-
PDF
- Split View
-
Views
-
Cite
Cite
F. Joel Fodrie, Kenneth W. Able, Fernando Galvez, Kenneth L. Heck, Olaf P. Jensen, Paola C. López-Duarte, Charles W. Martin, R. Eugene Turner, Andrew Whitehead, Integrating Organismal and Population Responses of Estuarine Fishes in Macondo Spill Research, BioScience, Volume 64, Issue 9, September 2014, Pages 778–788, https://doi.org/10.1093/biosci/biu123
Close - Share Icon Share
Abstract
Syntheses of research spanning diverse taxa, ecosystems, timescales, and hierarchies are crucial for understanding the cumulative impacts of the Macondo oil spill in the Gulf of Mexico. Four years after the spill, responses of estuarine fishes to oil pollution have been studied at organismal through population levels, and there is an emerging mismatch between consistent negative impacts detected among individual organisms and absence of measurable negative impacts among populations. To reconcile this apparent contradiction, we draw on lessons learned from this and previous spills to consider two classes of mechanisms: factors obscuring negative population impacts despite known organismal responses (e.g., high spatiotemporal variability, offsetting food-web cascades, fishery closures, temporal lags) and factors dampening population-level costs despite known organismal responses (e.g., behavioral avoidance, multiple compensatory pathways). Thus, we highlight critical knowledge gaps that should form the basis of current and future oil-spill research priorities to assess ecosystem responses to basin-scale disturbance.
The 2010 Macondo well blowout challenged the integrity and function of the Gulf of Mexico (GOM) ecosystem at an unprecedented scale. GOM fisheries, although stressed even before the spill, remain among the most productive in the world (commercial harvest, 600,000 metric tons per year, worth $600,000,000 per year dockside; recreational harvest, 25,000 tons per year and 25,000,000 trips per year; Lellis-Dibble et al. 2008), and there has been widespread concern regarding the impact of basin-scale marine oil pollution on fishes and shellfishes that support economies throughout the region (McCrea-Strub et al. 2011). Some project the potential overall economic impact of lost or degraded fisheries in the GOM to be $8.7 billion by 2020 (Sumaila et al. 2012). Given the economic stakes, there is broad-based interest among researchers, government agencies, fishermen, tourism sectors, and the oil industry to consider how science will support natural resource damage assessment and restoration plans for GOM ecosystems.
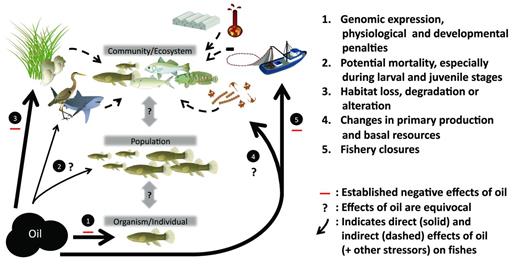
Petroleum hydrocarbons may injure fish through direct or indirect pathways, and via either acute or chronic effects (Peterson et al. 2003). These injuries may occur at organismal, population, or community levels, with symptoms that propagate or attenuate across these hierarchies (figure 1). Early life-history stages (e.g., embryo, larvae, juvenile) are often disproportionately susceptible to physiological stressors and have, therefore, been a primary focus of attention in previous spills. Indeed, embryos of pelagic species native to the GOM are sensitive to the toxic effects of spilled oil (Incardona et al. 2014). The direct oiling of eggs, embryos, or larvae in locations where surface slicks persisted or came ashore in the northern GOM could have killed animals through smothering of gas- and ion-exchange surfaces, ingestion of toxicants, or the loss of the epithelial mucus that protects fish from infections. As oil weathers, multiringed polycyclic aromatic hydrocarbons (PAHs) that accumulate in seawater can be toxic for fishes at even low concentrations (around 1 part per billion). For instance, embryos of Pacific herring (Clupea pallasii) and pink salmon (Oncorhynchus gorbuscha) exposed to Exxon Valdez (EV) oil exhibited elevated genetic damage, greater incidence of morphological deformities, reduced hatch sizes, premature hatching, and increased mortality (Kocan et al. 1996, Carls et al. 1999). For species that deposit benthic eggs or feed demersally, these injuries may last several years, as partially weathered oil becomes sequestered in sediments, thereby serving as a slow-release stressor (Culbertson et al. 2008). Manifestation of injuries can even be delayed across life stages: pink salmon fry appeared healthy following exposure to EV napthalenes but exhibited lower juvenile growth and subadult survivorship during the transition from estuarine to marine occupancy (Heintz et al. 2000).
Schematic of presumed and documented effects resulting from large-scale oil pollution at multiple hierarchical organization levels (i.e., organism, population, community) for fishes occupying estuarine environments. The symbols are courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (http://ian.umces.edu/symbols).
Negative impacts are not limited to the earliest life-history stages of fishes. Following the 1977 Tsesis oil spill in the Baltic Sea, flounder came into contact with oil mainly through the consumption of benthic filter feeders (Elmgren et al. 1983). Moreover, contaminated sediments may reduce the overall forage efficiency of benthic-feeding fishes (e.g., darter gobies, Gobionellus boleosoma; Gregg et al. 1997). PAH exposure can slow the metabolism and impair the swimming performance of juvenile and adult flatfishes and seabasses, which could subsequently affect foraging and predator-escapement rates (Claireaux et al. 2004, Gravato and Guilhermino 2009).
Oil pollution may also affect fishes indirectly, through food-web alterations or multiple-stressor syndromes (figure 1; Whitehead et al. 2013). These indirect effects are often associated with a notable time lag, because intermediate species or factors must be affected first with ensuing cascading effects through ecosystems. The diets of mummichog (Fundulus heteroclitus), for example, shifted following the Arthur Kill oil spill in 1990 from preferred prey, such as small shrimps, to less-nutritious detritus and algae (Brzorad and Burger 1994). In other polluted systems, these dietary shifts would be expected to result in reduced individual fitness through increased physiological costs or reduced vigilance to predators (Weis and Khan 1991) or other forms of narcosis (e.g., Gregg et al. 1997). Population models of the 1993 Pacific herring stock collapse in Prince William Sound suggest that sublethal effects among herring arising from oil ecotoxicity, combined with shifts in primary production, disease (viral hemorrhagic septicemia; Meyers et al. 1994), predation, or harvest pressure, interactively generated population-level instabilities that resulted in a delayed collapse years after the EV spill (Thorne and Thomas 2008). In contrast, acute mortality of sea otters (Enhydra lutris) after the EV oil spill did not result in the feared cascading effects on the abundance of sea urchins or the incidence of kelp overgrazing, which demonstrates that indirect effects are not easily predicted (Peterson et al. 2003).
