-
PDF
- Split View
-
Views
-
Cite
Cite
Ortal Danino, Shuli Svetitsky, Sarah Kenigsberg, Asaf Levin, Shani Journo, Aviram Gold, Michael Drexler, Nimrod Snir, Ori Elkayam, Bilha Fischer, Uri Arad, Inhibition of nucleotide pyrophosphatase/phosphodiesterase 1: implications for developing a calcium pyrophosphate deposition disease modifying drug, Rheumatology, Volume 57, Issue 8, August 2018, Pages 1472–1480, https://doi.org/10.1093/rheumatology/key092
Close - Share Icon Share
Abstract
Calcium pyrophosphate deposition (CPPD) is associated with osteoarthritis and is the cause of a common inflammatory articular disease. Ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (eNPP1) is the major ecto-pyrophosphatase in chondrocytes and cartilage-derived matrix vesicles (MVs). Thus, eNPP1 is a principle contributor to extracellular pyrophosphate levels and a potential target for interventions aimed at preventing CPPD. Recently, we synthesized and described a novel eNPP1-specific inhibitor, SK4A, and we set out to evaluate whether this inhibitor attenuates nucleotide pyrophosphatase activity in human OA cartilage.
Cartilage tissue, chondrocytes and cartilage-derived MVs were obtained from donors with OA undergoing arthroplasty. The effect of SK4A on cell viability was assayed by the XTT method. eNPP1 expression was evaluated by western blot. Nucleotide pyrophosphatase activity was measured by a colorimetric assay and by HPLC analysis of adenosine triphosphate (ATP) levels. ATP-induced calcium deposition in cultured chondrocytes was visualized and quantified with Alizarin red S staining.
OA chondrocytes expressed eNPP1 in early passages, but this expression was subsequently lost upon further passaging. Similarly, significant nucleotide pyrophosphatase activity was only detected in early-passage chondrocytes. The eNPP1 inhibitor, SK4A, was not toxic to chondrocytes and stable in culture medium and human plasma. SK4A effectively inhibited nucleotide pyrophosphatase activity in whole cartilage tissue, in chondrocytes and in cartilage-derived MVs and reduced ATP-induced CPPD.
Nucleotide analogues such as SK4A may be developed as potent and specific inhibitors of eNPP1 for the purpose of lowering extracellular pyrophosphate levels in human cartilage with the aim of preventing and treating CPPD disease.
A nucleotide analogue was developed as a specific inhibitor of ecto-nucleotide pyrophosphatase/phosphodiesterase 1.
An ecto-nucleotide pyrophosphatase/phosphodiesterase 1 inhibitor attenuates adenosine triphosphate-induced CPPD in osteoarthritic chondrocytes.
Ecto-nucleotide pyrophosphatase/phosphodiesterase 1 inhibitors may be developed to prevent and treat CPPD disease.
Introduction
Calcium pyrophosphate deposition (CPPD) associates with ageing and can manifest as a common asymptomatic radiological finding of cartilage calcification (chondrocalcinosis), but can also cause several clinical overlapping arthropathies [1]. Acute calcium pyrophosphate dihydrate (CPP) crystal arthritis (formerly termed pseudogout) is one of the most common inflammatory arthritides [2]. OA with CPPD may associate with more inflammatory features, with a more rapid progression and with an atypical distribution of affected joints (i.e. the radiocarpal joints and the elbows) [3]. Chronic and recurrent CPP crystal arthritis can lead to rapid joint destruction that is difficult to treat [4]. Unlike gout, there is no specific treatment to prevent CPP crystal deposition or to induce CPP crystal dissolution, and treatment with anti-inflammatory agents is mostly symptomatic [1].
