for women and 697–1094 ml for men].
Following fixation the cerebellum was cut off just below the inferior colliculus, the meninges and blood vessels were removed, and the brain was bisected in the midsagittal plane. Each hemisphere was weighed and volume obtained by water displacement. Cerebral weight and cerebral volume were defined as the sum of the scores of the right and left hemispheres. Cerebral weight correlated with fixed whole brain weight (intraclass correlation = 0.95, P < 0.0001). The intraclass correlation between cerebral volume and cerebral weight was r = 0.95 and 0.96 in women and men, respectively (P < 0.0001). In this study, volume measures are reported for comparison with the body of MRI studies relating structure and function.
Two-way ANOVAs indicated that for each brain-size measure men had larger brains (P < 0.0001) by ∼13%. There was no difference between left and right hemispheres on any brain measure for any hand/sex subgroup.
Cognitive measures
Hand preference
A measure of hand preference was used as an index of the pattern of functional aymmetry. A modified version of Annett's (1967) hand questionnaire was used, in which the subject was asked to demonstrate hand use, rather than to complete a questionnaire, for a series of 12 unimanual (e.g. tooth brushing) and bimanual (e.g. threading a needle) tasks. For each item, preference was recorded as right, either, or left, and scored as +1, 0, −1, respectively. The classification used in this study is based on Annett's (2002) model of the classification and genetics of hand preference and included two main categories: consistent-right-hand preference (CRH) (defined as all ‘right’ or ‘right’ with some ‘either’ preferences) and non-consistent-right-hand preference (non-CRH) (defined as left-hand preference on at least one of the twelve tasks, regardless of the hand used for writing). This dichotomous classification has proved to be sensitive to differences in the anatomy of the corpus callosum (Witelson, 1989) and in the Sylvian fissure (Witelson and Kigar, 1992), as well as by other researchers, e.g. motor cortex (Amunts et al., 2000) and in behavioural studies (McCormick et al., 1990). The non-CRH category included both consistent left-hand preference (CLH) (defined as all ‘left’ or ‘left’ with some ‘either’ preferences) and mixed-hand preference (MH) (defined as any combination including both right and left preferences). Among women there were 38 CRH and 20 non-CRH; among men, 20 CRH and 22 non-CRH. There were two CLH men. Educational histories revealed that four cases (all men) were forced to write with their right hand (forced dextral) but each of these tested as non-CRH. A handedness integer score was obtained which varied from +12 to −12. The mean scores for the hand/sex subgroups are given in Table 1.
Intelligence measures
The WAIS Verbal and Performance Scaled Scores were available for the majority of cases. These scores are measures of general verbal and visuospatial abilities. WAIS IQ scores are roughly age-corrected (by decade). For the purposes of this study, the Verbal Scaled Score (VSS, based on six subtests) and Performance Scaled Score (PSS, based on five subtests), which are not age-corrected, were used and then age was controlled statistically. Table 2 gives sample size and VSS and PSS means for each hand/sex subgroup. Two-way ANOVAs indicated no group differences for either VSS or PSS. In addition to the sample in the study proper, 10 cases were available that had not been included in the analyses because they did not complete all 11 WAIS subtests, but did have sufficient subtest data to allow for a prorated VSS or PSS. These cases were used as test cases to evaluate the regression models obtained in the results.
Statistical analyses
Pearson product–moment correlations and partial correlations (controlling for age or height) were performed. Age-adjusted values for both cerebral volume and cognitive scores in the scatterplots were obtained via regression analyses. Correlations were tested for influential or outlying (high residual) cases and correlations were done with these cases excluded and reported where relevant. Intraclass correlations were used to test the comparability between different brain size measures. Univariate linear regression analyses were used to fit models for the prediction of intelligence scores from cerebral volume. Although positive correlations have been consistently reported in the literature and one-tailed tests would have been appropriate, two-tailed tests of significance were used in this study, with significance level set at α = 0.05.
Results
Cerebral volume as a function of age
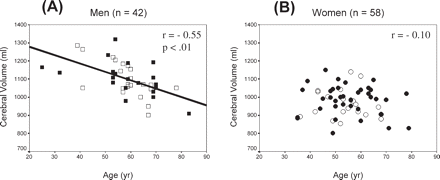
Table 3 presents the correlations of brain-size measures with age in each hand/sex subgroup. Cerebral volume was similarly associated with age in each male subgroup (r = −0.55, P < 0.001) (Fig. 1A). There is a decrease of ∼5 ml/year in men over the age span of this sample. In contrast, there was minimal decrease in cerebral volume with age in women (r = −0.12 and −0.08 for CRH and non-CRH subgroups, respectively) (Fig. 1B).
