-
PDF
- Split View
-
Views
-
Cite
Cite
Fu-Sheng Deng, Ching-Hsuan Lin, Cpp1 phosphatase mediated signaling crosstalk between Hog1 and Cek1 mitogen-activated protein kinases is involved in the phenotypic transition in Candida albicans, Medical Mycology, Volume 56, Issue 2, February 2018, Pages 242–252, https://doi.org/10.1093/mmy/myx027
Close - Share Icon Share
Abstract
Cellular signaling pathways involved in cell growth and differentiation mediated by mitogen-activated protein kinase (MAPK) cascades have been well characterized in fungi. However, the mechanisms of signaling crosstalk between MAPKs to ensure signaling specificity are largely unknown. Previous work showed that activation of the Candida albicans Cek1 MAPK pathway resulted in opaque cell formation and filamentation, which mirrored the phenotypes to hog1Δ. Additionally, deleting the HOG1 gene stimulated Cek1p. Thus, we hypothesized that an unknown factor could act as a bridge between these two MAPKs. In Saccharomyces cerevisiae, the dual-specificity phosphatase (DSP) Msg5 specifically dephosphorylates Fus3p/Kss1p. C. albicans Cpp1, an ortholog of Msg5, has been shown to be important in regulating Cek1p. Compared with the wild-type strain, hog1Δ shows a ∼40% reduction in CPP1 expression. Consistent with previous reports, CPP1 deletion also resulted in Cek1 hyperphosphorylation, implicating Cpp1 as a regulator of the Hog1 and Cek1 cascades. Interestingly, both cpp1Δ and hog1Δ induced 100% opaque colony formation in MTL-homozygous strains grown on N-acetylglucosamine (NAG) plates, whereas the wild-type and complemented strains exhibited 80.9% and 77.1% white-to-opaque switching rates, respectively. CPP1 gene deletion also caused hyperfilamentous phenotypes in both white and opaque cells. These phenomena may be due to highly phosphorylated Cek1p, as deleting CEK1 in the cpp1Δ background generated nonfilamentous strains and reduced opaque colony formation. Taken together, we conclude that cpp1Δ and hog1Δ exhibited comparable phenotypes, and both are involved in regulating Cek1 phosphorylation, implicating Cpp1 phosphatase as a key intermediary between the Hog1 and Cek1 signal transduction pathways.
Introduction
Like all living organisms, fungi must adapt to many environmental changes. Thus, external signals from the environment must effectively activate appropriate physiological changes. In eukaryotic cells, mitogen-activated protein (MAP) kinases play critical roles in the responses to various exterior stimuli and are involved in numerous cellular activities.1–3 In a MAP kinase cascade, a phosphorylated MAP kinase kinase kinase (MAPKKK or MEKK) phosphorylates a MAP kinase kinase (MAPKK or MEK), which triggers the phosphorylation of a MAP kinase (MAPK), which in turn activates downstream signaling.1,4 The MAPK-mediated signaling cascade is evolutionarily well conserved from yeasts to mammals. However, the biological function and ultimate response of each MAPK are diverse and depend on the individual species and their habitats.
In Saccharomyces cerevisiae, several MAPK-mediated pathways involved in mating responses (Fus3 MAPK), filamentous growth (Kss1 MAPK), cell integrity (Slt2 MAPK), and osmotic stress responses (Hog1 MAPK) have been well characterized.3 Interestingly, the Fus3 and Kss1 cascades, which are responsible for mating and filamentous growth, contain the same signaling components, such as MEKKK (Ste20), MEKK (Ste11), and MEK (Ste7).1,3 However, how cells decide which pathways must be activated to ensure signaling specificity remains largely unknown. To date, three potential strategies by which cells avoid erroneous crosstalk have been hypothesized: (1) temporal separation, (2) docking-specific binding, and (3) crosstalk inhibition.5–7 A report has demonstrated that docking interactions and pathway inhibition may play important roles in signaling specificity.8 For example, although the pheromone and filamentous growth pathways share some of the same signaling components, Ste11, Ste7, and Fus3, which are tethered to the scaffold protein Ste5, are utilized exclusively during the pheromone response.8,9 Alternatively, in crosstalk inhibition, the activation of one pathway causes the inactivation of another pathway.5 During the mating response, activated Fus3 kinase may inhibit the downstream Tec1 transcription factor that is specifically required for filamentous development.10 The formation of Tec1/Ste12 heterodimers is necessary during hyphal formation, whereas Ste12/Ste12 homodimers are required for yeast mating.11,12 Specifically, pheromone treatment triggers the ubiquitin-mediated degradation of Tec1, rather than blocking Tec1 synthesis.11,12
Similar to the mating response, the Hog1 signaling cascade also maintains pathway fidelity. In budding yeast, two major input branches, Sln1 and Sho1, are responsible for Hog1 activation. Mutations in the HOG1 or PBS2 gene have been shown to lead to osmolarity-induced activation of the mating pathway, suggesting that crosstalk between mating and osmotic signaling is prevented by inhibiting Sho1p, given that Sho1p is also a signaling component of the Fus3/Kss1 pathways.13,14