Despite these anticipated impacts, there remains no clear consensus regarding the immediate or long-term responses of fishes to the 87-day Macondo oil spill of 2010. Unlike previous spills occurring in shallow coastal environments dominated by surface slicks destined for shore, the Macondo spill presented novel challenges for assessing ecological resistance and recovery across multiple scales and hierarchies. The location of the blowout at a water depth of 1.5 kilometers (km) and the partial midwater retention of emulsified oil have made the difficult problem of detecting ecological impacts of human-induced disturbance even more challenging, and it remains unclear how representative previous models of oil ecotoxicity will be for this disaster (Peterson et al. 2012).
Here, we review the available literature addressing the responses of estuarine fishes to the Macondo spill. We focus on fishes associated with estuarine shorelines dominated by salt marsh (predominantly, Spartina alterniflora) or seagrasses (i.e., Thalassia testudinum, Halodule wrightii, Syringodium filiforme, Ruppia maritima). This reflects a primary focus of the available peer-reviewed literature, the importance of early life-history health and survival, and the economic value of estuarine-dependent fishes. In addition, these vegetated habitats are known to have been degraded by the grounding of surface oil slicks (Silliman et al. 2012, Mendelssohn et al. 2012), which provides further reason to explore the responses of fishes in these environments.
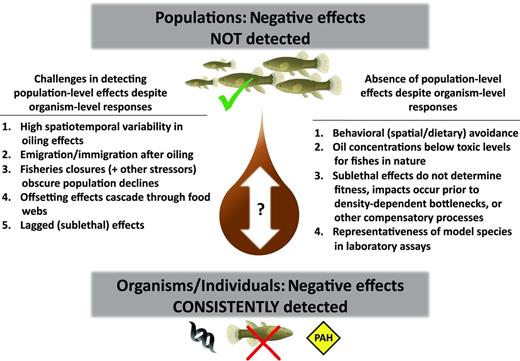
We searched the National Oceanic and Atmospheric Administration's Deepwater Horizon bibliography for all peer-reviewed literature relevant for gauging impacts of the Macondo spill (www.lib.noaa.gov/researchtools/subjectguides/dwh.html). We also searched multiple keyword combinations (e.g., oil spill*fish*GOM) using Web-based databases (e.g., Thomson Reuters Web of Knowledge) and supplemented this literature using published data sets known by the authors to contain information on marine oil spills. We found 16 papers in which the responses of estuarine fishes in the GOM to oil spills were explicitly examined at either an organismal (n = 10) or a population (n = 6) level (tables 1 and 2). Notably, studies at the organismal level have consistently documented negative effects of petroleum hydrocarbons on the ecophysiology of fishes, whereas studies at the population level have routinely failed to detect damages related to oil pollution. Below, we review the major findings of these studies and then consider potential mechanisms to account for this apparent paradox. We explore factors that could either obscure the detection of or simply dampen population-level effects despite known organismal responses (figure 2). These mechanisms highlight pressing gaps in our understanding of aquatic ecology in the northern GOM and guide the design of future research to explore the stability and recovery of estuarine ecosystems.
Potential mechanisms for the contrasting results (to date) of organismal (genomic, physiological, developmental) and population-level (densities, assemblage structure) investigations detailing the responses of fishes to the 2010 Macondo oil spill. The factors listed on the left highlight logistical and structural challenges in detecting population-level impacts following large-scale disturbances. The factors on the right are focused on why oil pollution may not have affected estuarine fishes at the population (or community) level, despite known responses at the organismal level. Abbreviation: PAH, polycyclic aromatic hydrocarbons. The symbols are courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (http://ian.umces.edu/symbols).
Characterization of studies of the organismal responses of estuarine fishes to Gulf of Mexico oil pollution.
| Citation . | Oil-spill context . | Organisms . | Lab or field . | Collection locations . | Genomic response . | Physiological response . | Morphological defects . | Increased mortality . | Population-level (fitness) impacts considered . |
|---|---|---|---|---|---|---|---|---|---|
| Ernst et al. 1977 | No. 2 fuel oil | Fundulus grandis | Lab | Texas | – | Yes | – | Yes | Presumed negative, not explicitly stated |
| Fucik et al. 1995 | Generic oil and Corexit | Atherinopsidae, Clupeidae, Sciaenidae | Lab | Western Gulf and Atlantic | – | – | – | Yes | Presumed negative, not explicitly stated |
| Gregg et al. 1997 | Diesel-fouled sediments | Gobionellus boleosoma | Lab | Louisiana | – | Reduced feeding | – | – | Not indicated |
| Whitehead et al. 2012 | Macondo | Fundulus grandis | Field | Louisiana, Mississippi, Alabama | Yes | Yes | – | – | Expected negative |
| De Soysa et al. 2012 | Macondo | Danio rerio (embryos) | Lab | – | – | – | Yes | – | Presumed negative, not explicitly stated |
| Garcia et al. 2012 | Macondo | Fundulus grandis | Field | Louisiana, Mississippi, Alabama | Yes | – | – | – | Presumed negative, not explicitly stated |
| Dubansky et al. 2013 | Macondo | Fundulus grandis (adults and embryos) | Lab and field | Texas, Louisiana, Mississippi, Alabama, Florida | Yes | Yes | Yes | – | Expected negative |
| Incardona et al. 2013 | Macondo | Danio rerio (embryos) | Lab | – | Yes | – | Yes | – | Presumed negative, not explicitly stated |
| Kuhl et al. 2013 | Macondo and Corexit | Fundulus grandis | Lab | – | – | – | – | Yes | Presumed negative, not explicitly stated |
