-
PDF
- Split View
-
Views
-
Cite
Cite
Wassilios Meissner, Arthur Leblois, David Hansel, Bernard Bioulac, Christian E. Gross, Abdelhamid Benazzouz, Thomas Boraud, Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations, Brain, Volume 128, Issue 10, October 2005, Pages 2372–2382, https://doi.org/10.1093/brain/awh616
Close - Share Icon Share
Abstract
High frequency stimulation (HFS) of the subthalamic nucleus (STN) is a well-established therapeutic approach for the treatment of late-stage Parkinson's disease. Although the underlying cause of this illness remains a mystery, changes in firing rate and synchronized activity in different basal ganglia nuclei have been related to its symptoms. Here we investigated the impact of STN-HFS on firing rate as well as correlated and oscillatory activity in the STN network in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned non-human primates by using simultaneous extracellular single-unit recordings. STN-HFS reduced (i) the firing rate of STN neurons, (ii) the oscillatory activity at an individual STN neuron level as well as (iii) the correlated and oscillatory activity between pairs of STN neurons, while contralateral rigidity was improved. A detailed analysis showed that the decrease of mean firing rate resulted from the resetting of firing probability to virtually zero by the stimulus pulse. Subsequently, STN neurons resumed their activity after a mean duration of 2.9 ± 0.1 ms and their firing probability returned to baseline values ∼7 ms after the onset of the stimulus pulse, the recovery of the firing probability being represented by a sigmoid function. Thus, the overall decrease of the mean firing rate resulted from the repetition of this dynamical process with a frequency of 130 Hz (interstimulus interval ∼7.7 ms), allowing the neuron to fire with its baseline firing rate only for a very short period. Although the mechanisms underlying the desynchronization of neuronal activity in the STN network remain unclear, the resetting of STN neuron firing probability by the electrical stimulus would rather be expected to increase oscillatory activity at an individual neuron level as well as correlated and oscillatory activity between pairs of STN neurons. However, assuming the resetting of firing rate to be the consequence of a transient GABAergic inhibition through excitation of presynaptic GABAergic axon terminals, different recovery periods of STN neurons might delay the appearance of synchronized oscillations, particularly if they are not generated locally. In conclusion, our study provides new evidence that STN-HFS decreases oscillatory activity in the STN network. Although the exact relation between oscillatory activity and Parkinson's disease symptoms remains to be determined, the present results suggest that STN-HFS might at least partially exert its beneficial effects through the reduction of oscillatory activity in the STN network and consequently in the entire cortex-basal ganglia-cortex network.
Introduction
High frequency stimulation (HFS) of the subthalamic nucleus (STN) has been established as a therapeutic approach for the treatment of late-stage Parkinson's disease improving rigidity, bradykinesia and tremor (Pollak et al., 1993; Limousin et al., 1998; Krack et al., 2003). Although there is growing clinical experience of STN-HFS, its mechanisms of action are not fully understood. Several experimental studies have suggested that STN-HFS reduces the activity of STN neurons (Benazzouz et al., 1993, 2004; Salin et al., 2002; Tai et al., 2003). According to the classical model of basal ganglia function (Alexander and Crutcher, 1990), this inhibition should decrease the activity of the internal segment of the globus pallidus (GPi), the main output nucleus of the basal ganglia, by relieving the GPi from its excitatory glutamatergic subthalamic input and subsequently normalize the disturbed thalamocortical information flow. Interestingly, Hashimoto et al. (2003) observed in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-(MPTP)-lesioned non-human primates that STN-HFS increases the mean firing rate of GPi neurons and modifies the firing pattern from spontaneously irregular into regular. This suggests that a combination of distinct effects on cell soma and axon might occur at effective stimulation parameters, i.e. STN-HFS might inhibit neuronal activity of the cell soma, while the axon might be excited.
Progress in electrophysiological recordings has allowed the assessment of correlated and oscillatory activity in the basal ganglia network in awake non-human primates (Nini et al., 1995; Raz et al., 2000). In this respect, it has been shown that STN neurons display oscillatory activity at an individual neuron level in MPTP-lesioned non-human primates (Bergman et al., 1994) and that pairs of STN neurons oscillate in phase with limb tremor in Parkinson's disease patients in the beta frequency band (Levy et al., 2000). Results obtained from recordings of local field potentials and unit activity in human STN, further suggest that increased oscillatory activity in the beta frequency band might be an important feature of the disease (Brown et al., 2001; Williams et al., 2002; Levy et al., 2002a; Brown, 2003; Kühn et al., 2004). Correlated and oscillatory activity have also been demonstrated between pairs of GPi neurons of MPTP-lesioned non-human primates and Parkinson's disease patients while they were virtually absent in the normal non-human primates (Nini et al., 1995; Raz et al., 2000; Levy et al., 2002b). Consequently, the setting of the basal ganglia network into a dynamical state with excessive correlated and oscillatory activity has been further related to Parkinson's disease motor symptoms (Bergman et al., 1998; Bar-Gad and Bergman, 2001).
Here we performed for the first time simultaneous extracellular single-unit recordings with four individually driven micro-electrodes in the STN network of MPTP-lesioned non-human primates during STN-HFS. The main goals were (i) to determine the impact of STN-HFS on the activity of STN neurons at an individual neuron level and (ii) to test the hypothesis of whether STN-HFS interferes with abnormal correlated and oscillatory activity in the basal ganglia (Bar-Gad and Bergman, 2001; Brown et al., 2001).
Material and methods
Animals
The study was conducted on two female rhesus macaques (Macaca mulatta, 2.3 and 4.1 kg) that were housed in a temperature (24 ± 1°C) and humidity (50 ± 5%) controlled facility with a 12 h light-dark cycle (lights on 8:00 a.m.). All experiments were performed during daytime. Food and water were available ad libitum, and a veterinarian skilled in the healthcare and maintenance of non-human primates supervised animal care. Experiments were performed in accordance with European Communities Council Directive of 24 November 1986 (86/609/EEC) and National Institutes of Health Guide for the Care and Use of Laboratory Animals. MPTP treatment and the surgical procedure to fix the recording chamber have been extensively described previously (Bezard et al., 2001; Boraud et al., 2001).