Candida albicans is an opportunistic fungal pathogen that can cause various superficial and systemic infections in humans.15 To achieve this, C. albicans has developed different strategies via distinct signaling pathways to colonize the host. Three major MAP kinase pathways, the Cek1/Cek2, Mkc1, and Hog1 cascades, have been extensively reported to modulate cell behavior, morphology, mating, virulence, and environmental adaptation.1,2,16,17 These pathways not only sense and respond to signals but also lead C. albicans to show prevalent morphologies, thereby contributing to the phenotypic plasticity of this pathogen in different niches.2 In C. albicans, yeast-hyphal transition and white-opaque switching, two major morphological conversions, have been known to contribute to fungal virulence and sexual behavior.18–22 The molecular mechanisms involved in the yeast-hyphal transition are very complicated. cAMP signaling and several MAPK pathways play important roles in hyphal formation.23–27 Deletion of a cAMP downstream effector, EFG1, or the CEK1 MAPK gene causes defects in hyphal development.23,28 Another phenotypic transition involved in white-opaque switching is mediated by Wor1, which coordinates with several other transcription factors.29,30 White and opaque cells typically exhibit differences in their morphologies, colonization sites, and sexual behavior.18,19,31,32 In particular, opaque cells are elongated and mating competent, whereas white cells are round and infertile.18,19,31
Interestingly, a recent study showed that the activation of Cph1, a downstream target of Cek1, induces C. albicans to switch to opaque cells.33 Furthermore, our previous work showed that deleting the HOG1 gene or mutating the conserved phosphorylation sites in Hog1 resulted in opaque cell formation.34,35 Therefore, we hypothesized that the Cek1 and Hog1 MAPK pathways undergo signaling crosstalk. In the present study, we first identified a dual-specificity phosphatase (DSP) Cpp1 that potentially acts as a bridge between the Cek1 and Hog1 MAPKs, as CPP1 deletion resulted in Cek1p hyperphosphorylation.36 Furthermore, cpp1Δ not only exhibits a hyperfilamentous phenotype36 but also induces C. albicans to form opaque cells. These phenotypes are similar to those of activated Cek1 and of hog1Δ in C. albicans.33–38 Together, our data imply that the phosphatase Cpp1 is involved in phenotypic switching by mediating signaling crosstalk between the Hog1 and Cek1 MAPKs.
Methods
Media and reagents
Yeast culture media were prepared as described previously with slight modifications.35,39 YPD medium, composed of 2% (w/v) peptone, 1% (w/v) yeast extract, and 2% (w/v) glucose, was used for cell growth. Nourseothricin-resistant strains were selected on YPD medium supplemented with 0.2 mg/ml nourseothricin (Werner BioAgents). Synthetic complete dextrose (SCD) consisted of 0.7% (w/v) yeast nitrogen base without amino acids, 0.17% complete amino acids powder, and 2% (w/v) glucose. Synthetic complete (SC) medium was formulated as SCD medium without the addition of glucose. Lee's N-acetylglucosamine (Lee's NAG) was prepared according to an established protocol and contained 1.25% N-acetylglucosamine (Alfa Aesar).40 SCD, SC, and Lee's NAG media were used for white-opaque phenotypic-transition assays. Spider medium (pH 7.2), containing 1% (w/v) mannitol, 1% (w/v) nutrient broth, and 0.4% (w/v) dipotassium phosphate (Showa Chemical Industry Co.), was used for opaque cell maintenance. Agar was added to a final concentration of 2% (w/v) to make solid plates. All chemicals were purchased from Sigma-Aldrich Chemical Co., unless otherwise stated.
Plasmid and strain construction
The yeast strains and primers used in this study are listed in Table S1 and Table S2. To generate cpp1Δ strains, the 5΄ and 3΄ flanking regions of CPP1 were PCR amplified using primers 197/198 and 199/200, respectively. The 5΄ and 3΄ PCR products were digested with ApaI/XhoI and SacII/SacI, respectively, and cloned into the plasmid pSFS2A41 to generate the plasmid pSFS-Cpp1 KO. This plasmid was digested with ApaI/SacI and transformed into MTLa/a, MTLα/α, and MTLa/α strains to generate heterozygous Δcpp1/CPP1 mutants. The SAT1 marker was recycled under treatment of 2% maltose, and the strains were re-transformed with the deletion construct to generate the cpp1Δ strains YL502/YL503, YL596/YL597, and YL598/YL599. The CPP1 complementation construct was made by amplification of its endogenous promoter and ORF using primer 253/254. The PCR product was digested with ApaI/XhoI and cloned into pSFS-Cpp1 KO to generate pSFS-CPP1 AB. The plasmid was digested with ApaI/SacI and transformed into mutants to create YL691/YL692, YL693/YL694, and YL695/YL696.
