-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher A. Nicolai, James S. Sedinger, David H. Ward, W. Sean Boyd, Mate loss affects survival but not breeding in black brant geese, Behavioral Ecology, Volume 23, Issue 3, May-June 2012, Pages 643–648, https://doi.org/10.1093/beheco/ars009
Close - Share Icon Share
Abstract
For birds maintaining long-term monogamous relationships, mate loss might be expected to reduce fitness, either through reduced survival or reduced future reproductive investment. We used harvest of male brant during regular sport hunting seasons as an experimental removal to examine effects of mate loss on fitness of female black brant (Branta bernicla nigricans; hereafter brant). We used the Barker model in program MARK to examine effects of mate loss on annual survival, reporting rate, and permanent emigration. Survival rates decreased from 0.847 ± 0.004 for females who did not lose their mates to 0.690 ± 0.072 for birds who lost mates. Seber ring reporting rate for females that lost their mates were 2 times higher than those that did not lose mates, 0.12 ± 0.086 and 0.06 ± 0.006, respectively, indicating that mate loss increased vulnerability to harvest and possibly other forms of predation. We found little support for effects of mate loss on fidelity to breeding site and consequently on breeding. Our results indicate substantial fitness costs to females associated with mate loss, but that females who survived and were able to form new pair bonds may have been higher quality than the average female in the population.
INTRODUCTION
Monogamy is the default mating system for most birds (Gowaty and Mock 1985). Long-term monogamy is widespread in birds and is associated with advantages of having a mate before the breeding season begins and during breeding (Choudhury 1995; Black 1996; McNamara and Forslund 1996), although mate change is widespread among monogamous species (Black 1996; Cézilly et al. 2000). Virtually all assessments of the advantages of monogamy across several avian species have been restricted to the reproductive period (Choudhury 1995; Black 1996; van de Pol et al. 2006), which reflects the difficulty of estimating survival when individuals can disperse outside the study system (Burnham 1993) after mate loss (Forero et al. 1999). Yet, it is certainly possible that if monogamy is beneficial for reproduction because paired individuals have greater access to resources, mate loss could reduce survival (McNamara and Forslund 1996) because of reduced access to food (Black 2001) or because individuals disperse after mate loss.
Although having a mate is essential for breeding, substantial evidence indicates that specific attributes of an individual's mate further affect fitness. Seabird pairs that are more similar to each other than average (assortitavely mated) are more likely to fledge chicks (Bridge and Nisbet 2004). Barnacle goose (Branta leucopsis) pairs matched in size or age were more likely to fledge young than less well-matched pairs (Black et al. 2007). Annual reproductive success (van de Pol et al. 2006) and fitness (Black 2001) both increase with the duration of pair bonds, suggesting that pairs better coordinate their behaviors as the duration of their pair bond increases. Loss of a mate, thus, could result in a reduction in fitness, even for individuals that are able to acquire another mate.
Among capital breeders (Drent and Daan 1980), nutrient storage before and during spring migration is essential for reproduction (Ankney and MacInnes 1978; Ebbinge and Spaans 1995; Morrison and Hobson 2004). In geese, pairs with offspring are dominant to other pairs, which are in turn dominate to single individuals in winter flocks (Raveling 1970; Black et al. 2007), enhancing the ability of females in family groups to acquire nutrients required for breeding. Social benefits of family groups are sufficiently strong that mate loss may produce effects that carryover into future years even for females that can acquire mates. Female black brant that did not breed in 1 year were also less likely to do so the next (Sedinger et al. 2008), consistent with being in a family group in 1 year positively influencing breeding effort the next year.
Mates are essential for adult female geese to acquire nutrient reserves before breeding (McLandress and Raveling 1981b; Akesson and Raveling 1982; Lamprecht 1987) because males provide vigilance and defend foraging patches allowing females to feed undisturbed. Indeed, having a mate is so important to reproductive success in geese that females do not undergo the physiological changes necessary for breeding if they do not have a mate (Black 2001). Male geese are, however, not essential for completing incubation or brood rearing (Prevett and MacInnes 1980; Cooke et al. 1981; McLandress and Raveling 1981a; Martin et al. 1985; Owen et al. 1988; LeSchack et al. 1998). Future costs to a female of incubating a clutch or rearing a brood alone has yet to be assessed, however. Divorce rates in geese are low (<0.05; Ens et al. 1996), suggesting that costs, including reduced survival, of finding new mates are substantial in relation to potential lifetime reproduction (Choudhury 1995; McNamara and Forslund 1996).
No study has yet examined survival costs of mate loss in long-lived monogamous birds. Absence of such studies results in part from the difficulty of distinguishing between mortality and dispersal away from a breeding area. Burnham (1993) and Barker (1997) capture–mark–recapture (CMR) models use encounters at the local scale (e.g., breeding location) and at the global scale (e.g., ring recoveries) to separate permanent emigration from mortality, thereby allowing estimation of true survival and fidelity to a breeding area. We used human harvest of brant during a long-term study of uniquely marked individuals to estimate the effects of mate loss on reproductive success, fidelity to the breeding area, and true survival of adult female brant. Adult female brant do not disperse from their breeding colony once they have nested there (Sedinger et al. 2008), so emigration was synonymous with nonbreeding. Because of the importance of mates for access to resources, we predicted that mate loss would affect both survival and reproductive success in brant.
