-
PDF
- Split View
-
Views
-
Cite
Cite
Ping K. Yip, Liang-Fong Wong, Damian Pattinson, Anna Battaglia, John Grist, Elizabeth J. Bradbury, Malcolm Maden, Stephen B. McMahon, Nicholas D. Mazarakis, Lentiviral vector expressing retinoic acid receptor β2 promotes recovery of function after corticospinal tract injury in the adult rat spinal cord, Human Molecular Genetics, Volume 15, Issue 21, 1 November 2006, Pages 3107–3118, https://doi.org/10.1093/hmg/ddl251
Close - Share Icon Share
Abstract
Spinal cord injury often results in permanent and devastating neurological deficits and disability. This is due to the limited regenerative capacity of neurones in the central nervous system (CNS). We recently demonstrated that a transcription factor retinoic acid receptor β2 (RARβ2) promoted axonal regeneration in adult sensory neurones located peripherally. However, it is not known if RARβ2 can promote axonal regeneration in cortical neurones of the CNS. Here, we demonstrate that delivery of RARβ2 via a lentiviral vector to adult dissociated cortical neurones significantly enhances neurite outgrowth on adult cortical cryosections, which normally provide an unfavourable substrate for growth. We also show that lentiviral-mediated transduction of corticospinal neurones resulted in robust transgene expression in layer V corticospinal neurones and their axonal projections in the corticospinal tract (CST) of the spinal cord. Expression of RARβ2 in these neurones enhanced regeneration of the descending CST fibres after injury to these axons in the mid-cervical spinal cord. Furthermore, we observed functional recovery in sensory and locomotor behavioural tests in RARβ2-treated animals. These results suggest that a direct and selective delivery of RARβ2 to the corticospinal neurones promotes long-distance functional regeneration of axons in the spinal cord and may thus offer new therapeutic gene strategy for the treatment of human spinal cord injuries.
INTRODUCTION
Currently, nearly 1000 new cases of spinal cord injury (SCI) occur annually in the UK each year (1). Patients with SCI are often left with permanent and devastating functional disabilities due to neurological deficits. Unfortunately, there are no effective therapies that promote regeneration of damaged axons of central nervous system (CNS) neurones and therefore this is still an area of unmet clinical need.
The retinoid signalling pathway is important in the survival and differentiation of the vertebrate CNS, and retinoic acid (RA) has been shown to stimulate both neurite number and length in embryonic cultures (2–4). The functions of retinoids, however, may extend beyond their well-documented roles in neurodevelopment and play important roles in adult neural function. For example, age-related downregulation of retinoid-mediated transcription events has been postulated to play a role in the cognitive decline observed in aged mice (5–8). A key observation in these studies is the downregulation of mRNA levels for the nuclear receptor RA receptor β (RARβ) in the brains of aged rodents. Retinoid ligand activation of RARβ (or other receptor subtypes) induces gene transcription by interacting with distinct promoter sequences in target genes (9). The highly conserved sequence homology of RARβ2 between species and not within other subtypes suggests it has a unique and important function regulating cell growth and differentiation.
We have previously shown that delivery of RARβ2 to adult rat sensory neurones in the dorsal root ganglion (DRG) located peripherally can stimulate functional axonal regeneration into the spinal cord (10), suggesting that RARβ2 induced growth programmes within the injured neurones. In adult spinal cord explants, lentiviral vector-mediated expression of RARβ2 also induced neurite outgrowth (11). However, the effects of RARβ2 on long-distance axonal regeneration in the adult CNS (known to be severely inhibitory to regrowing axons) have not yet been examined in vivo.
Here, we test whether RARβ2 can prime growth programmes within adult CNS neurones in order for the injured axons to overcome the inhibitory CNS environment and thereby promote repair. Using equine infectious anaemia virus (EIAV)-based lentiviral vectors to deliver RARβ2 to adult cortical neurones in vitro, we show that RARβ2 can induce neurite outgrowth from these neurones on different substrata, including substrates that are inhibitory to regeneration. In the sensorimotor cortex, efficient and long-term expression in corticospinal neurones was observed following lentiviral vector delivery. Treatment with EIAV vectors expressing RARβ2 in the cortex promoted axonal regeneration in the corticospinal tract (CST) carrying descending axons from the sensorimotor cortex to the spinal cord, and improved functional recovery after SCI. These data indicate that RARβ2 is capable of supporting long-distance axonal regeneration in the inhibitory adult CNS and may therefore have therapeutic applications for the treatment of human spinal cord injuries.
