-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas R. Meagher, Linking the Evolution of Gender Variation to Floral Development, Annals of Botany, Volume 100, Issue 2, August 2007, Pages 165–176, https://doi.org/10.1093/aob/mcm035
Close - Share Icon Share
Abstract
In the present review, I have endeavoured to conduct a joint assessment of the thinking underlying the evolutionary genetics of gender polymorphism and the developmental genetics of gender determination. It is my hope, through highlighting the historical development of ideas in two related but somewhat disparate sets of scientific literature, to encourage a synthetic perspective that integrates the two.
An overview is provided of various theories on the evolution of sex polymorphism and examples of evidence that has been brought to bear in support of them. Current knowledge on floral development is summarized, with an emphasis on gender variation. Finally, an attempt is made to integrate the two perspectives with the hope that it will encourage future research at the interface.
Evolutionary models of gender evolution have, of necessity, posited genetic effects that are relatively simple in their impacts. Emerging insights from developmental genetics have demonstrated that the underlying reality is a more complex matrix of interacting factors. The study of gender variation in plants is poised for significant advance through the integration of these two perspectives. Bringing genomic tools to bear on population-level processes, we may finally develop a comprehensive perspective on the evolution of floral gender.
The History of Science has suffered greatly from the use by teachers of second-hand material, and the consequent obliteration of the circumstances and the intellectual atmosphere in which the great discoveries of the past were made. A first-hand study is always instructive, and often … full of surprises.
INTRODUCTION
Following from Fisher's admonition above, a review is a guide into an area, not a substitute for the primary literature, and I encourage readers of this paper to delve into the scientific depth of gender variation in plants for themselves after consulting this guide.
There is a rich literature on gender variation in flowering plants, emphasizing two quite distinct perspectives: evolutionary genetics and developmental genetics. These have addressed such nuances as the evolution of mating systems (e.g. dioecy, etc.) (Bawa, 1980; Freeman et al., 1997; Barrett, 2002; Pannell, 2002), the evolutionary dynamics of inbreeding and inbreeding depression (Charlesworth and Charlesworth, 1987; Byers and Waller, 1999; Keller and Waller, 2002; Goodwillie et al., 2005), the evolution of sex chromosomes (Charlesworth, 1991, 2002; Matsunaga and Kawano, 2001; Lengerova et al., 2003; Vyskot and Hobza, 2004), the genetics of sex determination (Westergaard, 1958; Meagher, 1988; Dellaporta and Calderonurrea, 1993; Grant et al., 1994; Ainsworth, 1999) and the developmental genetics of floral organogenesis (Coen and Meyerowitz, 1991; Ng and Yanofsky, 2000; Goto et al., 2001; Jack, 2001, 2004; Theissen, 2001). Indeed, taking on the task of constructing an original review in this topic area is daunting.
In the present review, I have endeavoured to conduct a joint assessment of the thinking underlying the evolutionary genetics of gender polymorphism and the developmental genetics of gender expression. These two approaches are essentially tackling the same phenomenon, gender variation in plants, but from different angles and to an extent in scientific isolation from each other. It is my hope, through highlighting the historical development of ideas in the two related but somewhat disparate sets of scientific literature, to encourage a synthetic perspective that integrates the two.
Flowering plants are well known for the wide array of gender expression they exhibit, resulting in a complex array of terms to define specific gender states for both individuals and populations (Cruden and Lloyd, 1995; Neal and Anderson, 2005). Thus, a plant population may be: hermaphroditic, where female and male gender occur within the same flower; monoecious, where female and male gender occur within the same individuals, but within unisexual flowers; dioecious, where male and female gender are found in different plants; gynodioecious, where populations are polymorphic with either all female (male-sterile) or hermaphroditic individuals; or androdioecious, where populations are polymorphic with either all male (female-sterile) or hermaphroditic individuals. There are many additional variants on this theme! In the main, this terminology can be reduced to viewing flowering plant gender as a gradient from all female at one extreme, through varying levels of combined male and female gender (co-sexuality), to all male at the other extreme (Lloyd, 1980). There are also many flowering plant mating systems that do not entail gender asymmetries per se (e.g. heterostyly, self-incompatibility, etc.).
The general theme of the present review is evolutionary transitions among different gender states. One reason that the evolution of gender polymorphism has attracted so much attention is that the diversity of gender states found in flowering plants represents many independent evolutionary events. Moreover, gender variation in plants has a long history of scientific investigation. Darwin (1877) was among the first to focus attention on gender variation and its evolution in plants. In a comprehensive survey of the flowering plant groups as delineated at the time, Yampolsky and Yampolsky (1922) provided an early assessment of the frequency of different gender polymorphisms. Some of their observations have stood the test of time. For example, their estimates of the incidence of dioecy among genera of monocots (4 %) and dicots (7 %) were very close to more recent estimates obtained by Renner and Ricklefs (1995) (5 and 8 %, respectively). They reported only one genus that was androdioecious, and, to date, nearly one hundred years later, this gender polymorphism has still only been reported in a handful of species (Pannell, 2002). On the other hand, Yampolsky and Yampolsky (1922) reported low levels of gynodioecy and, although there are no recent estimates of the frequency of gynodioecy, it is almost certainly very widespread. Beyond merely accounting for the frequency and distribution of gender polymorphism in plants, there is also a well developed theoretical framework for interpreting gender variation.
A critical feature of the evolution of gender variation is the genetic mechanism underlying such variation. Theoretical and empirical work on the evolutionary biology of gender polymorphism has assumed genetic effects of a general nature, such as genes for ‘male sterility’ or ‘female sterility’. Indeed, there is a long tradition in evolutionary studies of emphasizing the nature of selection driving evolutionary change, assuming that response to selection will occur if there is appropriate underlying genetic variation. In parallel with such evolutionary studies, the discovery of homeotic genes underlying successive development of the different whorls within a flower (sepals, petals, stamens, carpels and ovules) (Coen and Meyerowitz, 1991) has revolutionized our understanding of the development of flowers over the past two decades. Such genes clearly underlie gender expression in plants, so that we are now in a position to be much more specific about the nature of the genetic effects involved in the evolution of gender polymorphism.
The overarching goal of the present review is to encourage a synthesis of evolutionary genetic and developmental genetic approaches to gender expression and variation. Thus, I will provide an overview of population genetic theory on the evolution of gender polymorphism and examples of evidence that have been brought to bear. I will then summarize the emerging developmental genetic model of floral development, with an emphasis on gender expression. Finally, I will attempt to integrate the two perspectives with the hope that it will encourage future research at the interface of these two areas.
EVOLUTIONARY GENETICS OF GENDER POLYMORPHISM
Theory
Much of the interest in studying gender variation has focused on evolutionary transitions between different population and individual gender states. At the core of such transitions is the evolution of asymmetries in male and female contribution to reproductive success. Such asymmetries can result in non-random mating patterns in a variety of ways; however, it needs to be borne in mind that sexual reproduction fundamentally involves equal contributions of male and female gametes to the offspring generation. Theory addressing the evolution of gender polymorphism has involved a number of evolutionary drivers, including frequency-dependent selection, avoidance of inbreeding and specialization due to resource limitation for gender expression.