Articular chondrocytes along with cartilage matrix vesicles (MVs) maintain physiological levels of extracellular pyrophosphate (PPi) that prevent mineralization of both CPP and basic calcium phosphate. The ratio of inorganic phosphate (Pi) to inorganic PPi in the SF or cartilage matrix controls tissue mineralization, such that low Pi:PPi ratio (<6.0) drives CPP crystal formation and a high Pi:PPi ratio (>140) favours formation of hydroxyapatite crystals [5]. In cartilage, extracellular PPi concentrations are tightly regulated by the balance between three key factors: (i) the activity of PPi producing enzymes, mainly ecto-nucleotide pyrophosphatase phosphodiesterase 1 (eNPP1); (ii) the activity of PPi-hydrolysing enzymes such as tissue non-specific alkaline phosphatase (TNAP) and others; and (iii) the extent of export of intracellular PPi via the multipass transmembrane protein ANK. Of note, depending upon the orientation with which the substrate approaches the catalytic site, eNPP1 can hydrolyse adenosine triphosphate (ATP) to either adenosine diphosphate (ADP) or adenosine monophosphate, releasing Pi or PPi accordingly. Moreover, ATP may also serve as a substrate for alkaline phosphatases [6, 7].
PPi concentrations are elevated (about 2-fold) in SFs of patients with CPPD [8–10] and cartilage of ageing individuals generates increased levels of PPi [11]. Although gain-of-function mutations in the ANKH gene have been demonstrated to cause rare syndromes of familial CPPD, and a functional polymorphism in the 5′-untranslated region of ANKH is associated with sporadic CPPD [12, 13], eNPP1 has more influence on the levels of PPi [14].
eNPP1 (formerly termed PC-1) generates PPi by hydrolysing extracellular ATP, as has been demonstrated in ex vivo human articular tissue and cellular models [14–17] and in osteoblasts and MVs derived from genetically altered murine models [18, 19]. Nucleotide pyrophosphatase (NPPase) activity is increased in human CPPD SFs and correlates with elevated PPi concentrations [10]. These findings implicate eNPP1 as a major contributor to the pathological NPPase activity and PPi levels observed in CPPD. Interestingly, one study concluded that in contrast to eNPP1’s role at the cell membrane, in MVs, eNPP1 does not appear to use ATP efficiently and instead TNAP, which is a major pyrophosphate hydrolysing enzyme, is also an ATPase and ADPase [20].
Recently, we have reported several promising eNPP1 inhibitors based on nucleotide and dinucleotide scaffolds [21, 22]. One of these compounds, ATP-α-thio-β,γ-CCl2, termed SK4A in the present article and analogue 2a in our previous report [22], was identified as a biocompatible, chemically and metabolically stable, water-soluble substance, and therefore deemed most suitable for further research. This inhibitor was not hydrolysed by eNPP1, NPP3 and ecto-nucleotidases nucleoside triphosphate (NTP) diphosphohydrolase (NTPDase) 1, 2, 3 and 8 (<5% hydrolysis), and barely affected the activity of NTPDase1, 2, 3 and 8 and NPP3. SK4A was not only a selective eNPP1 inhibitor with Ki of 0.68 μM and IC50 of 0.57 μM, but also a non-agonist of P2Y1/P2Y2/P2Y11 receptors.
Here, we utilized this promising eNPP1 inhibitor to study the role of eNPP1-mediated NPPase activity in affecting the levels of extracellular PPi in human osteoarthritic cartilage and to evaluate whether such nucleotide analogues could potentially be developed as disease-modifying drug candidates for CPPD-related arthritis.
Methods
Ethics approval
Cartilage was obtained from OA patients undergoing total knee or hip replacement surgery. The study was approved by the institutional ethics committee of the Tel Aviv Sourasky Medical Center, according to the Declaration of Helsinki. All patients provided written informed consent.
Chondrocyte, MVs and whole cartilage isolation and culture
Human articular chondrocytes were isolated from OA knee and hip joints by sequential digestion and cultured as follows: cartilage slices were removed and washed in phosphate-buffered saline (PBS). Tissues were then minced with a scalpel transferred into a digestion buffer containing Hanks’ balanced salt solution (HBSS) with 4 Wünsch units of LiberaseTM (Roche Applied Science, Mannheim, Germany) and incubated on a gyratory shaker (65 rpm) at 37°C overnight. Residual multicellular aggregates were removed by vortex and filtration through a 80 μm nylon mesh. The suspension was centrifuged (600 g, 10 min) to pellet the cells. The supernatant was kept at 4°C for MV isolation and the cell pellet was treated with trypsin (37°C for 10 min), washed three times with PBS and plated. In some cases the chondrocytes were subcultured once. The cells were grown in DMEM containing 10% fetal bovine serum and antibiotics until 80–90% confluence.