| Crowe et al. 2014 | Macondo | Fundulus grandis | Lab | – | Yes | Yes | – | – | Presumed negative, not explicitly stated |
| Citation . | Oil-spill context . | Organisms . | Lab or field . | Collection locations . | Genomic response . | Physiological response . | Morphological defects . | Increased mortality . | Population-level (fitness) impacts considered . |
|---|---|---|---|---|---|---|---|---|---|
| Ernst et al. 1977 | No. 2 fuel oil | Fundulus grandis | Lab | Texas | – | Yes | – | Yes | Presumed negative, not explicitly stated |
| Fucik et al. 1995 | Generic oil and Corexit | Atherinopsidae, Clupeidae, Sciaenidae | Lab | Western Gulf and Atlantic | – | – | – | Yes | Presumed negative, not explicitly stated |
| Gregg et al. 1997 | Diesel-fouled sediments | Gobionellus boleosoma | Lab | Louisiana | – | Reduced feeding | – | – | Not indicated |
| Whitehead et al. 2012 | Macondo | Fundulus grandis | Field | Louisiana, Mississippi, Alabama | Yes | Yes | – | – | Expected negative |
| De Soysa et al. 2012 | Macondo | Danio rerio (embryos) | Lab | – | – | – | Yes | – | Presumed negative, not explicitly stated |
| Garcia et al. 2012 | Macondo | Fundulus grandis | Field | Louisiana, Mississippi, Alabama | Yes | – | – | – | Presumed negative, not explicitly stated |
| Dubansky et al. 2013 | Macondo | Fundulus grandis (adults and embryos) | Lab and field | Texas, Louisiana, Mississippi, Alabama, Florida | Yes | Yes | Yes | – | Expected negative |
| Incardona et al. 2013 | Macondo | Danio rerio (embryos) | Lab | – | Yes | – | Yes | – | Presumed negative, not explicitly stated |
| Kuhl et al. 2013 | Macondo and Corexit | Fundulus grandis | Lab | – | – | – | – | Yes | Presumed negative, not explicitly stated |
| Crowe et al. 2014 | Macondo | Fundulus grandis | Lab | – | Yes | Yes | – | – | Presumed negative, not explicitly stated |
Characterization of studies of the organismal responses of estuarine fishes to Gulf of Mexico oil pollution.
| Citation . | Oil-spill context . | Organisms . | Lab or field . | Collection locations . | Genomic response . | Physiological response . | Morphological defects . | Increased mortality . | Population-level (fitness) impacts considered . |
|---|---|---|---|---|---|---|---|---|---|
| Ernst et al. 1977 | No. 2 fuel oil | Fundulus grandis | Lab | Texas | – | Yes | – | Yes | Presumed negative, not explicitly stated |
| Fucik et al. 1995 | Generic oil and Corexit | Atherinopsidae, Clupeidae, Sciaenidae | Lab | Western Gulf and Atlantic | – | – | – | Yes | Presumed negative, not explicitly stated |
| Gregg et al. 1997 | Diesel-fouled sediments | Gobionellus boleosoma | Lab | Louisiana | – | Reduced feeding | – | – | Not indicated |
| Whitehead et al. 2012 | Macondo | Fundulus grandis | Field | Louisiana, Mississippi, Alabama | Yes | Yes | – | – | Expected negative |
| De Soysa et al. 2012 | Macondo | Danio rerio (embryos) | Lab | – | – | – | Yes | – | Presumed negative, not explicitly stated |
| Garcia et al. 2012 | Macondo | Fundulus grandis | Field | Louisiana, Mississippi, Alabama | Yes | – | – | – | Presumed negative, not explicitly stated |
| Dubansky et al. 2013 | Macondo | Fundulus grandis (adults and embryos) | Lab and field | Texas, Louisiana, Mississippi, Alabama, Florida | Yes | Yes | Yes | – | Expected negative |
| Incardona et al. 2013 | Macondo | Danio rerio (embryos) | Lab | – | Yes | – | Yes | – | Presumed negative, not explicitly stated |
| Kuhl et al. 2013 | Macondo and Corexit | Fundulus grandis | Lab | – | – | – | – | Yes | Presumed negative, not explicitly stated |
| Crowe et al. 2014 | Macondo | Fundulus grandis | Lab | – | Yes | Yes | – | – | Presumed negative, not explicitly stated |
| Citation . | Oil-spill context . | Organisms . | Lab or field . | Collection locations . | Genomic response . | Physiological response . | Morphological defects . | Increased mortality . | Population-level (fitness) impacts considered . |
|---|---|---|---|---|---|---|---|---|---|
| Ernst et al. 1977 | No. 2 fuel oil | Fundulus grandis | Lab | Texas | – | Yes | – | Yes | Presumed negative, not explicitly stated |
| Fucik et al. 1995 | Generic oil and Corexit | Atherinopsidae, Clupeidae, Sciaenidae | Lab | Western Gulf and Atlantic | – | – | – | Yes | Presumed negative, not explicitly stated |
| Gregg et al. 1997 | Diesel-fouled sediments | Gobionellus boleosoma | Lab | Louisiana | – | Reduced feeding | – | – | Not indicated |
| Whitehead et al. 2012 | Macondo | Fundulus grandis | Field | Louisiana, Mississippi, Alabama | Yes | Yes | – | – | Expected negative |
| De Soysa et al. 2012 | Macondo | Danio rerio (embryos) | Lab | – | – | – | Yes | – | Presumed negative, not explicitly stated |
| Garcia et al. 2012 | Macondo | Fundulus grandis | Field | Louisiana, Mississippi, Alabama | Yes | – | – | – | Presumed negative, not explicitly stated |
| Dubansky et al. 2013 | Macondo | Fundulus grandis (adults and embryos) | Lab and field | Texas, Louisiana, Mississippi, Alabama, Florida | Yes | Yes | Yes | – | Expected negative |
| Incardona et al. 2013 | Macondo | Danio rerio (embryos) | Lab | – | Yes | – | Yes | – | Presumed negative, not explicitly stated |
| Kuhl et al. 2013 | Macondo and Corexit | Fundulus grandis | Lab | – | – | – | – | Yes | Presumed negative, not explicitly stated |
| Crowe et al. 2014 | Macondo | Fundulus grandis | Lab | – | Yes | Yes | – | – | Presumed negative, not explicitly stated |
Characterization of studies of population-level responses of estuarine fishes in the Gulf of Mexico (GOM) following oil spills.