For cek1 mutants, primers 501/502 and 503/504 were used to amplify the 5΄ and 3΄ regions of the CEK1 gene. The 5΄ and 3΄ PCR products were digested with ApaI/XhoI and SacII/SacI and cloned into pSFS2A to generate pSFS-Cek1 KO. The construct was linearized with ApaI/SacI and transformed into RBY717, YL8/YL9 (hog1Δ) and YL502/YL503 (cpp1Δ). The SAT1 marker was recycled by maltose induction, and the strains were again transformed with the deletion construct to generate YL824/YL825 (cek1Δ), YL857/YL858 (hog1Δ/cek1Δ), and YL855/YL856 (cpp1Δ/cek1Δ). The same protocol was used to generate cek2 mutants. Primers RB1/RB2 and RB3/RB4 were used to amplify the 5΄ and 3΄ DNA flanking fragments of CEK2, which were cloned into pSFS2A to generate pSFS-Cek2 KO. This plasmid was digested with ApaI/SacI and transformed into RBY717, YL8/YL9 (hog1Δ), YL824/YL825 (cek1Δ), and YL857/YL858 (cek1Δ/hog1Δ). After SAT marker removal, the strains were re-transformed with pSFS-Cek2 KO to generate YL785/YL786 (cek2Δ), YL1593/YL1594 (hog1Δ/cek2Δ), YL889/YL890 (cek1Δ/cek2Δ), and YL885/YL886 (hog1Δ/cek1Δ/cek2Δ). To maintain the strains in the opaque state, we overexpressed the WOR1 gene from the ACT1 promoter in each strain.42 The plasmid pSFS-pACT1-Wor1 was linearized with AatII and transformed into C. albicans. Confirmations of deletion mutants and the complementary strains were checked with PCR in each gene's open reading frame.
Protein extracts and Western blotting
To examine Cek1 phosphorylation in C. albicans, 200 μl of overnight culture was diluted into 10 ml of Spider broth and grown for 5 h at 25°C. The strains were collected by centrifugation and suspended in 800 μl of cold lysis buffer (50 mM Tris-HC l (pH 7.5), 10% glycerol, 1% Triton X-100, 0.1% SDS, 150 mM NaCl, 50 mM NaF, 1 mM sodium orthovanadate, 50 mM beta-glycerol phosphate, 5 mM sodium pyrophosphate, 5 mM EDTA, and the protease inhibitors: 1 mM PMSF (phenylmethylsulfonyl fluoride) and Protease/Phosphatase Inhibitor Cocktail (Cell Signaling Technology).43 The cells were broken by vigorous shaking with 0.5-mm glass beads three times in a fast-prep cell breaker (Thermolyne 37600 Mixer, highest level for 30 s). Cell extracts were separated from glass beads and cell debris, clarified by a 13,000-rpm spin for 10 min at 4°C and then transferred to new eppendorf tubes. Equal amounts of protein were mixed with 2 × Sample Dye, analyzed by SDS-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibody (Cell Signaling Technology, 4376) were used to detect phosphorylated Cek1. β-anti-actin antibody (GeneTex, Inc. Irvine, CA, ISA) was used as the internal control.
Quantitation of opaque-cell formation
Overnight cultures of C. albicans were plated onto SC, SCD, or Lee’ s NAG media supplemented with phloxine B and were incubated at 25°C for 5 days. Opaque colonies were stained red. The white-opaque switching efficiency was expressed as follows: (number of opaque colonies)/(total number of colonies) × 100%.
Transcriptional analysis by quantitative reverse transcription-PCR
Total RNA was extracted following the RiboPureTM Yeast Kit protocol (Thermo Fisher Scientific, Waltham, MA, USA) and treated with DNase I (Thermo Fisher Scientific, Waltham, MA, USA) to remove DNA. cDNA was synthesized using GoScript Reverse Transcriptase (Promega, Madison, WI, USA). Quantitative PCR was performed on a Bio-Rad CFX Manager (Bio-Rad Laboratories, Hercules, CA, USA). Signals from each sample were normalized to ACT1 gene expression. Experiments were repeated independently at least three times. Data are reported as the means from 2 to 3 independent biological replicates performed in triplicate. Expression differences between the reference and mutant strains were analyzed by Student's t test, with significance set at P < 0.05.
Determination of filamentation in the opaque state
Overnight cultures of the C. albicans wild-type and mutants were plated on Lee's NAG plates. Images were taken after 5-day incubation at 25°C. An Eclipse Ti inverted microscope (Nikon Instruments Inc., Melville, NY, USA) was used to examine the cell morphology.