MVs were isolated essentially as previously described [5]. In brief, after removal of the chondrocytes from the cartilage-digest, the supernatant was centrifuged at 13 000 g for 20 min to remove cellular debris. The pellet was discarded and the supernatant was submitted to a third centrifugation at 90 000 g for 1 h. The final pellet containing MVs was suspended in 300 μl of HBSS without phenol red and stored at 4°C. The protein concentration in the MV fraction was determined using the BCA assay kit (Pierce Biotechnology, Rockford, IL, USA).
Full thickness cartilage discs were prepared with a 5 mm diameter punch biopsy tool. The discs were weighed and placed at the well bottoms of a 96-well tissue culture plate and then overlaid with 100 μl DMEM without phenol red and incubated for 24 h in 37°C, 5% CO2.
The NPP1 inhibitor ATP-α-thio-β,γ-CCl2, termed SK4A in the present article, was used in further experiment and was synthesized, purified and characterized as we have described before [22].
Cell viability was determined by the sodium 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium inner salt (XTT) assay (Biological Industries Ltd, Beit Haemek, Israel) according to the manufacturer’s instructions.
Determination of metabolic stability of ATP in cultured chondrocytes
Chondrocytes were grown in DMEM and seeded into culture plates with 100 μM ATP for 0, 3, 6 and 8 h. After incubation, the samples were collected and heated at 80°C for 15 min and then centrifuged and extracted with chloroform at 1:1 v/v ratio. The aqueous layer was removed and freeze-dried. Each sample’s resulting residue was applied to an activated StrataTM X-AW weak anion exchange cartridge (Phenomenex, Torrence, CA) and then freeze-dried. The resulting residue was analysed by HPLC. The mixture was separated on a Gemini analytical column (5 u, C-18, 110 A; 150 × 4.60 mm) using isocratic elution with 50 mM triethylammonium acetate (TEAA):CH3CN, 96:4, and flow rate of 1 ml min−1. The enzymatic hydrolysis rate of ATP was determined by measuring the change in the integration of the respective HPLC peaks with time, as compared with controls (ATP and its hydrolysis products).
NPPase and TNAP colorimetric activity assays
For NPPase assays, cells (1.5 × 104 cells per well), cartilage discs or MV preparations (1–3 mg ml−1) were incubated in 200 μl (240 μl for cartilage discs) HBSS and 0.1 mM p-nitrophenyl-thymidine 5′-monophosphate (pnp-TMP; Sigma-Aldrich. cat. no. T4510) in 96-well plates at 37°C with gentle agitation [23]. SK4A was added at the concentrations indicated in each experiment. At the time points indicated, absorbance at 405 nm was measured with an ELISA reader. TNAP assays were similarly performed except that the substrate was p-nitrophenyl phosphate (Sigma-Aldrich, cat. no. P7998). A standard curve of 405 nm absorbance to p-nitrophenol (Sigma-Aldrich, cat. no. 73560) concentration was used to calculate enzymatic activity.
Immunoblotting
Cells were lysed after the indicated time points with ice-cold RIPA buffer [50 mM Tris–HCl, pH 7.2; 150 mM NaCl; 0.5% (v/v) Triton X-100; 5 mM EDTA] supplemented with protease inhibitor cocktail (Roche). Particulate matter was removed by centrifugation at 13 000 g, 10 min at 4°C and lysates stored at −80°C until use. Lysates were resolved by SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and subsequently probed with ENPP-1 antibody (Novusbio, Littleton, CO, cat. no. NBP2-27561) and actin antibody (Santa Cruz Biotechnology, Dallas, TX, USA, cat. no. sc-1616).