| Citation . | Oil-spill context . | Year of spill . | Assemblage surveyed . | Location . | Study design . | Density response . | Assemblage response . | Further description of results . |
|---|---|---|---|---|---|---|---|---|
| Rozas et al. 2000 | 3 spills, including Apex Houston spill | 1990, 1994, 1996 | Marsh-associated fishes | Texas | Stratified random with regression | None or positive | – | Stable or increasing densities (19 species) with increasing oil concentrations |
| Roth and Baltz 2009 | Unnamed 600-barrel spill | 2005 | Resident and transient marsh-associated nekton | Louisiana | BACI | None | None | Stable densities (10 species) after oiling; no change in assemblage |
| Fodrie and Heck 2011 | Macondo | 2010 | Seagrass-associated fishes | Louisiana, Mississippi, Alabama, Florida | Regional before–after comparisons | Positive | None | Stable or increasing densities after the oil spill; no change in assemblage |
| Chakrabarty et al. 2012 | Macondo | 2010 | 124 fishes | GOM | – | – | – | – |
| Moody et al. 2013 | Macondo | 2010 | Resident and transient marsh-associated nekton | Alabama | Single-site before–after | Few, temporary, negative | None | Temporary (12-month) decline in goby density and biomass; no change in assemblage |
| Able et al. 2014 | Macondo | 2010 | Resident marsh-associated fishes | Louisiana | Regional control–impact | None | None | No changes in densities or assemblage between unoiled and oiled sites |
| Citation . | Oil-spill context . | Year of spill . | Assemblage surveyed . | Location . | Study design . | Density response . | Assemblage response . | Further description of results . |
|---|---|---|---|---|---|---|---|---|
| Rozas et al. 2000 | 3 spills, including Apex Houston spill | 1990, 1994, 1996 | Marsh-associated fishes | Texas | Stratified random with regression | None or positive | – | Stable or increasing densities (19 species) with increasing oil concentrations |
| Roth and Baltz 2009 | Unnamed 600-barrel spill | 2005 | Resident and transient marsh-associated nekton | Louisiana | BACI | None | None | Stable densities (10 species) after oiling; no change in assemblage |
| Fodrie and Heck 2011 | Macondo | 2010 | Seagrass-associated fishes | Louisiana, Mississippi, Alabama, Florida | Regional before–after comparisons | Positive | None | Stable or increasing densities after the oil spill; no change in assemblage |
| Chakrabarty et al. 2012 | Macondo | 2010 | 124 fishes | GOM | – | – | – | – |
| Moody et al. 2013 | Macondo | 2010 | Resident and transient marsh-associated nekton | Alabama | Single-site before–after | Few, temporary, negative | None | Temporary (12-month) decline in goby density and biomass; no change in assemblage |
| Able et al. 2014 | Macondo | 2010 | Resident marsh-associated fishes | Louisiana | Regional control–impact | None | None | No changes in densities or assemblage between unoiled and oiled sites |
Abbreviation: BACI, before–after–control–impact.
Characterization of studies of population-level responses of estuarine fishes in the Gulf of Mexico (GOM) following oil spills.
| Citation . | Oil-spill context . | Year of spill . | Assemblage surveyed . | Location . | Study design . | Density response . | Assemblage response . | Further description of results . |
|---|---|---|---|---|---|---|---|---|
| Rozas et al. 2000 | 3 spills, including Apex Houston spill | 1990, 1994, 1996 | Marsh-associated fishes | Texas | Stratified random with regression | None or positive | – | Stable or increasing densities (19 species) with increasing oil concentrations |
| Roth and Baltz 2009 | Unnamed 600-barrel spill | 2005 | Resident and transient marsh-associated nekton | Louisiana | BACI | None | None | Stable densities (10 species) after oiling; no change in assemblage |
| Fodrie and Heck 2011 | Macondo | 2010 | Seagrass-associated fishes | Louisiana, Mississippi, Alabama, Florida | Regional before–after comparisons | Positive | None | Stable or increasing densities after the oil spill; no change in assemblage |
| Chakrabarty et al. 2012 | Macondo | 2010 | 124 fishes | GOM | – | – | – | – |
| Moody et al. 2013 | Macondo | 2010 | Resident and transient marsh-associated nekton | Alabama | Single-site before–after | Few, temporary, negative | None | Temporary (12-month) decline in goby density and biomass; no change in assemblage |
| Able et al. 2014 | Macondo | 2010 | Resident marsh-associated fishes | Louisiana | Regional control–impact | None | None | No changes in densities or assemblage between unoiled and oiled sites |
| Citation . | Oil-spill context . | Year of spill . | Assemblage surveyed . | Location . | Study design . | Density response . | Assemblage response . | Further description of results . |
|---|---|---|---|---|---|---|---|---|
| Rozas et al. 2000 | 3 spills, including Apex Houston spill | 1990, 1994, 1996 | Marsh-associated fishes | Texas | Stratified random with regression | None or positive | – | Stable or increasing densities (19 species) with increasing oil concentrations |
| Roth and Baltz 2009 | Unnamed 600-barrel spill | 2005 | Resident and transient marsh-associated nekton | Louisiana | BACI | None | None | Stable densities (10 species) after oiling; no change in assemblage |
| Fodrie and Heck 2011 | Macondo | 2010 | Seagrass-associated fishes | Louisiana, Mississippi, Alabama, Florida | Regional before–after comparisons | Positive | None | Stable or increasing densities after the oil spill; no change in assemblage |
| Chakrabarty et al. 2012 | Macondo | 2010 | 124 fishes | GOM | – | – | – | – |
| Moody et al. 2013 | Macondo | 2010 | Resident and transient marsh-associated nekton | Alabama | Single-site before–after | Few, temporary, negative | None | Temporary (12-month) decline in goby density and biomass; no change in assemblage |
| Able et al. 2014 | Macondo | 2010 | Resident marsh-associated fishes | Louisiana | Regional control–impact | None | None | No changes in densities or assemblage between unoiled and oiled sites |
Abbreviation: BACI, before–after–control–impact.