Correlations of age with brain size and with WAIS measures in the four hand/sex subgroups
| . | Women . | . | . | Men . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CRH . | Non-CRH . | Total . | CRH . | Non-CRH . | Total . | ||||||
| Brain size | ||||||||||||
| Fresh whole brain weight (g) | −0.23 | −0.06 | −0.17 | −0.12 | −0.47* | −0.25 | ||||||
| Cerebral weight (g) | −0.07 | −0.01 | −0.05 | −0.45* | −0.59** | −0.50** | ||||||
| Cerebral volume (ml) | −0.12 | −0.08 | −0.10 | −0.50* | −0.64** | −0.55** | ||||||
| WAIS | ||||||||||||
| VSS | −0.16 | −0.39 | −0.18 | −0.28 | 0.01 | −0.17 | ||||||
| PSS | −0.61** | −0.58 | −0.60** | −0.81** | −0.29 | −0.59** | ||||||
| . | Women . | . | . | Men . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CRH . | Non-CRH . | Total . | CRH . | Non-CRH . | Total . | ||||||
| Brain size | ||||||||||||
| Fresh whole brain weight (g) | −0.23 | −0.06 | −0.17 | −0.12 | −0.47* | −0.25 | ||||||
| Cerebral weight (g) | −0.07 | −0.01 | −0.05 | −0.45* | −0.59** | −0.50** | ||||||
| Cerebral volume (ml) | −0.12 | −0.08 | −0.10 | −0.50* | −0.64** | −0.55** | ||||||
| WAIS | ||||||||||||
| VSS | −0.16 | −0.39 | −0.18 | −0.28 | 0.01 | −0.17 | ||||||
| PSS | −0.61** | −0.58 | −0.60** | −0.81** | −0.29 | −0.59** | ||||||
CRH = consistent-right-handed; Non-CRH = non-consistent-right-handed; WAIS = Wechsler Adult Intelligence Scale; VSS = Verbal Scaled Score; PSS = Performance Scaled Score;
P < 0.05;
P < 0.01.
Correlations of age with brain size and with WAIS measures in the four hand/sex subgroups
| . | Women . | . | . | Men . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CRH . | Non-CRH . | Total . | CRH . | Non-CRH . | Total . | ||||||
| Brain size | ||||||||||||
| Fresh whole brain weight (g) | −0.23 | −0.06 | −0.17 | −0.12 | −0.47* | −0.25 | ||||||
| Cerebral weight (g) | −0.07 | −0.01 | −0.05 | −0.45* | −0.59** | −0.50** | ||||||
| Cerebral volume (ml) | −0.12 | −0.08 | −0.10 | −0.50* | −0.64** | −0.55** | ||||||
| WAIS | ||||||||||||
| VSS | −0.16 | −0.39 | −0.18 | −0.28 | 0.01 | −0.17 | ||||||
| PSS | −0.61** | −0.58 | −0.60** | −0.81** | −0.29 | −0.59** | ||||||
| . | Women . | . | . | Men . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CRH . | Non-CRH . | Total . | CRH . | Non-CRH . | Total . | ||||||
| Brain size | ||||||||||||
| Fresh whole brain weight (g) | −0.23 | −0.06 | −0.17 | −0.12 | −0.47* | −0.25 | ||||||
| Cerebral weight (g) | −0.07 | −0.01 | −0.05 | −0.45* | −0.59** | −0.50** | ||||||
| Cerebral volume (ml) | −0.12 | −0.08 | −0.10 | −0.50* | −0.64** | −0.55** | ||||||
| WAIS | ||||||||||||
| VSS | −0.16 | −0.39 | −0.18 | −0.28 | 0.01 | −0.17 | ||||||
| PSS | −0.61** | −0.58 | −0.60** | −0.81** | −0.29 | −0.59** | ||||||
CRH = consistent-right-handed; Non-CRH = non-consistent-right-handed; WAIS = Wechsler Adult Intelligence Scale; VSS = Verbal Scaled Score; PSS = Performance Scaled Score;
P < 0.05;
P < 0.01.
Scatterplots showing the relationship between cerebral volume and age in (A) men and in (B) women. Regression equations are: (A) Cerebral volume = −4.62 age + 1371; and (B) Cerebral volume = −0.78 age + 1020. Men: closed square = CRH; open square = non-CRH; women: closed circle = CRH; open circle = non-CRH.
The correlations of age with left or right hemisphere volume were very similar to each other and to total cerebral volume for each hand/sex subgroup. The same sex difference in the relationship of volume with age was obtained for each hemisphere.