ATP-induced CPPD in chondrocyte monolayers
Chondrocytes were seeded at 105 cells per well, in triplicate, in 96-well plates. Twenty-four hours before the beginning of each experiment the culture media were changed to DMEM without serum. The media were then replenished with 250 μM ATP and the indicated concentrations of SK4A and a control without ATP. After 5 days of incubation, calcium deposition in the cell monolayer was visualized and quantified with Alizarin red S (ARS) staining as previously described [24]. In brief, the cells were washed 3 times with PBS, fixed in 10% formalin, washed 3 times with dH2O and stained for 30 min with 40 mM ARS, pH 4.1. The monolayers were then extensively washed with dH2O, photographed and the remaining ARS stain was extracted with 10% acetic acid and quantified with an ELISA reader at 405 nm, relative to a standard curve.
Determination of the metabolic stability of SK4A in chondrocyte cell-cultures, human serum and human blood
For the determination of SK4A metabolic stability in chondrocyte cell-cultures medium, chondrocytes were grown in DMEM and seeded into medium in 24-well tissue culture plates for 24 h. The assay mixture, containing a final concentration of 0.1 mM SK4A or ATP in DMEM, was incubated at 37°C for 1 min and 1, 2, 4, 8 and 24 h. The assay mixture for human serum contained SK4A (0.2 mM) in 500 µl of DMEM and 200 µl human serum. The assay mixture for the evaluation of the stability of SK4A in human whole blood contained SK4A (final concentration of 0.2 mM) in 220 µl of DMEM and 400 µl human blood. After incubation at 37°C for indicated time points, the samples were collected and extracted with chloroform at 1:1 (v/v) ratio. The aqueous layer was removed and freeze-dried. Each sample’s resulting residue was applied to an activated Strata X-AW weak anion exchange cartridge and then freeze-dried. The resulting residue was analysed by HPLC; the mixture was separated on a Gemini analytical column (5 u, C-18, 110 A; 150 × 4.60 mm) using isocratic elution with 50 mM TEAA:CH3CN, 92:8 and flow rate of 1 ml min−1. The enzymatic hydrolysis rate of SK4A was determined by measuring the change in the integration of its HPLC peak with time, as compared with controls (SK4A and its hydrolysis products).
Statistical analysis
Colorimetric assays were performed in triplicate (chondrocytes and MVs) and quadruplicate (whole cartilage tissue) and repeated with samples from different patient donors (n = 10 for chondrocytes, n = 8 for MVs and n = 3 for whole cartilage tissue). Values of NPPase activity (increase in product concentration) were expressed as the mean (s.d.) of all experiments. A paired-samples two-sided t test was used to determine whether there was a statistically significant difference at 1 h.
For inhibition of ATP hydrolysis, six experiments were performed with primary chondrocytes from two different patient donors. Values of ATP remaining were expressed as mean (s.d.). An independent-samples t test was run to determine whether there were differences at 8 h. A value of P ⩽ 0.05 was considered statistically significant. Analyses were performed with SPSS Statistics software, version 24 (IBM Corp., Armonk, NY, USA).
Assays for ATP-induced CPPD were performed in triplicate. Four experiments were performed, each with chondrocytes derived from a different donor. Differences in the mean levels of ARS stain in SK4A-treated samples relative to samples treated with ATP only were analysed by Friedman’s test with Dunn’s multiple comparison post hoc test. A value of P ⩽ 0.05 was considered statistically significant. Analyses were performed with Prism software (GraphPad Software, La Jolla, CA, USA).
Results
eNPP1 is expressed in primary human chondrocytes
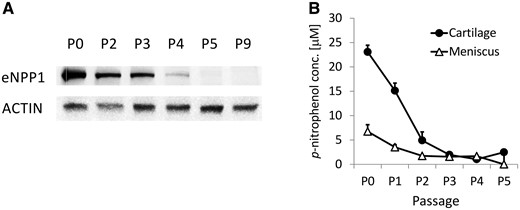
We first determined the expression of eNPP1 in primary human chondrocytes. For this purpose, chondrocytes were harvested from resected femoral condyle and tibial plateau cartilage obtained from patients with OA undergoing knee replacement surgery. The chondrocytes were cultured and samples were taken from each passage for analysis of eNPP1 expression by western blot. NPPase activity was determined with a colorimetric substrate, pnp-TMP. Abundant eNPP1 expression was observed in early-passage chondrocytes (Fig. 1A), but eNPP1 expression was lost upon repeated passaging, a well-known phenomenon for chondrocyte-specific genes [25]. Similarly, NPPase activity was evident only in P0 and P1 passages (Fig. 1B), especially in articular cartilage chondrocytes and barely in meniscus cells. All subsequent experiments were therefore preformed with cells at P0 or P1.