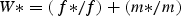
Asymmetries leading to the evolution of gender polymorphism may arise due to reproductive compensation in which loss of one sex function is favoured by selection due to increased fitness through the other sex function (Darwin, 1877; Costich and Meagher, 2001). Lewis (1940) formalized the conditions for the evolution of gynodioecy under the nuclear mode of inheritance by establishing that the female reproductive success of a female plant in a population that is otherwise co-sexual has to be twice as great in order for the trait to spread, thus: 1 + k > 2, where k is the added seed output of a female plant relative to a co-sexual. Similarly, the selection threshold for the evolutionary establishment of nuclear genes for androdioecy requires that the pollen fitness of a male (female-sterile) plant has to be at least twice as great as that for a co-sexual: 1 + K > 2, where K is the added pollen to seed output of a male plant relative to a co-sexual. These relationships set very high thresholds for the evolution of gynodioecy and androdioecy, but they fail to take into account the impact of inbreeding in co-sexuals. Lloyd (1975) introduced the notion of genealogical relationship and concomitant inbreeding into this theory; and Charlesworth and Charlesworth (1978) formally incorporated inbreeding (s) and inbreeding depression (δ) into Lewis's formulae, showing that the fitness advantage of female plants may be further enhanced in the presence of inbreeding in the co-sexuals with inbreeding depression, leading to: 1 + k > 2(1 – sδ). This lowers the threshold value of k required to enable gynodioecy as a stable gender polymorphism, and indeed gynodioecy is a widespread phenomenon among flowering plants. On the other hand, incorporating inbreeding and inbreeding depression into the conditions for the evolution of androdioecy raises the threshold conditions for the evolutionary maintenance of female sterility: 1 + K > 2(1 – sδ)/(1 – s). The reason that males are more difficult to maintain in a co-sexual background in the presence of inbreeding is that selfing of co-sexuals pre-empts potential mating with males, which are completely dependent on crossing with co-sexuals to produce offspring. Charlesworth and Charlesworth (1978) suggest that the added liability of selfing for maintenance of males in a co-sexual population background may account for the relative rarity of observed instances of androdioecy. This body of theory outlines a series of parameters (k or K, s, δ) that can be used to determine if observed gynodioecy or androdioecy is a stable polymorphism, and much of the empirical work since that time has been directed towards estimation of these parameters.
All of the above models are based on nuclear genetic control of gender polymorphism. In many cases, gynodioecy in particular is based on a balance between nuclear genes that promote male expression and cytoplasmic factors that promote female expression. The evolutionary implications of such ‘nucleo-cytoplasmic’ sex determination will be considered more fully below.
The evolution of inbreeding
The relationship of inbreeding to plant reproductive biology is complex. There is an extensive amount of work that has been done on inbreeding in natural populations, and I make no pretence at a comprehensive review of this topic. Rather, I present a few key points as they might bear on the subsequent discussion of the evolution of gender polymorphism. Lande and Schemske (1985) outlined theory suggesting bimodality in plant mating strategy between being predominantly inbred vs. predominantly outbred. They went on to present a survey of studies of plant mating systems that appeared to support their theory (Schemske and Lande, 1985), which was widely criticized on the grounds of probable bias in the species studied (Waller, 1986). However, Vogler and Kalisz (2001) conducted a similar survey 15 years later, and they found a bimodality in plant mating strategies for wind-pollinated species, though not for animal-pollinated species. Whether there is an inbreeding: outbreeding bimodality or a continuum of mixed mating in plants, the key to the evolution of mating systems is the extent to which species show inbreeding depression. Thus, one would expect strong selection against inbreeding in a species with high levels of inbreeding depression, whereas inbreeding may be favoured when inbreeding depression is low.
A major factor governing the interplay between inbreeding and inbreeding depression is the history of a population (Charlesworth and Charlesworth, 1987). On the one hand, inbreeding exposes deleterious recessive genetic effects, leading to inbreeding depression. Under this scenario, there will be selection favouring the establishment of outcrossing mechanisms, such as the evolution of dioecy (Stebbins, 1957; Baker, 1984). On the other hand, the ability to self may facilitate the establishment of new populations within an island (Baker, 1955, 1967) or metapopulation context (Pannell and Barrett, 1998, 2001; Pannell et al., 2005). A species with a history of inbreeding, such that deleterious recessive effects have been purged by natural selection by being exposed in homozygous form, may not suffer the consequences of inbreeding depression as severely as a species with a history of being outcrossed (Byers and Waller, 1999).
The evolution of dioecy
The evolution of dioecy has been a focus of considerable attention for some very good reasons. Although it is relatively rare in flowering plants, it is widespread, suggesting that it has undergone independent evolution in many lineages. Thus, even though dioecy itself is uncommon, there is a useful sample of the evolutionary process that has given rise to dioecy. Historically, dioecy was perceived as a relatively straightforward mechanism by which a species could evolve to promote outbreeding in response to inbreeding depression (Stebbins, 1957; Baker, 1984). From a genetic point of view, the intermediate steps involved in the evolution of dioecy are more likely than the intermediate steps for the evolution of other outbreeding mechanisms, such as self-incompatibility. Genetic changes resulting in gender specialization are typically sterility mutations, and such mutations are considered relatively common (Charlesworth and Charlesworth, 1978). An alternative perspective on the evolution of dioecy is that gender specialization enhances female or female fitness sufficiently to lead to separation of gender into different individuals (Charnov, 1982; Charlesworth and Morgan, 1991). Thus, the two main drivers proposed for the evolution of dioecy are promotion of outbreeding on the one hand and fitness benefits of gender specialization on the other.
For a long time, outbreeding as a driver in the evolution of dioecy remained a speculation, though it was typically treated as fact. With the advent of molecular genetic markers, the measurement of inbreeding levels in plant populations has become standard practice. Even so, direct tests of the relationship between inbreeding and the occurrence of dioecy remain limited. Two species that exhibit intraspecific variation in gender expression with dioecious and monoecious populations, Ecballium elaterium and Saggittaria latifolia, have been the object of a comparative assessment of inbreeding (Table 1). In both cases, monoecious populations exhibit higher levels of inbreeding than dioecious populations. Although this does not prove that dioecy has evolved to promote outcrossing in these species, it does demonstrate a correlation between inbreeding levels and dioecy that is consistent with the assumption that the evolution of dioecy is a response to selection to promote outcrossing.