MATERIALS AND METHODS
Field methods
Brant are long-distance migrants that breed in high latitude (>60° N) coastal habitats from the mid-Canadian arctic, west to Russia, and south to the Yukon–Kuskokwim Delta, Alaska (Reed et al. 1998). Brant winter in coastal lagoons from the Alaska peninsula in the north, to mainland Mexico in the south (Reed et al. 1998; Ward et al. 2004). We conducted the nesting component of this study at the Tutakoke River brant colony (hereafter TRC; Sedinger et al. 1993) on the Yukon–Kuskokwim Delta, Alaska from 1987 through 2005. Winter resighting efforts occurred during winter from 1989 through 2006 at coastal lagoons primarily in Mexico, California, and British Columbia (Ward et al. 2004). Pair status and mate identity only occurred at TRC because pairs are easily identifiable, whereas in the winter, aggregations are dense and dynamic.
Adult and young brant were captured during the adult remigial molt by herding them into corral traps in late July 1986 through 2007 (Sedinger et al. 1997). On capture, individuals were fitted with a standard U.S. Geological Survey (USGS) metal tarsal ring and a uniquely coded plastic tarsal ring (2.5 cm tall). We measured diagonal tarsus using dial calipers (±0.1 mm; Dzubin and Cooch 1992) for most adult brant (≥2 years old).
Forty-nine random 50 m radius plots were monitored throughout the TRC from egg laying through hatch to record clutch size and nest initiation dates. Marked individuals nesting within these plots were recorded. In addition, we searched the remainder of the colony for nests where one or both individuals of the attending pair were marked. Marked individuals were identified using 20–60× spotting scopes. We recorded clutch size for nests found during egg laying. We estimated nest initiation date by assuming one egg was laid per day (Nicolai et al. 2004). We backdated 26 days from hatch dates to estimate nest initiation dates if egg laying was not observed. In addition to observing pairs nesting, we classified females as breeding if they were captured during ringing operations with a brood patch.
Field crews on wintering and staging areas examined brant flocks when birds came out of the water after high tide to rest, preen, and acquire grit. Questar 35–120× telescopes were used to read rings in winter (Ward et al. 2004).
Removal of male member of pair
We obtained ring recovery information (e.g., date recovered and ring number) from the Bird Banding Laboratory (USGS) for individuals that were shot throughout the entire year from 1987 through 2005 (97% of recoveries were between mid-September and mid-February). We limited this analysis to females for whom the male mate was shot because: 1) we were interested in effect of mate loss on female and 2) most males paired with females from other colonies after mate loss and followed them to their home colonies after loss of mates from TRC (Lindberg et al. 1998). We use the term year to begin with nesting (when marked pairs were identified) and end with the closing of hunting season (typically mid-February). We assigned all individuals to the following 2 treatment levels: 1) control: individuals where the male member of the pair was not shot and reported in either year t0 or t1 after observation as a marked pair or (2) treatment: individuals for which only the male mate was shot and reported in year i or i + 1 after a marked pair was first observed.
Analysis
We assigned a treatment level to each pair based on the following criteria: 1) control—neither member of the pair was shot in the nonbreeding season immediately following detection as a pair on the breeding colony; 2) shot year i—the male of the pair was shot and reported during the nonbreeding season immediately following being detected as breeding at TRC, and; 3) shot year i + 1—the male of the pair was shot and reported during the nonbreeding season 14–20 months following being detected breeding at TRC. This latter group was not detected breeding in the summer before the male was reported dead, but they were detected the previous summer. We pooled these last 2 treatments due to small sample size, under the assumptions that annual survival rates are high (∼0.90; Sedinger et al. 2007) and divorce rates are low (Black et al. 2007). That is, we assumed both members of the pair were still alive and together during the breeding season immediately preceding the male being shot.
We calculated return rates of individual females where the male was not removed following being detected as a marked pair for 18 breeding cohorts (individuals observed breeding in a given year). We also calculated return rates for a pooled sample of females whose mates were shot. We calculated return rates by dividing the number observed breeding each year after treatment by the total number in the breeding cohort. We calculated a cumulative return rate for each breeding cohort and for the pooled sample of treated females.