RESULTS
EIAV-RARβ2 induces neurite outgrowth in adult rat cortical neurones
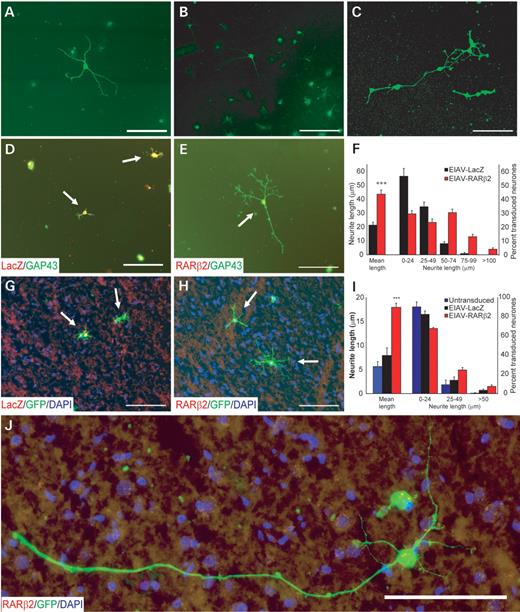
Dissociated neurones were prepared from adult rat cortex and cultured on a limiting substrate (0.01% poly-l-lysine) that was unfavourable for neurite outgrowth. Plated cells were identified as neurones by immunohistochemical staining with known neuronal markers such as MAP2 (Fig. 1A), NeuN (Fig. 1B) and βIII tubulin (Fig. 1C). Efficient transduction of cortical neurones was observed with EIAV vectors expressing either a reporter gene LacZ (EIAV-LacZ; Fig. 1D) or RARβ2 (EIAV-RARβ2; Fig. 1E) at a multiplicity of infection (MOI) of 100; 71.0±3.4% and 74.4±2.1% of the cortical neurons were transduced in EIAV-LacZ and EIAV-RARβ2 cultures, respectively. EIAV-RARβ2 increased neurite outgrowth in cortical neurones, compared with control EIAV-LacZ-transduced neurones (Fig. 1D and E; P<0.001, Student's t-test, from three independent experiments, at least 150 transduced neurones immunostained with the regeneration marker GAP 43 were counted per experiment). Neurite outgrowth from transduced cortical neurones was assessed by measuring the length of the longest neurite from the neurones. In EIAV-RARβ2-transduced neurones, the length of the longest neurite was 43.6±3.0 µm (mean±SEM) compared with 21.3±2.2 µm in EIAV-LacZ neurones (Fig. 1F). Further analysis of neurite lengths from cortical neurones revealed that there was an increase in the percentage of transduced neurones with neurite lengths of >50 µm in EIAV-RARβ2 cultures, compared with EIAV-LacZ cultures, where 91% of transduced neurones retained neurites of <50 µm. Furthermore, 17% of EIAV-RARβ2-transduced neurones produced neurites that were >75 µm, whereas in EIAV-LacZ cultures, only 0.8% of neurones produced neurites >75 µm. These data demonstrate that RARβ2 has the capacity to promote neurite outgrowth in adult cortical neurones in a serum-free medium without any neurotrophic support.
EIAV-RARβ2 promoted neurite outgrowth in adult cortical neurones in vitro. Adult cortical cultures were immunostained for neuronal markers such as MAP2 (A), NeuN (B) and βIII tubulin (C) in order to verify that cultures were enriched with neurones. Neurite outgrowth from cortical neurones was assessed on poly-l-lysine (D–F) or on cortical sections that provide a non-permissive substrate (G–J). Adult cortical neurones transduced with either control EIAV-LacZ (D and G) or EIAV-RARβ2 (E and H) were identified by mouse anti-β-galactosidase (1:300, Promega) or mouse anti-RARβ (1:200, Chemicon), indicated in red, respectively. Neurite outgrowth was detected, in green, by rabbit anti-GAP 43 (D and E; 1:1000, Chemicon), a regeneration marker. (F) In cultures on poly-l-lysine, measurement of neurite lengths from transduced neurones indicated that the average longest neurite from EIAV-RARβ2-transduced neurones was longer compared with control EIAV-LacZ-transduced neurones, with an increase in percentage of transduced neurones with neurite lengths greater than 50 µm. (I) EIAV-RARβ2-transduced neurones also extended longer neurites on cortical cryosections compared with EIAV-LacZ neurones and control-untreated neurones. (I) The percentage of transduced neurones with neurite lengths greater than 25 µm was also increased in EIAV-RARβ2 cultures, with long neurites observed from a few neurones (J). Results are the mean±SEM of at least 150 neurones per experiment, and from three independent experiments. Scale bar: 100 µm.
Cryosections of the cerebrum provide a poor substrate for the outgrowth of adult rodent cortical neurones (12). We tested whether expression of EIAV-RARβ2 could induce outgrowth by plating adult rat green fluorescent protein (GFP+) rat cortical neurones that were transduced with EIAV-RARβ2 on adult coronal cerebral cryosections. The GFP+ neurones enabled us to differentiate between the transduced dissociated cortical neurones and neurones within the cerebrum cryosections. EIAV-RARβ2-transduced neurones were able to extend longer neurites on the grey matter compared with control vector treated or untreated in the presence of 10−11 mtrans-RA (Fig. 1G–I; P<0.001, Student's t-test, from three independent experiments, at least 150 transduced neurones were counted per experiment). The mean length of the longest neurite was 18.0±0.9 µm (mean±SEM) in EIAV-RARβ2 neurones compared with 7.9±1.6 µm in non-transduced neurones and 5.6±1.0 µm in EIAV-LacZ neurones (Fig. 1I). As with cortical neurones grown on poly-l-lysine substrates, we observed an upward shift in the percentage of transduced neurones with longer neurites (>25 µm) in EIAV-RARβ2 cultures compared with untransduced and control EIAV-LacZ cultures. There was an increase in the number of neurones with neurite lengths of greater than 50 µm in EIAV-RARβ2 cultures; in a few cases, neurones were observed to extend neurites of up to 300 µm (Fig. 1J) In contrast, this was not observed in EIAV-LacZ cultures.
Transduction of pyramidal neurones in the sensorimotor cortex
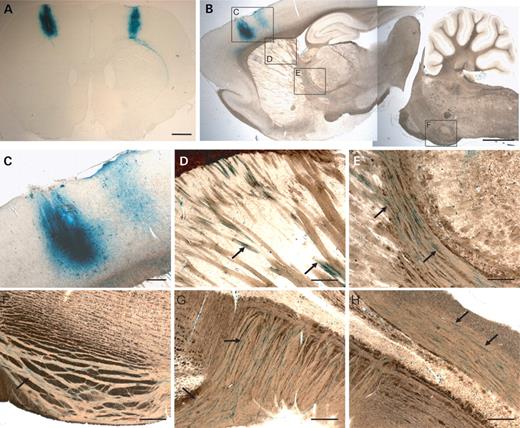
To transduce corticospinal neurones in vivo, EIAV vectors pseudotyped with the VSV-G glycoprotein were injected into the sensorimotor cortex. Using an EIAV vector expressing the reporter gene LacZ, we observed strong vector expression in the layer V pyramidal neurones at the site of injection (Fig. 2A–C). Positive X-gal staining was also detected in axons as they course through the corpus callosum (Fig. 2D), internal capsule (Fig. 2E), the cerebral peduncle of the midbrain and the ventral pons (Fig. 2F). Positively stained fibres were also observed as the corticospinal axons crossed over at the pyramidal decussation (Fig. 2G) and along the CST in the spinal cord (Fig. 2H). These data provide evidence that following transduction of corticospinal neurones, β-galactosidase from the EIAV vectors was anterogradely transported along the fibres in the CST to the spinal cord.