Comparative population genetic assessment of inbreeding in dioecious and closely related non-dioecious taxa
| . | FIS* . | FIT† . | F‡ST . |
|---|---|---|---|
| Ecballium elaterium (Costich and Meagher, 1992) | |||
| Monoecious | 0·43 ± 0·31 | 0·98 ± 0·03 | 0·96 ± 0·04 |
| Dioecious | 0·23 ± 0·08 | 0·41 ± 0·08 | 0·23 ± 0·03 |
| Sagittaria latifolia (Dorken et al., 2002) | |||
| Monoecious | 0·29 ± 0·16 | 0·50 ± 0·11 | 0·29 ± 0·07 |
| Dioecious | 0·11 ± 0·06 | 0·28 ± 0·08 | 0·19 ± 0·07 |
| . | FIS* . | FIT† . | F‡ST . |
|---|---|---|---|
| Ecballium elaterium (Costich and Meagher, 1992) | |||
| Monoecious | 0·43 ± 0·31 | 0·98 ± 0·03 | 0·96 ± 0·04 |
| Dioecious | 0·23 ± 0·08 | 0·41 ± 0·08 | 0·23 ± 0·03 |
| Sagittaria latifolia (Dorken et al., 2002) | |||
| Monoecious | 0·29 ± 0·16 | 0·50 ± 0·11 | 0·29 ± 0·07 |
| Dioecious | 0·11 ± 0·06 | 0·28 ± 0·08 | 0·19 ± 0·07 |
* Inbreeding within local populations.
† Inbreeding overall.
‡ Differentiation among populations.
Comparative population genetic assessment of inbreeding in dioecious and closely related non-dioecious taxa
| . | FIS* . | FIT† . | F‡ST . |
|---|---|---|---|
| Ecballium elaterium (Costich and Meagher, 1992) | |||
| Monoecious | 0·43 ± 0·31 | 0·98 ± 0·03 | 0·96 ± 0·04 |
| Dioecious | 0·23 ± 0·08 | 0·41 ± 0·08 | 0·23 ± 0·03 |
| Sagittaria latifolia (Dorken et al., 2002) | |||
| Monoecious | 0·29 ± 0·16 | 0·50 ± 0·11 | 0·29 ± 0·07 |
| Dioecious | 0·11 ± 0·06 | 0·28 ± 0·08 | 0·19 ± 0·07 |
| . | FIS* . | FIT† . | F‡ST . |
|---|---|---|---|
| Ecballium elaterium (Costich and Meagher, 1992) | |||
| Monoecious | 0·43 ± 0·31 | 0·98 ± 0·03 | 0·96 ± 0·04 |
| Dioecious | 0·23 ± 0·08 | 0·41 ± 0·08 | 0·23 ± 0·03 |
| Sagittaria latifolia (Dorken et al., 2002) | |||
| Monoecious | 0·29 ± 0·16 | 0·50 ± 0·11 | 0·29 ± 0·07 |
| Dioecious | 0·11 ± 0·06 | 0·28 ± 0·08 | 0·19 ± 0·07 |
* Inbreeding within local populations.
† Inbreeding overall.
‡ Differentiation among populations.
There is also evidence that the evolution of dioecy is brought about by selection favouring gender specialization in the event of resource limitation, in particular drought stress. An increase in the incidence of dioecy along moisture gradients has been observed at a variety of levels, from changes in the frequency of dioecious species to variation in sex ratios. A general pattern that has emerged is that drier sites along a moisture gradient are associated with increased gender specialization, with males increasing in frequency as sites become more xeric. The impact of moisture as a limiting resource has also been observed at the intraspecific level. Costich (1995) set up a series of experimental plots of monoecious E. elaterium across a moisture gradient from North (mesic) to South (xeric) Spain, and found that plants in the more xeric plots showed more specialized gender expression than plants in the mesic plots. Thus, plants that share a common genetic background show more specialized gender expression under drought stress. A similar phenomenon has been observed in Wurmbea dioica, where Case and Barrett (2001, 2004) found that dioecious populations predominate in drier sites whereas co-sexual populations predominate on wetter sites. Xeric sites also favour loss of female function in the gynodioecious species Ochradenus baccatus, leading to dioecy (Wolfe and Shmida, 1997). Such effects could result in frequency-dependent selection that would favour a genetic polymorphism for sex determination. For example, Ashman (1999) found that populations of the gynodioecious species Fragaria virginiana occupying relatively dry sites showed reduced female reproduction in hermaphroditic plants accompanied by a higher frequency of female plants.
Another long-standing assumption regarding the evolution of dioecy, arising from its sporadic occurrence among flowering plants, is that it represents an evolutionary dead end. More recently, Heilbuth (2000) used a phylogenetic approach to confirm that dioecious clades are less species rich than non-dioecious clades, suggesting that dioecious taxa experience either a higher rate of extinction or a lower rate of speciation than non-dioecious taxa. Vamosi [nee Heilbuth] and Vamosi (2005) went on to explore the possibility of increased extinction risk in contemporary dioecious taxa by evaluating the relative representation of families containing dioecious species vs. those that do not in the higher risk categories of the IUCN Red List, finding that dioecious species are disproportionately represented. The higher risk of extinction of dioecious species in contemporary populations suggests that dioecious taxa experience higher extinction rates. Whether or not dioecious taxa experience a lower speciation rate is still an open question, but the comparative assessments of genetic variation in intraspecific dioecious and monoecious populations mentioned above (Table 1) are suggestive. In both species listed, the magnitude of differentiation between populations was higher for monoecious than for dioecious populations. It may be that dioecy, by promoting outcrossing that maintains high levels of within-population genetic variation, reduces genetic differentiation between populations, which in the long term would result in reduced rates of speciation.
The evolution of gynodioecy and androdioecy
Given the above discussion, and indeed much of the theoretical literature on this subject, one could get the impression that all evolutionary trajectories lead to dioecy. This is clearly not the case, and indeed the various gender polymorphisms mentioned up to this point all potentially represent evolutionary end-points with trajectories of their own.
Consider the evolution of gynodioecy. The theoretical framework established by Charlesworth and Charlesworth (1978) identified a set of parameters that, if measured, should predict not only the evolutionary stability of gynodioecy, but also the equilibrium frequency of females that should occur. Since that time, there have been many studies that have measured these parameters. Shykoff et al. (2003) conducted a metanalysis encompassing studies of 54 taxa over a 20 year time frame, in order to determine the impacts of male sterility on reproductive characters. They found that female plants generally had greater seed set and produced larger seed with a higher establishment rate, in all likelihood owing to the combined effects of increased availability of resources due to gender specialization as well as enhanced genetic capacity due to outbreeding. These characters address parameter ‘k’ of the Charlesworth and Charlesworth (1978) model, and show that enhanced maternal fitness of female plants is a common feature of gynodioecious species. Similarly, Collin and Shykoff (2003, Supplementary data) constructed a table of reported measurements of outcrossing rates for 23 gynodioecious taxa, demonstrating that in general progeny from hermaphroditic individuals are substantially more inbred than progeny from females in the same populations. Thus, two of the theoretical conditions for the evolutionary maintenance of gynodioecy, enhanced seed set and less inbreeding in females, are manifested in natural populations.