The behaviour of both non-human primates was assessed on a parkinsonian non-human primate rating scale (Benazzouz et al., 1995) using videotape recordings of non-human primates as previously described (Boraud et al., 2001; Bezard et al., 2003). This rating scale, shown to be a non-human primate equivalent of the Unified Parkinson's Disease Rating Scale (Imbert et al., 2000) assesses tremor, posture, general activity, vocalization, freezing, rigidity and arm movements. A score of 0 corresponds to a normal animal and a score above 6 to a parkinsonian animal.
The clinical efficacy of STN-HFS was verified by assessing contralateral rigidity during all stimulation sessions. This complies with the surgical procedure in Parkinson's disease patients, where contralateral rigidity is assessed while stimulating through micro-electrodes of the tracking system to ensure the correct placement of the macro-electrode within STN (Benazzouz et al., 2002; Pollak et al., 2002). The severity of rigidity (0 = none, 1 = mild, 2 = moderate, 3 = severe) was rated as described above by two independent skilled raters that were blinded to the experimental condition.
Recordings and data collection
Recordings were performed in the left STN in both normal and MPTP-lesioned conditions. Single-unit activity was recorded using four individually driven glass-coated tungsten micro-electrodes (impedance 0.5 MΩ at 1 kHz). The micro-electrodes were initially placed in a guide with an inner diameter of 1.3 mm in a rectangular configuration, i.e. the maximum distance between two electrodes in the horizontal plane was ∼1.8 mm. Before starting the recordings, the guide was lowered to a depth of 8 mm below the dura. Subsequently, the electrodes were individually lowered until the typical signal of STN neurons was detected. The maximal distance in the vertical plane was never >2 mm and thus the distance between the tips never exceeded 2.7 mm. The signal of each electrode was first amplified (Multi Channel Processor, Alpha Omega, Nazareth Illit, Israel) and then bifurcated into two different channels. One channel was filtered (high pass: 300 Hz, low pass: 3 kHz) and sorted online (Multi Spike Detector, Alpha Omega, for details see Raz et al., 2000). Interspike intervals were then stored with 12 kHz sampling rate through alpha map. The other channel was stored as unfiltered raw data for further off-line analysis of the stimulation artefact (see below) with a sampling rate of 24 kHz through alpha map. The Multi Spike Detector allowed isolation up to three units from single electrodes. Baseline activity was recorded for at least 10 min and only stable spike trains were further recorded and analysed. In the MPTP-lesioned condition, baseline recordings were followed by STN-HFS as described below.
STN-HFS
STN-HFS was performed in the stable parkinsonian condition through one of the four micro-electrodes after recording STN baseline activity as previously reported for Parkinson's disease patients (Benazzouz et al., 2002). The other three electrodes have been used for recordings during HFS. Extracellular recordings guided the correct placement of the stimulation micro-electrode in the STN. For STN-HFS the following stimulation parameters were applied: frequency 130 Hz, pulse width 60 μs and intensity 100 μA, using a linear stimulus isolator (WPI Instruments, Sarasota, FL, USA). Current intensity was limited to 100 μA due to the physical properties of the stimulus isolator and the risk of tissue damage when using higher current intensities. Each stimulation session included 6 min of recording before stimulation (pre), 6 min of recording during stimulation (HFS) and 9 min of recording after HFS (post1 = 3 min, post2 = 6 min).
Stimulation artefact
Alpha Omega's Multi Spike Detector recognizes action potentials of individual neurons by using a template-matching algorithm. A template can be defined online for each neuron. Proper recognition of individual spike trains by the Multi Spike Detector while performing STN-HFS was verified and confirmed by inspection of digital and analogue raw data of 10 representative neurons with NeuroExplorer (Version 3.124, Nex Technologies, Littleton, MA, USA). In a second approach, the stimulation artefact was subtracted from unfiltered raw data of 10 representative neurons (see further Fig. 1A) as described previously (Tai et al., 2003). A subsequent comparison between unsubtracted and subtracted spike trains resulted in a 98% match.
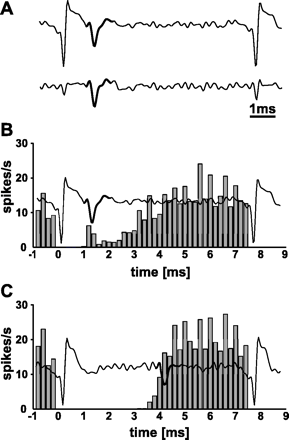
Stimulation artefact and STN neuronal activity while performing STN-HFS. (A) Example of representative filtered analogue recordings before (above) and after subtraction of the stimulation artefact (below). (B, C) Two representative filtered analogue recordings, each showing the stimulation artefact and a representative spike (bold) that was recognized by Alpha Omega's Multi Spike Detector. The filtered analogue signal of each example is superimposed to a peristimulus histogram of the respective STN neuron that is aligned to the onset of the stimulation artefact (t = 0 ms) in 0.2 ms bins. The spike count of each bin is normalized by the total number of electrical stimuli and the bin size. Note that the analogue signal shows only one representative spike between two stimulation artefacts, while the peristimulus histogram represents the firing probability over the entire 6 min of HFS. (B) The close relation of the spike to the stimulation artefact underlines that the template-matching algorithm reliably recognized spikes during STN-HFS except for the period of complete saturation. (C) Most STN neurons (29/43) resumed their neuronal activity over 3 ms after the onset of the electrical stimulus, i.e. long after the end of complete saturation of the recording signal.
The duration of total saturation of the recording signal by the stimulation artefact was determined for each neuron from unfiltered analogue recordings and is indicated in the results.