Transduction of the adult sensorimotor cortex and CST by EIAV-LacZ. Robust and efficient expression from a lentiviral vector encoding a reporter gene (EIAV-LacZ) was observed in the sensorimotor cortex following injection, as illustrated in coronal (A) and sagittal (B) sections. (C–H) Higher magnification pictures demonstrating transduction of cortical neurones, including layer V corticospinal neurones (C), and anterograde transport of reporter gene product (LacZ; indicated in blue) to the corpus callosum (D), internal capsule and thalamus (E), pons (F), pyramidal tract decussation in the caudal medulla (G) and at the base of the dorsal columns of the spinal cord (H). Scale bar: (A and B) 1 mm; (C–H) 100 µm.
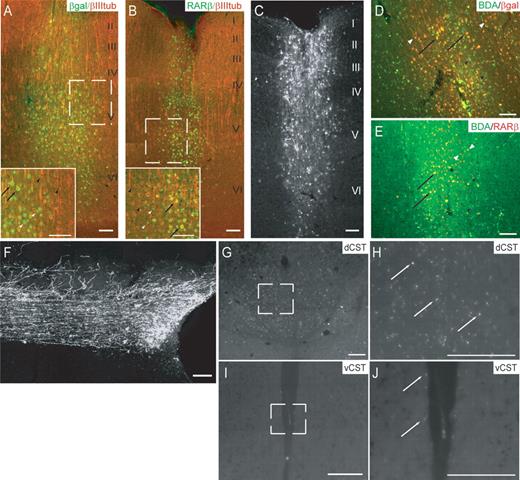
Colocalization studies revealed that injection of EIAV-LacZ into the sensorimotor cortex resulted in transduction of 74.6±1.9% of βIII tubulin-positive neurones in the layer V region at the site of injection, whereas EIAV-RARβ2 infusion led to transduction of 62.4±4.6% of βIII tubulin-positive neurones (Fig. 3A and B). These data demonstrate that targeting of the lentiviral vectors to forelimb sensorimotor cortex enabled efficient transduction of the corticospinal neurones at the site of injections. We were also able to demonstrate efficient labelling of pyramidal neurones with biotinylated dextran amine (BDA, MW 10 000) using the same stereotaxic coordinates (Fig. 3C). We performed immunohistochemistry to determine whether there were differences in the spread of the lentiviral vectors and BDA tracer. We observed that 46.4±2.2% of β-galactosidase (β-gal)-positive neurones were labelled with BDA in control injected animals, whereas 50.2±4.3% of RARβ2-positive neurones were labelled with BDA (Fig. 3D and E). BDA-labelled axons from the cortical neurones could be traced from the cortex through to the pyramidal and CST (Fig. 3F). In the spinal cord, numerous BDA-labelled fibres were detected in the dorsal CST (Fig. 3G and H), and a small number of labelled fibres were observed in the ventral CST (Fig. 3I and J).
Lentiviral expression in the sensorimotor cortex and BDA labelling of corticospinal axons. (A, B) βGal or RARβ expression (green) was colocalized with βIII tubulin (red) in layer V corticospinal neurones in the cortex in animals after EIAV transduction (inset represents magnified area of boxed region in the main picture). Colocalization of βgal or RARβ with βIII tubulin is highlighted with black arrows, whereas βgal- or RARβ-positive and βIII tubulin-positive neurones are indicated by white and black arrowheads, respectively. (C) Efficient anterograde labelling of CST was also achieved by administering BDA (MW 10 000) to the cortex, using identical stereotaxic coordinates as those used for lentiviral transduction. In (A–C), roman numerals represent cortical layers within the cortex. (D, E) βGal- or RARβ-overexpressing layer V neurones (red) were also colocalized with BDA-labelled cells (in green) in the cortex. Colocalization of βgal or RARβ with BDA is highlighted with black arrows, whereas βgal- or RARβ-positive and BDA-positive neurones are indicated by white and black arrowheads, respectively. Corticospinal fibres were also extensively labelled at the spinal level, and individual fibres with arbours can be visualized, allowing for quantification of axonal regeneration in the CST (F). (G and H) The majority of BDA-labelled fibres (indicated by white arrows) were present in the dorsal CST (dCST), whereas in (I and J), a few fibres (white arrows) could be detected in the ventral CST (vCST). (H and J) represent magnified areas of the boxed regions in (G and I), respectively. Scale bar: 100 µm.
Enhanced axonal regeneration with EIAV-RARβ2 transduction in vivo
In order to test the hypothesis that overexpression of RARβ2 can enhance the regeneration potential of corticospinal neurones, we delivered EIAV-RARβ2 to the sensorimotor cortex in a rat model of SCI. Following expression of EIAV-RARβ2 in the corticospinal neurones, SCI was induced in adult Wistar rats by performing a dorsal column lesion at the level of the C4 spinal cord. Anatomical regeneration of injured CST axons was assessed by labelling neurones in the motor cortex with BDA, as described previously. To ensure complete transection of the CST, we observed an absence of immunoreactivity for protein kinase C (PKC)-γ (13,14) in the dorsal columns of lumbar cord segments in both EIAV-LacZ and EIAV-RARβ2 animals (Fig. 4). Animals with incomplete dorsal column crush lesions, as demonstrated by the presence of dorsal funicular PKCγ, were removed from analysis.