As noted above, inbreeding only impacts on fitness in the presence of inbreeding depression; and, indeed, the Charlesworth and Charlesworth model includes parameters for both. Selected examples of studies that measure all of the parameters underlying the Charlesworth and Charlesworth model are summarized in Table 2. Inbreeding is generally inferred in these studies from genetic marker analyses that take into account both selfing and biparental inbreeding, which is why females have non-zero inbreeding estimates. For two of the five examples, the combined effect of inbreeding and inbreeding depression is sufficient to give females a selective advantage even if they only produce the same seed output as hermaphrodites. Thus, inbreeding effects can substantially lower the selection threshold for the establishment and maintenance of nuclear genes resulting in gynodioecy. Schiedea is an interesting case in point, because this genus shows a range of breeding systems from hermaphroditism to dioecy (Sakai et al., 2006). In addition to the two species listed in Table 2, breeding system parameters for the hermaphroditic species S. menziesii suggest that the current hermaphroditic breeding system is evolutionarily unstable and that the evolution of gynodioecy is likely (Rankin et al., 2002).
Impacts of inbreeding and inbreeding depression on the maintenance of gender polymorphism in gynodioecious populations
| Species . | Co-sexual inbreeding (sc)* . | Female inbreeding (sf) . | Inbreeding depression (δ) . | Female advantage threshold [2(1–scδ)] . |
|---|---|---|---|---|
| Bidens sandvicensis (Schultz and Ganders, 1996) | ||||
| 0·71 | – | 0·60 | 1·15 | |
| 0·67 | – | 0·60 | 1·20 | |
| 0·45 | – | 0·60 | 1·46 | |
| 0·51 | – | 0·60 | 1·39 | |
| Chionographis japonica var. kurohimensis (Maki, 1993) | ||||
| 0·94 | 0·03 | 0·34 | 1·36 | |
| 1·00 | – | 0·24 | 1·52 | |
| Schiedea adamantis (Sakai et al., 1997) | ||||
| 0·68 | – | 0·60 | 1·18 | |
| Schiedea salicaria (Weller and Sakai, 2005)† | ||||
| 0·70 | 0·00 | 0·72 | 0·99 | |
| 0·69 | 0·21 | 0·72 | 1·00 | |
| 0·72 | 0·16 | 0·72 | 0·96 | |
| 0·67 | 0·02 | 0·72 | 1·03 | |
| 0·63 | 0·13 | 0·72 | 1·10 | |
| 0·56 | 0·19 | 0·72 | 1·20 | |
| 0·60 | 0·00 | 0·72 | 1·14 | |
| 0·92 | 0·35 | 0·72 | 0·67 | |
| 0·67 | 0·08 | 0·72 | 1·03 | |
| Wurmbea biglandulosa (Ramsey et al., 2006) | ||||
| 0·10 | – | 0·86 | 1·83 | |
| 0·53 | – | 0·86 | 1·10 | |
| 0·96 | 0·22 | 0·86 | 0·34 | |
| 0·89 | 0·12 | 0·87 | 0·46 | |
| Species . | Co-sexual inbreeding (sc)* . | Female inbreeding (sf) . | Inbreeding depression (δ) . | Female advantage threshold [2(1–scδ)] . |
|---|---|---|---|---|
| Bidens sandvicensis (Schultz and Ganders, 1996) | ||||
| 0·71 | – | 0·60 | 1·15 | |
| 0·67 | – | 0·60 | 1·20 | |
| 0·45 | – | 0·60 | 1·46 | |
| 0·51 | – | 0·60 | 1·39 | |
| Chionographis japonica var. kurohimensis (Maki, 1993) | ||||
| 0·94 | 0·03 | 0·34 | 1·36 | |
| 1·00 | – | 0·24 | 1·52 | |
| Schiedea adamantis (Sakai et al., 1997) | ||||
| 0·68 | – | 0·60 | 1·18 | |
| Schiedea salicaria (Weller and Sakai, 2005)† | ||||
| 0·70 | 0·00 | 0·72 | 0·99 | |
| 0·69 | 0·21 | 0·72 | 1·00 | |
| 0·72 | 0·16 | 0·72 | 0·96 | |
| 0·67 | 0·02 | 0·72 | 1·03 | |
| 0·63 | 0·13 | 0·72 | 1·10 | |
| 0·56 | 0·19 | 0·72 | 1·20 | |
| 0·60 | 0·00 | 0·72 | 1·14 | |
| 0·92 | 0·35 | 0·72 | 0·67 | |
| 0·67 | 0·08 | 0·72 | 1·03 | |
| Wurmbea biglandulosa (Ramsey et al., 2006) | ||||
| 0·10 | – | 0·86 | 1·83 | |
| 0·53 | – | 0·86 | 1·10 | |
| 0·96 | 0·22 | 0·86 | 0·34 | |
| 0·89 | 0·12 | 0·87 | 0·46 | |
* Co-sexual inbreeding is corrected to take into account underestimates due to inbreeding depression (Maki, 1993).
† Corrected co-sexual inbreeding as reported in the original paper contained typographic errors. Numbers presented here were obtained directly from S. Weller.
Impacts of inbreeding and inbreeding depression on the maintenance of gender polymorphism in gynodioecious populations
| Species . | Co-sexual inbreeding (sc)* . | Female inbreeding (sf) . | Inbreeding depression (δ) . | Female advantage threshold [2(1–scδ)] . |
|---|---|---|---|---|
| Bidens sandvicensis (Schultz and Ganders, 1996) | ||||
| 0·71 | – | 0·60 | 1·15 | |
| 0·67 | – | 0·60 | 1·20 | |
| 0·45 | – | 0·60 | 1·46 | |
| 0·51 | – | 0·60 | 1·39 | |
| Chionographis japonica var. kurohimensis (Maki, 1993) | ||||
| 0·94 | 0·03 | 0·34 | 1·36 | |
| 1·00 | – | 0·24 | 1·52 | |
| Schiedea adamantis (Sakai et al., 1997) | ||||
| 0·68 | – | 0·60 | 1·18 | |
| Schiedea salicaria (Weller and Sakai, 2005)† | ||||
| 0·70 | 0·00 | 0·72 | 0·99 | |
| 0·69 | 0·21 | 0·72 | 1·00 | |
| 0·72 | 0·16 | 0·72 | 0·96 | |
| 0·67 | 0·02 | 0·72 | 1·03 | |
| 0·63 | 0·13 | 0·72 | 1·10 | |
| 0·56 | 0·19 | 0·72 | 1·20 | |
| 0·60 | 0·00 | 0·72 | 1·14 | |
| 0·92 | 0·35 | 0·72 | 0·67 | |
| 0·67 | 0·08 | 0·72 | 1·03 | |
| Wurmbea biglandulosa (Ramsey et al., 2006) | ||||
| 0·10 | – | 0·86 | 1·83 | |
| 0·53 | – | 0·86 | 1·10 | |
| 0·96 | 0·22 | 0·86 | 0·34 | |
| 0·89 | 0·12 | 0·87 | 0·46 | |
| Species . | Co-sexual inbreeding (sc)* . | Female inbreeding (sf) . | Inbreeding depression (δ) . | Female advantage threshold [2(1–scδ)] . |
|---|---|---|---|---|
| Bidens sandvicensis (Schultz and Ganders, 1996) | ||||
| 0·71 | – | 0·60 | 1·15 | |
| 0·67 | – | 0·60 | 1·20 | |
| 0·45 | – | 0·60 | 1·46 | |
| 0·51 | – | 0·60 | 1·39 | |
| Chionographis japonica var. kurohimensis (Maki, 1993) | ||||
| 0·94 | 0·03 | 0·34 | 1·36 | |
| 1·00 | – | 0·24 | 1·52 | |
| Schiedea adamantis (Sakai et al., 1997) | ||||
| 0·68 | – | 0·60 | 1·18 | |
| Schiedea salicaria (Weller and Sakai, 2005)† | ||||
| 0·70 | 0·00 | 0·72 | 0·99 | |
| 0·69 | 0·21 | 0·72 | 1·00 | |
| 0·72 | 0·16 | 0·72 | 0·96 | |
| 0·67 | 0·02 | 0·72 | 1·03 | |
| 0·63 | 0·13 | 0·72 | 1·10 | |
| 0·56 | 0·19 | 0·72 | 1·20 | |
| 0·60 | 0·00 | 0·72 | 1·14 | |
| 0·92 | 0·35 | 0·72 | 0·67 | |
| 0·67 | 0·08 | 0·72 | 1·03 | |
| Wurmbea biglandulosa (Ramsey et al., 2006) | ||||
| 0·10 | – | 0·86 | 1·83 | |
| 0·53 | – | 0·86 | 1·10 | |
| 0·96 | 0·22 | 0·86 | 0·34 | |
| 0·89 | 0·12 | 0·87 | 0·46 | |
* Co-sexual inbreeding is corrected to take into account underestimates due to inbreeding depression (Maki, 1993).