To further determine the temporal relationship between the stimulation artefact and the firing probability, the spike count of each STN neuron was aligned to the onset of the stimulation artefact with a bin width of 0.2 ms, giving a standard peristimulus time histogram (Fig. 1B and C). The spike count of each bin was normalized by the total number of electrical stimuli and the bin size, i.e. the value in each bin represents the mean firing probability in that bin over the entire stimulation period. Results are expressed in spikes/s to allow an easy comparison with baseline values.
Data analysis
Off-line analysis included firing rate as well as correlated and oscillatory activity of STN neurons. For this purpose, auto-correlograms (ACs) of spike trains and cross-correlograms (CCs) of pairs of spike trains were assessed for each observation period. To ensure that changes in firing rate did not influence the bandwidth of the detected oscillatory activity of STN neurons, the same number of spikes were taken from pre, HFS, post1 and post2 for the calculation of AC and CC. Thus, the number of analysed spikes of a single neuron was the same for all four experimental conditions, while the number of analysed spikes between different neurons could have been different.
AC: Single-cell oscillatory activity was detected by calculating AC for all recorded spike trains (1000 ms offset with a bin width of 5 ms). After removing the trough of the refractory period around time 0 in the AC (reducing high-frequency noise) and after subtracting the average firing rate of the cell (reducing DC offset) the calculated power spectra of each AC allowed a 1 Hz resolution in the frequency band between 2 and 100 Hz. For every peak in this range we calculated a signal-to-noise ratio (SNR) as the difference between the maximum power and the average of the power spectrum between 3 and 30 Hz, divided by the SD of the entire power spectrum (0–100 Hz, in this case) (Lenz et al., 1988). Additionally, an oscillation index (OI) was calculated for all peaks. The OI was defined as the area under the peak, divided by the total power over the whole spectrum (2–100 Hz). A peak in the AC was considered significant if the SNR was >5 and if it had an OI of >15%. In respect to oscillatory activity, we focused on the 4–30 Hz range, since MPTP treatment induces almost exclusively significant oscillations in that frequency band (Bergman et al., 1994). Thus, a neuron was considered oscillatory if a significant peak was found in the power spectrum between 4 and 30 Hz. For neurons with more than one significant peak in the power spectrum, power and frequency of all peaks were assessed and the neuron was considered to be oscillatory in all of these frequencies.
CC: Correlated and synchronized oscillatory activity between neurons was detected by calculating CC of all pairs of spike trains (1000 ms offset with a bin width of 5 ms, centred around the spike). Although the Multi Spike Detector allowed isolation of more than one unit from single electrodes, only pairs that were recorded by different electrodes were analysed to avoid a shadowing effect and artificial correlation (Bar-Gad et al., 2001). We considered pairs of neurons to be correlated if they had (i) a significant peak or trough in their CC or (ii) a significant peak in the power spectrum of their CC. A CC was considered to have a significant peak if there were more than three consecutive bins with a value higher than the baseline firing rate by at least 2.5 SD. Baseline firing rate and SD were estimated using the first and last 250 ms of the CC. The same analysis was repeated to find significant troughs. The power spectra of all CC were calculated after subtracting the mean baseline firing rate of the two assessed neurons (reducing the DC offset). Furthermore, the same analysis as for AC power spectra was applied to CC power spectra to find significant peaks. A pair of STN neurons was considered to have synchronized oscillatory activity in all frequencies that corresponded to significant peaks in the power spectrum.
Histology
The recording tracks and the extent of the lesion were verified post-mortem as previously described (Bezard et al., 2001; Boraud et al., 2001) to ensure (i) proper STN targeting while recording and (ii) homogeneous lesioning of the substantia nigra pars compacta (SNc) in both animals. Briefly, cresyl violet staining was performed on 20 μm coronal sections containing the STN and adjacent structures for the reconstruction of the recording tracks. Mesencephalic sections were processed for tyrosine hydroxylase immunohistochemistry. Cell counts were performed using a computer-based image analyser (Visioscan version 4.12; Biocom, Les Ulis, France). The boundaries of the SNc were chosen on three consecutive sections corresponding to a representative median plane of the SNc by examining the size and shape of the different tyrosine hydroxylase immunoreactive (TH-IR) neuronal groups, cellular relationships to axonal projections, and nearby fibre bundles. The number of TH-IR per SNc representative plane was calculated three times by one examiner blinded with regard to the experimental condition. Mean cell number per plane and SEM were then calculated. To allow a comparison with the normal condition, control values obtained from five normal animals of our brain bank that were matched for age, sex and weight are also indicated in the results.
Statistical analysis
Statistical analysis of firing rate was performed with the Sigma Stat software (Version 2.03; SPSS Inc, Chicago, IL, USA) using a t-test (normal versus MPTP) and a one-way repeated measures ANOVA (comparison between pre, HFS, post1, and post2) followed by post hoc multiple comparisons using the method of Bonferroni. AC and CC were analysed by using χ2 tests (normal versus MPTP) and McNemar's tests (comparison between pre, HFS, post1 and post2). For the comparison of AC and CC with oscillatory activity in the beta frequency band (14–30 Hz), χ2 tests or Fisher exact tests were used where appropriate. The mean frequency of oscillating AC and CC was assessed by using t-tests (normal versus MPTP) and one-way ANOVAs (comparison between pre, HFS, post1 and post2). A probability level of 5% (P < 0.05) was considered significant.
The firing rate, the frequency of oscillating AC and CC in addition to the number of TH-IR neurons in the SNc are shown as mean ± SEM. AC as well as CC are presented as percentage of total AC and CC, respectively.
Results
The same animals were recorded before (normal condition) and after MPTP treatment. According to a non-human primate rating scale (Benazzouz et al., 1995) both non-human primates were moderately parkinsonian (median MPTP = 9, range 8–10). Immunohistochemistry showed a decrease in the number of TH-IR neurons in the SNc of both non-human primates (150.4 ± 21.9) in comparison with control values (961.6 ± 15.7).