To examine effects of mate loss on subsequent survival and fidelity of females to TRC, we used the Barker CMR model implemented in program MARK (Barker 1997; White and Burnham 1999). The Barker model incorporated observations from the breeding colony as well as recoveries from brant shot by hunters and observations of individuals during winter. We used a Barker model because it allowed permanent emigration to be separated from mortality, using reporting of harvested individuals and winter observations of individuals away from TRC. Harvested individuals and those observed away from the breeding area were representative of the global brant population, including nonbreeders, whereas those observed at TRC were representative of the local breeding population (e.g., Burnham 1993). Taken together, encounters of individuals both at TRC and away from TRC allowed us to estimate both true annual survival and fidelity to TRC (Burnham 1993; Barker 1997; White and Burnham 1999). Because adult females are nearly completely faithful to TRC (fidelity ∼ 1.0; Sedinger et al. 2008), “dispersal” after mate loss was almost certainly associated with nonbreeding by females that lost their mates. The Barker model, thus allowed us to distinguish between survival and breeding costs of mate loss. We conditioned the initial release into the study on the first time females were seen nesting and their marked mate was identified. We used subsequent encounters on breeding areas (nesting and/or brood rearing), reports of harvested birds from USGS-Bird Band Laboratory, and resightings from wintering areas to construct encounter histories. We excluded pairs where both individuals were shot in the same day to eliminate confounding between a shared mortality risk and fitness costs to females of losing their mates. We report the following parameters from the Barker analysis: S (true annual survival), p (encounter probability at the TRC), r (reporting rate, the probability that an individual died [including being shot] during the nonbreeding season and the ring was reported), R (probability an individual alive during the breeding season was observed during the preceding nonbreeding period), F (fidelity of individuals to the TRC). All individuals that were “reported” in this study were shot by hunters; thus, differences in reporting rates reflected differences in the underlying mortality process, combined with differences in harvest rates. Ring recovery rates (as distinguished from reporting rates) represent the probability that an individual was shot by a hunter, and the ring was reported to the U.S. Geological Survey Bird Banding Laboratory (Brownie et al. 1985). Recovery rates, thus represent a direct index of harvest rate, which can transformed to an estimate of harvest rate if ring reporting rates (as a component of recovery rates) are known (Nichols et al. 1995). The terminology here can be confusing. Reporting rates that are components of ring recovery rates are different parameters from the reporting rates we estimated in our analyses. Ring recovery rates can be approximated from estimates of Barker reporting rates (r) using the formula f = r × (1 – S) (Barker 1997; White and Burnham 1999; Nicolai et al. 2005).
We used 2 separate sets of year-specific individual covariates in which individuals were assigned 0 for no mate loss and 1 for mate loss. For the first covariate (cov1), females received a 1 in the year their mate was shot and a 0 in all other years. This covariate allowed us to assess the impact of mate loss on survival and fidelity of females to TRC only in the year after mate loss. In the second covariate (covF), we assigned females a 1 in the year of mate loss and in all subsequent years. This second covariate allowed us to examine lifetime effects of mate loss. We only considered models that allowed encounter probability (p) at TRC and resighting probability (R) in winter to vary by year. We constrained parameters in the Barker model so our estimates of fidelity to TRC were derived only from effects of permanent emigration (Barker 1997; Nicolai 2010).
Our modeling approach had 3 steps. First, we considered all possible models that allowed survival (S), reporting rate (r), and fidelity (F ) to TRC to vary by year (t), or we constrained parameters to be constant across years. Second, we used the best supported model in the first stage and considered models in which cov1 was used to explain variation in survival and reporting rate, and covF was used to explain variation in fidelity to TRC. These models allowed us to examine whether loss of a mate influenced either survival or fidelity to TRC. We only allowed covF rather than cov1 to explain variation in fidelity to TRC because for females that were alive but never returned, we could not determine when permanent emigration actually occurred. Last, in the best supported model from level 2, we tested models where we replaced cov1 with covF for explaining survival and reporting rate to examine the hypothesis that loss of a mate had lifetime consequences for survival. At this stage, we also considered models in which nest initiation date relative to other clutches in the same year, clutch size relative to other clutches in the same year, and tarsus length were considered as explanatory variables for reporting rate and fidelity to TRC. Our rationale for these models was that individual quality is associated with nesting date, clutch size, and body size (Sedinger et al. 1995).
We did not include an estimate of c (overdispersion parameter, ĉ ) in our variance estimates or model selection because no method currently exists for estimation of c for the Barker estimator. However, c was likely near 1.0, given that this analysis only includes females and not pairs (Schmutz et al. 1995), and the ĉ from a Brownie estimator in Sedinger et al. (2007) was near 1.0 (∼1.1). We present model-averaged parameter estimates ±standard error derived from models with a ΔAICc ≤ 4.0.
RESULTS
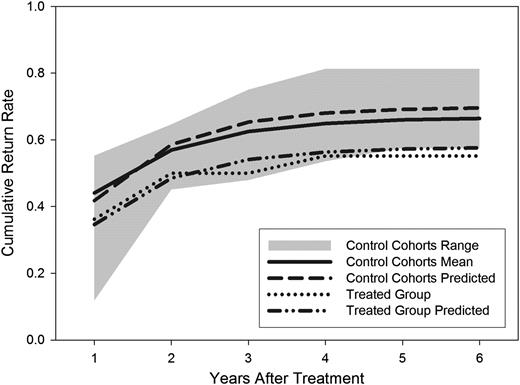
Two thousand four hundred and ninety-eight unique females identified with a marked mate were observed nesting at TRC from 1987 to 2005 (Table 1). These initial releases resulted in 4117 subsequent resightings at TRC, 330 ring recoveries, and 1237 resightings on wintering areas with 2338 control and 58 treatment individual females (Table 1). Twenty-three additional pairs had both members shot in the same year as each other and in 15 of these, both mates were shot within 1 day of each other. These 15 pairs were removed from all further analyses because of the potential that both members of the pair were shot together, in which case death of the female did not represent a cost of mate loss. Asymptotic return rate was 0.55 for treatment females. Asymptotic return rate for 18 control cohorts ranged from 0.57 to 0.82 (mean = 0.66) (Figure 1).