Passage-dependent eNPP1 expression and NPPase activity
Primary human chondrocytes were cultured and at each passage a sample was analysed for eNPP1 expression by western blot (A) and for NPPase activity by hydrolysis of p-nitrophenyl-thymidine 5′-monophosphate (B). eNPP1, ecto-nucleotide pyrophosphatase/phosphodiesterase 1; NPPase, nucleotide pyrophosphatase.
SK4A is metabolically stable and non-toxic to primary human chondrocytes
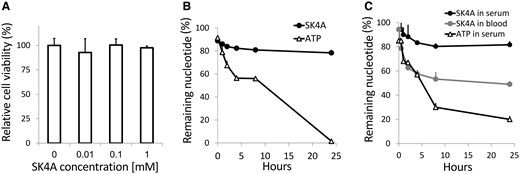
A prerequisite for the application of inhibitor SK4A as a therapeutic agent is the lack of toxicity. For this purpose, primary human chondrocytes were cultured with increasing concentrations of SK4A up to 1 mM for 24 h. Thereafter, cell viability was measured relative to untreated controls by the XTT assay (Fig. 2A). No decrease in cell viability was discernible at concentrations of up to 1 mM.
Inhibitor SK4A is metabolically stable and not toxic to primary human chondrocytes
(A) Chondrocytes were incubated with SK4A at the indicated concentrations for 24 h, and then cell viability was assessed by the XTT assay. (B) Chondrocytes were cultured with DMEM supplemented with 0.1 mM ATP or SK4A. At the time points indicated, the samples were collected and analysed by HPLC. The hydrolysis rate of the SK4A was determined as above. Values represent mean (s.d.) of three experiments. (C) Assay mixtures containing 30% human serum in DMEM or 35% whole human blood in DMEM were incubated with 0.2 mM SK4A or 0.2 mM ATP, at 37°C for 0.5–24 h. At various time intervals the aqueous layer of each sample was collected and analysed by HPLC. The hydrolysis rate of the nucleotide analogues was determined by measuring the change in the integration of the respective HPLC peaks with time. Values represent mean (s.d.) of three experiments. ATP, adenosine triphosphate; SK4A, ATP-α-thio-β,γ-CCl2; XTT, sodium 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium inner salt.
We verified that SK4A is metabolically stable in chondrocyte cell cultures. Chondrocytes were incubated in medium containing SK4A or ATP for 0.01–24 h and the nucleotide’s stability was evaluated and monitored by HPLC for dephosphorylation products. SK4A was highly stable compared with ATP; the inhibitor levels decreased by only 8% after 8 h and by 10% after 24 h (Fig. 2B). In contrast, ATP was rapidly degraded and its levels decreased by 35 and 98% after 8 and 24 h, respectively.
We also evaluated the metabolic stability of SK4A in human blood and serum (Fig. 2C). Blood serum contains dephosphorylating enzymes and therefore provides a good model system to study the stability of nucleotide analogues for future in vivo studies [26, 27]. SK4A was found to be highly stable in both serum and whole blood; in serum, the t½ of SK4A was 40 h as compared with ATP with a t½ of 4.5 h. The t½ of SK4A in whole blood was 22 h.