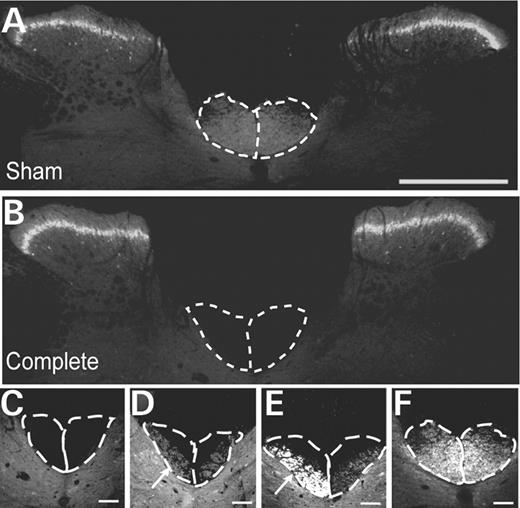
Detection of PKCγ in the lumbar spinal cord. Immunostaining for PKCγ was carried out in the lumbar spinal cord to ensure that the CSTs were completely lesioned and did not result in spared axon(s) along the CST. (A) In sham (unlesioned) animals, intense labelling of PKCγ in the lamina II and at the base of the dorsal columns was observed, whereas animals with complete lesions demonstrated lack of PKCγ labelling at the base of the dorsal columns (B). Detection of PKCγ in lamina II of the spinal cord was unchanged in these animals. (C–F) Higher magnification micrographs of dorsal columns of animals with (C) complete, (D and E) incomplete and (F) sham lesions. In animals with incomplete lesions, some PKCγ staining at the base of the dorsal columns was observed (indicated by white arrows). These animals were subsequently excluded from the study. Scale bar: (A and B) 500 µm; (C–F) 100 µm.
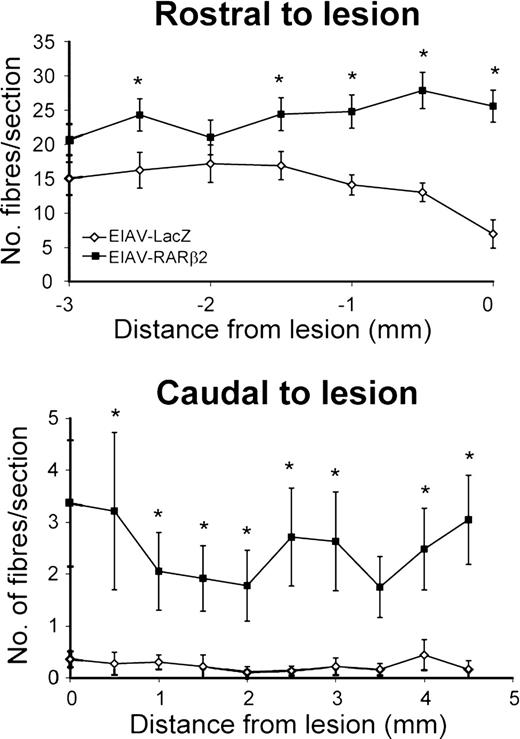
In control-treated animals (EIAV-LacZ), retraction of labelled fibres was observed, and few fibres with bulbous endings approached the lesion (Figs. 5A and B). Few, if any, labelled fibres were present in the immediate vicinity of the lesion or extended beyond the lesion site (Fig. 5C and D). In contrast, rats which received EIAV-RARβ2 treatment showed thick-labelled fibre bundles that approached the proximal edge of the lesion (Fig. 5E–G). At higher magnification, we could detect BDA-labelled axons around the lesion site (Fig. 5L). Furthermore, labelled fibres were detected beyond the lesion (Fig. 5H–K), and some axons were observed to extend arborizing collaterals from the white matter into the grey matter (Fig. 5K, indicated by white arrowhead). We also detected some axons with growth cone-like endings in the spinal cord (Figs. 5H and M, indicated by white arrowheads). Quantification of the number of BDA-labelled fibres present in the CST immediately rostral to the lesion showed that there were significantly more labelled fibres in EIAV-RARβ2 compared with control EIAV-LacZ animals (Fig. 6; P<0.05, two-way RM ANOVA). In the spinal cord caudal to the lesion, the number of BDA-labelled fibres in the CST was also significantly higher in EIAV-RARβ2 animals compared with EIAV-LacZ animals (Fig. 6; P<0.05, two-way RM ANOVA).
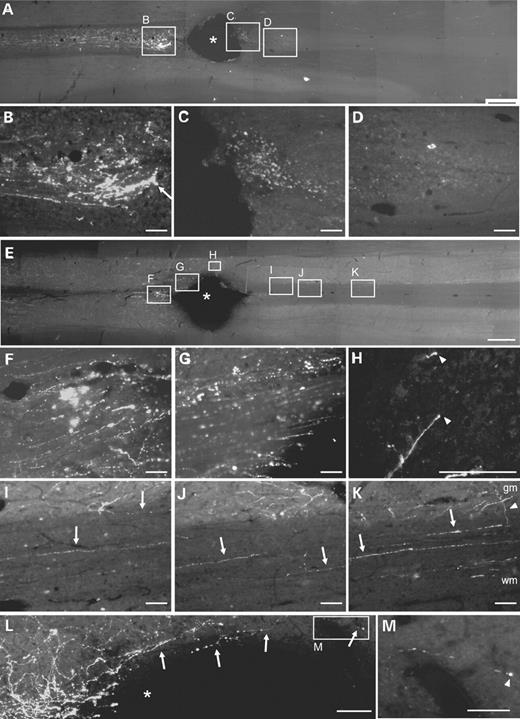
Axonal regeneration in the CST after spinal cord lesion. Longitudinal sections of adult rat spinal cord were labelled with BDA to determine fibre regeneration in the CST after spinal cord lesion. Photomicrographs were captured in the rostral–caudal orientation from left to right. (A–D) In control EIAV-LacZ animals, the lesion resulted in Wallerian degeneration of CST axons and few fibres with bulbous endings (B, indicated by white arrow) approached the proximal edge of the lesion. BDA-labelled axons could not be detected in the CST surrounding the lesion (C) or distal to the lesion (D). In contrast, EIAV-RARβ2-treated animals did not exhibit extensive degeneration of fibres (E and F), and labelled fibre bundles were detected up to the edge of the lesion (G). Labelled axons were also detected at the distal edge of the lesion (H) and in the CST at various distances caudal to the lesion (I–K). (H) At the tips of some axons, growth cone-like endings were present (indicated by white arrowheads). In (K), axons appear to send collaterals (indicated by white arrowhead) from the white matter (wm) to the grey matter (gm). (L) In a higher magnification image of the lesion site, labelled axons could be detected around the lesion site in the spinal cord; in (M), a labelled axon with growth-cone like endings is present. In (A), (E) and (L), asterisk denotes lesion site. Scale bars: (A and E) 1 mm; (B–D, F–L) 100 µm; (M) 50 µm.