Results
Deletion of CEK1, but not CEK2, inhibits opaque cell formation
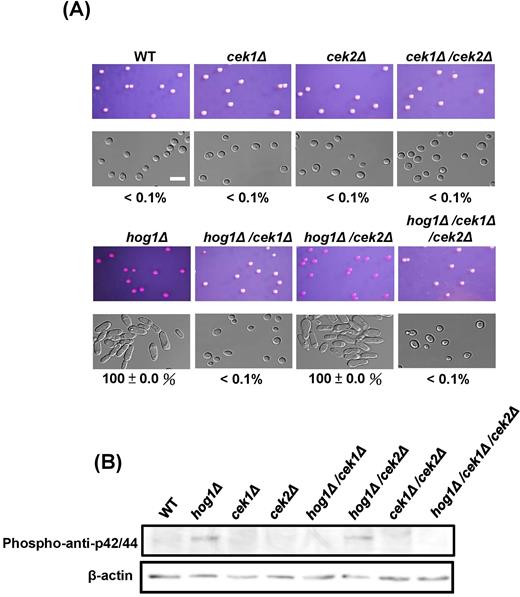
We have shown that the lack of the HOG1 gene or a functional Hog1 protein in C. albicans MTL homozygous strains stimulated opaque cell formation at a frequency of 100%.34,35 Furthermore, a recent study showed that Cph1 activation promoted white-to-opaque switching.33 Cph1 is a transcription factor that is required for sexual mating in C. albicans and is controlled by the Cek1/Cek2 MAPK.33,44 Several review articles have revealed that the signaling crosstalk among MAP kinases in the model organism S. cerevisiae exists.3,5,6,45 We therefore tested whether the signaling crosstalk between Cek1/Cek2 and Hog1 MAP kinases is related to the white-to-opaque transition. To this end, we generated cek1Δ, cek2Δ, cek1Δ/cek2Δ, hog1Δ,35hog1Δ/cek1Δ, hog1Δ/cek2Δ, and hog1Δ/cek1Δ/cek2Δ mutant strains (Fig. 1A). As expected, the wild-type strain (MTLa/a), as well as cek1Δ, cek2Δ, and cek1Δ/cek2, displayed white colonies, whereas hog1Δ strains promoted opaque colony formation. Interestingly, deleting CEK1 in a hog1Δ background suppressed the ability to switch from white cells to opaque cells, as the hog1Δ mutant displayed a 100% white-to-opaque switching on SC medium (Fig. 1A).35hog1Δ/cek2Δ also maintained a 100% switching rate (Fig. 1A), suggesting that the crosstalk is restricted to the Cek1 and Hog1 signaling pathways. hog1Δ/cek1Δ/cek2Δ strains also suppressed opaque cell formation. We then performed Western blotting to understand whether crosstalk exists between the Hog1 and Cek1 MAP kinases. Consistent with the previous study,38 we showed that Cek1p, but not Cek2p, was highly phosphorylated in the absence of HOG1 (Fig. 1B) because the phosphorylated signal disappeared in the hog1Δ/cek1Δ mutant strain. The following experiments of this study will therefore focus on Cek1p, rather than Cek2p. These results showed that HOG1 gene deletion promotes Cek1p phosphorylation in C. albicans,38 suggesting the involvement of an unknown factor in the signaling crosstalk.
Signaling crosstalk between Hog1 and Cek1. (A) Deleting CEK1, but not CEK2, inhibits white-to-opaque switching in the MTLa/ahog1Δ background. White cells from YPD medium were plated onto SC plates supplemented with phloxine B and incubated at 25°C for 5 days. Opaque cells stained red. At least 400 colonies were examined for each experiment. The switching frequency was represented as a percentage of the mean ± SD and is shown below each image. (B) Western blotting revealed that HOG1 gene deletion activated Cek1p phosphorylation. Phospho-anti-p42/44 and β-actin antibodies were used to detect Cek1p and as an internal control, respectively. Scale bar, 20 μm.
cpp1Δ mutant strains cannot undergo white-to-opaque switching on SC medium
NAG (N-acetylglucosamine) has been shown to induce opaque cell formation.46 Similar to hog1Δ mutant strains, the white-to-opaque switching rate of cpp1Δ (100%) was significantly higher than that of the wild-type strain (80.9%) on NAG plates (Table 1 and Fig. 3A). Furthermore, given that HOG1 deletion strains had a high white-to-opaque switching competency on SC medium (Fig. 1),35 we want to understand whether high switching rates can also be observed in cpp1Δ mutant strains under the same culture condition. As shown in Table 1, however, deletion of CPP1 did not promote white cells to switch to opaque cells on SC medium, whereas hog1Δ exhibits 100% opaque-cell formation on SC medium.35 These data suggest that carbohydrate metabolism affects the switching ability of C. albicans. Furthermore, the utilization of different sugars supplemented in the culture medium might influence the capacity for white-opaque switching. Indeed, our previous work demonstrated that the high rates of switching observed with hog1Δ mutants on SC medium were not observed on medium supplemented with glucose (SCD) (Table 1),35 suggesting that glucose negatively regulates opaque cell formation. This finding also implies that Hog1, rather than Cpp1, has more impact on nutrient metabolism, thereby causing different outcomes. Indeed, a large subset of genes involved in carbohydrate metabolism and energy reserve metabolism in C. albicans are highly depended on Hog1 SAPK.47
White-to-opaque switching efficiencies of C. albicans MTLa/a cells under different culture conditions.