Numbers of marked black brant pairs identified by reading coded tarsal rings at the TRC, Alaska, and recovered in either the immediately following or subsequent hunting season
| Number of marked pairs | |||||||
| Reported year i | Reported year i + 1 | ||||||
| Year (i) | Control | Both | Female only | Male only | Both | Female only | Male only |
| 1987 | 16 | 1 | 1 | 1 | 1 | ||
| 1988 | 41 | 1 | 1 | 1 | |||
| 1989 | 95 | 1 | 2 | 3 | 1 | ||
| 1990 | 146 | 2 | 2 | ||||
| 1991 | 132 | 1 | 2 | 1 | 2 | ||
| 1992 | 206 | 1 | 7 | 1 | 2 | 2 | |
| 1993 | 110 | 1 | 2 | 2 | 1 | 2 | |
| 1994 | 167 | 3 | 2 | 4 | 2 | ||
| 1995 | 196 | 3 | 2 | 3 | 3 | 1 | |
| 1996 | 158 | 3 | 2 | 2 | 2 | 1 | 4 |
| 1997 | 110 | 1 | 2 | 3 | 2 | 2 | |
| 1998 | 133 | 3 | 6 | 1 | 3 | 2 | |
| 1999 | 174 | 1 | 4 | 2 | 4 | 1 | |
| 2000 | 169 | 1 | 1 | 4 | 2 | ||
| 2001 | 71 | 1 | 1 | ||||
| 2002 | 128 | 3 | 1 | 1 | |||
| 2003 | 110 | 3 | 1 | ||||
| 2004 | 107 | 2 | 3 | 3 | 3 | ||
| 2005 | 119 | 3 | 3 | 1 | 4 | ||
| TOTAL | 2338 | 15 | 41 | 29 | 8 | 38 | 29 |
| Number of marked pairs | |||||||
| Reported year i | Reported year i + 1 | ||||||
| Year (i) | Control | Both | Female only | Male only | Both | Female only | Male only |
| 1987 | 16 | 1 | 1 | 1 | 1 | ||
| 1988 | 41 | 1 | 1 | 1 | |||
| 1989 | 95 | 1 | 2 | 3 | 1 | ||
| 1990 | 146 | 2 | 2 | ||||
| 1991 | 132 | 1 | 2 | 1 | 2 | ||
| 1992 | 206 | 1 | 7 | 1 | 2 | 2 | |
| 1993 | 110 | 1 | 2 | 2 | 1 | 2 | |
| 1994 | 167 | 3 | 2 | 4 | 2 | ||
| 1995 | 196 | 3 | 2 | 3 | 3 | 1 | |
| 1996 | 158 | 3 | 2 | 2 | 2 | 1 | 4 |
| 1997 | 110 | 1 | 2 | 3 | 2 | 2 | |
| 1998 | 133 | 3 | 6 | 1 | 3 | 2 | |
| 1999 | 174 | 1 | 4 | 2 | 4 | 1 | |
| 2000 | 169 | 1 | 1 | 4 | 2 | ||
| 2001 | 71 | 1 | 1 | ||||
| 2002 | 128 | 3 | 1 | 1 | |||
| 2003 | 110 | 3 | 1 | ||||
| 2004 | 107 | 2 | 3 | 3 | 3 | ||
| 2005 | 119 | 3 | 3 | 1 | 4 | ||
| TOTAL | 2338 | 15 | 41 | 29 | 8 | 38 | 29 |
Numbers of marked black brant pairs identified by reading coded tarsal rings at the TRC, Alaska, and recovered in either the immediately following or subsequent hunting season
| Number of marked pairs | |||||||
| Reported year i | Reported year i + 1 | ||||||
| Year (i) | Control | Both | Female only | Male only | Both | Female only | Male only |
| 1987 | 16 | 1 | 1 | 1 | 1 | ||
| 1988 | 41 | 1 | 1 | 1 | |||
| 1989 | 95 | 1 | 2 | 3 | 1 | ||
| 1990 | 146 | 2 | 2 | ||||
| 1991 | 132 | 1 | 2 | 1 | 2 | ||
| 1992 | 206 | 1 | 7 | 1 | 2 | 2 | |
| 1993 | 110 | 1 | 2 | 2 | 1 | 2 | |
| 1994 | 167 | 3 | 2 | 4 | 2 | ||
| 1995 | 196 | 3 | 2 | 3 | 3 | 1 | |
| 1996 | 158 | 3 | 2 | 2 | 2 | 1 | 4 |
| 1997 | 110 | 1 | 2 | 3 | 2 | 2 | |
| 1998 | 133 | 3 | 6 | 1 | 3 | 2 | |
| 1999 | 174 | 1 | 4 | 2 | 4 | 1 | |
| 2000 | 169 | 1 | 1 | 4 | 2 | ||
| 2001 | 71 | 1 | 1 | ||||
| 2002 | 128 | 3 | 1 | 1 | |||
| 2003 | 110 | 3 | 1 | ||||
| 2004 | 107 | 2 | 3 | 3 | 3 | ||
| 2005 | 119 | 3 | 3 | 1 | 4 | ||
| TOTAL | 2338 | 15 | 41 | 29 | 8 | 38 | 29 |
| Number of marked pairs | |||||||
| Reported year i | Reported year i + 1 | ||||||
| Year (i) | Control | Both | Female only | Male only | Both | Female only | Male only |
| 1987 | 16 | 1 | 1 | 1 | 1 | ||
| 1988 | 41 | 1 | 1 | 1 | |||
| 1989 | 95 | 1 | 2 | 3 | 1 | ||
| 1990 | 146 | 2 | 2 | ||||
| 1991 | 132 | 1 | 2 | 1 | 2 | ||