SK4A does not inhibit alkaline phosphatase activity in human chondrocytes and cartilage MVs
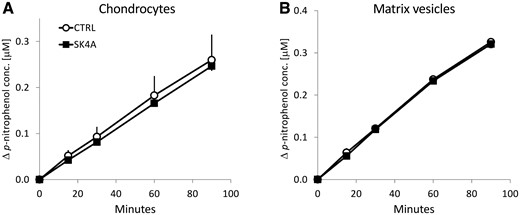
In our previous study we found that SK4A was stable to hydrolysis by NTPDase1, 2, 3, 8, eNPP1, NPP3 and TNAP, did not inhibit ATP hydrolysis by NTPDase1, 2, 3, 8 and exhibited limited inhibition of TNAP and NPP3, as opposed to efficient eNPP1 inhibition, proving to be a selective eNPP1 inhibitor [22]. To confirm that SK4A does not interfere with alkaline phosphatase activity in adult human cartilage, experiments were performed in primary human chondrocytes and cartilage-derived MVs. SK4A did not inhibit alkaline phosphatase activity (0% inhibition) as measured by p-nitrophenyl phosphate hydrolysis (Fig. 3).
SK4A does not inhibit alkaline phosphatase activity
Human-derived primary chondrocytes (A) and matrix vesicles (B) were cultured and incubated with or without SK4A (0.1 mM) and assayed for alkaline phosphatase activity by hydrolysis of p-nitrophenyl phosphate. A representative experiment of five assays, each from a different patient donor is shown. SK4A, adenosine triphosphate-α-thio-β,γ-CCl2.
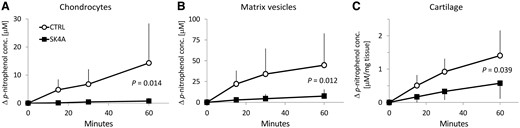
SK4A inhibits NPPase activity in primary human chondrocytes, human cartilage MVs and in intact cartilage tissue
We next evaluated the ability of SK4A to inhibit NPPase activity in primary chondrocytes cultured in monolayers. At equimolar concentrations (0.1 mM) SK4A inhibited the hydrolysis of pnp-TMP by about 88% (Fig. 4A). Similarly, SK4A also inhibited the NPPase activity of isolated cartilage MVs (Fig. 4B). In addition we evaluated whether SK4A can readily diffuse into intact cartilage tissue and reach the target sites of NPPase activity. As is demonstrated in Fig. 4C, SK4A inhibited NPPase activity in intact human cartilage by about 60%.
SK4A inhibits NPPase activity
The eNPP1 inhibitor, SK4A, inhibits NPPase activity in primary chondrocytes (A), cartilage-derived matrix vesicles (MVs) (B) and whole cartilage tissue explants (C) NPPase activity was assayed by measuring the hydrolysis of the chromogenic substrate, p-nitrophenyl-thymidine 5′-monophosphate. SK4A was added at equimolar concentrations to the substrate (0.1 mM). The experiments was performed in triplicate (chondrocytes, MVs) or quadruplicate (cartilage explants) with samples from different patient donors (n = 10 for chondrocytes, n = 8 for MVs and n = 3 for whole cartilage tissue). Data represents mean (s.d.) values of all experiments. eNPP1, ecto-nucleotide pyrophosphatase/phosphodiesterase 1; NPPase, nucleotide pyrophosphatase; SK4A, adenosine triphosphate-α-thio-β,γ-CCl2.
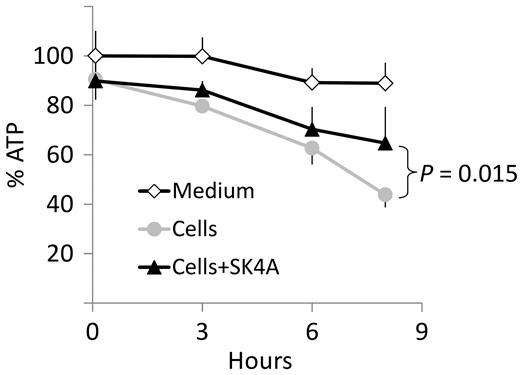
SK4A inhibits extracellular ATP hydrolysis in primary human chondrocytes
After finding that SK4A successfully inhibited NPPase activity by measuring the hydrolysis of pnp-TMP, we used an analytical HPLC to determine whether SK4A can inhibit the hydrolysis of ATP in primary human chondrocytes. ATP and SK4A each at a final concentration of 0.1 mM were incubated in the absence or the presence of chondrocytes for 0, 3, 6 and 8 h (Fig. 5). After 8 h, 11% of the ATP had been non-enzymatically degraded in the absence of cells, while in the presence of chondrocytes 51% of the ATP was degraded. In cultures supplemented with both ATP and SK4A, only 28% of ATP was degraded. Therefore, SK4A successfully inhibited 46% of enzymatic ATP degradation, apparently by inhibiting the eNPP1-mediated hydrolysis of ATP.