Quantification of labelled fibres in the CST after spinal cord lesion. EIAV-RARβ2-treated animals showed an increase in the number of BDA-labelled fibres in the CST rostral and caudal to the lesion when compared with the EIAV-LacZ-treated animals. In EIAV-LacZ animals, the number of labelled fibres in the CST decreased as the CST approached the lesion (14.1±1.5 per section at 1 mm), whereas in EIAV-RARβ2 animals, the number of labelled fibres in the CST did not decrease (24.8±2.4 per section at 1 mm from lesion). In the CST caudal to the lesion, few BDA-labelled fibres were observed in EIAV-LacZ animals (0.3±0.1 per section at 1 mm caudal to lesion), whereas, significantly, more fibres were detected in the EIAV-RARβ2 group (2.1±0.7 per section at 1 mm caudal to lesion; P<0.05, two-way RM ANOVA).
Functional recovery associated with histological regeneration
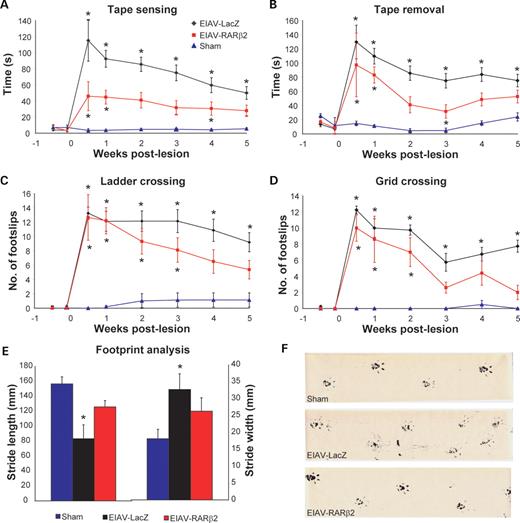
The presence of arborizing collaterals in the grey matter suggested that the regenerated CST axons may establish functional connections with post-synaptic neurones. Dorsal column projections are important for discriminative touch and proprioception, and therefore we undertook appropriate behavioural tests to examine functional recovery. Rats were assessed for sensorimotor function by testing for their ability to sense and remove an adhesive tape attached onto the plantar surface of their forepaw. Unlesioned sham controls performed these tasks quickly (5.6±1.2 s and 24.0±5.7 s to sense and remove tape, respectively), whereas EIAV-LacZ-treated animals were significantly impaired throughout testing (Fig. 7A and B; 50.1±8.0 s and 75.3±8.8 s, respectively, at 5 weeks' post-lesion; P<0.001, two-way RM ANOVA). In contrast, EIAV-RARβ2-treated animals showed recovery of function; at 6 weeks' post-lesion, the time taken to sense and remove tape in these animals was 28.0±6.8 s and 52.7±9.0 s respectively). However, the recovery in EIAV-RARβ2 was not complete, as latencies for tape sensing and removing were significantly different from sham controls (P<0.05 and P<0.01, respectively, two-way RM ANOVA). EIAV-RARβ2 treatment also consistently improved recovery in motor function in a ladder- and grid-crossing task, in which rats were assessed for the number of foot slips as they crossed a horizontal ladder or grid (Fig. 7C and D). In both tasks, unlesioned sham controls made few foot slips but EIAV-LacZ-treated rats were severely and significantly impaired throughout testing. At 5 weeks' post-lesion, the number of foot slips made by EIAV-LacZ rats in ladder- and grid-crossing was 9.2±1.3 and 7.8±0.8, respectively, compared with 1.1±1.0 and 0±0, respectively, in sham controls (P<0.001, two-way RM ANOVA). The EIAV-RARβ2 group showed improvement in task performance from 3 weeks' post-lesion; and at 5 weeks' post-lesion the number of foot slips made was 5.4±1.3 and 2.0±0.8 in ladder- and grid-crossing tasks, respectively. The improvement demonstrated by EIAV-RARβ2 rats was partial, as the number of foot slips was significantly different from sham controls (P<0.01, two-way RM ANOVA). Walking patterns were also assessed by analysing footprint spacing. At 5 weeks' post-lesion, EIAV-LacZ-treated rats took shorter and wider strides (65.6±8.4 mm and 37.3±1.3 mm, respectively) compared with sham unlesioned controls (Fig. 7E and F; 156±9.5 mm and 18.1±2.8 mm, respectively; P<0.01 and P<0.05, respectively, Student's two-tailed unpaired t-test). In contrast, the EIAV-RARβ2 group did not show a significant change in stride length and width (125.8±7.7 mm and 26.3±4.0 mm, respectively) compared with sham controls, suggesting that these rats made a significant recovery to their walking patterns.
Assessment of functional regeneration using behavioural tests. Control and treated groups were tested for sensory and locomotor function in order to assess functional regeneration. (A and B) In the tape sensing and removal tests, control EIAV-LacZ animals showed severe deficits compared with the sham controls (P<0.001, two-way RM ANOVA), whereas EIAV-RARβ2 showed partial recovery of function (P<0.05 and P<0.01 for tape sensing and removal, respectively, compared with sham controls, two-way RM ANOVA). (C and D) In the ladder and grid crossing tasks, EIAV-LacZ made an increased number of foot slips throughout the testing period compared with sham controls (P<0.001, two-way RM ANOVA); in contrast, the EIAV- RARβ2 group showed improvement in task performance from 3 weeks' post-lesion; however, full recovery was not observed, as these animals were significantly different from sham controls (P<0.01, two-way RM ANOVA). (E and F) In footprint analyses, EIAV-LacZ animals took shorter and wider strides compared with sham controls (P<0.01 for stride length and P<0.05 for stride width, Student's two-tailed unpaired t-test), whereas EIAV-RARβ2 took longer and narrower strides that were not significantly different from sham controls.