According to clinical practice during the surgery of Parkinson's disease patients, rigidity was chosen to verify clinical efficacy of STN-HFS. In contrast to other items of the primate rating scale, rigidity can be easily assessed while the non-human primate is placed in a primate chair. In agreement with previous data (Benazzouz et al., 1993), STN-HFS improved contralateral rigidity (median MPTP = 1, range 1–2; median STN-HFS = 0, range 0–1, n = 13, P < 0.05, Wilcoxon-signed rank test) as observed in patients when stimulating the STN during the surgery through micro-electrodes (Benazzouz et al., 2002; Pollak et al., 2002; Filali et al., 2004).
Stimulation artefact
The saturation of the recording signal due to the stimulation artefact is a major concern of electrophysiological studies that investigate mechanisms of HFS. This stimulation artefact consists of an interval where the amplifier is saturated and an interval where the recording signal returns to the baseline. In the present study, the complete saturation of the recording signal due to the stimulation artefact was 0.9 ± 0.1 ms, similar to what has been reported for the globus pallidus (GP) (Bar-Gad et al., 2004). It is thus not possible to report the activity of STN neurons during that period. In most cases, the stimulation artefact was shorter than the duration of the electrical pulse (60 μs) plus an action potential of STN neurons (Bergman et al., 1994). Thus, it seems to be very unlikely that a spike might have been triggered by the stimulus. However, we cannot rule out that some evoked spikes might have been obscured by the artefact in recorded neurons that had a total saturation over 0.9 ms. In 29 of the 43 neurons, the latency of the first detected spike after onset of the stimulus was over 3 ms and in the remainder it was less (Figs 1B, C and 2E). This suggests that (i) the stimulation artefact might not have masked neuronal activity and that (ii) time-locked triggering of recurrent bursts of spikes by the electrical stimulus, as previously described for in vitro STN preparations (Garcia et al., 2003), might not occur in the STN of awake non-human primates.
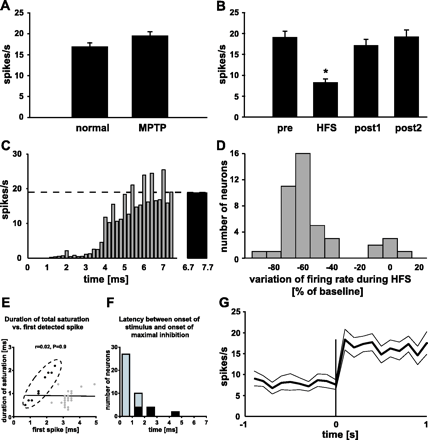
Firing rate of STN neurons. (A) The firing rate was not modified by MPTP treatment. (B) STN-HFS in the MPTP-lesioned condition decreased significantly the firing rate of subthalamic neurons. *P < 0.05 versus pre, post1 and post2. (C) Peristimulus histogram representing the mean firing probability of the population of recorded STN neurons (n = 43) aligned to the onset of the stimulation artefact. The dashed line indicates the mean firing rate before STN-HFS (pre) and the black bar shows the mean firing probability during the last ms preceding the next electrical stimulus while performing STN-HFS. (D) Distribution of the variation of firing rate during STN-HFS in comparison with baseline. STN-HFS decreased the mean firing rate of most subthalamic neurons (37/43), while firing rate was not significantly modified in 6/43 neurons. (E) Scatter diagram plotting the duration of total saturation of the recording signal versus the latency of the first detected spike after the onset of the stimulus pulse. A linear regression including all recorded neurons (black and grey dots) was used for its representative value (r = 0.02), while a Pearson correlation was performed to ascertain the nature of the relationship (P = 0.9). Eleven neurons (black dots encircled by the dashed line) displayed firing activity after the end of total saturation of the recording signal, while the remainder (32/43, grey dots) did not. (F) Histogram showing the latency between the onset of the electrical stimulus and the onset of maximal inhibition. The neurons represented by the black dots in E correspond to the black bars. The latency of maximal inhibition for the majority of STN neurons (grey dots in E) coincided with the end of total saturation, i.e. the latency of maximal inhibition comprised in these neurons at the most the duration of complete saturation of the recording signal by the stimulation artefact. (G) Mean firing rate (bold line) ± SEM (regular lines) of the population of stimulated STN neurons during the last second of HFS and the first second of post1 showing a rapid return to baseline firing rate after the end of STN-HFS.
STN-HFS decreases firing rate of STN neurons
STN firing rate in the normal condition was 16.9 ± 0.9 spikes/s (n = 41) and 19.6 ± 0.9 spikes/s (n = 97) in the stable parkinsonian state (Fig. 2A). This difference was not significant (P > 0.05). Among all neurons recorded in the stable parkinsonian condition, 43 have been further tested for their response to stimulation. STN-HFS decreased STN firing rate in these neurons from 19.0 ± 1.5 spikes/s (pre) to 8.3 ± 0.9 spikes/s (Fig. 2B). The adjusted mean firing rate excluding the saturation time of the amplifier by the stimulation artefact was 9.3 ± 1.0 spikes/s. An overall estimation revealed significant differences between the four conditions (pre, during, post1 and post2; F(3,171) = 48.7, P < 0.05; F(3,171) = 38.1, P < 0.05 for adjusted mean firing rate). Further post hoc analysis showed a significant decrease in firing rate during HFS (non-adjusted and adjusted) compared with pre, post1 and post2. No significant difference in firing rate was observed when the recordings during HFS were divided in three clusters of 2 min duration each (0–2 min: 9.0 ± 1.0 spikes/s; 2–4 min: 8.5 ± 0.9 spikes/s; 4–6 min: 7.5 ± 0.8 spikes/s, F(2,128) = 0.9, P > 0.05). Values for adjusted mean firing rate were 10.2 ± 1.2 spikes/s, 9.6 ± 1.0 spikes/s and 8.4 ± 0.8 spikes/s, F(2,128) = 0.9, P > 0.05.