SK4A inhibits extracellular ATP hydrolysis in primary human chondrocytes
Adenosine triphosphate (ATP) was added to serum-free culture medium and incubated with and without equimolar concentration (0.1 mM) of SK4A. As a control for background non-enzymatic hydrolysis, ATP was incubated with the culture media but without cells. At the indicated time points, the concentration of ATP was measured by HPLC. Data are mean (s.d.) of six experiments performed with chondrocytes obtained from two different patient donors. SK4A, ATP-α-thio-β,γ-CCl2.
SK4A inhibits ex vivo ATP induced CPPD
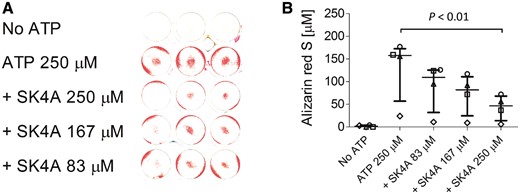
Biochemical, morphological [28] and spectroscopic [29] methods have been previously used to demonstrate that incubating freshly isolated chondrocytes in the presence of high concentrations of ATP results in the deposition of CPPD in the cell layer [30]. We used this model to assess whether the inhibition of ATP hydrolysis by SK4A translates into inhibition of CPPD. Primary chondrocytes were incubated with 250 μM ATP and various concentrations of SK4A for 5 days, after which calcium deposition was visualized and quantified by ARS staining. The cells were visually inspected by microscopy at the end of the incubation period, and appeared viable and attached to the plastic surface. Cell viability, as assayed by the XTT method, showed that addition of ATP increased viability by about 20% and that SK4A at concentrations of up to 250 μM was not detrimental to the cells (data not shown). Figure 6 demonstrates that accumulation of calcium deposition was dependent upon the presence of ATP and that SK4A inhibited the accumulation of calcium deposition in a dose-dependent manner.
SK4A inhibits ATP-induced CPPD in vitro
Human chondrocytes were incubated with medium containing 250 μM ATP and the indicated concentrations of SK4A. After 5 days the cell monolayers were stained with Alizarin red S, photographed (A, a representative experiment), and the dye was extracted from each well with 10% acetic acid and quantified by absorbance at 405 nm (B). Bars indicate median (s.d.) of four experiments, each performed in triplicate with chondrocytes from a different patient donor. ATP, adenosine triphosphate; SK4A, ATP-α-thio-β,γ-CCl2.
Pharmacological properties of SK4A
The metabolic stability of SK4A was tested at liver microsomes and the t½ was determined at >145 min. We found that 74.2% of SK4A binds to human plasma proteins. Caco-2 permeability assays, which serve as an indicator of intestinal bio-absorption, revealed that SK4A exhibited a very low permeability (mean Papp < 0.04) and was not a substrate of efflux transporters (mean Papp < 0.03). When administered intravenously in rats, the rate of clearance of SK4A was 0.1 ml min−1 kg−1, resulting in a reasonable half-life of 0.7 h. SK4A was undetectable in blood following 24 h indicating its complete elimination during this period.
Discussion
eNPP1 protein levels and NPPase activity decreased with progressive passaging of primary human chondrocytes similarly to other chondrocyte-specific proteins, such as collagen II and SOX9 [25], implying chondrocyte differentiation is required to maintain high levels of eNPP1 expression. This is also evident from the higher NPPase activity in articular chondrocytes relative to meniscus cells. An association between eNPP1 expression and NPPase activity has been demonstrated in human chondrocytes and cartilage-derived MVs in earlier work [14–17].