Organism-level effects associated with the Macondo oil spill
Controlled laboratory investigations or field collections combined with molecular, physiological, or developmental assessments are commonly employed to determine the acute and chronic injuries that organisms face following oil spills (table 1). In particular, these tests are designed to establish cause–effect relationships between organismal health and exposure to oil contamination in at-risk habitats (Whitehead 2013).
The Gulf killifish (Fundulus grandis) has been used as a sentinel species of Macondo oil spill ecotoxicity because of its close life-history association with salt marshes, its likely site fidelity, and the pollution sensitivity of an Atlantic congener (F. heteroclitus). Whitehead and colleagues (2012) conducted a before–after–control–impact (BACI) field experiment, in which serial collections of adult Gulf killifish were made from Louisiana (oiled) and from Mississippi and Alabama (unoiled references) before the Macondo oil reached shore, during the peak of shoreline oiling, and months after visible surface oil had disappeared. At the oiled site in Louisiana but not at the unoiled reference sites in Mississippi and Alabama, Gulf killifish responded through increased expression of genes that are transcriptionally regulated by pollutant-activated aryl hydrocarbon receptors (AHRs) and that are diagnostic for developmental abnormalities in exposed embryos. Other transcriptional changes observed at the oiled field site, coincident with oil contamination, included genes responsible for stress responses (DNA repair), apoptosis, estrogen signaling, and immune response. Within the gills of Gulf killifish at the oiled site, cytochrome CYP1A protein (used in xenobiotic metabolism) production was elevated. The increased CYP1A levels correlated with structural damage of gill epithelia, including hyperplasia, thus decreasing the total effective surface area for gas and waste exchange. Garcia and colleagues (2012) employed a high-throughput sequencing approach to quantify mRNA expression and showed that AHR and cytochrome P450 genes were upregulated in Gulf killifish collected at oil-affected sites in Louisiana. These data also revealed that genes associated with hypoxic stress (perhaps in response to gill impairment) and immune response were differentially regulated in exposed individuals. More than a year after the spill, upregulation of cytochrome P450 protein in gill, liver, intestine, and kidney tissues was recorded in fishes collected at oiled sites in Louisiana, but not unoiled reference sites in Texas, Mississippi, and Alabama (Dubansky et al. 2013). Further laboratory trials have confirmed that CYP1A upregulation was correlated with oxidative stress (i.e., radicals formed during PAH detoxification; Crowe et al. 2014).
Similar negative physiological outcomes were found when developing embryos were incubated in field-collected water or over sediments collected from contaminated versus unoiled field sites (for a pre-Macondo study with similar findings, see Ernst et al. 1997). Whitehead and colleagues (2012) exposed fertilized Gulf killifish embryos to Louisiana (oiled) or Mississippi (unoiled) marsh waters for 24 days and found increased CYP1A production in fish exposed to Macondo-derived PAHs (at relatively low concentrations). Although the anticipated cardiovascular abnormalities were not observed in these embryos, adults collected from oil-affected sites showed an altered regulation of genes associated with blood and blood vessel maintenance (Whitehead et al. 2012). Gulf killifish embryos exposed for 21 days to oiled sediments collected more than a year after the spill were also characterized by upregulation of CYP1A, as well as reduced hatch rates, a smaller size at hatch, reduced heart rates, and poor vigor (Dubansky et al. 2013).
Adult Gulf killifish were also used as a model species to assess the environmental impacts of chemical dispersants (e.g., Corexit) applied to reduce the environmental half-life of spilled oil. Alarmingly, oil treated with dispersant in laboratory trials was consistently more lethal than undispersed oil, and Corexit alone could induce complete mortality within the first week of its application (Kuhl et al. 2013). Notably, PAHs are more soluble in fresh water than in salt water, which suggests a potential for greater deleterious impacts in estuarine systems than in offshore waters.
The potential ecotoxicity of oil for GOM fishes has been demonstrated across several families, including the Cyprinidae, Atherinopsidae, Clupeidae, Sciaenidae, and Fundulidae. Although it is not native to the GOM, the zebrafish (Danio rerio) is regularly used as a model species for toxicology testing (Van Veld and Nacci 2008). Zebrafish embryos and larvae incubated in baths containing the water-accumulated fraction (WAF) of Macondo oil exhibited neural crest-cell defects, craniofacial deformities, and circulatory impairment, as well as errors in programmed cell death, biomechanical function, and sensory abilities (de Soysa et al. 2012). Macondo oil that was both weathered and fresh and EV oil were all equally toxic for zebrafish embryogenesis via cardiotoxicity, CYP1A expression, and phototoxicity (Incardona et al. 2013). Before the Macondo spill, Fucik and colleagues (1995) considered the effects of generic western and central GOM oil and Corexit dispersant on inland silversides (Menidia beryllina), Atlantic menhaden (Brevoortia tyrannus), spot (Leiostomus xanthurus), and red drum (Sciaenops ocellatus). For all four species and regardless of the oil source, WAF exposures significantly decreased hatch success, as well as early (less-than-96-hour) survival of all species except red drum.
Collectively, these data demonstrate impacts for multiple individual-level vital rates and, therefore, predict significant population-level fitness consequences for fishes exposed to oiled water or sediments in estuarine systems of the northern GOM (table 1). The toxic components of oil were bioavailable to resident fish, and exposed fish revealed biochemical responses consistent with exposure for more than a year.
Population- and assemblage-level effects associated with the Macondo oil spill
In five published studies, the response of marsh- or seagrass-associated fishes was explored at population and assemblage levels following the Macondo spill (n = 3) or in reference to smaller spills (n = 2) in the northern GOM (table 2). The distribution of nearly all species included in these studies intersected the trajectory of spilled Macondo oil (Chakrabarty et al. 2012). A combination of regression, before–after comparisons (multiple and single sites), control–impact comparisons, and BACI designs were employed to examine the immediate consequences of hydrocarbon pollution.
