-
PDF
- Split View
-
Views
-
Cite
Cite
Marc Théry, Martine Debut, Doris Gomez, Jérôme Casas, Specific color sensitivities of prey and predator explain camouflage in different visual systems, Behavioral Ecology, Volume 16, Issue 1, Jan./Feb. 2005, Pages 25–29, https://doi.org/10.1093/beheco/arh130
Close - Share Icon Share
Abstract
In situations of aggressive mimicry, predators adapt their color to that of the substrate on which they sit for hunting, a behavior that is presumed to hide them from prey as well as from their own predators. Females of few crab-spider species encounter such situations when lying on flowers to ambush pollinators. To evaluate the efficiency of spider camouflage on flowers, we measured by spectroradiometry adult female Thomisus onustus and marguerite daisies, Leucanthemum vulgare. We compared chromatic contrast (color used for short-range detection) of each pair of spider and flower to detection thresholds computed in the visual systems of both Hymenopteran prey and passerine bird predator. We also computed achromatic contrast (brightness) used for long-range detection. In both visual systems, each individual spider was efficiently matching the precise color of the flower center on which it was hunting. Being significantly darker than flowers, crab-spiders could in theory be detected at long range by either predator or prey using achromatic contrast. However, long-range detection is unlikely, owing to small spider size. Spiders also generated significant chromatic and achromatic contrasts to both Hymenoptera and bird when moving on flower periphery. Our study is the first to identify which photoreceptors of both prey and predator are involved in camouflage. The analysis suggests more research on bird predation and vision to determine to which extent bird predators effectively constrain spider crypsis.
In animal communication systems, color displays are understood as resulting from a compromise between conspicuousness to conspecifics and crypsis to predators or prey. A different case occurs when a predator uses aggressive mimicry to hide both from prey and from his own predator. However, color display as well as camouflage is likely to involve visual signals, ambient light, and/or photoreceptors notably active in the near ultraviolet to which humans are not sensitive (UV-B 320–400 nm; Cuthill et al., 2000). Therefore, measuring the efficiency of visual camouflage requires objective quantification of coloration (Bennett et al., 1994). Recent studies of color contrast on flowers used spectroradiometry and physiological models of color vision instead of subjective human vision, and considered two types of visual contrast (see Chittka, 2001; Heiling et al., 2003; Théry and Casas, 2002): (1) brightness contrast, used for long-range or small-target detection, is generally considered as relayed at short distance by (2) color contrast (Osorio et al., 1999a,b; Spaethe et al., 2001). For a honeybee approaching a flower, brightness contrast is relayed by color contrast when the target subtends an area of at least 15° (a flower measuring 26 cm in diameter seen from the distance of 1 m; Spaethe et al., 2001). This distance has not been measured in birds, but achromatic contrast is also known to be used by domestic chicks to identify small objects and patterns, whereas chromatic contrast is used to detect larger targets (Osorio et al., 1999a,b).
Females of few crab-spider species (Thomisidae) adapt their entire body color to that of flowers on which they sit for hunting, a behavior that is presumed to hide them from predators and from visiting pollinators that constitute their main prey (Oxford and Gillespie, 1998). This has been confirmed for two species: Misumena vatia, seen by Hymenopteran prey (Chittka, 2001), and Thomisus onustus, seen by both Hymenopteran prey and bird predator (Théry and Casas, 2002). To date, however, there is no explanation on how these spiders succeed in fooling simultaneously such different types of visual systems.
In this article, we investigated the coloration of crab-spiders, Thomisus onustus, seen by Hymenopteran prey and insectivorous bird predators on flowers of the marguerite daisy, Leucanthemum vulgare (Asteridae). To evaluate individual camouflage efficiency, we measured chromatic and achromatic contrasts of each pair of spider and flower and determined short- and long-range detection abilities in both visual systems. Our goal was to add to previous studies that only considered mean but not individual values of contrast (Chittka, 2001; Théry and Casas, 2002). In addition, we analyzed excitation values of prey and predator photoreceptors to explain how spiders managed to appear simultaneously cryptic with respect to color in different visual systems.
METHODS
Spider and flower collection
We collected 10 adult female crab-spiders, Thomisus onustus, sitting on the yellow central part of 10 flowers of Leucanthemum vulgare. No female spider was found on the white peripheral part of that flower species. All individuals were collected with their flower in the same meadow at Chambray-lès-Tours, France (47°20′18″ N, 00°42′52″ E), from 25 May–1 June 2001. Each spider was kept in a closed plastic box with the flower it was sitting on and brought to the laboratory at Tours to be measured the following day. When arriving at the laboratory, spiders were fed with wild Drosophila melanogaster, and the flower was lightly sprayed with water.