SK4A is a specific nucleotide analogue inhibitor of eNPP1-mediated NPPase activity relative to other NTP-hydrolysing enzymes including NTPDase1, 2, 3, 8, NPP3 and TNAP [22]. The ability to specifically inhibit the eNPP1 enzymatic activity with SK4A enabled us to validate the hypothesis that eNPP1 is the major NTP hydrolysing and PPi generating enzyme in adult human osteoarthritic cartilage. Indeed SK4A was effective in inhibiting NPPase activity as measured by pnp-TMP hydrolysis in chondrocytes, MVs and intact cartilage (∼80% inhibition at 0.1 mM). On the other hand, in both chondrocytes and MVs, SK4A did not inhibit alkaline phosphatase activity. Using ATP as a substrate, we also demonstrated that SK4A could effectively inhibit ATP hydrolysis in chondrocytes, implying that indeed the majority of ATP hydrolysis in chondrocytes, and most probably also in MVs, was mediated by eNPP1. Together these results do not support a role for TNAP in PPi production in human chondrocytes or human cartilage MVs, in contrast to a previous study of MVs derived from calviral osteoblasts from genetically modified mice [20].
Elevated PPi concentration in the extracellular SF is thought to be the major determinant for CPP articular precipitation in humans. Therefore, we confirmed that NPPase inhibition results in reduced levels of CPP deposition in a cell culture based model of CPPD. Indeed, our results demonstrate that SK4A inhibited the ATP-driven accumulation of calcium deposition in a dose-dependent manner, providing evidence that eNPP1 inhibitors can prevent mineralization of osteoarthritic chondrocytes. Notably, in the absence of ATP, we could not detect considerable CPPD suggesting that in our experimental conditions ANK did not substantially contribute to CPPD.
Our findings indicated favorable pharmacological properties of SK4A such as relatively high metabolic stability in whole blood and liver microsomes, no toxic effects in chondrocyte cell cultures (at least up to 1000 µM), and reasonable binding to plasma proteins. In contrast, the compound’s limited predicted absorption in the digestive tract and the half-life of 0.7 h in rats pose obstacles in its development as a therapeutic drug. SK4A is a competitive inhibitor of eNNP1 and its effects are expected to be concentration dependent. We have observed that washing chondrocytes that were pre-incubated with SK4A before addition of the pnp-TMP substrate, diminished NPPase inhibition by about 80% relative to cultures that were not washed before the addition of the substrate (data not shown).
Excessive eNPP1 inhibition may raise the Pi:PPi concentration to levels that could promote formation of hydroxyapatite crystals in joints and blood vessels, as is exemplified in the tip toe walking mouse strain, which harbours a nonsense mutation in the eNpp1 gene [31], and in cases of ‘idiopathic’ infantile arterial calcification [32]. Therefore, a therapeutic window based on Pi:PPi ratios that do not favour pathological calcification would need to be determined. On the other hand, eNPP1 overexpression and overactivity are associated with insulin resistance and type 2 diabetes, and thus eNPP1 inhibition might have favourable metabolic consequences [33].
Being ATP-based, SK4A is not a classical drug-like compound, although related compounds such as thiazole-4-carboxamide adenine dinucleotide and denufosol have found their way into clinical trials [34, 35]. Developing SK4A into a drug may require pro-drug approaches [35], appropriate formulations and/or administration modes other than oral, such as the sub-lingual route or intra-articular injection to susceptible or affected joints. Liposome encapsulation may perhaps substantially increase the intra-articular retention time of such compounds. However, even if SK4A will not be eventually developed into a drug it can still serve as an important mechanistic tool for the study of the complex process of mineralization.
Our study demonstrates the proof-of-concept that nucleotide analogues may be developed as potent and specific inhibitors of eNNP1 for the purpose of lowering extracellular PPi levels in human cartilage with the aim of preventing and treating CPPD.
Funding: This work was supported by the Kamin Fund of the Israel Ministry of Trade and Industry (grant number 56136).
Disclosure statement: The authors have declared no conflicts of interest.
References
Author notes
O. Danino and S. Svetitsky contributed equally to this study.
B. Fischer and U. Arad contributed equally to this study.




Comments