DISCUSSION
We have investigated the effects of EIAV-RARβ2 on axonal regeneration of adult cortical neurones in vitro and in an in vivo rat model of SCI. Overexpression of RARβ2 in cortical neurones enabled significant neurite outgrowth in a non-permissive environment and on cortical cryosections compared with that of control neurones. In vivo, efficient transduction of the sensorimotor cortex was demonstrated and resulted in good labelling of the CST. Using this delivery method, we showed that overexpression of RARβ2 in adult rat corticospinal neurones increased CST axonal outgrowth up to and beyond a CNS lesion, and improved functional recovery in sensory and locomotor behavioural tasks.
Previous studies in adult spinal cord regeneration have used embryonic cells, transformed cells or perinatal cells to evaluate neurite outgrowth due to the ease of culture of these cellular types. However, in these studies, the use of neurones derived from the adult brain is more relevant. Dissociated adult cortical neurones have been previously employed in other studies in mice (12,15) and rats (16,17), and Tom et al. (12) have demonstrated that adult cortical neurones were normally unable to produce long neurites on CNS cryosections. In our experiments, RARβ2-transduced adult cortical neurones when seeded onto adult cortical cryosections did not demonstrate significant neurite outgrowth compared with controls (data not shown). This was surprising because RARβ2-transduced neurones were able to extend neurites in the absence of exogenous ligand on a different substrate such as poly-l-lysine (Fig. 1F). The presence of retinol and RA-binding proteins, such as cellular retinol-binding protein-1 (CRBP-1) and cellular RA-binding protein-1 (CRABP-1), as well as the RA-synthesizing enzyme retinaldehyde dehydrogenase-3 (RALDH-3), has been demonstrated in the adult rodent cortex, suggesting that there are internal retinoid reservoirs within the adult cortical neurones (18–20). In the case of cortical neurones grown on the cryocultures, the endogenous levels of RA may not be sufficient in cortical neurones to overcome the inhibitory environment of the grey matter of the CNS, hence neurite growth was attenuated without additional ligand supply. Therefore, when we provided exogenous ligand in the form of trans-RA at a concentration of 10−11 m, there was a significant increase in neurite outgrowth from RARβ2-transduced adult cortical neurones on cryosections, but not from control cultures. The concentration (10−11 m) of trans-RA used in the cryoculture experiments was 100-fold less than previously observed in vivo and it is likely that in vivo sources of RA, for example, the cerebrospinal fluid or other brain regions such as the striatum, will be sufficient to activate EIAV-RARβ2 in the in vivo experiments.
Following spinal cord lesions, signals derived from myelin such as Nogo, myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (OMgp) (21–27) as well as extracellular matrix molecules within the glial scar such as chondroitin sulphate proteoglycans (CSPGs) (28) prevent regeneration of damaged axons. Many studies have shown some functional recovery after SCI by neutralizing these inhibitory signals with treatments including Nogo-blocking peptides (22,29), infusions of chondroitinase ABC (13) or transplantation of olfactory ensheathing cells (30,31). These strategies make the injured spinal cord less hostile to regeneration, either from lesioned fibres that attempt to repair axonal connections or from surviving intact axons that promote functional recovery through plasticity mechanisms. Our approach in this study was to use an EIAV-based lentiviral vector encoding RARβ2 to target the CNS corticospinal neurones in order to increase their regenerative capacity despite the adverse environment in the spinal cord. The nuclear transcription factor RARβ2 is not normally detectable in corticospinal neurones, as demonstrated by Zetterstrom et al. (19), and in our study, by the absence of positive RARβ immunostaining in areas distant from the injection site. This indicates that low, if any, amounts of RARβ2 protein is present in these cells. By targeting the sensorimotor cortex with EIAV-RARβ2 and efficiently labelling CST axons through the use of precise stereotaxic coordinates, we were able to increase RARβ2 levels in a large number of corticospinal neurones and study its effects on CST regeneration. We demonstrated that numerous transduced layer V cortical neurones were also labelled with the BDA anterograde tracer, indicating that vector transduction and tracer spread were not mutually exclusive. This suggested that many of the anterogradely labelled fibres in the spinal cord are likely to arise from transduced neurones. By studying this sample population of cells, we observed that RARβ2 treatment increased axonal regeneration in the CST and improved functional recovery after SCI.
Assessment of axonal regeneration in the spinal cord using the seven-point criteria proposed by Steward et al. (32) indicated that several of the criteria were fulfilled. We detected labelled axons in the immediate vicinities of the lesion in the RARβ2 spinal cord, whereas few, if any, were observed in control LacZ cord (Fig. 5L). Astrogliosis in the immediate tissue surrounding the lesion site often generates an environment inhibitory to regeneration. Therefore, Criteria I and III were fulfilled, where the axon was shown to extend from the site of injury into the tissue environment of the scar that develops at the injury site. Furthermore, we show unusual tracking and morphology of regenerated axons, for example, traversing of axons from the white matter into the grey matter and axon branching in the grey matter (Fig. 5I–K; Criteria IV and VII). We have also determined that the observed axonal regeneration in the RARβ2 cord did not exceed plausible regeneration rates, as labelled fibres were observed at <10 mm from the lesion site (on the basis of a maximal regeneration rate of 1 mm per day in the CNS, the maximal regeneration distance was 35 mm in the experimental period) (criterion V). Finally, we detected labelled axons around the lesion site in the spinal cord, which had growth cone-like endings (Fig. 5H and M; criterion VI). These observations suggest that the labelled fibres in the RARβ2 cord are likely to be regenerated, but not spared axons.