† Corrected co-sexual inbreeding as reported in the original paper contained typographic errors. Numbers presented here were obtained directly from S. Weller.
In addition to inbreeding effects, other factors contribute to female advantage in gynodioecious populations. Additional resources available for female function when male function is lost can enhance seed set in female plants (see references in Table 2; Talavera et al., 1996; Orellana et al., 2005). Another potential benefit to females from loss of male function is attraction of fewer insects (Asikainen and Mutikainen, 2005) or additional resource input into chemical defences (Alonso et al., 2005), leading to reduced insect predation on female plants (Ashman, 2002).
In contrast to gynodioecy, male advantage has not been found to be sufficient to account for the evolutionary maintenance of androdioecy. For example, in the androdioecious shrub Phillyrea angustifolia, Vassiliadis et al. (2002) measured enhanced male reproductive success in female steriles as 1·4 times that of co-sexuals, well below the K threshold for evolutionary stability of androdioecy required by the Charlesworth and Charlesworth (1978) model. The missing piece to this puzzle can be found by considering metapopulation dynamics, characterized by local extinction and colonization events. It has long been suggested that colonization events are more likely to be successful for co-sexual plants because a single founder could give rise to a new population (Baker, 1955). On the other hand, once such a population is established, high levels of inbreeding should favour the subsequent evolution of gender polymorphism to promote outcrossing. Collectively, this process has been dubbed Baker's law (Stebbins, 1957; Baker, 1967). More recently, Pannell (2000) has pointed out that the early stages of population colonization will tend to result in selection favouring a population imbalance towards female expression through the process of local mate competition, in which a single founder or small number of founders results in mating between closely related individuals in the early stages of a population. In general, there are many more pollen grains produced than are strictly necessary to fertilize all of the available ovules in a population, such that if all of the mating individuals are closely related, an individual that produces offspring that show an excess of female function will have a higher long-term fitness because its offsprings' gametes make up the majority of the gene pool for both genders. As the newly established population grows, the imbalance towards female gender will result in frequency-dependent selection favouring male function. Pannell (2000) has suggested that this process may account for the evolution of androdioecy, and indeed it may account for other gender polymorphisms that sporadically emphasize male function in co-sexual plants, such as andromonoecy (e.g. Elle and Meagher, 2000).
Other aspects of metapopulation dynamics are also important factors in the evolution of genetic control mechanisms for gender variation in gynodioecious species. Gynodioecy is often based on cytoplasmic male sterility (CMS) factors that drive individuals towards female gender expression. For example, an association between mitochondrial markers and gender expression has been found in Silene vulgaris (McCauley and Olson, 2003), although the extent of this association varies across a metapopulation landscape (Olson and McCauley, 2002). A CMS factor will increase if there is even a slightly enhanced seed output for male-sterile individuals. As originally noted by Lewis (1940), the selection threshold for maintenance of females under CMS reduces to k > 1. In the extreme, this would lead to an excess of female expression that is unsustainable and, as the population becomes predominantly female, there will be increasing selection favouring genes that restore male function. Thus, CMS is often found in association with nuclear genes that restore male function, leading to what is known as nucleocytoplasmic control of gynodioecy based on an interaction between one to several CMS factors and corresponding nuclear restorer genes. The interplay of CMS restorer genes could result in heterogeneity in female: co-sexual ratios among populations.
Variation in CMS factors and interactions with different nuclear restorers have been demonstrated empirically by examining sex ratio variation in metapopulation systems (Charlesworth and Laporte, 1998; Laporte et al., 2001; Byers et al., 2005) as well as by performing crosses within and among local populations (Taylor et al., 1999; Dudle et al., 2001). In crosses involving gynodioecious species with nucleocytoplasmic sex determination, cytoplasmic effects will drive progeny sex ratios towards all female for progeny of female plants and all hermaphroditic for progeny from hermaphroditic plants. The presence of male restorer effects is demonstrated by varying proportions of hermaphroditic progeny from female parents, reflecting the frequency of restorer alleles in the pollen pool as well as segregation of females in progeny from hermaphroditic parents that are heterozygous for restorer alleles. The evolutionary maintenance of nucleocytoplasmic sex determination is further complicated by local inbreeding, which can enhance loss through genetic drift of male restorer alleles in small populations (Byers et al., 2005).
Summary
In summary, we arrive at a general picture of the various interacting factors that lead to the evolution of gender polymorphism. A major factor is the impact of inbreeding depression manifested due to inbreeding in a population, providing strong selection favouring the evolution of an outbreeding mechanism. One mechanism to promote outbreeding is gender polymorphism: female plants in a gynodioecious population are obligately outbred, as are males in an androdioecious population. Indeed, the evolution of dioecy has been perceived by some authors as driven primarily by selection favouring outbreeding (e.g. Stebbins, 1957). The other major factor is reproductive compensation (Darwin, 1877; Charnov, 1982), in which male or female reproductive success is sufficiently enhanced by gender specialization to offset the loss of the other sex function. Although the theory developed to explain the evolution of gender polymorphism has been very effective at making predictions about observed gender dynamics in plant populations, it still treats underlying genetic effects as a black box.
DEVELOPMENTAL GENETICS OF GENDER EXPRESSION
The ABC(D)E model of floral development
One of the momentous discoveries in developmental evolution is the existence of major gene systems that control the development of entire organs. Some of these gene systems are extremely ancient and have a long evolutionary history; for example, the MADS box genes, named for some of the genes (MCM1, AGAMOUS, DEFICIENS and SRF) first identified as sharing the MADS transcription sequence. The MADS box has been found as part of gene transcription regulation sequences in a wide range of eukaryotes, and appears to be ubiquitous (Shore and Sharrocks, 1995; Theissen et al., 1996). In the flowering plants, the MADS genes are a critical component of the emerging genetic model of flower development.