Three separate oil spills affected salt-marsh habitat within Galveston Bay, Texas, during the 1990s. Over 2,700,000 liters of oil were spilled because of ship–barge collisions (1990; partially refined crude oil), pipeline ruptures (1994; diesel and crude oil), and barge equipment failures (1996; fuel oil). Subsequently, Rozas and colleagues (2000) sampled the interface between salt marsh and open water at 100 stations (oiled and reference sites in a stratified random approach) in Galveston Bay during 1995 and 1996 to survey fishes and collect marsh sediments for hydrocarbon analyses. The catch rates for 10 out of 10 fish species (members of Gobiidae, Clupeidae, and Sciaenidae) were unrelated to gradients in the concentration of total petroleum hydrocarbons, which were generally low throughout their sampling sites (75% were lower than 200 parts per million) and within background levels observed in urbanized estuaries.
The responses of marsh-associated fishes were also evaluated in Barataria Bay, Louisiana, following a 100,000-liter spill in 2005 that interrupted an ongoing faunal survey. Roth and Baltz (2009) employed drop traps in a BACI design to investigate the response of mobile nekton in the months following the grounding of oil on a marsh island (Mendicant Island). A marginally significant time × site interaction (p = .09) suggested that oiling did affect (i.e., a 3.5% decrease) the catch rates of all fishes at the control (18.2 individuals per trap) versus oiled sites (17.6 individuals per trap). At the individual species level, however, the catch rates of the control and affected sites were not different. The most common species found at both affected and control sites belonged to the Gobiesocidae, Sciaenidae, Gobiidae, Engraulidae, and Cynoglossidae because oiling had no detectable effect on assemblage composition.
Surveys of fishes residing in seagrass and salt-marsh habitats after the Macondo spill showed no measurable impacts on the integrated survival of eggs, larvae, and juveniles. Fodrie and Heck (2011) constructed a 5-year (2006–2010) trawl survey data set (12 sites from the Chandeleur Islands, Louisiana, to Saint Joseph Bay, Florida) to investigate early-stage survival of fish species inhabiting seagrass habitat in the months after the spill. Although many of these seagrass-associated fishes spawned during spring and summer and produced larvae vulnerable to oil-polluted water, overall and species-by-species catch rates were higher in 2010, after the spill (approximately 2000 fishes per km towed), than in the previous 4 years (about 1000 fishes per km towed). Twelve of the 20 most commonly encountered fishes were characterized by significantly higher catch rates in 2010 than in 2006–2009, whereas the remaining 8 taxa had prespill catch rates that were statistically indistinguishable from postspill catches. Furthermore, the species composition and diversity of juvenile fishes were unaltered in the months following the Macondo spill.
Alabama beaches were among the first and most heavily oiled following the Macondo spill, and light oiling of Alabama marshes was reported throughout the summer of 2010 (Beazley et al. 2012), although neither visible oiling nor detectable PAHs were recorded at the Mississippi and Alabama sites visited by Whitehead and colleagues (2012). The effects of this disturbance on resident and transient marsh nekton were investigated by Moody and colleagues (2013), who conducted fyke-net sampling before (2009) and after (2010–2011) the spill. Catches of resident species (Fundulidae, Poeciliidae, Cyprinodontidae) were similar before (June catch rates, around 5 individuals per tidal cycle) and after (about 4 individuals per tidal cycle in 2010, approximately 8 individuals per tidal cycle in 2011) the Macondo spill. The abundance of gobies (Gobiidae) did decline in 2010 (approximately 1 individual per tidal cycle) relative to 2009 (around 3 individuals per tidal cycle) and then rebounded in 2011 (about 3 individuals per tidal cycle). Goby dry-weight biomass, however, remained depressed in both postspill sampling years (about 0.1–0.2 gram per tidal cycle in 2010–2011 versus about 0.6 gram per tidal cycle in 2009). Although the density of the most common invertebrate (Palaemonetes pugio) did decrease after the spill, the overall community composition during the sampled years was statistically indistinguishable.
Barataria and Terrebonne Bays, in Louisiana, were among the most heavily oiled salt marshes along the northern GOM coast following the spill. Within these bays, Able and colleagues (2014) sampled multiple paired oiled and unoiled sites in 2012–2013 and documented no differences in the densities, sizes, or assemblage structures of seven Cyprinodontiform fishes (including F. grandis). Although Able and colleagues (2014) acknowledged several confounding factors, such as microhabitat differences among sites and the absence of before-spill information, these data exemplify the general mismatch between organismal and population-level assessments of oil-spill impacts despite significant geographic overlap among studies. Therefore, an important step for guiding injury assessment in the GOM is uncovering the logistical and biological factors that contribute to this disconnect.
Factors obscuring population-level responses despite organism-level ecotoxicity
Estuarine fishes are, in general, characterized by high spatial and temporal population variability, which imposes inherent challenges in isolating the effects of oil pollution in field assessments from other environmental factors.
Spatiotemporal variability
For these “noisy” populations, planned perturbations that allow for adequate sampling designs are exceedingly rare, and in the case of oil spills, even BACI designs are generally not able to anticipate the timing and location of disturbance far enough in advance to sufficiently constrain variability associated with complex recruitment and food-web oscillations (Underwood 1994). As a result, many attempts to detect environmental damages simply do not have sufficient statistical power to reject a null hypothesis even when it is false (Peterson et al. 2001), nor can these studies mechanistically connect observed decreases in densities following anthropogenic disturbance with any specific event. Natural variability at the population level is significantly greater than for individual-based parameters (Osenberg et al. 1994), which potentially contributes to the apparent disconnect between organismal and population-level studies in the wake of the Macondo spill.
Although variability at the population level promotes challenges for assigning cause-and-effect relationships in ecotoxicological damage assessments, community composition is typically more stable and can, therefore, be a more reliable proxy of ecosystem resilience (Osenberg et al. 1994). Therefore, it is notable that in each GOM study in which the effects of oil pollution on fish assemblage patterns were examined using multivariate approaches, detectable impacts were negligible (table 2). Indeed, across a broad spectrum of taxa, only one taxon among all the species examined exhibited a significant decrease in abundance (table 1). Conversely, statistical power was great enough to reveal a significant increase in population densities for several taxa (e.g., Fodrie and Heck 2011). These positive density responses may, themselves, be an indication of disturbance if changes in population age structure released juveniles from competition or predation from older or larger individuals (Brzorad and Burger 1994; sensu Moody et al. 2013 for gobies).