During assessment of functional regeneration using behavioural tasks, we observed that the EIAV-RARβ2 group made partial recoveries in some of the sensory-locomotor tasks compared with sham controls and showed a modest increase in the number of labelled fibres in the CST after spinal cord lesion. The effects of EIAV-RARβ2 we have seen may be limited by the partial transduction of corticospinal neurones we were able to produce. We showed that 60–75% of the layer V cortical neurones were transduced by the lentiviral vectors at the site of injections (Fig. 3A and B); however, fewer neurones were transduced in the cortical regions distant from the injection sites (data not shown). It has been shown that three unilateral injections into the cortex lead to a tracing efficiency of 1.5% of total CST fibres (33). Given that we injected at six sites, we are likely to have transduced only a modest fraction of all CST neurones. Nevertheless, the partial recovery that was observed in these studies was significant.
The effects of RARβ2 overexpression on axonal regeneration in the inhibitory environment of the injured spinal cord may be a result of transcriptional changes that were induced within the corticospinal neurones. We have previously shown that EIAV-RARβ2 transduction of DRG neurones increases cAMP levels in a model of peripheral nerve regeneration into the CNS (10), and cAMP has been demonstrated to promote regeneration of lesioned dorsal column fibres in rats (34,35). It is therefore possible that EIAV-RARβ2 can activate cAMP-signalling systems in corticospinal neurones to increase the intrinsic regenerative capacity of these neurones. In this study, we observed that EIAV-RARβ2 mediated long-distance regeneration in a modest number of fibres. EIAV-RARβ2 was administered to the sensorimotor cortex while the lesion was induced at the C4 level of the spinal cord, and therefore any RARβ2-mediated effects are likely to occur at the level of the cell bodies of the corticospinal neurones and not locally at the lesion site in the spinal cord. We detected a small number of BDA-labelled fibres in the ventral CST in the spinal cord (Fig. 3I and J) and it is thus possible that the functional recovery we observed may be due to spontaneous sprouting from spared fibres in the ventral CST (36). Spared ventral corticospinal fibres have the propensity to extend branching collaterals into the grey matter areas, as demonstrated by Brosamle and Schwab (33). However, in our studies, we have only analysed BDA-labelled fibres in the white matter area representing the main CST (in the dorsal columns) and not in the grey matter. Furthermore, the population of BDA fibres in the ventral CST represents a small fraction of the total labelled fibres (1.8%) and therefore we feel the majority of the labelled fibres that are present in the RARβ2-treated spinal cords, particularly those that are located in the dorsal columns, are likely to originate from regenerated axons and not from the ventral CST.
Although gene therapy approaches are being developed in the clinic for other neurodegenerative diseases such as Parkinson's and motor neurone diseases, gene therapy for spinal cord injuries has not been widely studied and is still in its infancy. Our study provides a proof-of-concept that genetic manipulation of specific neurones can enhance neuronal function and improve functional regeneration, thereby paving the way for further experimentation with molecules in the treatment of spinal cord injuries. This approach may be used in combination with current strategies that aim to provide a favourable environment in the lesioned spinal cord for functional regeneration, such as digesting inhibitory proteoglycans or providing artificial growth support matrices.
MATERIALS AND METHODS
Minimal lentiviral vector construction and production
EIAV-RARβ2 was based on the SMART2 series of EIAV vectors. EIAV-RARβ2 comprises minimal long-terminal repeat sequences, a 5′ central polypurine tract element, minimal CMV promoter-driving expression of mouse RARβ2 and a 3′ woodchuck post-transcriptional regulatory element (WPRE) sequence. The RARβ is ∼100% conserved among mouse, rat and human when its protein homology was compared between species using the BLAST 2 sequences in the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi) (37).
Adult cortical neurone cultures
Adult male Wistar rats (220–250 g) (n=3) were sacrificed and part of their cortices were dissected out for dissociation. The adult cortical neurones were prepared as previously published (15,38). Briefly, the animal was transcardially perfused with heparinized saline, the cortices removed with as little white matter attached as possible. The cortices were cut into 0.5 mm longitudinal sections using a McIlwain tissue chopper, white matter was trimmed away before dissociation in 2 mg/ml papain at 30°C for 30 min. The cortical neurones were mechanically dissociated with a glass Pasteur pipette and separated from debris by centrifugation in four 1 ml steps of Optiprep in B27/HibernateA medium (7.5, 10, 12.5 and 17.5%) at 600 g for 15 min. Fractions containing neurones were collected, washed and resuspended in B27/NeurobasalA medium with gentamycin, ready for plating at a density of approximately 2000 neurones per well on pre-treated cover slips (0.01% poly-l-lysine). The neurones were allowed to settle onto the poly-l-lysine substrate for 18 h, after which EIAV-RARβ2 pseudotyped with vesicular stomatitis virus (VSV-G) envelope was added to the media at MOI 100 for the cortical neurones. The neurones were left for a further 3 days in vitro before immunohistochemistry.
Adult cortical neurone cryocultures
Studies involving adult cortical neuron cryocultures have been limited by the inability to visualize the dissociated neurones seeded on top of the adult cortical cryosections by immunocytochemistry. To overcome this in this study, the dissociated adult cortical neurones were prepared from enhanced green florescent protein (GFP)-expressing rats obtained from Professor M. Okabe (Sprague Dawley strain, Osaka University, Japan) (39). Adult GFP-expressing rats (250–300 g) (n=3) were sacrificed, cortices removed and neurones prepared as described earlier. For preparation of cortical cryosections, wild-type Sprague Dawley were perfused as described earlier, brains were removed, sectioned coronally and quickly frozen on dry ice. Prior to culture, 12 µm coronal sections of cortex were cut on a cryostat and thaw-mounted onto pre-treated poly-l-lysine cover slips. Cryosections were left to dry in culture hood for at least 30 min but no more than 3–4 h before addition of dissociated adult cortical neurones.