Early on, studies of floral development converged on the ABC model (Coen and Meyerowitz, 1991; Ng and Yanofsky, 2000; Jack, 2001), with a cascade of MADS box genes regulating successive whorls of the flower: sepals (calyx) arise from A-function genes, petals (corolla) arise from A-function and B-function genes, stamens (androecium) arise from B-function and C-function genes, and carpels (gynoecium) arise from C-function genes. The ABC model has an essential elegance, but it only encompasses a subset of the various interacting MADS box genes contributing to floral development. Colombo et al. (1995) identified a MADS box gene involved in ovule development in Petunia and proposed an ABCD model, in which D-function genes are solely involved in ovule development. Another class of MADS genes found in Arabidopsis, SEPELLATA (Pelaz et al., 2000), appear to provide another layer of developmental control, and have been labelled ‘E’ class genes; assuming lack of generality of D-function genes, we are left with the ABCE model (Theissen, 2001).
Model organisms, such as Arabidopsis thaliana and Antirrhinum majus, have played a critical role in identifying regulatory pathways in floral development, with specific genes isolated representing every level of the ABCE model (Table 3). Blazquez (2000) summarized a range of environmental inputs and genetic regulatory effects underlying floral development in A. thaliana, highlighting the role of MADS box genes. The level of detail on the genetic developmental network that provides the interface between environmental stimuli and floral development is extensive. As in the original ABC model, the current view of A. thaliana floral development shows the four major floral whorls as the end-points, and it still roughly corresponds to an updated ABC sequence of MADS box gene effects. DNA sequences from MADS box genes in these species have been used to identify MADS box genes with homologous function across a wide range of flowering plants (e.g. Frohlich and Meyerowitz, 1997; Kim et al., 2004).
Floral development genes identified in model plant species
| Species . | A function . | B function . | C function . | E function . | References . |
|---|---|---|---|---|---|
| Arabidopsis thaliana | APETALA1, APETALA2 | APETALA3, PISTILLATA | AGAMOUS | SEPALLATA1, SEPALLATA2, SEPALLATA3 | (Bowman et al., 1989; Pelaz et al., 2000) |
| Antirrhinum majus | SQUAMOSA | DEFICIENS, GLOBOSA | PLENA | (Schwarz-Sommer et al., 1990) |
| Species . | A function . | B function . | C function . | E function . | References . |
|---|---|---|---|---|---|
| Arabidopsis thaliana | APETALA1, APETALA2 | APETALA3, PISTILLATA | AGAMOUS | SEPALLATA1, SEPALLATA2, SEPALLATA3 | (Bowman et al., 1989; Pelaz et al., 2000) |
| Antirrhinum majus | SQUAMOSA | DEFICIENS, GLOBOSA | PLENA | (Schwarz-Sommer et al., 1990) |
Floral development genes identified in model plant species
| Species . | A function . | B function . | C function . | E function . | References . |
|---|---|---|---|---|---|
| Arabidopsis thaliana | APETALA1, APETALA2 | APETALA3, PISTILLATA | AGAMOUS | SEPALLATA1, SEPALLATA2, SEPALLATA3 | (Bowman et al., 1989; Pelaz et al., 2000) |
| Antirrhinum majus | SQUAMOSA | DEFICIENS, GLOBOSA | PLENA | (Schwarz-Sommer et al., 1990) |
| Species . | A function . | B function . | C function . | E function . | References . |
|---|---|---|---|---|---|
| Arabidopsis thaliana | APETALA1, APETALA2 | APETALA3, PISTILLATA | AGAMOUS | SEPALLATA1, SEPALLATA2, SEPALLATA3 | (Bowman et al., 1989; Pelaz et al., 2000) |
| Antirrhinum majus | SQUAMOSA | DEFICIENS, GLOBOSA | PLENA | (Schwarz-Sommer et al., 1990) |
Floral origins
The predominant role of MADS box genes in floral development suggests that their gene genealogies would provide insight into the evolutionary origin of the flower. Indeed, B-function MADS box genes in particular have been shown to have undergone evolutionary radiation within the flowering plants, with an earlier divergence through a common ancestor from a gymnosperm predecessor (Frohlich and Meyerowitz, 1997; Kim et al., 2004; Stellari et al., 2004). Gender specificity of MADS box genes may lie at the heart of floral origins in that the B-function MADS box genes appear to be ancestral to modern C-function genes in flowering plants (Kramer et al., 1998).
On the basis of MADS box gene phylogenies, as well as morphological evidence, it has been suggested that the modern flower is derivative of male strobili in the common ancestor of modern gymnosperms and angiosperms (Frohlich and Parker, 2000). Under this scenario, the genes for female expression would have been derived evolutionarily from genes for male expression. It has also been noted, independently, that much of the selection driving floral evolution is based on male function. Invoking Bateman's Principle, male reproductive success is perceived to be limited by opportunities for mating, which in turn is based on frequency of floral visitation by pollinators, whereas female reproductive success is perceived to be limited by resources required for seed set (Bell, 1985, and many papers since). Collectively, this leads to the prediction that floral attractiveness to pollinators is primarily driven by selection favouring male reproductive success because male success is enhanced by multiple visits whereas a single visit can saturate potential female success. Obviously, the female function of flowers is also critical to overall reproductive success, but the nature of selection driving female function is likely to be based on frequency-dependent resource allocation internal to the plant rather than interaction with external pollinators as such.
Gender polymorphism
Given the role of B-function and C-function MADS box genes in the development of the androecium and gynoecium, these developmental genes seem a likely candidate for the cause of gender expression, and indeed could play a role in gender asymmetry, such as the separation of male and female function in dioecious species. The catalogue of dioecious species in which these genes have been identified is growing (Table 4). Two plant species that have been the object of considerable investigation of gender differentiation, from sex chromosomes to floral evolution, are Silene latifolia and Rumex acetosa. I will address each species in turn.