Other factors affecting variation in cerebral volume
There were only small statistically non-significant relationships between cerebral volume and height (with age partialled out) for each hand/sex subgroup (rage = 0.02 to 0.17). The correlations of cerebral volume and age with height controlled were almost identical to the zero order correlations (change = ±0.01 to 0.07). PMI also showed no correlation with cerebral volume in either sex when the factor of age was partialled out (rage ≤ 0.12).
Cerebral volume and intelligence
Intelligence scores as a function of age
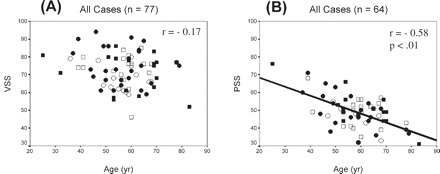
VSS showed only small statistically non-significant correlations with age in all four hand/sex subgroups and no effect in non-CRH men. Analysis revealed that no influential cases or outliers accounted for the zero correlation in non-CRH men (Table 3, Fig. 2A). In contrast, PSS decreased with advancing chronological age in women (r = −0.60, P < 0.001) and in men (r = −0.59, P < 0.001) (Table 3, Fig. 2B). These results are very consistent with reports in the literature (e.g. Kaufman et al., 1991). Because of the relationship of age with cerebral volume and cognitive scores (even though some correlations were low), both cerebral volume and cognitive scores were age-corrected for subsequent analyses.
Scatterplots showing the relationship of age with (A) VSS and (B) PSS for the total sample. Regression equations are: (A) VSS = −0.16 cerebral volume + 81 and (B) PSS = −0.50 cerebral volume + 78. Men: closed square = CRH; open square = non-CRH; women: closed circle = CRH; open circle = non-CRH.
Verbal ability
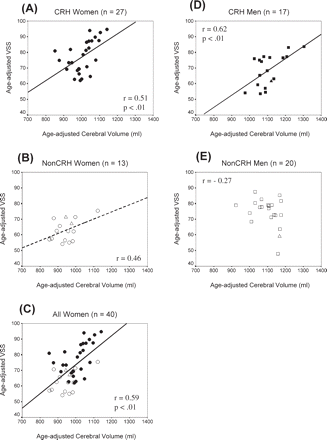
Figure 3 presents the scatterplots, correlations and regression lines for VSS as a function of cerebral volume in the hand/sex subgroups. Age-adjusted values were used for both variables. For CRH women, r = 0.51 (P = 0.007); for non-CRH women, r = 0.46 (P = 0.11). The slopes of the regression lines were similar between the two female subgroups: for the total group of women the correlation was r = 0.59 (P < 0.001) (Fig. 3A–C; legend). With height partialled out the correlation barely changed: rheight = 0.62 (P < 0.001).
Scatterplots and regression lines showing the relationship between age-adjusted VSS and age-adjusted cerebral volume. (A) CRH women, (B) non-CRH women, (C) all women, (D) CRH men and (E) non-CRH men. Correlations and regression lines do not include the plotted test cases. Regression equations are (for age-adjusted scores): (A) VSS = 0.07 cerebral volume + 2; (B) VSS = 0.04 cerebral volume + 20; (C) VSS = 0.09 cerebral volume − 19; (D) VSS = 0.08 cerebral volume − 18; (E) VSS = −0.03 cerebral volume + 113. Women: closed circle = CRH; open circle = non-CRH; men: closed square = CRH; open square = non-CRH. Test cases: closed triangle = CRH (n = 1), open triangle = Non-CRH (n = 3). Dashed regression line indicates that r is not statistically significant but may reflect insufficient power.
For CRH men, the correlation was r = 0.62 (P = 0.008), and the slope of the regression line was similar to that of CRH or non-CRH women (Fig. 3; legend). With height controlled, rheight = 0.59 (P = 0.02). Non-CRH men showed no evidence of a positive relationship; in fact r = −0.27 (P = 0.24) (Fig. 3E). With height controlled, rheight = −0.26.
The correlations of VSS with left or right hemisphere volume were not different from each other or from the correlations of VSS with total cerebral volume in any hand/sex subgroup.
Performance ability
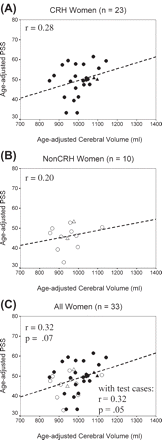
Figure 4 presents the scatterplots, correlations and regression lines for PSS as a function of cerebral volume in women. Age-adjusted values were used for both variables. For each subgroup of women the correlations and the slope of the regression lines were similar (Fig. 4A and B; legend). For the total group r = 0.32 (P = 0.07) (Fig. 4C) and with height controlled, the correlation was rheight = 0.36 (P = 0.05).