Spectroradiometric measurements
We measured spider and flower colors with a spectroradiometer (Ocean Optics S2000 calibrated from 200–850 nm). Illumination was provided with a deuterium-halogen lamp (DH-2000 emitting from 215–1500 nm) connected with a 1.5-mm-diameter sensor. Spiders were briefly anesthetized by using a piece of cotton wool impregnated with three drops of ether, and placed on an adjustable mounting stand. Three reflectance spectra of both abdomen and cephalothorax were taken at 90° relative to a 99% reflectance standard (300–700 nm, Spectralon) and to the dark current. A reference and dark current calibration were taken before measuring each spider. For each individual, the mean reflectance spectrum of abdomen and cephalothorax measurements was used in statistical analyses. Flowers were laid flat on the mounting stand, and three reflectance spectra of both yellow center and white periphery were measured by using the same protocol. Although all females were found at the flower center, we also studied their camouflage on flower periphery because they sometimes move rapidly within and between flowers in case prey is visually detected (Chittka, 2001; data not shown). Mean reflectance spectra of each flower center and periphery were used in analyses.
Modeling visual systems and contrasts
The computed color contrasts were compared to optimal discrimination thresholds of bird and Hymenoptera in their particular color space. For the most comprehensively studied bird, the pigeon Columbia livia (Kelber et al., 2003), color discrimination is a function of wavelength with an optimal resolution of 4 nm around 540 nm (Neumeyer, 1991). The minimal Euclidean distance of color contrast discrimination was computed as the minimal distance generated between two normal spectra separated by 4 nm in the blue tit color tetrahedron, that is, a contrast threshold of 0.06. We proceeded similarly in the color hexagon of Hymenoptera by computing the minimal color distance allowing to discriminate two object spectra differing by 5 nm, which is the optimal resolution around 500 nm for a honeybee (von Helversen, 1972). This distance was measured as 0.05 (Théry and Casas, 2002). A color contrast of 0.1 is considered as equivalent to about 70% discriminability for bees (Chittka, 1996, 2001). For each pair of spider and flower, computed color contrasts were compared to the Hymenopteran prey and bird predator discrimination thresholds, providing measures of individual color mimicry in both visual systems.
Honeybees and birds are known to use achromatic (brightness) contrast at long range or to detect small targets (Osorio et al., 1999a,b; Spaethe et al., 2001). At longer distances, bees use green receptors, whereas birds use double-cones, which combine absorbance spectra of the medium- and long-wavelengths sensitive photoreceptors (Hart et al., 2000; Spaethe et al., 2001). Achromatic contrasts, computed as the values of green or double-cone photoreceptor signals when excited by spiders divided by the corresponding values for flowers, were thus compared with the value of 1.0 predicted for equal brightness. Therefore, values of achromatic contrast higher than 1.0 indicate that spiders are brighter than are flowers, values lower than 1.0 that spiders are darker than are flowers.
Statistical procedures
All statistical analyses were performed with version 9.01 of Systat (SPSS, 1998). We tested the frequency distribution of each variable for normality, and used normal log-transformed data. Each individual value of chromatic contrast of a spider on its flower was compared with detection thresholds of both prey and predator using one-sample t tests with the Bonferroni correction (Rice, 1989). We compared individual values of achromatic contrasts to the value predicted for equal brightness by using the same statistics.
For each visual system, we examined if the excitation of each photoreceptor by spider reflectance could explain chromatic contrasts of individual spiders on “their” flowers by using one-way ANOVA models. In both ANOVAs, chromatic contrasts generated by spiders on flower centers and peripheries were dependent variables, and Hymenopteran or bird photoreceptor excitations were independent variables. We are aware that color perception notably depends on combined photoreceptor excitations and not on individual receptor signal (Kelber et al., 2003; Peitsch et al., 1992; Vorobyev et al., 1998). Despite this limitation, specific photoreceptor excitations are regularly used in studies of color camouflage to determine if a particular receptor signal is more critical than another to allow efficient color mimicry or display (see Chittka, 2001; Heiling et al., 2003).