The decrease of the mean firing rate of all tested STN neurons resulted from the resetting of the firing probability of STN neurons to virtually zero by each stimulus pulse (Fig. 2C). Subsequently, STN neurons resumed their activity after a mean duration of 2.9 ± 0.1 ms and the firing probability returned to baseline values ∼7 ms after the onset of the electrical stimulus (last ms before next stimulus: 18.8 ± 0.3 spikes/s versus pre: 19.0 ± 1.5 spikes/s; Fig. 2C). The recovery of the mean firing probability between two stimuli is represented by a sigmoid function: f(x) = F0/[1 +exp(−k (x − x0))], where F0 is the baseline firing rate, x is the time in seconds, k = 4.5 ± 0.6 ms−1 and x0 = 4.4 ± 0.2 ms.
The mean firing rate was reduced in 37/43 neurons (Fig. 2D). Most of these neurons (23/37) showed a stereotypical response to stimulation consisting of an initial complete inhibition followed by a recovery of firing probability to baseline values before the occurrence of the next stimulus pulse. Two neurons showed baseline activity and seven more neurons showed firing below baseline values just after the end of complete saturation of the recording signal due to the stimulation artefact. In these nine neurons, the maximal inhibition occurred with a certain latency after the end of the stimulus pulse followed by the recovery of firing probability to baseline values (Fig. 2F). One neuron showed a constant reduction of firing rate to 30% of baseline firing rate. The remaining four neurons with reduced mean firing rate during STN-HFS displayed multi-phasic responses consisting of either excitation-inhibition-recovery of baseline firing to baseline values (one neuron) or inhibition-baseline-inhibition-recovery of baseline firing to baseline values (three neurons). STN-HFS did not modify the mean firing rate in 6/43 neurons (Fig. 2D). Interestingly, all of these neurons showed an initial inhibition followed by an excitation, i.e. their firing probability between two electrical stimuli was also represented by a sigmoid function.
STN-HFS decreases correlated and oscillatory activity
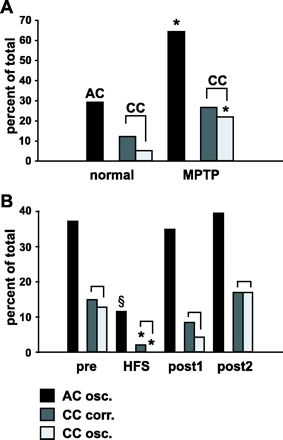
MPTP treatment increased significantly the number of individual STN neurons with oscillatory activity from 29.3% (12/41) in the normal condition to 64.3% (63/98) in the parkinsonian state (χ2 = 4.1, df = 1, P < 0.05, Fig. 3A). The mean frequency of oscillations significantly increased from 5.7 ± 0.5 Hz (n = 15) in the normal condition to 7.6 ± 0.3 Hz (n = 107) after MPTP treatment (P < 0.05). In the parkinsonian state, STN-HFS decreased the number of individual oscillatory STN neurons [pre: 37.2% (16/43), HFS: 11.6% (5/43), post1: 34.9% (15/43), and post2: 39.5% (17/43), Figs 3B and 4]. This decrease was significant (HFS versus pre, χ2 = 9.1, df = 1, P < 0.05; HFS versus post1, χ2 = 8.1, df = 1, P < 0.05; and HFS versus post2, χ2 = 10.1, df = 1, P < 0.05). In contrast, the mean frequency of individual STN neuron oscillations remained unchanged while performing STN-HFS [pre: 8.3 ± 0.5 Hz (n = 31), HFS: 7.4 ± 0.9 Hz (n = 7), post1: 7.6 ± 0.5 Hz (n = 22), and post2: 8.9 ± 1.1 Hz (n = 28); F(3,87) = 0.6, P > 0.5]. The analysis of the frequency band showed that oscillatory activity mainly occurred in the theta and alpha frequency band. Accordingly, oscillations in the beta frequency band (14–30 Hz) were absent (0/15) in the normal condition and occurred only in 4.7% (5/107) in the stable parkinsonian state. This difference was not significant (χ2 = 0.0, P > 0.5). Oscillations in the beta frequency band were not different before, during and after HFS [pre: 6.5% (2/31), HFS: 0% (0/7), post1: 0.0% (0/22), post2: 7.1% (2/28), P = 1.0 for HFS versus pre, post1 and post2].
Correlated and oscillatory activity in the STN network. Values are expressed as percentage of all assessed auto-correlograms (AC) or cross-correlograms (CC). Black bars = AC with oscillatory activity at an individual neuron level, dark grey = CC with correlated activity between pairs of STN neurons and light grey = CC with synchronized oscillations between pairs of STN neurons. (A) MPTP treatment increased the number of AC with oscillatory activity and the number of CC with synchronized oscillations. *P < 0.05 versus normal. (B) STN-HFS reduced oscillatory activity at an individual STN neuron level as well as the correlated and oscillatory activity between pairs of STN neurons. §P < 0.05 versus pre, post1 and post2. *P < 0.05 versus pre and post2.
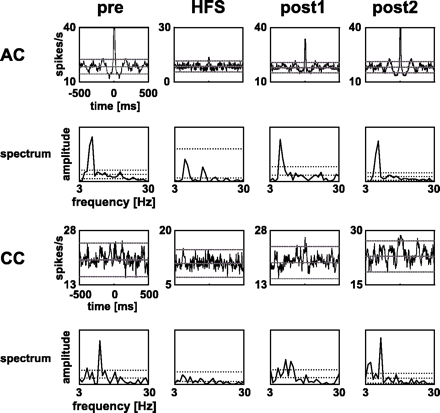
Example of auto-correlograms (AC) of one STN neuron and cross-correlograms (CC) of one pair of simultaneously recorded STN neurons with their power spectra before (pre), during and after HFS (post1 and post2). The lines in the ACs and CCs indicate mean ± 2.5 SD, while they represent mean ± 5 SD in the power spectra. Upper and lower lines correspond to the threshold of significance in ACs and CCs, while peaks in power spectra were considered to be significant if their values exceeded mean + 5 SD. The first and second rows provide data of one STN neuron that displayed oscillatory activity before and after HFS (post1 and post2), but not while HFS was applied (see power spectra). The third and fourth rows represent one pair of STN neurons that showed synchronized oscillations (significant peak in power spectra) before and after HFS (post1 and post2) that were suppressed by HFS.