| Strain (medium) . | Switch Freq. (%) . |
|---|---|
| WT (SCD) | <0.01 |
| cpp1Δ (SCD) | <0.01 |
| hog1Δ (SCD) | <0.01 |
| WT (SC) | <0.01 |
| cpp1Δ (SC) | <0.01 |
| hog1Δ(SC) | 100 ± 0.0 |
| WT (NAG) | 80.9 ± 11.7 |
| cpp1Δ (NAG) | 100 ± 0.0 |
| hog1Δ (NAG) | 100 ± 0.0 |
| Strain (medium) . | Switch Freq. (%) . |
|---|---|
| WT (SCD) | <0.01 |
| cpp1Δ (SCD) | <0.01 |
| hog1Δ (SCD) | <0.01 |
| WT (SC) | <0.01 |
| cpp1Δ (SC) | <0.01 |
| hog1Δ(SC) | 100 ± 0.0 |
| WT (NAG) | 80.9 ± 11.7 |
| cpp1Δ (NAG) | 100 ± 0.0 |
| hog1Δ (NAG) | 100 ± 0.0 |
Synthetic complete (SC) medium was formulated as SCD medium without the addition of glucose as described previously in Methods.
NAG, N-acetylglucosamine; SCD, synthetic complete dextrose.
White-to-opaque switching efficiencies of C. albicans MTLa/a cells under different culture conditions.
| Strain (medium) . | Switch Freq. (%) . |
|---|---|
| WT (SCD) | <0.01 |
| cpp1Δ (SCD) | <0.01 |
| hog1Δ (SCD) | <0.01 |
| WT (SC) | <0.01 |
| cpp1Δ (SC) | <0.01 |
| hog1Δ(SC) | 100 ± 0.0 |
| WT (NAG) | 80.9 ± 11.7 |
| cpp1Δ (NAG) | 100 ± 0.0 |
| hog1Δ (NAG) | 100 ± 0.0 |
| Strain (medium) . | Switch Freq. (%) . |
|---|---|
| WT (SCD) | <0.01 |
| cpp1Δ (SCD) | <0.01 |
| hog1Δ (SCD) | <0.01 |
| WT (SC) | <0.01 |
| cpp1Δ (SC) | <0.01 |
| hog1Δ(SC) | 100 ± 0.0 |
| WT (NAG) | 80.9 ± 11.7 |
| cpp1Δ (NAG) | 100 ± 0.0 |
| hog1Δ (NAG) | 100 ± 0.0 |
Synthetic complete (SC) medium was formulated as SCD medium without the addition of glucose as described previously in Methods.
NAG, N-acetylglucosamine; SCD, synthetic complete dextrose.
cpp1Δ, but not ptp2Δ and ptp3Δ, stimulate Cek1 activation
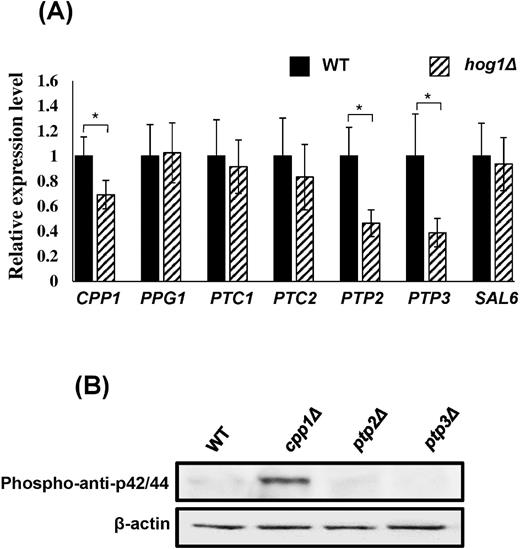
As described previously, a protein phosphatase plays a role in shaping the outcome of MAPK signaling.48 To understand whether the highly phosphorylated Cek1p observed in hog1Δ mutant strains was related to phosphatases, we selected several potential phosphatase genes based on literature searches.48,49 The phosphatases include the type 1 serine/threonine phosphatase SAL6, the type 2A serine/threonine phosphatase PPG1, the type 2C serine/threonine phosphatases PTC1 and PTC2, the PTP family phosphatases PTP2 and PTP3, and the dual-specificity phosphatase CPP1,36,49 all of which have been reported to be potentially involved in MAPK signaling in budding yeast or in C. albicans, particularly in the filamentation/mating and Hog1 signaling pathways.36,49,50 Here, we found that CPP1, PTP2, and PTP3 expression levels exhibited a 40–65% reduction in the hog1Δ strain compared with those in the wild-type strain (Fig. 2A).
Cpp1 mediates signaling crosstalk between Hog1 and Cek1 MAPKs. (A) The expression of each gene in hog1Δ (striped) was analyzed and compared with the response in wild-type cells (black). Cpp1, PTP2, and PTP3 expression was reduced in hog1Δ strains. Expression levels were normalized to the ACT1 gene. The values are the means ± SD of at least three replicates. (B) CPP1 gene deletion elevated the intensities of Cek1p. Phospho-anti-p42/44 and β-actin antibodies were used to detect Cek1p and as an internal control, respectively.