RESULTS
Individual visual contrasts
There is appreciable variation of color between flowers (mean ± SE of flower centers coordinates in the Hymenopteran color hexagon: x = 0.8054 ± 0.0912, y = 0.0030 ± 0.0252; in the bird color tetrahedron: x = 0.0030 ± 0.0007, y = −0.8265 ± 0.1128, z = 0.2930 ± 0.0445), and spider individually adjust their color accordingly (Table 1). Chromatic contrasts of individual crab-spiders on flower centers do not significantly differ from detection thresholds of either Hymenoptera or bird (Table 1). Therefore, female spiders are unlikely to be detected at short distance on flower centers by either Hymenopteran prey or bird predator. On the contrary, color contrasts of individual spiders on flower peripheries strongly exceed detection thresholds of both Hymenoptera and bird (Table 1). As a consequence, both prey and predator would easily detect spiders at short range on flower periphery. This color contrast on flower periphery may well be perceived by spiders, as we observed them invariably at the center of flowers.
Summary of one-sample t tests of chromatic and achromatic contrasts of crab-spiders on marguerite daisies
. | Mean ± SD . | t . | df . | p . |
|---|---|---|---|---|
| Hymenoptera vision | ||||
| Chromatic contrast with center | 0.061 ± 0.024 | 0.913 | 9 | .385 |
| Chromatic contrast with periphery | 0.624 ± 0.045 | 109.951 | 9 | <.0001 |
| Achromatic contrast with center | 0.402 ± 0.111 | −10.213 | 9 | <.0001 |
| Achromatic contrast with periphery | 0.161 ± 0.092 | −11.958 | 9 | <.0001 |
| Bird vision | ||||
| Chromatic contrast with center | 0.056 ± 0.034 | −1.202 | 9 | .520 |
| Chromatic contrast with periphery | 0.867 ± 0.017 | 418.757 | 9 | <.0001 |
| Achromatic contrast with center | 0.474 ± 0.164 | −6.885 | 9 | <.0001 |
| Achromatic contrast with periphery | 0.280 ± 0.133 | −8.732 | 9 | <.0001 |
. | Mean ± SD . | t . | df . | p . |
|---|---|---|---|---|
| Hymenoptera vision | ||||
| Chromatic contrast with center | 0.061 ± 0.024 | 0.913 | 9 | .385 |
| Chromatic contrast with periphery | 0.624 ± 0.045 | 109.951 | 9 | <.0001 |
| Achromatic contrast with center | 0.402 ± 0.111 | −10.213 | 9 | <.0001 |
| Achromatic contrast with periphery | 0.161 ± 0.092 | −11.958 | 9 | <.0001 |
| Bird vision | ||||
| Chromatic contrast with center | 0.056 ± 0.034 | −1.202 | 9 | .520 |
| Chromatic contrast with periphery | 0.867 ± 0.017 | 418.757 | 9 | <.0001 |
| Achromatic contrast with center | 0.474 ± 0.164 | −6.885 | 9 | <.0001 |
| Achromatic contrast with periphery | 0.280 ± 0.133 | −8.732 | 9 | <.0001 |
Sequential Bonferroni correction (Rice, 1989) has been conducted on the p values. Bold type indicates efficient mimicry.
Summary of one-sample t tests of chromatic and achromatic contrasts of crab-spiders on marguerite daisies
. | Mean ± SD . | t . | df . | p . |
|---|---|---|---|---|
| Hymenoptera vision | ||||
| Chromatic contrast with center | 0.061 ± 0.024 | 0.913 | 9 | .385 |
| Chromatic contrast with periphery | 0.624 ± 0.045 | 109.951 | 9 | <.0001 |
| Achromatic contrast with center | 0.402 ± 0.111 | −10.213 | 9 | <.0001 |
| Achromatic contrast with periphery | 0.161 ± 0.092 | −11.958 | 9 | <.0001 |
| Bird vision | ||||
| Chromatic contrast with center | 0.056 ± 0.034 | −1.202 | 9 | .520 |
| Chromatic contrast with periphery | 0.867 ± 0.017 | 418.757 | 9 | <.0001 |
| Achromatic contrast with center | 0.474 ± 0.164 | −6.885 | 9 | <.0001 |
| Achromatic contrast with periphery | 0.280 ± 0.133 | −8.732 | 9 | <.0001 |
. | Mean ± SD . | t . | df . | p . |
|---|---|---|---|---|
| Hymenoptera vision | ||||
| Chromatic contrast with center | 0.061 ± 0.024 | 0.913 | 9 | .385 |
| Chromatic contrast with periphery | 0.624 ± 0.045 | 109.951 | 9 | <.0001 |
| Achromatic contrast with center | 0.402 ± 0.111 | −10.213 | 9 | <.0001 |
| Achromatic contrast with periphery | 0.161 ± 0.092 | −11.958 | 9 | <.0001 |
| Bird vision | ||||
| Chromatic contrast with center | 0.056 ± 0.034 | −1.202 | 9 | .520 |
| Chromatic contrast with periphery | 0.867 ± 0.017 | 418.757 | 9 | <.0001 |
| Achromatic contrast with center | 0.474 ± 0.164 | −6.885 | 9 | <.0001 |
| Achromatic contrast with periphery | 0.280 ± 0.133 | −8.732 | 9 | <.0001 |
Sequential Bonferroni correction (Rice, 1989) has been conducted on the p values. Bold type indicates efficient mimicry.