Floral development genes identified in selected dioecious plant species.
| Species . | A function . | B function . | C function . | E function . | References . |
|---|---|---|---|---|---|
| Silene latifolia | SLM4, SLM5 | SLM2, SLM3 | SLM1 | SISEP1, SISEP3 | (Hardenack et al., 1994; Matsunaga et al., 2004) |
| Rumex acetosa | RAD1, RAD2 | RAP1 | (Ainsworth et al., 1995) | ||
| Thalictrum dioicum | ThdPI-1, ThdPI-2, ThdAP3-1, ThdAP3-2a, ThdAP3-2b | ThdAG-1, ThdAG-2, ThtAG-1, ThtAG-2 | (Di Stilio et al., 2005) | ||
| (T. thalictroides) | ThtPI, ThtAP3-1, ThtAP3-2a, ThtAP3-2b | ||||
| Asparagus officinalis | AODEF | AOM1 | (Caporali et al., 2000; Park et al., 2003) | ||
| Populus | PTAG1, PTAG2 | (Brunner et al., 2000) | |||
| Spinacea oleracea | SpAPETALA3, SpPISTILLATA | SpAGAMOUS | (Pfent et al., 2005; Sather et al., 2005) |
| Species . | A function . | B function . | C function . | E function . | References . |
|---|---|---|---|---|---|
| Silene latifolia | SLM4, SLM5 | SLM2, SLM3 | SLM1 | SISEP1, SISEP3 | (Hardenack et al., 1994; Matsunaga et al., 2004) |
| Rumex acetosa | RAD1, RAD2 | RAP1 | (Ainsworth et al., 1995) | ||
| Thalictrum dioicum | ThdPI-1, ThdPI-2, ThdAP3-1, ThdAP3-2a, ThdAP3-2b | ThdAG-1, ThdAG-2, ThtAG-1, ThtAG-2 | (Di Stilio et al., 2005) | ||
| (T. thalictroides) | ThtPI, ThtAP3-1, ThtAP3-2a, ThtAP3-2b | ||||
| Asparagus officinalis | AODEF | AOM1 | (Caporali et al., 2000; Park et al., 2003) | ||
| Populus | PTAG1, PTAG2 | (Brunner et al., 2000) | |||
| Spinacea oleracea | SpAPETALA3, SpPISTILLATA | SpAGAMOUS | (Pfent et al., 2005; Sather et al., 2005) |
Floral development genes identified in selected dioecious plant species.
| Species . | A function . | B function . | C function . | E function . | References . |
|---|---|---|---|---|---|
| Silene latifolia | SLM4, SLM5 | SLM2, SLM3 | SLM1 | SISEP1, SISEP3 | (Hardenack et al., 1994; Matsunaga et al., 2004) |
| Rumex acetosa | RAD1, RAD2 | RAP1 | (Ainsworth et al., 1995) | ||
| Thalictrum dioicum | ThdPI-1, ThdPI-2, ThdAP3-1, ThdAP3-2a, ThdAP3-2b | ThdAG-1, ThdAG-2, ThtAG-1, ThtAG-2 | (Di Stilio et al., 2005) | ||
| (T. thalictroides) | ThtPI, ThtAP3-1, ThtAP3-2a, ThtAP3-2b | ||||
| Asparagus officinalis | AODEF | AOM1 | (Caporali et al., 2000; Park et al., 2003) | ||
| Populus | PTAG1, PTAG2 | (Brunner et al., 2000) | |||
| Spinacea oleracea | SpAPETALA3, SpPISTILLATA | SpAGAMOUS | (Pfent et al., 2005; Sather et al., 2005) |
| Species . | A function . | B function . | C function . | E function . | References . |
|---|---|---|---|---|---|
| Silene latifolia | SLM4, SLM5 | SLM2, SLM3 | SLM1 | SISEP1, SISEP3 | (Hardenack et al., 1994; Matsunaga et al., 2004) |
| Rumex acetosa | RAD1, RAD2 | RAP1 | (Ainsworth et al., 1995) | ||
| Thalictrum dioicum | ThdPI-1, ThdPI-2, ThdAP3-1, ThdAP3-2a, ThdAP3-2b | ThdAG-1, ThdAG-2, ThtAG-1, ThtAG-2 | (Di Stilio et al., 2005) | ||
| (T. thalictroides) | ThtPI, ThtAP3-1, ThtAP3-2a, ThtAP3-2b | ||||
| Asparagus officinalis | AODEF | AOM1 | (Caporali et al., 2000; Park et al., 2003) | ||
| Populus | PTAG1, PTAG2 | (Brunner et al., 2000) | |||
| Spinacea oleracea | SpAPETALA3, SpPISTILLATA | SpAGAMOUS | (Pfent et al., 2005; Sather et al., 2005) |
Silene latifolia has a long history or exploration of sex determination. It was the first plant species in which sex chromosomes were identified (Westergaard, 1958), and it is an object of ongoing investigation into the structure and evolution of sex chromosomes and their role in gender expression. This species has an XX female XY male chromosomal sex determination, with a Y chromosome that is greatly enlarged relative to the X, which in turn is substantially larger than any of the autosomes. Silene latifolia has been widely used to investigate the evolutionary mechanisms of sex chromosome evolution, through theoretical models (Charlesworth, 1991), analysis of sequence divergence in homologous genes in recombining and non-recombining regions (Atanassov et al., 2001), and genetic and cytological analysis of chromosome rearrangement (Moore et al., 2003). It was proposed early on, through analysis of plants showing deletions of portions of the Y chromosome (Westergaard, 1958; Donnison et al., 1996), that genes located on the Y chromosome serve to suppress gynoecium development and promote anther development. Thus, the classical view of sex determination in this species is that genes that result in male or female function are present in both sexes, indicating a significant role for the X chromosomes, but are differentially expressed due to impacts of Y-linked genes (Charlesworth, 2002). More recently, Lebel-Hardenack et al. (2002) used an amplified fragment length polymorphism (AFLP) based map of the Y chromosome to locate specific chromosomal deletions that confirm the classical view of three male-determining factors on Y chromosomes of S. latifolia: carpel suppression, early stamen development and late stamen development.
In order to identify more specifically genes associated with gender expression in S. latifolia, sex-specific cDNA libraries have been constructed (Matsunaga et al., 1996; Barbacar et al., 1997; Hinnisdaels et al., 1997). One class of genes identified by such methods includes MROS1, MROS2, MROS3 and MROS4 that are expressed exclusively in the development of the androecium (Matsunaga et al., 1996, 1997). The placement of these loci corresponds to the classical view of genetic control of sex expression in that they are all either X-linked (MROS3) or autosomal (MROS1, MROS2 and MROS4), and hence present in both sexes but differentially expressed in males (Kejnovsky et al., 2001). Another gene, MEN-9, with a DNA sequence indicating a close relationship to MROS3, has been found to be correlated with androecium development (Robertson et al., 1997; Scutt et al., 1997). This gene starts out being expressed in flower primordia of both sexes, but then drops out during female flower development at the stage that stamen development is arrested. Interestingly, this gene is also expressed later in flower development in female flowers in which stamen development has been induced by the smut Microbotryum violaceum (Robertson et al., 1997).
The ABCE model has also been explored in S. latifolia (see Table 4). Hardenack et al. (1994) constructed cDNA libraries and screened them for sequence homology with MADS box genes from A. majus. They subsequently used in situ hybridization to identify expression patterns of these sequences in different floral tissues, concluding that apparent B-function genes (SLM2 and SLM3) showed differential expression in male and female organs, and hence were a potential candidate for sex-determining loci. E-function genes have also been isolated from S. latifolia, but their relationship to gender expression is not clear (Matsunaga et al., 2004).