Scatterplots and regression lines showing the relationship between age-adjusted PSS and age-adjusted cerebral volume. (A) CRH women, (B) non-CRH women and (C) all women. Correlations and regression lines do not include the plotted test cases. Regression equations are (for age-adjusted scores): (A) PSS = 0.03 cerebral volume + 17; (B) PSS = 0.02 cerebral volume + 31; (C) PSS = 0.03 cerebral volume + 17. Closed circle = CRH; open circle = non-CRH; test cases: closed triangle = CRH (n = 2); open triangle = non-CRH (n = 2). Dashed regression lines indicate that r is not statistically significant but may reflect insufficient power.
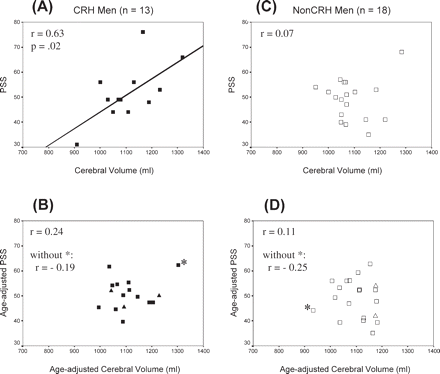
Figure 5A and B presents the scatterplots, correlations and regression lines for PSS and cerebral volume in CRH men. Without correction for age, PSS correlated with cerebral volume (r = 0.63, P = 0.02) (Fig. 5A), but with age controlled, the correlation decreased to r = 0.24 (P = 0.44) (Fig. 5B), indicating that the correlation between PSS and non-age-adjusted cerebral volume was mediated in part by the factor of age. In Fig. 5B, one case was influential and with its removal the correlation was r = −0.19.
Scatterplots and regression lines showing the relationship between PSS and cerebral volume. (A) CRH men, (B) CRH men with age controlled, (C) non-CRH men, (D) non-CRH men with age controlled. Asterisk (*) denotes influential cases. Correlations do not include the plotted test cases. closed square = CRH; open square = non-CRH. Test cases shown in (B) and (D): closed triangle = CRH (n = 3); open triangle = non-CRH (n = 2).
Figure 5C and D presents the results for non-CRH men. There was no evidence of a relationship between PSS and cerebral volume without age controlled (r = 0.07). With age controlled, r = −0.11. However, one case was influential and with its removal r = −0.25 (P = 0.33). With the influential cases removed, the slopes of the regression lines for age-adjusted scores were similar for the two male groups. Analysis of the two groups combined (n = 31) revealed that the same two cases were influential (those with the smallest and largest cerebral volume) and with their removal, r = −0.23 (P = 0.24). With height controlled, the correlation was rheight = −0.32 (P = 0.11).
As in the case of VSS, the correlations of PSS with left or right hemisphere volumes were not different from each other or from the correlations of PSS with total cerebral volume for each hand/sex subgroup.
Cerebral weight revealed almost identical correlations with test scores as did cerebral volume for each analysis.
Regression models evaluated with test cases
The linear regression models were obtained for each hand/sex subgroup using age-adjusted WAIS scaled scores as the criterion variable and age-adjusted cerebral volume as the predictor variable. The obtained prorated and age-adjusted VSS or PSS for the 10 test cases were plotted and evaluated in relation to predicted scores. Observed and predicted values were compared using the SD of the mean of the predicted values.
Verbal ability
Two test cases were available, both non-CRH. Their age-adjusted observed VSS are plotted against their age-adjusted cerebral volume in Fig. 3C. Each VSS is within 1 SD of the predicted value, providing support for the validity of this model. The correlation between VSS and cerebral volume including the two test cases remained the same (r = 0.59, P < 0.001).
One test case was available and his age-adjusted observed VSS is plotted (Fig. 3D) and is within 2 SDs of the predicted value. The correlation including this test case remained similar (r = 0.60, P = 0.008).
In non-CRH men, age-adjusted cerebral volume was not a significant predictor of age-adjusted VSS (Fig. 3E). There was one test case and his age-adjusted score is plotted. The correlation including this test case remained statistically non-significant (r = −0.34, P = 0.14).
Performance ability
For all women, cerebral volume accounted for about 10% of the variance in PSS (r = 0.32, P = 0.07) (Fig. 4C). There were four test cases (two CRH, two non-CRH) and their age-adjusted observed PSS scores are plotted (Fig. 4C). Three are within 1 SD and one within 2 SD of the predicted values, supporting the validity of this model. The correlation between PSS and cerebral volume including these four cases was r = 0.32 (P = 0.05).