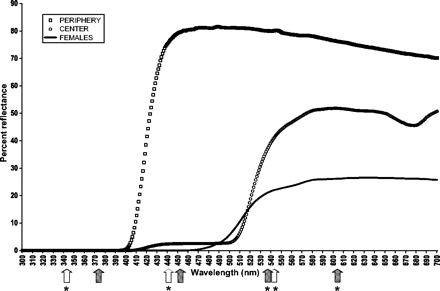
With reference to achromatic contrast perceived by the visual systems of both Hymenopteran prey and bird predator, spiders are significantly darker than are yellow flower centers (Figure 1 and Table 1). Because whitish flower periphery is brighter than is yellow flower center (Figure 1), achromatic contrast would be stronger if spiders were seen on flower periphery than on flower center. At long range, both predator and prey using achromatic contrast would in theory detect spiders on either flower center or periphery.
Mean reflectance spectra of crab-spiders and flowers. Spectral locations of peaks of sensitivity are shown for Hymenopteran photoreceptors (empty arrows) and blue tit photoreceptors (filled arrows). Stars indicate significant effects of photoreceptors on crab-spider camouflage.
Color-receptor excitation values and chromatic contrasts
When the Hymenopteran visual system is excited by spiders' reflectance spectra, low chromatic contrast on yellow flower centers depends on the relative excitation signals of the three photoreceptor types sensitive to blue, green, and UV-B wavelengths (Table 2). Even though this camouflage is efficient in the bird visual system (Table 1), none of the excitation signals of the four bird photoreceptors explain the efficient color matching (Table 2). On the contrary, high chromatic contrast of spiders on whitish flower periphery is well explained by excitation signals of two birds photoreceptors, in the red and green wavelengths, whereas Hymenopteran photoreceptor signals do not explain chromatic contrast on this flower part (Table 2).
ANOVAs of the effects of photoreceptor stimulations of both prey and predator on chromatic contrasts of crab-spiders on marguerite daisies
. | F . | df . | p . |
|---|---|---|---|
| Hymenoptera vision | |||
| Contrast with center | |||
| EUV | 6.943 | 1 | .039 |
| EBlue | 12.119 | 1 | .013 |
| EGreen | 8.838 | 1 | .025 |
| Contrast with periphery | |||
| EUV | 0.280 | 1 | .616 |
| EBlue | 1.558 | 1 | .258 |
| EGreen | 0.546 | 1 | .488 |
| Bird vision | |||
| Contrast with center | |||
| EUV | 0.834 | 1 | .403 |
| EBlue | 1.000 | 1 | .363 |
| EGreen | 0.099 | 1 | .766 |
| ERed | 0.156 | 1 | .709 |
| Contrast with periphery | |||
| EUV | 0.040 | 1 | .850 |
| EBlue | 0.028 | 1 | .874 |
| EGreen | 6.941 | 1 | .021 |
| ERed | 16.691 | 1 | .009 |
. | F . | df . | p . |
|---|---|---|---|
| Hymenoptera vision | |||
| Contrast with center | |||
| EUV | 6.943 | 1 | .039 |
| EBlue | 12.119 | 1 | .013 |
| EGreen | 8.838 | 1 | .025 |
| Contrast with periphery | |||
| EUV | 0.280 | 1 | .616 |
| EBlue | 1.558 | 1 | .258 |
| EGreen | 0.546 | 1 | .488 |
| Bird vision | |||
| Contrast with center | |||
| EUV | 0.834 | 1 | .403 |
| EBlue | 1.000 | 1 | .363 |
| EGreen | 0.099 | 1 | .766 |
| ERed | 0.156 | 1 | .709 |
| Contrast with periphery | |||
| EUV | 0.040 | 1 | .850 |
| EBlue | 0.028 | 1 | .874 |
| EGreen | 6.941 | 1 | .021 |
| ERed | 16.691 | 1 | .009 |
Bold type indicates significant effect on chromatic contrast. The number of spiders and flowers analyzed is 10.