The correlated activity between pairs of STN neurons was not modified by MPTP treatment [normal condition: 12.3% (7/57), MPTP condition: 26.7% (40/150), χ2 = 2.6, df = 1, P > 0.05, Fig. 3A]. In the parkinsonian state, STN-HFS reduced correlated activity between STN neurons [pre: 14.9% (7/47), HFS: 2.1% (1/47), post1: 8.3% (4/47), and post2: 17.0% (8/47), Fig. 3B]. This reduction was significant (HFS versus pre, χ2 = 4.2, df = 1, P < 0.05; HFS versus post2, χ2 = 5.1, df = 1, P < 0.05).
While 5.3% (3/57) of pairs of STN neurons showed oscillatory activity in the normal condition, MPTP treatment significantly increased the number of these pairs to 22.0% (33/150) (χ2 = 5.1, df = 1, P < 0.05, Fig. 3A). The mean oscillatory frequency was 6.3 ± 1.3 Hz (n = 4) in the normal condition and 7.5 ± 0.4 Hz (n = 42) after MPTP treatment, which is not significantly different (P > 0.05). In the parkinsonian state, STN-HFS reduced the abnormal population oscillatory activity [pre: 12.8% (6/47), HFS: 0.0% (0/47), post1: 4.3% (2/47), and post2: 17.0% (8/47), Figs 3B and 4]. This decrease was significant (HFS versus pre, χ2 = 4.2, df = 1, P < 0.05; HFS versus post2, χ2 = 6.1, df = 1, P < 0.05). The mean oscillatory frequency was similar between pre, post1 and post2 (pre: 8.5 ± 1.4 Hz (n = 6), post1: 8.2 ± 1.0 Hz (n = 5), post2: 7.3 ± 0.9 Hz (n = 9); F(2,19) = 0.4, P > 0.5). A more detailed analysis of the frequency band showed that oscillatory activity mainly occurred in the theta and alpha frequency band. Accordingly, oscillations in the beta frequency band (14–30 Hz) were absent (0/4) in the normal condition and occurred only in 2.4% (1/42) in the stable parkinsonian state. This difference was not significant (P = 1.0). Oscillations in the beta frequency band were not different before, during and after HFS [pre: 16.7% (1/6), HFS: 0% (0/0), post1: 0% (0/5), post2: 0.0% (0/9), P = 1.0 for HFS versus pre, post1 and post2].
Discussion
The present results afforded three main findings. STN-HFS reduced (i) the firing rate of STN neurons, (ii) the oscillatory activity at an individual STN neuron level as well as (iii) the correlated and oscillatory activity between pairs of STN neurons, while contralateral rigidity was improved.
Methodological considerations
We used micro-recordings to determine neuronal activity of STN neurons in relation to STN-HFS with high impedance micro-electrodes. This is different from clinical practice since STN-HFS in Parkinson's disease patients is applied through implanted macro-electrodes. However, before implantation of these macro-electrodes, the clinical efficacy is tested by stimulation through micro-electrodes that allow the induction of similar clinical effects as macro-electrodes (Benazzouz et al., 2002; Pollak et al., 2002; Filali et al., 2004). Accordingly, STN-HFS through micro-electrodes improved contralateral rigidity in both non-human primates in the present study.
STN-HFS decreases firing rate of STN neurons
MPTP treatment did not significantly modify STN firing rate. This contrasts with previously reported data (Bergman et al., 1994; Bezard et al., 1999). The differences might be due to the fact that in the present study both non-human primates had only moderate parkinsonian symptoms.
As observed in 6-hydroxydopamine-lesioned rats (Benazzouz et al., 2000; Tai et al., 2003) and Parkinson's disease patients (Filali et al., 2004; Welter et al., 2004) STN-HFS decreased the firing rate of STN neurons. There was no difference in firing rate over time while performing STN-HFS. A decrease in firing rate has also been reported for pallidal neurons while performing HFS of the GP in patients and non-human primates (Boraud et al., 1996; Dostrovsky et al., 2000; Bar-Gad et al., 2004), suggesting that a common mechanism might underlie the inhibition of subthalamic and pallidal neuronal activity.
Dynamics of the recovery of firing rate
One of the key findings of this study is that the decrease of mean firing rate resulted from the resetting of the firing probability of STN neurons to virtually zero by the stimulus pulse (Fig. 2C). If each stimulus resets the firing probability of STN neurons for a certain duration one could speculate that the higher the stimulation frequency the stronger the inhibitory effect of STN-HFS on firing rate, because of the shortening of the interval between two electrical stimuli. Subsequently, STN neurons resumed their activity after a mean duration of 2.9 ± 0.1 ms and the firing probability returned to baseline values ∼7 ms after the onset of the electrical stimulus (Fig. 2C), the recovery of firing probability being represented by a sigmoid function. Thus, the overall decrease of the mean firing rate resulted from the repetition of a dynamical process with a frequency of 130 Hz (interstimulus interval ∼7.7 ms), allowing the neuron to fire with its baseline firing rate only for a very short period. This observation confirms the hypothesis that STN-HFS does not impose a depolarizing block on STN neurons (Tai et al., 2003) or triggers recurrent bursts of spikes by each electrical stimulus (Garcia et al., 2003). Similar conclusions have been drawn from the results of a study that investigated the mechanisms of GP-HFS (Bar-Gad et al., 2004). In this study, HFS induced a multi-phasic change in the firing rate of GP neurons, while the majority of the neurons displayed partial inhibition. The authors concluded that HFS might (i) facilitate the activation of excitatory and inhibitory axons that target the GP, or (ii) simply excite inhibitory inputs to GP neurons followed by the resetting of their activity and the creation of a multi-phasic synchronized response (Kita, 2001).