In both CRH and non-CRH groups of men, with age controlled, cerebral volume was not significantly correlated with PSS (Fig. 5B and D). There were five test cases (three CRH and two non-CRH) and their age-adjusted observed PSS scores are plotted. The same two cases remained influential and when they were removed, the correlation for the combined group including the five test cases remained the same (r = −0.23, P = 0.24).
Discussion
In this study of 100 cognitively normal men and women, a general measure of verbal intelligence was positively related to postmortem cerebral volume in both right and non-right-handed women. In contrast, the same relationship was found for right-handed men, but not for non-right-handed men, suggesting that functional asymmetry may be a factor in the neural substrate of verbal ability in men. Visuospatial intelligence was also related positively to cerebral volume but only in women and less strongly. In men, regardless of handedness, there was a suggestion of a statistically non-significant trend for a negative relationship. This sex difference suggests the possibility of different neural substrates of visuospatial intelligence between men and women.
In addition, a sex difference was observed in the effect of chronological age on cerebral volume over the age span of 25–83 years. In men, cerebral volume correlated with age (r = −0.55), with brain size decreasing by 50 ml/decade. In women, there was only minimal decrease in brain size with advancing age, with change of ∼6 ml/decade. This sex difference is similar to that reported elsewhere (e.g. Ho et al., 1980; Witelson, 1991; Murphy et al., 1996).
Verbal ability
In all women and in right-handed men, verbal ability was positively correlated with cerebral volume accounting for ∼36% of the variation in verbal ability. The regression model for each of the three groups was similar. Verbal Scaled Score (VSS) increased by 9 points for each 100 ml of brain volume with age controlled in women and in right-handed men. The validity of this relationship was supported by the test cases fitting the regression models. A few MRI studies found similar but less strong relationships between various measures of verbal ability and brain volume in women (r = 0.4 in each: Andreasen et al., 1993; Wickett et al., 1994; Gur et al., 1999). The stronger relationship observed in our study may be related in part to the direct measurement of cerebral volume by the water displacement method. In men, most MRI studies reported correlations of r ∼ 0.3 (Andreasen et al, 1993; Gur et al., 1999; Wickett et al., 2000), although one recent study combining the sexes found no correlation of the WAIS Verbal Comprehension subtest with either grey or white matter volume (Posthuma et al., 2003). Previous MRI studies did not consider hand preference as an independent variable. The stronger relationship observed for right-handed men in our study may be related to the adjustment of cerebral volume to account for the age-associated decline, and to the consideration of functional asymmetry, as indexed by hand preference, as a factor.
Although the correlation and slope of the regression lines were similar between women and right-handed men, it is evident from inspection of the graphs (Fig. 3A and D) that the intercepts were different: that a similar VSS score, for example 80, was associated with a cerebral volume of 1100 ml in women, but close to 1300 ml in men. This suggests that at some level the neural substrate of verbal ability is different between the sexes in spite of similar relationships between overall brain size and ability.
In recent MRI studies of male twins, cerebral volume was shown to have high heritability (67%, Carmelli et al., 2002; 65%, Geschwind et al., 2002), which we interpret as evidence that verbal ability and brain size may be directed and linked to some degree by common genetic factors. This suggestion is further supported by the study of Thompson et al. (2001) who found that brain regions under genetic control (e.g. 90–95% heritability for volume of frontal grey matter) were correlated with Spearman's general intelligence ‘g’ (r = 0.40), a measure of general intelligence which they showed also to be highly heritable (70%). However, our finding that cerebral volume accounted for only 36% of the variation in verbal ability clearly points to a likely significant role for environmental factors, as concluded in a study of the relationship between verbal ability and brain size within families (Schoenemann et al., 2000).
In contrast to the findings for women and right-handed men, there was no evidence of a positive relationship between verbal ability and cerebral volume in our group of non-right-handed men but rather a statistically non-significant trend of a negative relationship. These results suggest that functional asymmetry, as indexed by hand preference, may be a factor in the relationship between verbal intelligence and brain size in men but not in women. Handedness was found to be a relevant factor in the one other study considering it as a factor in the relationship between IQ and brain size (Tan et al., 1999). The determinants and correlates of brain size may differ between right and non-right-handed men. In the heritability study of male twins (Geschwind et al., 2002), non-right-handers showed less genetic control of brain volume than right-handed men (70 versus 90%, respectively). This suggests that there is a greater contribution of non-genetic, random environmental factors on brain size in non-right-handed men, which we suggest may be one factor for the small relationship between verbal ability and brain size in our sample of non-right-handed men. Additionally, midsagittal area of the corpus callosum is greater in non-right-handers than right handers, particularly in men. This may reflect less pruning or axon loss, associated with less strong lateralization of verbal functions to the left hemisphere (Witelson, 1989; Witelson and Goldsmith, 1991). Size of the corpus callosum was also found to decrease with advancing age at a significantly faster rate among non-right-handed than right-handed men (Unsal et al., 1995). There may be less genetic guidance of callosal development in non-right-handed men. These findings suggest that different principles of brain development may be involved in right versus non-right-handed men and that aspects of brain development related to interhemispheric connectivity may be a factor in determining the neural substrate of verbal intelligence in men.