ANOVAs of the effects of photoreceptor stimulations of both prey and predator on chromatic contrasts of crab-spiders on marguerite daisies
. | F . | df . | p . |
|---|---|---|---|
| Hymenoptera vision | |||
| Contrast with center | |||
| EUV | 6.943 | 1 | .039 |
| EBlue | 12.119 | 1 | .013 |
| EGreen | 8.838 | 1 | .025 |
| Contrast with periphery | |||
| EUV | 0.280 | 1 | .616 |
| EBlue | 1.558 | 1 | .258 |
| EGreen | 0.546 | 1 | .488 |
| Bird vision | |||
| Contrast with center | |||
| EUV | 0.834 | 1 | .403 |
| EBlue | 1.000 | 1 | .363 |
| EGreen | 0.099 | 1 | .766 |
| ERed | 0.156 | 1 | .709 |
| Contrast with periphery | |||
| EUV | 0.040 | 1 | .850 |
| EBlue | 0.028 | 1 | .874 |
| EGreen | 6.941 | 1 | .021 |
| ERed | 16.691 | 1 | .009 |
. | F . | df . | p . |
|---|---|---|---|
| Hymenoptera vision | |||
| Contrast with center | |||
| EUV | 6.943 | 1 | .039 |
| EBlue | 12.119 | 1 | .013 |
| EGreen | 8.838 | 1 | .025 |
| Contrast with periphery | |||
| EUV | 0.280 | 1 | .616 |
| EBlue | 1.558 | 1 | .258 |
| EGreen | 0.546 | 1 | .488 |
| Bird vision | |||
| Contrast with center | |||
| EUV | 0.834 | 1 | .403 |
| EBlue | 1.000 | 1 | .363 |
| EGreen | 0.099 | 1 | .766 |
| ERed | 0.156 | 1 | .709 |
| Contrast with periphery | |||
| EUV | 0.040 | 1 | .850 |
| EBlue | 0.028 | 1 | .874 |
| EGreen | 6.941 | 1 | .021 |
| ERed | 16.691 | 1 | .009 |
Bold type indicates significant effect on chromatic contrast. The number of spiders and flowers analyzed is 10.
DISCUSSION
Visual contrasts
Each individual female Thomisus onustus is highly cryptic at short range on the flower center where it is hunting, when both Hymenopteran prey and bird predator use chromatic contrast to detect color patterns. This efficient camouflage, here demonstrated for individual crab-spiders sitting on yellow corollas of marguerite daisies, reinforces previous results obtained with mean values of chromatic contrast of the same crab-spider species seen by both predator and prey hunting on pink corollas of Mentha spicata and yellow corollas of Senecio jacobea (Théry and Casas, 2002). Color mimicry has also been shown using mean values of chromatic contrast of females Misumena vatia seen by bees hunting on white Chaerophyllum temulum (Chittka, 2001). This efficient camouflage is confirmed by recent field experiments on females Misumena vatia, notably showing that Hymenopteran prey do not detect crab-spiders at their first visit on flowers (Dukas and Morse, 2003). Individual color camouflage appears efficient for bird predators, which is consistent with the particular danger represented for flower crab-spiders by sight-hunting predators (Oxford and Gillespie, 1998).
Recent studies have shown that the minimum separable distance in bee color space may be smaller than what has been previously suggested (Chittka et al., 2003; Dyer and Chittka, 2004). In essence, if bees are penalized for errors, they might be able to detect much smaller color differences than those taken into account in our model. The predation risk encountered by bees when visiting flowers could indeed induce finer color discrimination than expected from previous laboratory experiments. However, this minimum color distance separable by bees is presently unknown.