Movement
Shoreline cleanup assessments (i.e., “SCAT” surveys) and chemical analyses of sediments show that the grounding of oil was highly patchy (Mendelssohn et al. 2012), and oiling at the marsh–water interface could vary from heavy to absent over 100-meter scales. For many fishes using salt-marsh or seagrass habitat, movements across diel cycles, seasons, and life stages occur over hundreds of meters and even kilometers. For instance, pinfish (Lagodon rhomboides) is a common estuarine fish, and daily movement can range over 10–50 meters along marsh–seagrass interfaces (Irlandi and Crawford 1997). Even-larger-scale migrations are possible for species that eventually make ontogenetic shifts to other (offshore) habitats. Therefore, immigration from unaffected clean patches could offset local changes in densities within oiled patches, such that catch-per-unit-effort data show no differences among sites. Roth and Baltz (2009) specifically cited immigration into affected sites from unoiled marshes as a potential mechanism for why they observed no differences in fish densities in oiled marshes on Mendicant Island, Louisiana.
Fishery closures
The National Marine Fisheries Service enacted large-scale fishery closures throughout the northern GOM exclusive economic zone in May 2010 in response to food-safety concerns (Ylitalo et al. 2012). These closures persisted throughout the year, and typical harvests and bycatch mortalities were effectively eliminated for at least 6 months, with potential increased survival across a broad range of species (e.g., Diamond et al. 2000). Although rigorous tests regarding the release from fishing pressure are confounded by multiple stressors and environmental noise, Fodrie and Heck (2011) considered the evidence of fishing release within seagrass systems. Spotted sea trout (Cynoscion nebulosis), for example, spawn during summer, and many adult individuals are typically removed by recreational fishing before reproducing. In 2010, when the harvest of spawning adults was precluded, catch rates of juvenile spotted sea trout in Louisiana and Mississippi were an order of magnitude higher than during the previous 4 years. Therefore, the effects of fishing closures were superimposed on the acute effects of oil pollution throughout the northern GOM, with potentially equal or larger positive effects on the abundances of some estuarine (juvenile) fishes in the aftermath of the spill.
Offsetting food-web effects
For many species, oil pollution may affect individuals and populations indirectly through food-web interactions propagating up or down trophic levels or through gross alterations of the environment (figure 1). Food-web and habitat alterations seemed pervasive in the aftermath of the Macondo spill that could ultimately have negative, positive, or offsetting effects on local and regional abundances of fishes. Stable isotope ratios in plankton have confirmed that oil entered the coastal food web in the summer of 2010 (Graham et al. 2010), and mesocosm trials indicate that phytoplankton taxa such as diatoms, euglenophytes, and chlorophytes may have increased in relative (if not total) abundance in oil-contaminated coastal waters (Gilde and Pinckney 2012). Changes in the assemblages of primary producers and zooplankton likely affected the survival of fish eggs and larvae through an impairment of resource acquisition (presumed negative effects) and avoidance of predators in the water column (presumed positive effects). Benthic infauna, including polychaetes, oligochaetes, and meiofauna, respond positively to oil enrichment, which, for many fishes, likely increases available prey resources (DeLaune et al. 1984, Brzorad and Burger 1994). However, it remains questionable whether benthic-feeding fishes forage effectively within contaminated sediments (Gregg et al. 1997). Within salt-marsh and seagrass ecosystems, shrimps, crabs, insects, and spiders are all highly sensitive to PAH toxicity and exhibited short-term decreases in densities following oiling, with recovery apparent by the summer of 2011 (McCall and Pennings 2012, Moody et al. 2013). Therefore, food sources for fishes—but also potential intraguild predators—were altered with latent cascading effects on the population ecology of fishes in oil-affected areas. At higher trophic levels, oil pollution likely affected bird-egg hatching success (Finch et al. 2011) and could also have interacted with thermal stress to increase neonatal dolphin (Tursiops truncatus) mortality (Carmichael et al. 2012). A reduction in bird or dolphin foraging would ultimately decrease natural mortality of some estuarine fishes and would potentially offset, in part, the detectable, direct injuries associated with oil toxicity.
Lagged effects
The importance of sublethal and chronic effects following oil spills is well appreciated (Peterson et al. 2003) but may not have been detectable in species abundance surveys conducted within 1–2 years after the Macondo spill. As Pacific herring exemplify following the EV spill, populations can harbor instabilities over protracted periods that may eventually result in delayed collapses (Thorne and Thomas 2008). Fishery production in the GOM is tightly linked to coastal vegetated habitats that serve as spawning habitat, foraging areas, or nursery grounds (Peterson and Turner 1994). Salt-marsh and seagrass habitat loss associated with oiling may have negative effects on regional productivity over multiple generations (Mendelssohn et al. 2012), although such losses may not cause detectable site-specific decreases in fish density in the short term. Beyond habitat loss, oil contamination in sediments can remain elevated for decades in some habitats, with subtle, long-term effects on the fitness of sediment-associated species (Culbertson et al. 2008).
Factors dampening population-level responses despite organismal ecotoxicity
Despite the significant lethal and sublethal threats posed by oil pollution, the life histories of estuarine fishes in the GOM may have promoted avoidance behaviors or compensatory mechanisms that reduced the overall population impacts of the Macondo spill in salt-marsh and seagrass habitats.