| 1992 | 206 | 1 | 7 | 1 | 2 | 2 | |
| 1993 | 110 | 1 | 2 | 2 | 1 | 2 | |
| 1994 | 167 | 3 | 2 | 4 | 2 | ||
| 1995 | 196 | 3 | 2 | 3 | 3 | 1 | |
| 1996 | 158 | 3 | 2 | 2 | 2 | 1 | 4 |
| 1997 | 110 | 1 | 2 | 3 | 2 | 2 | |
| 1998 | 133 | 3 | 6 | 1 | 3 | 2 | |
| 1999 | 174 | 1 | 4 | 2 | 4 | 1 | |
| 2000 | 169 | 1 | 1 | 4 | 2 | ||
| 2001 | 71 | 1 | 1 | ||||
| 2002 | 128 | 3 | 1 | 1 | |||
| 2003 | 110 | 3 | 1 | ||||
| 2004 | 107 | 2 | 3 | 3 | 3 | ||
| 2005 | 119 | 3 | 3 | 1 | 4 | ||
| TOTAL | 2338 | 15 | 41 | 29 | 8 | 38 | 29 |
Cumulative return rates for adult female black brant nesting at the Tutakoke River Colony in which either 1) the male member of the pair was removed (dotted line) or 2) 18 cohorts of control individuals (shaded area). The shaded region depicts the range of cumulative return rates for females for which we have no record of mate loss; the thick black line is the mean estimate for this group. We provide predicted cumulative return rates based on our estimates of survival, fidelity, and mean capture probability for the control and treatment groups.
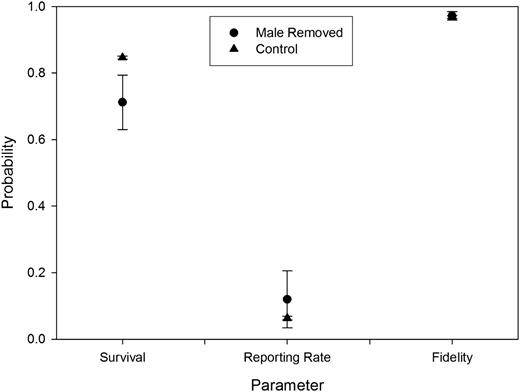
In our analysis using the Barker model, we first identified the best performing model that allowed survival, reporting rate, and fidelity to TRC to remain constant across years (Table 2). The overall best supported model allowed survival and reporting rate to differ in the year of mate loss (cov1) and constrained fidelity to TRC to remain constant. We found substantial support for models containing cov1; sum of Akaike weights (Σwi) = 0.83 and 0.55 for effects of cov1 on survival and reporting rate, respectively. Overall, we found little support for annual variation in annual survival, reporting rate, and fidelity to TRC: Σwi = 0.01, 0.00, and 0.00, respectively. Model averaged estimates of annual survival in the year after treatment were lower for treated (0.71 ± 0.081) than control (0.85 ± 0.004) individuals (Figure 2). We found no support for lifelong effects (covF) on annual survival. Treated individuals experienced increased reporting rates (0.12 ± 0.086) compared with controls (0.06 ± 0.006) (Figure 2) in the year of mate loss (cov1). Because a model containing lifelong effects of mate loss (covF) on reporting rate had little support (wi = 0.18), we derived model-averaged parameter estimates for this model for the first year after treatment only. Converting reporting rates to ring recovery rates produced estimates of ring recovery rates of 0.03 and 0.01 for treated and control individuals, respectively. This allowed for a direct comparison with concurrent estimates of ring recovery rates for the same population (Sedinger et al. 2007). We found little support for treatment effects on fidelity to TRC as estimates were nearly identical for the 2 groups (Σwi = 0.12; F = 0.97 ± 0.013 and 0.97 ± 0.004, for treatment and control females, respectively) (Figure 2), indicating that treatment and control females returned to breed at the same rates if they survived. We found no support for effects of relative clutch size before treatment, nest initiation date before treatment, or diagonal tarsus length on either reporting rate or fidelity to TRC after mate loss (ΔAICc > 10.0; Nicolai 2010).