To further determine whether Cpp1, Ptp2, and Ptp3 are required for the regulation of Cek1 phosphorylation, deletion mutants were made. Consistent with the previous report,36 Figure 2B showed that in the absence of CPP1, Cek1p phosphorylation was greatly elevated, whereas ptp2Δ and ptp3Δ showed no change. This result indicates that Cpp1 potentially functions as an intermediary between Cek1 and Hog1 signaling.
Although expression levels of PTP2 and PTP3 were significantly reduced in hog1Δ in Spider medium at 30°C, deletion of each gene did not affect the Cek1 activity. Indeed, Ptp2 and Ptp3 belong to tyrosine phosphatases and are responsible for regulation of the phosphorylation activity of Hog1p.50,51 A previous report has shown that PTP3 in C. albicans can be induced in serum-containing medium at 37°C.51 Furthermore, expression levels of PTP2 and PTP3 were significantly induced under the culture condition of rapamycin treatment at 37°C, thereby inhibiting the basal activity of Hog1p.50 These indicate that although Ptp2 and Ptp3 target on Hog1 phosphorylation, Hog1 also regulates the PTP2 and PTP3 in certain culture conditions, suggesting that they might have a sophisticated regulatory system between the Hog1p, PTP2, and PTP3.
CPP1 deletion induces white-to-opaque switching
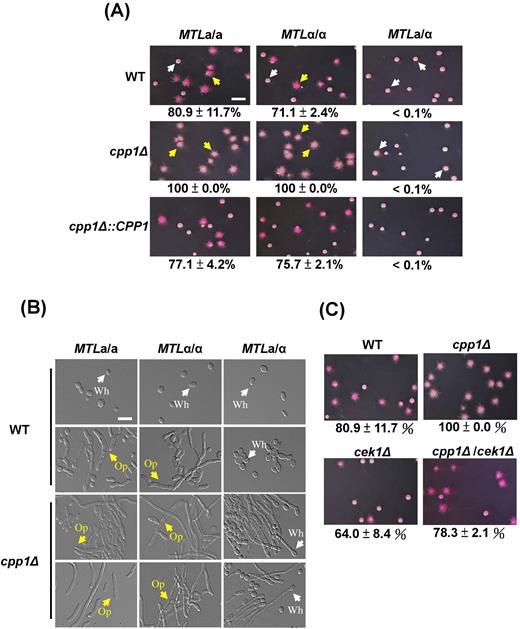
To understand whether the role of Cpp1 as a bridge between these two MAP kinase pathways is also involved in phenotypic switching, mutants were created and tested for their white-opaque switching efficiencies on NAG (N-acetylglucosamine) medium. Interestingly, among the mutants, cpp1Δ in the MTLa/a and MTLα/α homozygous strains resulted in 100% white-to-opaque switching, whereas the wild-type strains showed 80.9% and 71.1% opaque colonies in a/a and α/α, respectively (Fig. 3A and 3B). Reintroducing CPP1 into each mutant repressed the efficiencies of opaque cell formation (Fig. 3A).
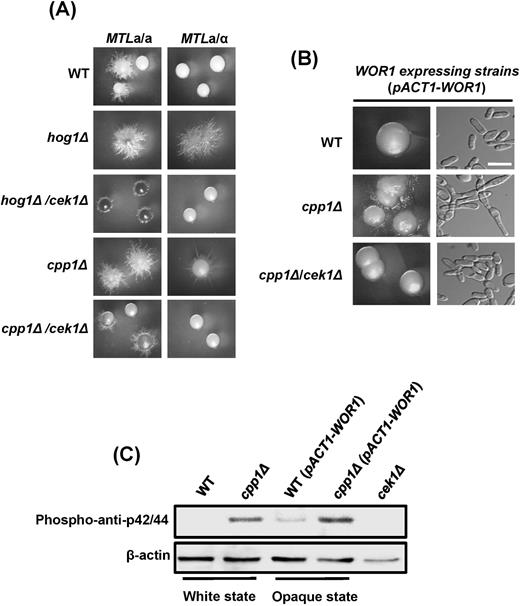
The dual-specificity phosphatase Cpp1, involved in white-opaque switching, is closely linked to Cek1 MAPK. (A) Deletion of the CPP1 gene induces white-to-opaque switching in MTLa/a and MTLα/α C. albicans strains on NAG plates. The white-to-opaque switching rate of each strain is expressed as the mean ± SD. White (white colonies) and yellow (opaque colonies) arrows were the represented colonies for panel (B). Scale bar, 0.4 cm. (B) Images of individual cells from represented colonies from the panel (A) of the wild-type and cpp1Δ strains are shown. Wh: white (white arrows); Op: opaque (yellow arrows). Scale bar, 20 μm. (C) Loss of the CEK1 gene in a cpp1 mutant background suppresses opaque cell formation in C. albicans MTLa/a.
We next tested whether the high-switching phenotype of cpp1Δ is related to Cek1 MAPK. cpp1Δ/cek1Δ double-knockout mutants exhibited a reduced opaque switching rate of 78.3%, similar to that of the wild-type strain (Fig. 3C). These data suggest that the DSP Cpp1, which is regulated by Hog1 MAPK, has a negative regulation on Cek1p phosphorylation.