The prevalence of left-handedness is lower in women than men among singletons (Oldfield, 1971) and twins (Annett, 2003). Annett accounted for this sex difference in her model of the genetics of brain lateralization by suggesting that the right-shift (RS+) gene for left-hemisphere dominance for language and hand preference has a stronger expression among women. In fact our group of non-CRH women tended to have a higher mean handedness score than our non-CRH men (Table 1). In this context, our phenotypically defined non-CRH women may have a greater genetic expression for right-handedness compared to our non-CRH men. The greater genetic similarity between right and non-right-handed women may contribute to our finding that right and non-right-handed women showed similar relationships between verbal intelligence and brain size. In contrast, the different genetic contribution to brain size in right and non-right-handed men (Geschwind et al., 2002) may contribute to our finding that the two groups of men showed dissimilar relationships between verbal intelligence and brain size.
Performance ability
In each hand subgroup of women, performance ability showed a non-significant trend for a similar, small positive relationship with cerebral volume that accounted for ∼10% of the variance which represents a less tight fit of PSS with cerebral volume than in the case of VSS. PSS increased by 3 points/100 ml of brain volume which is less of a change with variation in volume compared to the situation for VSS. The four test cases of women fit the regression model of the combined group of women yielding a correlation of r = 0.32 (P = 0.05). This lower relationship may be reliable as other studies have also found relatively lower correlations between visuospatial measures and measures of brain size (Yeo et al., 1987, r = 0.06; Andreasen et al., 1993, r = 0.31; Wickett et al., 1994, r = 0.28; Posthuma et al., 2003, r = 0.39). However, it is possible that tighter function-structure relationships may exist for more specific tests of visuospatial cognition.
The situation in men is more complex, since cerebral volume is more strongly correlated with age in men than in women. Three previous studies reported positive relationships between performance measures and brain size in men without controlling for the common factor of age in each variable (Andreasen et al., 1993; Egan et al., 1994; Gur et al., 1999). In our right-handed group, without age controlled, we also found that PSS correlated highly with cerebral volume (r = 0.63). However, with age controlled, this relationship was diminished, showing that it was largely determined by the correlation of each of these variables with age. For the non-right-handers, there was no reliable relationship whether age was controlled or not.
However, with age controlled, each of our male subgroups had one influential case which when removed resulted in a non-significant trend of a negative relationship between visuospatial ability and cerebral volume which was similar in both groups. The finding that the right and non-right-handed men showed a similar relationship supports the notion that our non-CRH male subgroup may be representative of non-CRH men even though it differs from all other subgroups in its relationship of verbal ability with brain size.
These results for visuospatial intelligence suggest a sex difference: for women, regardless of handedness, a modest positive relationship was observed; for men, regardless of handedness, a non-significant minimal trend of a negative relationship. The positive relationship in women may be related to their use of verbal strategies in visuospatial tasks more than in the case of men. Further work is needed to clarify the neural substrate of visuospatial ability in men. A stronger relationship may be found with more functionally specific brain regions. For example, in the case of Albert Einstein, overall brain weight was within normal limits for his age, but unique morphology and expanded size of his inferior parietal region was found which was suggested to underlie his extraordinary spatial cognitive ability (Witelson et al., 1999).
In sum our results support a model in which the type of cognition, sex, functional asymmetry and ageing are all relevant factors in the relationship between intelligence and brain size, involving an interaction of sex and functional asymmetry for verbal intelligence, a sex factor in spatial intelligence and a sex factor for brain changes with age.
Sex difference in brain change with age
We found a strong negative relationship between cerebral volume and age in men, but minimal in women. In our study all women were post-menopausal and were not on oestrogen hormone replacement therapy suggesting that stability of brain size is not dependent on any neuroprotective effects of oestrogen. Although VSS did not decrease with age, PSS decreased with age similarly in men and women, as reported in the literature (Kaufman et al., 1991). In men, particularly right-handed men, the decrease in PSS with age appears tied to the decrease in cerebral volume with age. Whatever neural changes underlie the decrease in performance ability in women, decrease in cerebral volume does not appear to be a major factor.