Models of survival (S), encounter probability (p), reporting rate (r), resighting probability (R), and fidelity (F) for black brant nesting at the TRC, Alaska, 1987–2007
| Modela | AICc | ΔAICc | Model weight | K |
| S(cov1) p(t) r(cov1) R(t) F(.) | 24428.4 | 0.0 | 0.30 | 40 |
| S(cov1) p(t) r(.) R(t) F(.) | 24428.7 | 0.4 | 0.25 | 39 |
| S(cov1) p(t) r(covF) R(t) F(.) | 24429.4 | 1.1 | 0.18 | 40 |
| S(cov1) p(t) r(.) R(t) F(cov1) | 24430.5 | 2.2 | 0.10 | 40 |
| S(.) p(t) r(cov1) R(t) F(.) | 24431.2 | 2.8 | 0.07 | 39 |
| S(.) p(t) r(.) R(t) F(.) | 24432.3 | 4.0 | 0.04 | 38 |
| S(.) p(t) r(.) R(t) F(cov1) | 24433.9 | 5.6 | 0.02 | 39 |
| S(covF) p(t) r(.) R(t) F(.) | 24434.0 | 5.7 | 0.02 | 39 |
| S(t) p(t) r(.) R(t) F(.) | 24435.3 | 6.9 | 0.01 | 56 |
| S(.) p(t) r(t) R(t) F(.) | 24446.4 | 18.1 | 0.00 | 56 |
| S(t) p(t) r(.) R(t) F(t) | 24447.1 | 18.8 | 0.00 | 73 |
| S(.) p(t) r(.) R(t) F(t) | 24452.1 | 23.8 | 0.00 | 55 |
| S(t) p(t) r(t) R(t) F(.) | 24452.8 | 24.4 | 0.00 | 74 |
| S(t) p(t) r(t) R(t) F(t) | 24464.4 | 36.1 | 0.00 | 91 |
| S(.) p(t) r(t) R(t) F(t) | 24464.9 | 36.6 | 0.00 | 73 |
| Modela | AICc | ΔAICc | Model weight | K |
| S(cov1) p(t) r(cov1) R(t) F(.) | 24428.4 | 0.0 | 0.30 | 40 |
| S(cov1) p(t) r(.) R(t) F(.) | 24428.7 | 0.4 | 0.25 | 39 |
| S(cov1) p(t) r(covF) R(t) F(.) | 24429.4 | 1.1 | 0.18 | 40 |
| S(cov1) p(t) r(.) R(t) F(cov1) | 24430.5 | 2.2 | 0.10 | 40 |
| S(.) p(t) r(cov1) R(t) F(.) | 24431.2 | 2.8 | 0.07 | 39 |
| S(.) p(t) r(.) R(t) F(.) | 24432.3 | 4.0 | 0.04 | 38 |
| S(.) p(t) r(.) R(t) F(cov1) | 24433.9 | 5.6 | 0.02 | 39 |
| S(covF) p(t) r(.) R(t) F(.) | 24434.0 | 5.7 | 0.02 | 39 |
| S(t) p(t) r(.) R(t) F(.) | 24435.3 | 6.9 | 0.01 | 56 |
| S(.) p(t) r(t) R(t) F(.) | 24446.4 | 18.1 | 0.00 | 56 |
| S(t) p(t) r(.) R(t) F(t) | 24447.1 | 18.8 | 0.00 | 73 |
| S(.) p(t) r(.) R(t) F(t) | 24452.1 | 23.8 | 0.00 | 55 |
| S(t) p(t) r(t) R(t) F(.) | 24452.8 | 24.4 | 0.00 | 74 |
| S(t) p(t) r(t) R(t) F(t) | 24464.4 | 36.1 | 0.00 | 91 |
| S(.) p(t) r(t) R(t) F(t) | 24464.9 | 36.6 | 0.00 | 73 |
Analysis based on Barker parameterization of CMR models. The Barker model provides estimates of true annual survival, fidelity to the breeding colony, and ring reporting rate.
Model notation is as follows: 1) the 5 parameters of interest are: S = true annual survival, p = encounter probability at TRC, r = ring reporting rate, R = encounter probability on wintering areas, and F = fidelity to TRC; 2) parameter structure: (.) = constant, t = varies annually, cov1 = individual binomial covariate depicting a single year effect of mate loss, and covF = individual binomial covariate depicting a lifelong effect of mate loss.