When considering achromatic (brightness) contrast used for discrimination from larger distances, females are darker than flowers and thus in theory conspicuous to both prey and predator. However, because of their small size (about 8 mm maximal body length without legs for adult females; Jones, 2001), it is unlikely that either Hymenoptera or bird can indeed detect female Thomisus onustus at long range. For example, honeybees require a minimum visual angle of 5° to detect a stimulus presenting chromatic and achromatic contrasts (Giurfa et al., 1996, 1997), an angle that would not be filled by a crab-spider hiding at more than 10 cm from the bee. Small size and the fact that color contrast is used independently from brightness contrast most likely explain why female crab-spiders appear highly cryptic on the mimicked flowers. How crab-spiders perceive and adjust their body coloration to that of flowers remains unknown and is presently studied.
Camouflage in two visual systems
Our results indicate that photoreceptors of both Hymenopteran prey and bird predator are differentially involved in detecting the degree of camouflage of crab-spiders on different parts of the same flower. All three types of Hymenopteran photoreceptors appear to explain low color contrast of crab-spiders at the center of marguerite daisies, whereas the two bird photoreceptors sensitive to long and medium wavelengths are involved in detecting higher color contrast of spiders on flower periphery. The relative constraints of the two visual systems appear complementary at spectral scale, with Hymenopteran photoreceptors explaining spider coloration from UV-B to green wavelengths, and bird photoreceptors from green to red. However, the medium- and long-wavelengths sensitive photoreceptors of birds, here identified in detecting chromatic contrast on flower periphery, are also combined as double cones involved in detection of achromatic contrast (Hart et al., 2000). Therefore, an alternative explanation to the contribution of bird photoreceptors in the detection of chromatic contrast at flower periphery may simply be related to the use of double cones for achromatic contrast. More research on spider predation and respective use of chromatic and achromatic contrasts by birds will be necessary to determine to which extent bird predators are constraining spider camouflage.
Integrating specific photoreceptor peak sensitivities and type numbers in both predator and prey visual systems was the aim of the present study, and is a requisite for understanding the bottom-up and top-down evolutionary forces acting on animal coloration. We also demonstrated that this approach is necessary but insufficient to explain the efficient mimicry of spiders located in flower centers in the bird vision system. Indeed, optimal color discrimination always differs from photoreceptor maximal absorbance (Chittka, 1992; Chittka and Waser, 1997). This has also been studied thoroughly in the pigeon (see Bowmaker et al., 1997; Neumeyer, 1991), an approach that needs now to be extended to many other organisms and framed in ecological settings of relevance.
M. Debut is now at the Institut National de la Recherche Agronomique, Station de Recherches Avicoles, F-37380 Nouzilly, France.
We thank Nathan Hart for kindly providing blue tit photoreceptors photon catches. This study was funded by the Institut Fédératif d'Ecologie Fondamentale et Appliquée (IFR 101), by the Groupement de Recherche en Ecologie Comportementale (CNRS GDR 2155), and by the Centre National de la Recherche Scientifique and the Muséum National d'Histoire Naturelle through UMR 5176 and USM 301.
References
Bennett ATD, Cuthill IC, and Norris KJ,
Bowmaker JK, Heath LA, Wilkie SE, and Hunt DM,
Chittka L,
Chittka L,
Chittka L,
Chittka L, Dyer AG, Bock F, and Dornhaus A,
Chittka L, Schimda A, Troje N, and Menzel R,
Chittka L, and Waser NM,
Cuthill IC, Partridge JC, Bennett ATD, Church SC, Hart NS, and Hunt S,
Dyer AG, and Chittka L,
Giurfa M, Vorobyev M, Brandt R, Posner B, and Menzel R,
Giurfa M, Vorobyev M, Kevan P, and Menzel R,
Goldsmith TH,
Hart NS, Partridge JC, Cuthill IC, and Bennett ATD,
Heiling AM, Herberstein ME, and Chittka L,
Kelber A, Vorobyev M, and Osorio D,
Maier EJ, and Bowmaker JK,
Neumeyer C,
Osorio D, Miklósi A, and Gonda Z,
Osorio D, Vorobyev M, and Jones CD,
Oxford GS, and Gillespie RG,
Peitsch D, Fietz A, Hertel H, de Souza J, Ventura DF, and Menzel R,
Spaethe J, Tautz J, and Chittka L,
von Helversen O,
Author notes
aMuséum National d'Histoire Naturelle, Département d'Ecologie et de Gestion de la Biodiversité, CNRS UMR 5176, 91800 Brunoy, France
bInstitut de Recherche sur la Biologie de l'Insecte, Université de Tours, CNRS UMR 6035, F-37200 Tours, France