Cpp1 is involved in yeast-hyphae transition in both white and opaque states
Apart from white-opaque switching, yeast and hyphae are another major phenotypic transition in which the state of each developmental morphology is crucial for the pathogenesis of C. albicans. Disruption of the CPP1 gene in C. albicans is known to promote the yeast-to-hyphal transition due to increased Cek1p phosphorylated,36 as cpp1Δ/cek1Δ suppresses the morphological effects of cpp1Δ.36 Besides, deletion of CPP1 gene not only induced white-to-opaque switching (Fig. 3A) but also promoted filamentation in both white and opaque cells (Fig. 3B). To step further to understand the mechanisms in details, several mutants of the MTL homozygous and heterozygous strains were generated and evaluated for the ability of filamentation. As shown in Figure 4A, deleting the HOG1 or CPP1 gene yielded a comparable phenotype, in which both mutants promoted hyphal development compared with the wild-type strain in both MTLa/a (opaque cells) and MTLa/α (white cells). The hyperfilamentous morphology was repressed by deleting the CEK1 gene in the hog1Δ or cpp1Δ background (Fig. 4A); the results of hyphal formation are consistent with a previous description.36 To ensure that the filaments of cpp1Δ observed in MTLa/a (Fig. 4A) were not due to a few white cells, we overexpressed WOR1 from the ACT1 promoter in wild-type, cpp1Δ and cpp1Δ/cek1Δ cells (Fig. 4B). The results indicate that cpp1Δ also stimulated filamentation in C. albicans opaque cells, and the phenotype can be reverted by the further deletion of CEK1 (Fig. 4B). Western blotting also revealed that cpp1Δ mutants in both white and opaque cells (ACT1-WOR1 expression strains) activated Cek1p phosphorylation (Fig. 4C). These data indicate that, in addition to white-opaque switching, Cpp1 is involved in the yeast-hyphae transition via the signaling cross-inhibition.
Cpp1 is also involved in the yeast-hyphae phenotypic transition in both white and opaque cells. (A) cpp1Δ and hog1Δ in both the white (MTLa/α) and opaque (MTLa/a) states exhibited hyperfilamentous morphologies. The vegetative growth phenotypes in cpp1Δ and hog1Δ were suppressed if CEK1 was further deleted. (B) Constitutively expressed WOR1 gene in C. albicans MTLa/a opaque cells demonstrated that cpp1Δ promotes hyphal growth in opaque cells. The hyperfilamentous phenotype disappeared in cpp1Δ/cek1Δ double-knockout strains. Scale bar, 20 μm. (C) Western blotting revealed that cpp1Δ activated Cek1p phosphorylation in both white cell and opaque cells (ACT1-WOR1 expression strains in MTLa/a). A phospho-anti-p42/44 antibody was used to detect C. albicans Cek1p. cek1Δ served as the negative control.
Discussion
The MAPK crosstalk first identified in budding yeast evolved to ensure pathway specificity.3,5,36 Different MAPK pathways may interact antagonistically or synergistically to help cells execute appropriate responses.3,5,6,8,10,48,52–54 Additionally, the FUS3 and KSS1 MAPK pathways share several upstream components.5,6,55 However, in each of these pathways, divergent components have evolved to ensure pathway specificity. For example, trimeric G proteins, Ste5, and Fus3 are exclusively used for mating but not for the filamentous (Kss1) pathway.56 Moreover, Hog1 MAPK is specifically required for the response to osmotic stress.57 Scaffold proteins that can bind two or more signaling components in a pathway are widely thought to promote signaling specificity and prevent pathway crosstalk. The Ste5 and Pbs2 scaffold proteins, which act in the FUS3 and HOG1 MAPK pathways, respectively, appear to regulate and maintain response specificity.56,57 Finally, the activation of one pathway can cause the inactivation of another pathway. During the mating response, activated Fus3 can inhibit the downstream Tec1 transcription factor that is specifically required for filamentous development.10,12 Similarly, the HOG1 MAPK may activate the expression of Msg5, which encodes a phosphatase that dephosphorylates Fus3 and Kss1, thereby suppressing their functions.52,53
Another possible mechanism to ensure efficient and accurate, but not hyperstimulated, MAPK activation is to modulate the phosphorylation level of each MAPK. Thus, the precise regulation of each signaling component requires protein phosphatases to act as a counterbalance to highly activated MAPKs.48 Three types of MAPK phosphatases provide a complex negative feedback network to down-regulate MAPKs, including tyrosine phosphatases, serine/threonine phosphatases and DSPs.48 In S. cerevisiae, Msg5 specifically dephosphorylated phosphotyrosine on the pheromone responsive protein kinase Fus3p.36 Moreover, overexpression of MSG5 exhibited inhibitory effects on pheromone-inducible promoter activities and recover faster from pheromone-induced G1 arrest,58 suggesting that the presence of MSG5 is required for the adaptation to pheromone response in budding yeast. Interestingly, Msg5 comprises two isoforms of different length, the short Msg5 and long Msg5. Two Msg5 isoforms showed different binding affinities for Fus3 and Slt2 in order to specifically modulate yeast mating and cell wall integrity, respectively.52,53 These data suggest that posttranscriptional regulation of MSG5 is crucial to direct the outputs to maintain signaling specificity and avoid unwanted responses. The C. albicans CPP1 gene, an ortholog of MSG5 in S. cerevisiae, has been shown to be involved in Cek1p phosphorylation, and it is a dual specificity protein tyrosine phosphatase, too.36 Interestingly, although cpp1Δ mutant strains highly activated Cek1p phosphorylation and were hyper-filamentous under Spider medium, loss of CPP1 gene in C. albicans reduced the fungal burden in kidneys and its virulence.36 The causes of lower virulence of cpp1Δ mutant strains possibly are due to a growth rate defect in mycelia and formation of fewer lateral buds.23,36 These results indicate that the MAP kinase action counterbalanced by specific phosphatases is required for maintenance of an appropriate response to an environment and a host niche. Nevertheless, whether Cpp1 in C. albicans exists two isoforms in order to interact with specific MAP kinase proteins to promote signaling specificity needs to be determined.