Models of survival (S), encounter probability (p), reporting rate (r), resighting probability (R), and fidelity (F) for black brant nesting at the TRC, Alaska, 1987–2007
| Modela | AICc | ΔAICc | Model weight | K |
| S(cov1) p(t) r(cov1) R(t) F(.) | 24428.4 | 0.0 | 0.30 | 40 |
| S(cov1) p(t) r(.) R(t) F(.) | 24428.7 | 0.4 | 0.25 | 39 |
| S(cov1) p(t) r(covF) R(t) F(.) | 24429.4 | 1.1 | 0.18 | 40 |
| S(cov1) p(t) r(.) R(t) F(cov1) | 24430.5 | 2.2 | 0.10 | 40 |
| S(.) p(t) r(cov1) R(t) F(.) | 24431.2 | 2.8 | 0.07 | 39 |
| S(.) p(t) r(.) R(t) F(.) | 24432.3 | 4.0 | 0.04 | 38 |
| S(.) p(t) r(.) R(t) F(cov1) | 24433.9 | 5.6 | 0.02 | 39 |
| S(covF) p(t) r(.) R(t) F(.) | 24434.0 | 5.7 | 0.02 | 39 |
| S(t) p(t) r(.) R(t) F(.) | 24435.3 | 6.9 | 0.01 | 56 |
| S(.) p(t) r(t) R(t) F(.) | 24446.4 | 18.1 | 0.00 | 56 |
| S(t) p(t) r(.) R(t) F(t) | 24447.1 | 18.8 | 0.00 | 73 |
| S(.) p(t) r(.) R(t) F(t) | 24452.1 | 23.8 | 0.00 | 55 |
| S(t) p(t) r(t) R(t) F(.) | 24452.8 | 24.4 | 0.00 | 74 |
| S(t) p(t) r(t) R(t) F(t) | 24464.4 | 36.1 | 0.00 | 91 |
| S(.) p(t) r(t) R(t) F(t) | 24464.9 | 36.6 | 0.00 | 73 |
| Modela | AICc | ΔAICc | Model weight | K |
| S(cov1) p(t) r(cov1) R(t) F(.) | 24428.4 | 0.0 | 0.30 | 40 |
| S(cov1) p(t) r(.) R(t) F(.) | 24428.7 | 0.4 | 0.25 | 39 |
| S(cov1) p(t) r(covF) R(t) F(.) | 24429.4 | 1.1 | 0.18 | 40 |
| S(cov1) p(t) r(.) R(t) F(cov1) | 24430.5 | 2.2 | 0.10 | 40 |
| S(.) p(t) r(cov1) R(t) F(.) | 24431.2 | 2.8 | 0.07 | 39 |
| S(.) p(t) r(.) R(t) F(.) | 24432.3 | 4.0 | 0.04 | 38 |
| S(.) p(t) r(.) R(t) F(cov1) | 24433.9 | 5.6 | 0.02 | 39 |
| S(covF) p(t) r(.) R(t) F(.) | 24434.0 | 5.7 | 0.02 | 39 |
| S(t) p(t) r(.) R(t) F(.) | 24435.3 | 6.9 | 0.01 | 56 |
| S(.) p(t) r(t) R(t) F(.) | 24446.4 | 18.1 | 0.00 | 56 |
| S(t) p(t) r(.) R(t) F(t) | 24447.1 | 18.8 | 0.00 | 73 |
| S(.) p(t) r(.) R(t) F(t) | 24452.1 | 23.8 | 0.00 | 55 |
| S(t) p(t) r(t) R(t) F(.) | 24452.8 | 24.4 | 0.00 | 74 |
| S(t) p(t) r(t) R(t) F(t) | 24464.4 | 36.1 | 0.00 | 91 |
| S(.) p(t) r(t) R(t) F(t) | 24464.9 | 36.6 | 0.00 | 73 |
Analysis based on Barker parameterization of CMR models. The Barker model provides estimates of true annual survival, fidelity to the breeding colony, and ring reporting rate.
Model notation is as follows: 1) the 5 parameters of interest are: S = true annual survival, p = encounter probability at TRC, r = ring reporting rate, R = encounter probability on wintering areas, and F = fidelity to TRC; 2) parameter structure: (.) = constant, t = varies annually, cov1 = individual binomial covariate depicting a single year effect of mate loss, and covF = individual binomial covariate depicting a lifelong effect of mate loss.
Model averaged estimates of annual survival, reporting rate, and fidelity (±standard error) of female black brant for whom the male member of the pair was removed compared with control pairs for all models with ▵AICc ≤ 4.0.
DISCUSSION
Our results provide strong evidence that survival of female brant declines substantially when they lose their mates. Lower survival after mate loss might be expected in species where paired individuals enjoy higher social status (Black et al. 2007) and loss of a mate reduces access to food or other resources (Lamprecht 1987; Choudhury 1995). By not having a mate to provide vigilance during feeding bouts, body condition may be impacted and makes female brant more vulnerable to other mortality events such as predation and increased likelihood of disease or parasitism. Additionally, increased investment in rearing offspring after mate loss could also result in lower survival of the surviving mate (Daan et al. 1996). Lower survival of treatment females cannot be attributable to harvest because: 1) we removed females from the analysis that were shot and reported within 1 day of the date their mate was reported and 2) the harvest rate we estimated is not sufficient even under a fully additive harvest model (Anderson and Burnham 1976) to account for the reduction in survival we observed.
Change or loss of mate is typically followed by lower reproductive investment or success (Ens et al. 1996; Catry et al. 1997; van de Pol et al. 2006). We found no support for reduced breeding performance for females that survived after the loss of a mate. Widowed females, if they survived, returned to breed at the same rate as control females and actually laid larger clutches than control females (Nicolai 2010). We interpret the latter result as an indication that only higher quality individuals survived, formed new pair bonds, and resumed breeding. That is, females who were of higher quality and laid larger clutches (e.g., Daan et al. 1990) before they lost their mates were more likely to survive loss of their mates and return to the breeding population. Both control and treatment groups averaged greater than 5 years old (mean ages were 6.2 ± 3.3, 4.9 ± 2.5, 7.1 ± 3.3, and 6.0 ± 3.1 for control known age, control minimum age [ringed as unknown age adults], treatment known age, and treatment minimum age, respectively). Because clutch size does not increase with age beyond 5 years of age (Sedinger et al. 1998), larger clutch sizes for females that lost their mates cannot be attributed to their being slightly older than control females. We also found no relationship between age or structural size (total tarsus length) and probability of being harvested or the probability of returning to breed after mate loss (Nicolai 2010), and therefore, suggest that variation in quality must be related to factors other than age or size.