Height
There is clear documentation that cerebral volume or cerebral weight is greater in men than in women by ∼12–15% in our study and in previous postmortem studies (Pakkenberg and Voigt, 1964; Ho et al., 1980) and MRI studies (Gur et al., 1991; Mayhew and Olsen, 1991). Brain size was minimally correlated with body height in our study, with height accounting for 1–4% of the variance within each sex. Adjusting brain size for any effect of height in our analyses made little difference. During development the sex difference in brain size begins to appear by around 3 years of age but in height only by 8 years (Dekaban and Sadowsky, 1978). These results question the justification of using height as a means to statistically control for sex differences in brain size as is frequently done (e.g. Andreasen et al., 1993; Flashman et al., 1998). Moreover, Ankney (1992) argued that when the appropriate ANCOVA (versus ratio scores) method is used to control for height or body size, the sex difference in brain size remains. At the theoretical level, these results leave unresolved the basis and consequences, if any, of the sex difference in brain size.
Limitations
Cerebral volume was necessarily determined from formalin-fixed brain specimens, but formalin fixation has not been found to result in consistent significant increase or decrease in weight or volume (Frydl et al., 1989; Quester and Schroder, 1997). We found that the correlations between fresh (unfixed) brain weight and brain volume (fixed) were high (intraclass correlation = 0.87, P < 0.001).
The sample of research subjects was not randomly selected. Only metastatic patients who presented at cancer clinics and whom the oncologists knew had accepted the seriousness of their prognosis were approached and only some of this subset agreed to participate in the study. The mean IQ of this sample was 115, which is one standard deviation above the population mean. Although all subjects were ambulatory and well functioning, they were on medication. It is possible that some aspect of their illness or treatment may have influenced brain size or test performance. In some cases, the patients survived a few years after completion of the cognitive tests, resulting in an interval between time of testing and time of brain measurements. Our subjects varied in age. Age was found to be a relevant variable for variation in brain size and intelligence. Although a longitudinal study would be preferential to assess the effects of age, only cross-sectional data were available for this study.
The statistically non-significant negative trends in structure–function relationships found for various samples of men are inconclusive and further work with larger samples is needed to address the reliability of the present results.
We demonstrated that general multifactorial measures of intelligence were correlated with cerebral volume, a gross measure of brain size. It would seem likely that since there is marked cortical localization of function in the human brain, that even stronger or clearer relationships might be found if size of functionally specific brain regions was studied in relation to specific cognitive skills. There are only a few such studies to date. In one example, among musicians, perfect pitch was found to be associated with the anatomy of the superior temporal gyrus independent of the extent of musical experience (Keenan et al., 2001).
Our study stops short in addressing which neural features of larger brains are correlated with greater ability. Variation in cerebral volume may reflect variation in amount of grey or white matter. Cajal (1989) speculated that variation in axonal connectivity may be a correlate of intelligence. Grey and white matter were considered in some MRI studies but the findings have been inconsistent. Gur et al. (1999) found that verbal and visuospatial measures correlated more strongly with volume of white than grey matter among women, whereas men showed similar relationships between ability with either white or grey matter volume. In an MRI study of only women, performance on the Stroop test (dependent on frontal regions) was correlated with volume of prefrontal white matter (r = 0.52) (Schoenemann et al., 2000). Andreasen et al. (1993) reported stronger correlations for IQ and grey matter volume but no significant correlations with white matter volume. Numerous histological features (such as the ratio of neurons to glia, cortical columnar spacing, amount of neurotransmitters or their receptors) not reflected in cerebral volume measures are also possibly relevant to variation in intelligence. New and emerging technology, such as diffusion tensor imaging (DTI) will allow for further investigation of the neural substrates of cognitive abilities.
Ethics
These findings raise questions about future ability and practice of predicting cognitive ability based on structural measures of the brain that are now easily obtained with the technical advances in neuroimaging. In our study, the simple measure of cerebral volume could predict as much as 36% of the variation in verbal cognition. It is highly likely that future research will reveal even stronger relationships between more specific cognitive skills and measures of neuroanatomical structures. The importance of considering responsible application of these findings is a current issue (Garland, 2004).
This work was supported by grants from US NIH NS62344 and NS18954, and MA-10610 from MRC (Canada), and the Albert Einstein/Irving Zucker Chair in Neuroscience to SFW. We are indebted to the patients and their families for their contribution and to the oncologists, pathologists, medical and nursing staff and administrators of McMaster University and Hamilton Health Sciences for their support and assistance of the research. A preliminary report was presented at the Society for Neuroscience Annual Meeting, 2002 (Abstract no. 182.15). We thank the two anonymous reviewers for their insightful comments.