Mechanisms of STN-HFS
Although STN-HFS is believed to inhibit the activity of STN neurons (Salin et al., 2002; Tai et al., 2003; Benazzouz et al., 2004; Filali et al., 2004; Welter et al., 2004), there is currently a large amount of speculation whether HFS inhibits or excites STN efferents. In this view Hashimoto et al. (2003) observed in MPTP-lesioned non-human primates that STN-HFS increases the mean firing rate of GPi neurons and modifies the firing pattern from spontaneously irregular into regular, suggesting that stimulation might excite STN neurons or STN efferents that project to GPi. In support of this idea it has been shown that STN-HFS enhances the concentration of extracellular glutamate in the SNr of normal rats (the main basal ganglia output nucleus in rodents) (Windels et al., 2000), but no modification of extracellular glutamate levels has been observed in the SNr in a rat model of Parkinson's disease (Windels et al., 2005). A recent study that investigated the effects of STN-HFS on the activity of SNr neurons might provide the key for the understanding of these results (Maurice et al., 2003). The authors observed a decrease in SNr activity while STN-HFS was performed with lower intensity and an increase of SNr activity while the STN was stimulated at higher intensity, suggesting that a combination of distinct effects on somatic and axonal activity might occur above a certain threshold. A model-based analysis was used to assess further if such a combination of distinct effects on cell soma and axon could explain the paradoxical experimental results (McIntyre et al., 2004). The authors observed that subthreshold stimulation for direct activation of thalamocortical relay neurons suppressed intrinsic firing activity through activation of presynaptic terminals. In contrast, suprathreshold stimulation suppressed the intrinsic firing in the soma, but generated efferent output at the stimulus frequency in the axon (McIntyre et al., 2004).
Taken together, this suggests that STN-HFS might decrease the activity of STN neurons by exciting presynaptic GABAergic afferent fibres and increase the activity of GPi neurons by exciting postsynaptic glutamatergic efferent fibres. Our data provide an additional argument supporting the hypothesis that STN-HFS excites presynaptic GABAergic axon terminals: (i) in 10/43 neurons the inhibition of neuronal activity occurred with a latency after the end of the stimulus artefact (Figs 1B, 2E and F) that complies with those of GABAergic inhibitory postsynaptic currents (Edwards et al., 1990) and (ii) the recovery kinetics of the firing rate after each stimulus pulse are also in line with such a mechanism. Since slice preparations are disconnected from their GABAergic axonal inputs, this hypothesis would also reconcile the results of an in vitro study showing a time-locked triggering of recurrent bursts of spikes by the electrical stimulus (Garcia et al., 2003).
Recovery of firing rate after the end of STN-HFS
Once STN-HFS has been stopped, the return to baseline firing rate between HFS and post1 occurred within 100 ms (Fig. 2G), i.e. spiking activity of STN neurons did not increase linearly through the entire post1 time interval. This contrasts with data obtained in anaesthetized rat and slice preparations, where the return to baseline firing rate after the end of STN-HFS took between 30 s and several minutes (Benazzouz et al., 2000; Beurrier et al., 2001; Garcia et al., 2003). However, a prolonged return of spiking activity to baseline firing rate exceeding more than several hundreds of milliseconds has not been observed in STN neurons of parkinsonian patients (Filali et al., 2004; Welter et al., 2004), in GPi neurons of awake MPTP-lesioned non-human primates (Hashimoto et al., 2003) and in GPi neurons following HFS of the same nucleus (Boraud et al., 1996; Bar-Gad et al., 2004). The differences might be related to anaesthesia in rodent studies or methodological particularities of slice preparations, as well as the recruitment of adjacent areas in rodents, given the small size of the STN that could imply additional mechanisms of action.
Impact of STN-HFS on correlated and oscillatory activity
While MPTP treatment increased oscillatory activity at an individual STN neuron level, the amount of correlated activity between pairs of STN neurons remained unchanged. However, when separating correlated non-oscillatory and oscillatory activity between these pairs increased oscillatory activity could be detected. Changes in oscillatory activity between normal and MPTP-lesioned conditions were mainly observed in the theta and alpha frequency band, while MPTP treatment induced only a small non-significant increase in oscillatory activity in the beta frequency band. This contrasts with the results of different studies that recorded local field potentials or unit activity in human STN (Brown et al., 2001; Levy et al., 2000, 2002a; Williams et al., 2002; Kühn et al., 2004), which suggest that increased oscillatory activity in the beta frequency band might be an important feature of Parkinson's disease. However, beta band oscillatory activity seems not to be a significant feature of the parkinsonian syndrome in MPTP-lesioned non-human primates, in which oscillations occur mainly at lower frequencies (Bergman et al., 1994; Nini et al., 1995; Raz et al., 1996, 2000, 2001; Heimer et al., 2002; Bar-Gad et al., 2004; Goldberg et al., 2004).
The present results provide new evidence that STN-HFS reduces abnormal oscillatory activity at an individual STN neuron level as well as correlated and abnormal oscillatory activity between pairs of STN neurons. Consequently, this could result in a reduction of abnormal oscillatory activity in the cortex–basal ganglia–cortex network. The recent finding that STN-HFS decreases abnormal oscillatory local field potential activity in human GPi further supports this hypothesis (Brown et al., 2004). Since abnormal synchronized oscillatory activity in the STN and GPi of MPTP-lesioned non-human primates and Parkinson's disease patients has been further related to symptoms of the illness (Bergman et al., 1994; Nini et al., 1995; Levy et al., 2000, 2002a, b; Raz et al., 2000; Brown et al., 2001; Williams et al., 2002; Brown, 2003; Silberstein et al., 2003; Kühn et al., 2004), the present data support the hypothesis that a reduction of abnormal synchronized oscillatory activity could underlie clinical efficacy of STN-HFS.