Immunohistochemistry
After 3 days in culture, neurones were fixed with 4% paraformaldehyde for 20 min and then permeabilized with cold methanol for 3 min. The neurons were washed with 3×5 min with PBS before 2 h incubation with rabbit anti-GAP43 (1:1500, Chemicon), mouse anti-RARβ (1:200, Chemicon), mouse anti-β-galactosidase (1:500, Sigma) and rabbit anti-GFP (1:1000, Molecular Probes). The cover slips were washed 3×5 min with PBS and then incubated 45 min with donkey anti-rabbit 488 and -mouse 546, secondary antibodies (1:2000, Molecular Probes). After 3×5 min PBS washes, the cover slips were embedded in Flurosave reagent (Calbiochem) with 0.5 µl DAPI (10 µg/ml) to visualize cell nuclei. For quantification of neurite outgrowth, the length of the longest neurite for the first 150 neurones encountered when scanning in a systematic manner was determined using image analysis software (SigmaScan Pro 4.01) and was expressed as mean±SEM.
Viral vector delivery to the sensorimotor cortex
Adult male Wistar rats (n=8–9 per group) were anaesthetized using a combination of ketamine and medetomidine and fixed in a stereotaxic frame. The skull was exposed and injections were made at a depth of 2 mm dorsoventrally into the sensorimotor cortex region using the following injection coordinates as determined from a microstimulation mapping study (40): (in reference to bregma; AP, anterior–posterior; L, lateral) AP −1.5 mm, L 2.5 mm; AP −0.5 mm, L 3.5 mm; AP +0.5 mm, L 3.5 mm; AP +1.0 mm, L 1.5 mm; AP +1.5 mm, L 2.5 mm; AP +0.5 mm, L 3.5 mm. EIAV-RARβ2 pseudotyped with VSV-G was directly injected at 1 µl per site via a finely pulled glass micropipette at a rate of 0.2 µl/min, using a microinfusion pump and left for a further 1 min. EIAV vector pseudotyped with a VSV-G envelope produced strong anterograde transgene expression (41,42). Three weeks after viral injection, SCI was induced by performing a bilateral dorsal column crush at the level of C4 using fine forceps (13). Following injury, regeneration of the descending motor system was assessed behaviourally (13,43).
Behavioural assessment
The behavioural tests used were grid- and ladder-walking, sticky tape removal and footprint analysis (13,44). Assessment of all parameters was collected weekly before lesion and for 5 weeks post-lesion and compared with the appropriate groups such as a control vector (EIAV-LacZ)-treated group as well as a sham-operated group. At the end of the behavioural assessment, the animals were sacrificed and perfused transcardially with 4% paraformaldehyde and tissue collected for histology.
Detection of vector transduction and tracer labelling
Thirty-five micrometre coronal sections of the cortex were obtained on a vibratome and randomly chosen for X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) staining or immunohistochemistry. Using standard immunohistochemistry protocols, sections were doubly incubated with either mouse anti-βIII tubulin (1:1000, Promega) and rabbit anti-βgal/RARβ or extrAvidin-FITC (to detect BDA, 1:400, Sigma) and rabbit anti-βgal/RARβ, followed by detection with secondary antibodies. Dual colour images were obtained at 10× magnification of the layer V cortical region and analysed using the AxioVision LE 4.2 program. At least six transduced sites were analysed and quantified per animal (n=6 in each group).
Assessment of axonal regeneration
Regeneration of the descending corticospinal axons was assessed by injecting 1 µl 10% BDA (MW 10 000, Molecular Probes) bilaterally into the sensorimotor cortex 4 weeks before termination of experiment using the same coordinates as those used to administer the lentiviral vector. Longitudinal sections of the spinal cord (30 µm) were collected on a vibratome in cold 0.01 m PBS with 0.1% sodium azide. The sections were rinsed in 3×5 min PBS-T (0.01 m PBS with 0.3% Triton X-100), then incubated with 0.3% H202 to remove endogenous peroxidases. After a further 3×5 min wash with PBS-T, the sections were incubated in avidin–biotin peroxidase complex (Vectastain ABC Elite Kit, Vector Laboratories) for 30 min at room temperature. After a 3×5 min washing in PBS-T, sections were incubated in tyramide (1:75) for 10 min. After 3×5 min wash, the sections were incubated with extra-avidin FITC (1:400, Sigma) for 2 h before the sections were washed and mounted onto slides and embedded with Flurosave reagent (Calbiochem). For quantitative analysis, four sections per animal containing the CST were selected blindly and stained for BDA. Fibres were counted rostral and caudal to the lesion site at every 0.5 mm under the microscope at 20× magnification. Only main fibres that were running longitudinally in the white matter along the cord were counted and the fibres tangent to these were not counted given that these are collateral fibres, not the main CST fibres. The number of fibres at the rostral end of the lesion represents the quality and quantity of labelled fibres before the injury. For detection of BDA-labelled fibres in the ventral CST, 30 µm thick transverse sections of the spinal cord were obtained and stained for BDA, as described previously. At least six random sections from each animal were selected and the number of BDA fibres in the contralateral dorsomedial funiculus (i.e. one side of the CST) and the ipsilateral ventromedial funiculus were counted (n=6).
Histological confirmation of CST lesion
To determine whether the CST lesion was complete, transverse sections of the lumbar cord were stained for protein kinase γ (PKCγ). Animals with staining of PKCγ at the base of the dorsal columns were excluded from the study.
ACKNOWLEDGEMENTS
The authors wish to thank Professor M. Okabe (Osaka University, Japan) for providing the GFP rats and Professor W.F. Blakemore (University of Cambridge, U.K.) for supplying the GFP rats. The work was supported by Christopher Reeve Paralysis Foundation and Medical Research Council.
Conflict of Interest statement. The authors wish to declare competing financial interests.
REFERENCES
Author notes
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint First Authors.
Present Address Department of Gene Therapy, Section of Infectious Diseases, Division of Medicine, Wright–Fleming Institute, Imperial College London, St Mary's Campus, Norfolk Place, London W2 1PG, UK.