References
Amunts K, Jancke L, Mohlberg H, Steinmetz H, Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender.
Andreasen NC, Flaum M, Swayze V II, O'Leary DS, Alliger R, Cohen G, et al. Intelligence and brain structure in normal individuals.
Ankney CD. Sex differences in relative brain size: the mismeasure of woman, too?
Annett M. The binomial distribution of right, mixed and left handedness.
Annett M. Cerebral asymmetry in twins: predictions of the right shift theory.
Baaré WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJ, Schnack HG, et al. Quantitative genetic modeling of variation in human brain morphology.
Carmelli D, Swan GE, DeCarli C, Reed T. Quantitative genetic modeling of regional brain volumes and cognitive performance in older male twins.
Coffey CE. Anatomic imaging of the aging human brain: computed tomography and magnetic resonance imaging. In: Coffey CE, Cummings JL, editors. Textbook of geriatric neuropsychiatry. Arlington, VA: American Psychiatric Press;
Coren S, Halpern DF. Left-handedness: a marker for decreased survival fitness.
Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes.
Dekaban AS, Sadowsky D. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights.
Egan V, Chiswick A, Santos C, Naidu K, Rimmington JE, Best JJK. Size isn't everything: a study of brain volume, intelligence and auditory evoked potentials.
Flashman LA, Andreasen NC, Flaum M, Swayze VW II. Intelligence and regional brain volumes in normal controls.
Frydl V, Koch R, Zavodska H. The effect of formalin fixation on several properties of the brain.
Garland B, editor. Neuroscience and the law. Brain, mind and the scales of justice. New York: Dana Press;
Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness.
Gur RC, Mozley PD, Resnick SM, Gottlieb GL, Kohn M, Zimmerman R, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging.
Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance.
Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults.
Ho KC, Roessmann U, Straumfjord JV, Monroe G. Analysis of brain weight. I. Adult brain weight in relation to sex, race, and age.
Kaufman AS, Kaufman-Packer JL, McLean JE, Reynolds CR. Is the pattern of intellectual growth and decline across the adult life span different for men and women?
Keenan JP, Thangaraj V, Halpern AR, Schlaug G. Absolute pitch and planum temporale.
Mayhew TM, Olsen DR. Magnetic resonance imaging (MRI) and model-free estimates of brain volume determined using the Cavalieri principle.
McCormick CM, Witelson SF, Kingstone E. Left-handedness in homosexual men and women: neuroendocrine implications.
Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging.
Oldfield RC. The assessment of handedness: the Edinburgh inventory.
Pakkenberg H, Voigt J. Brain weight of the Danes: a forensic material.
Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DN, Simon JH, et al. A twin MRI study of size variations in human brain.
Posthuma D, Baaré WF, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJ. Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed.
Quester R, Schroder R. The shrinkage of the human brain stem during formalin fixation and embedding in paraffin.
Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter.
Rushton JP, Ankney CD. Brain size and cognitive ability: correlations with age, sex, social class, and race.
Scamvougeras A, Kigar DL, Jones D, Weinberger DR, Witelson SF. Size of the human corpus callosum is genetically determined: an MRI study in mono and dizygotic twins.
Schoenemann PT, Budinger TF, Sarich VM, Wang WS. Brain size does not predict general cognitive ability within families.
Spitzka EA. A study of the brains of six eminent scientists belonging to the American Anthropometric Society: together with a description of the skull of Professor E. D. Cope.
Tan U, Tan M, Polat P, Ceylan Y, Suma S, Okur A. Magnetic resonance imaging brain size/IQ relations in Turkish university students.
Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure.
Unsal A, Witelson SF, Kigar DL, Steiner M. Sex and handedness differences in change of size of the corpus callosum during normal aging.
Wickett JC, Vernon PA, Lee DH. In vivo brain size, head perimeter, and intelligence in a sample of healthy adult females.
Wickett JC, Vernon PA, Lee DH. Relationships between factors of intelligence and brain volume.
Willerman L, Schultz R, Rutledge JN, Bigler ED. In vivo brain size and intelligence.
Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study.
Witelson SF, Goldsmith CH. The relationship of hand preference to anatomy of the corpus callosum in men.
Witelson SF, Kigar DL. Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men.
Witelson SF, Kigar DL, Harvey T. The exceptional brain of Albert Einstein.
Witelson SF, McCulloch PB. Premortem and postmortem measurement to study structure with function: a human brain collection.
Author notes
1Albert Einstein/Irving Zucker Chair in Neuroscience and 2Department of Psychiatry and Behavioural Neurosciences, Michael G. DeGroote School of Medicine, McMaster University, Hamilton, ON, Canada