We found support for the hypothesis that ring reporting rate increased after mate loss. Transforming ring reporting rate to a ring recovery rate (Williams et al. 2002), produced estimates of recovery rate for the control birds in this study were similar to those presented in Sedinger et al. (2007). Ring recovery rates for individuals who lost their mates were approximately 3 times higher than for control individuals. Because ring recovery rates provide an index to harvest rate (Reynolds and Sauer 1991), our results suggest that individuals that lost their mates were more vulnerable to harvest after mate loss. Because we removed from the analysis all individuals that were shot within 1 day of their mates, higher harvest rates for females that lost their mates were not an artifact of them being shot with their mates. Even if harvest was completely additive to other forms of mortality, however, increased risk of harvest accounted for only about 20% (0.03/0.14) of the increased mortality experienced by females that lost their mates. Thus, our results show that a substantial cost of mate loss in brant is manifested in reduced survival of females after loss of their mates.
A reasonable hypothesis, to explain increased mortality after mate loss, is that females must forage without the vigilance and defense of foraging space offered by their mate (Gauthier and Tardif 1991), resulting in lower body condition, which has been shown to increase vulnerability to harvest (Hepp et al. 1986) and potentially other forms of predation in ducks. Because we used a capture–recapture model that allowed for encounters away from the marking area (e.g., winter encounters and hunter recoveries), we were able to control for individuals that permanently emigrated from our study site (Barker 1997). Consequently, the additional mortality experienced by females that lost their mates represented higher true mortality and could not have been influenced by dispersal away from the breeding colony.
We used fidelity to TRC as a surrogate for breeding propensity (Sedinger et al. 2008) because breeding brant show nearly absolute fidelity to the TRC once they have nested there (Lindberg et al. 1998; Sedinger et al. 2008), and a significant proportion of “dispersers” are actually permanent nonbreeders away from the TRC colony (Lindberg et al. 1998; Sedinger et al. 2008). We, therefore, interpreted differences in fidelity to TRC between females that lost their mates and controls as evidence that mate loss resulted in permanent nonbreeding. Our data did not allow for examination of temporary nonbreeding (too few treated females). We expected to detect differences in fidelity to TRC (associated with nonbreeding) between control females and those that lost their mates. We did not, however, detect a difference in breeding (as measured by fidelity to TRC) between treated and control groups, and our results indicate that if females that lost their mates survived the initial year after mate loss, they formed new pair bonds and resumed breeding at the same rate as control females.
Fitness is a function of adult survival and recruitment of offspring into the breeding population. Recruitment is, in turn, the product of numerous life history traits, including adult breeding propensity, clutch size, nest success, and survival of young until age of breeding. Therefore, studies attempting to estimate overall fitness must take into account all of these factors to obtain an unbiased estimate of fitness. We believe our study is the first to fully characterize affects of mate loss on traits determining fitness in precocial birds. We show that reduced annual survival of adult female brant after loss of their mate is the principal mechanism by which fitness is reduced for females that lost their mates.
FUNDING
Many individuals assisted with observations of marked brant. Logistic support in Mexico was provided by San Diego National Wildlife Refuge, D. and R. Wheeler, Kuyima Inc., Ducks Unlimited de Mexico, and La Compania de Exportadora de Sal. S. A. Funding for work in Mexico was provided by U.S. Geological Survey-Alaska Science Center, Ducks Unlimited de Mexico, Ducks Unlimited Inc., and the U.S. Fish and Wildlife Service-Migratory Bird Division, Region 7 and through the North American Wetland Conservation Fund. Primary funding sources for Canadian ring resighting were Environment Canada, The Nature Trust of British Columbia, and Arctic Goose Joint Venture. Ringing and nesting studies were supported by both the Alaska Science Center, United States Geological Survey, Ducks Unlimited, The Black Brant Group (Morro Bay, CA), Phil Jebbia (in memory of Marnie Shepherd), and the National Science Foundation (OPP 92 14970, DEB 98 15383, OPP 99 85931, OPP 01 96406) with logistical support from Yukon Delta National Wildlife Refuge. Additional student support was provided by Ducks Unlimited through the Futch Scholarship, Jay Dow Sr. Wetland Scholarship, and the Dennis Raveling Scholarship. Dave Koons, Lew Oring, Gordon Orians, and 2 anonymous reviewers reviewed earlier drafts. Brant were ringed under U.S. Geological Survey Bird Banding Laboratory permit number 22666. Animal procedures were approved by the University of Alaska Fairbanks and University of Nevada Reno Institutional Animal Care and Use Committees (most recent protocol number 00056).