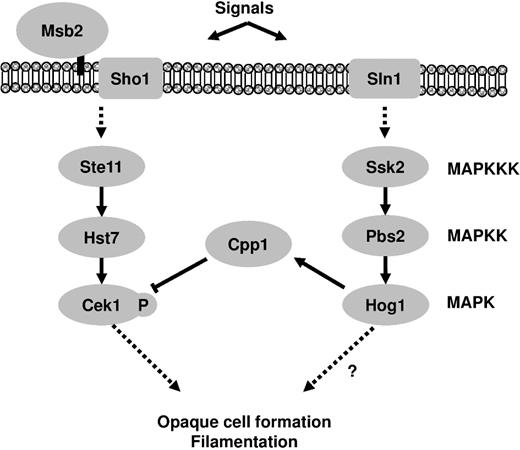
C. albicans, a diploid fungus, is the most important human fungal pathogen and can survive in different host niches owing to strong adaptation, divergent life styles and phenotypic plasticity.31,51,59–61 Despite major advances in study of the MAPK signaling pathways responsible for C. albicans environmental adaptation and morphological transition,2,16,17 the interconnections between these signaling pathways are not yet clear. Two reports have shown that Hog1 signaling inhibits Cek1p, but the presence of Hog1 promotes the activation of Mkc1p, a MAP kinase of the cell-wall integrity pathway.37,62 These results therefore raise additional questions regarding the potential mechanisms by which factors control what and when signals are activated to determine cell fates and pathway specificity. Furthermore, unlike S. cerevisiae, Hog1p activation of C. albicans is fully dependent on an osmosensor, Sln1, because the deletion of the transmembrane adaptors SHO1 or MSB2 had no effect on Hog1 phosphorylation.43 However, sho1Δ, msb2Δ, or sho1Δ/msb2Δ mutant strains showed reduced Cek1 activation.43 Therefore, the potential mode of interaction between the Hog1 and Cek1 MAPKs in C. albicans may be different from that in S. cerevisiae. Here, we demonstrated that crosstalk between two MAPK signaling pathways in C. albicans is mediated by the DSP Cpp1 (Fig. 5) based on the following reasons: (1) either cpp1Δ or hog1Δ could stimulate Cek1p activation; (2) both cpp1Δ and hog1Δ exhibit the same phenotypic transition under certain conditions; and (3) CPP1 expression is positively regulated by Hog1.
Proposed model of Hog1 and Cek1 pathway cross-inhibition in C. albicans. The dual-specificity phosphatase Cpp1 is a key connecting factor to modulate signaling specificity and avoid unwanted responses.
The gene expression profiles of white and opaque cells have shown that Efg1 and Ume6 play crucial roles in hyphal formation.42 Although the transcriptional regulation of filamentous growth is highly similar between white and opaque cells, the Cek1/Cek2 MAPK pathway plays no obvious role in filamentous growth under specific culture conditions, such as sorbitol and low-phosphate media.42 Different environmental cues are likely to affect the transcriptional regulatory network, indicating that C. albicans has evolved more sophisticated mechanisms than we can currently imagine to regulate MAPK activations for environmental adaptation and differentiation.
Taken together, our study showed that Cpp1 expression is positively regulated by Hog1 MAPK. The Cpp1 phosphatase specifically targets Cek1 phosphorylation to shape the outcome of Cek1 signaling, thereby affecting phenotypic development in both yeast-hyphae transition and white-opaque switching. Most importantly, this study illustrates that the modulation of each MAPK activity for environmental adaptation and differentiation is important to help cells to maintain pathway specificity and to prevent unwanted responses.
Supplementary material
Supplementary data are available at MMYCOL online.
Acknowledgements
This work was supported by MOST103-2628-B-002-003-MY3 and NTU103R7787 from the Ministry of Science and Technology and National Taiwan University, respectively.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.