Initially, the resetting of STN neuron firing probability by the electrical stimulus (Fig. 2C) would rather be expected to increase synchronized oscillatory activity between pairs of STN neurons. However, this reset occurred with a period of 7.7 ms, which is much shorter than the observed period of the oscillations (∼8 Hz, period ∼125 ms) and beyond the bandwidth of our analysis (2–100 Hz). Moreover, assuming the resetting of firing rate to be the consequence of a transient GABAergic inhibition, different recovery periods of STN neurons might delay the appearance of synchronized oscillations, particularly if they are not generated locally.
Conclusion
Although the underlying mechanisms of action remain to be clarified, our study provides new evidence that STN-HFS decreases abnormal oscillatory activity in the STN. Consequently, this could result in a reduction of abnormal oscillatory activity in the whole cortex–basal ganglia–cortex network. Since abnormal synchronized oscillations are supposed to be at least partially at the origin of parkinsonism, the reduction of oscillatory activity might be as important as the impact on firing rate for the beneficial effects of STN-HFS.
W.M. was a Marie Curie Fellow of the European Community (HPMF-CT-2001-01300). The authors would like to thank E. Bezard for the helpful discussion and P. Ravenscroft for critical reading of the manuscript. This study was supported by the Centre National de la Recherche Scientifique, Université Victor Ségalen Bordeaux 2, Région Aquitaine, IFR de Neurosciences (INSERM N° 8; CNRS N°13) and by grants from the Fédération Française des Groupements des Parkinsoniens and the Fondation pour la Recherche Médicale.
References
Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing.
Bar-Gad I, Bergman H. Stepping out of the box: information processing in the neural networks of the basal ganglia.
Bar-Gad I, Ritov Y, Vaadia E, Bergman H. Failure in identification of overlapping spikes from multiple neuron activity causes artificial correlations.
Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6–tetrahydropyridine-treated primate in response to pallidal microstimulation.
Benazzouz A, Gross C, Feger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys.
Benazzouz A, Boraud T, Dubedat P, Boireau A, Stutzmann JM, Gross C. Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: a pilot study.
Benazzouz A, Gao DM, Ni ZG, Piallat B, Bouali-Benazzouz R, Benabid AL. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat.
Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease.
Benazzouz A, Tai CH, Meissner W, Bioulac B, Bezard E, Gross C. High-frequency stimulation of both zona incerta and subthalamic nucleus induces a normalization of basal ganglia metabolic activity in experimental parkinsonism.
Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism.
Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates.
Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons.
Bezard E, Boraud T, Bioulac B, Gross C. Involvement of the subthalamic nucleus in glutamatergic compensatory mechanisms.
Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6–tetrahydropyridine-lesioned macaque model of Parkinson's disease.
Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, et al. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function.
Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal globus pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey.
Boraud T, Bezard E, Bioulac B, Gross CE. Dopamine agonist-induced dyskinesias are correlated to both firing pattern and frequency alterations of pallidal neurons in the MPTP-treated monkey.
Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease.
Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease.
Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, et al. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson's disease.
Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. Microstimulation-induced inhibition of neuronal firing in human globus pallidus.
Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study.
Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus.
Garcia L, Audin J, D'Alessandro G, Bioulac B, Hammond C. Dual effect of high-frequency stimulation on subthalamic neuron activity.
Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H. Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials.
Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons.
Heimer G, Bar-Gad I, Goldberg JA, Bergman H. Dopamine replacement therapy reverses abnormal synchronization of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6–tetrahydropyridine primate model of parkinsonism.
Imbert C, Bezard E, Guitraud S, Boraud T, Gross CE. Comparison between eight clinical rating scales used for the assessment of MPTP-induced parkinsonism in the macaque monkey.
Kita H. Neostriatal and globus pallidus stimulation induced inhibitory postsynaptic potentials in entopeduncular neurons in rat brain slice preparations.
Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease.
Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance.
Lenz FA, Tasker RR, Kwan HC, Schnider S, Kwong R, Murayama Y, et al. Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic ``tremor cells'' with the 3–6 Hz component of parkinsonian tremor.
Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor.
Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease.
Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. Synchronized neuronal discharge in the basal ganglia of parkinsonian patients is limited to oscillatory activity.
Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease.
Maurice N, Thierry AM, Glowinski J, Deniau JM. Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus.
McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition.
Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism.
Pollak P, Benabid AL, Gross C, Gao DM, Laurent A, Benazzouz A, et al. Effects of high frequency stimulation of subthalamic nucleus in Parkinson's disease.
Pollak P, Krack P, Fraix V, Mendes A, Moro E, Chabardes S, et al. Intraoperative micro- and macrostimulation of the subthalamic nucleus in Parkinson's disease.
Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates.
Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6–tetrahydropyridine vervet model of parkinsonism.
Raz A, Frechter-Mazar V, Feingold A, Abeles M, Vaadia E, Bergman H. Activity of pallidal and striatal tonically active neurons is correlated in MPTP-treated monkeys but not in normal monkeys.
Salin P, Manrique C, Forni C, Goff LK. High-frequency stimulation of the subthalamic nucleus selectively reverses dopamine denervation-induced cellular defects in the output structures of the basal ganglia in the rat.
Silberstein P, Kühn AA, Kupsch A, Trottenberg T, Krauss JK, Wöhrle JC, et al. Patterning of globus pallidus local field potentials differs between Parkinson's disease and dystonia.
Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata.
Welter ML, Houeto JL, Bonnet AM, Bejjani PB, Mesnage V, Dormont D, et al. Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients.
Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, et al. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans.
Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, Feuerstein C, et al. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat.
Author notes
1Basal Gang, Laboratoire de Neurophysiologie, CNRS UMR 5543, Université Victor Ségalen, Bordeaux, 2Neurophysique et Physiologie du Système Moteur, CNRS UMR 8119, Université Paris V, Paris, France and 3Neurologische Klinik, Charité Campus Virchow-Klinikum, Humboldt Universitaet, Berlin, Germany