The genus Rumex has also been well studied with regard to sex chromosomes and sex determination (Westergaard, 1958; Smith, 1969). For example, R. acetosa has two Y chromosomes, resulting in XX females and XY1Y2 males. Unlike S. latifolia, in which the Y chromosome is active in sex determination, R. acetosa exhibits sex expression based on X: autosome balance and dosage compensation (Parker and Clark, 1991). As in S. latifolia, analogues of B-function and C-function MADS genes have been identified in R. acetosa and their relationship to floral gender expression explored (Ainsworth et al., 1995). The B-function genes (RAD1 and RAD2) were found to be associated with androecium development. A C-function gene (RAP1) was expressed in early development of both floral genders, but then dropped out as either androecium or gynoecium development was arrested in female and male flowers, respectively. Subsequent investigation of the C-function gene (Ainsworth et al., 2005) showed that it is unlikely to be a causal factor in gender identification. Thus, there are sex-specific patterns of expression of B- and C-function genes in R. acetosa, but their role as a causal basis for gender differentiation is uncertain.
Summary
The ABC(D)E model has provided a useful framework for interpreting floral development. This model was originally based on specific developmental genes, MADS box genes, identified in the model species A. thaliana and A. majus by observation of developmental anomalies associated with different mutant types. Through identification of genes in other species that show sequence homology with the relevant MADS box genes in these model species, it has been shown that such MADS box genes are associated with floral development across flowering plant species. B-function and C-function MADS box genes are specifically associated with floral whorls involved in male expression (androecium) and female expression (gynoecium), and indeed differential expression of such genes has been demonstrated in a number of plant species showing gender polymorphism. At present, it is not known whether such correlated expression patterns imply a causal role for B-function or C-function genes in gender expression, or whether differential expression reflects higher order genetic effects underlying gender variation.
SYNTHESIS
There is a constructive dynamic between the evolutionary theory regarding gender polymorphism and empirical confirmation of its premises. This situation has developed in large part because of the ongoing development and deployment of genetic marker-based assessment of plant breeding systems. Similarly, models of flower morphogenesis and the underlying developmental genetics have expanded greatly, to the point where it should be feasible to study such phenomena at a population genetic level. Integration of insights and methods from these disparate lines of investigation holds great promise for increasing our understanding of floral gender evolution. I highlight a few selected examples of potential lines of investigation below.
Floral origins
To some extent, developmental genetics has already had a significant impact on our understanding of the evolutionary origin of the flower. As noted above, analysis of gene genealogies of B-function genes has contributed to our understanding of the evolution of the flower. Using such methods, it has been suggested that the earliest flowers may have been derived from predominantly male strobili, with the C-function genes that regulate female gender expression in flowers being derivative of B-function genes. It might be useful to take this a step further in order to interpret the evolutionary pressures that may have generated flowers in the first place. Specifically, work on gene genealogies suggests that the evolutionary sequence in the development of the ancestral flower involved dioecy → gynodioecy → hermaphroditism or monoecy → gynomonoecy → hermaphroditism from a common ancestor with gymnosperms. Certain steps in these potential pathways are well understood, such as transitions between gynodioecy and hermaphroditism, but others are not. The transition from dioecy to gynodioecy has not been explored on theoretical grounds, and there has been no empirical work done on this transition either. Moreover, the majority of the empirical work that has been done on the evolution of gender polymorphisms has focused on modern angiosperms. Little is known of how well existing theory addresses gender polymorphisms in gymnosperms. Studies of breeding system evolution in conifers most closely related to flowering plants, for example the Gnetales, would be useful in interpreting the ecological conditions under which flowers might have evolved. This would nicely complement ongoing studies based on morphological and phylogenetic evidence.
Genetic effects underlying gender
Models for the evolution of gender polymorphism typically invoke major genes with specific effects (e.g. male sterility or female sterility) as an approximation to the underlying genetics. The developmental models that have emerged in recent years suggest a more complex mechanism of gender control, involving an interacting network of environmental and genetic effects. Although developmental genetics of gender is still based on correlations of gene expression with morphology, it is rapidly approaching a model in which specific causal factors in gender expression will be apparent. Current models for the evolution of gender in plants define thresholds for the evolutionary maintenance of gender polymorphism, but they are unable to provide insight into the evolutionary trajectory leading to such polymorphism. There is scope for the development of evolutionary models that take on board the complexity of gender expression as articulated in model species. Such models would provide a new search image for analysis of variation in gender, such as candidate genes for population level assessment of variation in expression patterns associated with gender polymorphism.
Integration with quantitative genetics
Although gender expression is generally regarded as a qualitative trait, other traits associated with gender, such as flower size and resource allocation patterns, are typically interpreted as quantitative traits. Quantitative genetic analyses of sexually dimorphic species have been conducted in only a few dioecious species (Meagher, 1999). Among these, the best studied is S. latifolia (Meagher, 1992, 1994; Delph et al., 2002, 2004). The studies have shown that, although there are gender differences in flower size (male flowers are larger), the genetic variation underlying flower size in male and female flowers is tightly correlated. Thus, the genetic effects involved in gender differentiation are distinct from those that drive flower size evolution. Indeed, recent work in my laboratory has shown that genetic effects involved in flower size evolution may be the result of gene regulatory impacts of variation in repetitive DNA, manifested as a negative correlation between genome size and flower size (Meagher and Costich, 1996, 2004; Meagher et al., 2005).
The underlying premise of quantitative genetics is the infinitesimal model in which quantitative variation is due to additive effects of allelic substitution across a large number of loci (Bulmer, 1980; Falconer and Mackay, 1996). This approach has proven useful in interpreting the genetic properties of sex differences in dioecious and gynodioecious species (Meagher, 1992, 1999; Ashman, 2003; Culley et al., 2006); but with more detailed genomic analyses, such as those involving genome size and composition as well as those involving developmental genetic effects, it has become clear that the quantitative genetic model for character expression is only an approximation. These is a need for a comprehensive model that takes into account the various levels of interaction between environmental stimuli and genome organization, incorporating into the latter impacts of MADS box genes as well as repetitive DNA on patterns of gene expression.
CONCLUSION
Evolutionary models of gender evolution have, of necessity, posited genetic effects that are relatively simple in their impacts. The emerging insights from developmental genetics have demonstrated that the underlying reality is a more complex matrix of interacting factors. The study of gender variation in plants is poised for significant advance through the integration of these two perspectives. Bringing genomic tools to bear on population level processes, we may finally develop a comprehensive perspective on the evolution of floral gender.
I have highlighted above a few areas of potential synthesis between evolutionary genetics and developmental genetics of gender polymorphism. These are not meant to limit the synthesis to just these areas, but rather to suggest only a few of many possible directions. I look forward to what the literature on plant gender evolution will bring forth as this synthesis moves forward.
ACKNOWLEDGEMENTS
I thank the Editorial Board of Annals of Botany for inviting this review and thus encouraging me to develop my thinking in this area. I thank input from my colleagues with whom I have discussed the premise of this review during its preparation, including R. Abbott, J. Antonovics, T.-L. Ashman, D. Charlesworth, D. Costich, E. Elle, A. Hudson, M. Looseley, E. Nic Lughadha, L. Meagher, S. Weller, J. Wright and R. Yahr. I also thank the members of the University of St Andrews Evolution Genes & Genomics Forum for their input.