Behavioral avoidance
Many fishes are highly mobile and are likely capable of fleeing oil-affected shorelines, given the scales and spatial gradients of disturbance in coastal habitats following the Macondo spill. For marine fishes, the ability to seek refugia confers resilience against the effects of hydrocarbon pollution associated with offshore petroleum production platforms, despite quantifiable impacts for less-mobile invertebrates (e.g., crustaceans, echinoderms, other nonselective deposit feeders; Peterson et al. 1996). Indeed, flatfishes and sciaenids (e.g., spot, Leiostomus xanthurus) are capable of detecting and avoiding heavily oiled sediments, although they do not necessarily avoid lightly oiled sediments or food items (Moles et al. 1994, Hinkle-Conn et al. 1998). Furthermore, long-term periodic exposures to hydrocarbons in regions with natural background seepage such as the northern GOM may prime adaptive avoidance behaviors or tolerance in resident species (sensu Rozas et al. 2000, Van Veld and Nacci 2008).
Dilution
A significant fraction of the oil released from the Macondo well did not rise to the surface and enter coastal environments (Peterson et al. 2012). As the novel impacts of this spill in the deep sea are realized, estuarine systems seem less affected relative to early concerns promoted by previous marine oil spills. Still, significant amounts of oil reached coastal ecosystems in patches—especially Louisiana salt marshes—and at concentrations high enough to affect fishes. PAH concentrations in the coastal waters of Louisiana, Mississippi, Alabama, and Florida were elevated throughout 2010 (more than 1 part per billion; Allan et al. 2012), which mirrored surface-oil trajectories observed along the coastline. Several hundred kilometers of salt marsh were visibly oiled, and biogeochemical analyses of the sediments have confirmed the activity of oil-degrading microorganisms within these marshes (Beazley et al. 2012). Despite low hydrocarbon concentrations in the water or fish tissues of coastal Louisiana, Whitehead and colleagues (2012) documented complex genomic and physiological responses of Gulf killifish to the spill. These data confirm that oil reached coastal environments at concentrations sufficient to cause biological responses in resident fishes and that dilution cannot explain the divide between organismal and population-level findings.
Compensatory processes
Density-mediated responses in vital rates, such as juvenile and adult survival and growth rates, may often be sufficient to overcome the impacts of oil exposure, which may result in little change at the population level. Such compensatory responses are frequently quite strong, and they underlie the resilience of marine fish populations to additional mortality sources, such as harvest (Neubauer et al. 2013). For example, a meta-analysis of stock–recruitment relationships (i.e., the survival rate between egg and juvenile stages) showed that fish families that are well represented in GOM estuaries (Sciaenidae and Pleuronectidae) are capable of significant surplus production (Myers et al. 1999). Indeed, even when adult biomass has been reduced to 20% of carrying capacity, these stocks are capable of producing 80% of the recruitment (i.e., surviving eggs and juveniles) that adult biomass functioning at carrying capacity would generate. The mechanisms underlying this compensatory increase in egg to juvenile survival are poorly understood but likely involve reduced competition for food and predation refuges. Even highly effective targeted removals of adult fish can fail to reduce recruitment, so it is perhaps not surprising that oiling impacts at the population level are much weaker than those observed at the individual level.
Representativeness of controlled laboratory trials
Press experiments in which fishes are exposed to weathered oil or WAFs may underestimate or overestimate ecotoxicological risk. For instance, the chronic aspects of oil contact are often not evaluated in these trials and, therefore, these studies do not account for potential total harm (Peterson et al. 2003). Relative to the absence of population-level effects in the literature (table 2), model species, such as Gulf killifish and zebrafish, potentially fail to accurately reflect the likelihood of oil contact or impacts for other taxa, given interspecific variation in longevity, microhabitat use, diet, mobility, and stress tolerance (e.g., killifishes are relatively tolerant of salinity, temperature, and oxygen fluctuations but are relatively sensitive to organic toxicants; Van Veld and Nacci 2008). In addition, choices regarding hydrocarbon concentrations, trial durations, and weathering factors in oil-exposure assays may all beget laboratory-based conclusions regarding oil ecotoxicity, even if they fail to reflect field conditions. That aside, concerns of publication bias against negative results (no difference) in laboratory trials are minimal, given the high-profile nature of the Macondo spill. Furthermore, field-based assays have complemented and expanded the findings from laboratory trials demonstrating consistent genomic and physiological effects, which suggests that laboratory artifacts are not a major concern.
Recommendations for ongoing and future research
Cumulatively, the mismatch between organismal and population-level studies of ecotoxicology in the GOM highlights multiple—potentially interacting—logistical challenges and ecological data needs for improved assessments regarding the consequences of basin-scale oil pollution. First, development and integration of long-term observation networks recording basic physiochemical and biological data appear essential to implement BACI surveys with adequate statistical power to evaluate future environmental (oil- and other-stressor-related) impacts at the population level. Second, population-level surveys should incorporate collections of individuals to simultaneously investigate genomic, physiological, and demographic (growth, reproduction) responses. The integration of demographic and abundance measures is particularly important for gauging sublethal impacts, indirect effects, and the potential for lagged responses of estuarine fishes. Dynamic energy budget theory provides a coherent framework for integrating suborganismal, organismal, fitness, and potential demographic effects of stressors (Nisbet et al. 2000). This approach also allows researchers to consider whether resident, seasonally resident, and transient estuarine fish life histories are equally affected by oil pollution. Third, there is a great need for information regarding many aspects of the basic ecology and early life-history dynamics of estuarine fishes. For instance, there is a dearth of information on the movements, diet, habitat dependency, and longevity of most species, including the Gulf killifish, which has been used as a sentinel of oil pollution at both organismal and population levels. These data gaps hinder any attempt to evaluate the potential for behavioral, physiological, or demographic compensation that could determine the extent of pollution impacts. Furthermore, the efficacy of proposed restoration plans may be weakened by uncertainty regarding the basic ecology of harvested or endangered species. Notably, these research priorities already align with the rationale and objectives of the Magnuson–Stevens Fishery Conservation and Management Acts. We conclude that industry-funded trusts (e.g., BP penalties) and natural resource agencies should focus attention on research that will address the needs cited above, thereby allowing improved assessments of why individuals and populations have or have not changed in response to the Macondo oil spill and might do so in response to future environmental disasters.
Major financial support was provided by BP and the Gulf of Mexico Research Initiative through the Coastal Waters Consortium at the Louisiana Universities Marine Consortium. Financial sources played no role in the design or interpretation of this review. Abigail Poray and three anonymous reviewers provided helpful editorial feedback.