-
PDF
- Split View
-
Views
-
Cite
Cite
Shannon A Novosad, Sridhar V Basavaraju, Pallavi Annambhotla, Marika Mohr, Alison Laufer Halpin, Linda Foy, Richard Chmielewski, Jonas M Winchell, Alvaro J Benitez, Shatavia S Morrison, Taccara Johnson, Donna M Crabb, Amy E Ratliff, Ken Waites, Matthew J Kuehnert, Mycoplasma hominis Infections Transmitted Through Amniotic Tissue Product, Clinical Infectious Diseases, Volume 65, Issue 7, 1 October 2017, Pages 1152–1158, https://doi.org/10.1093/cid/cix507
Close - Share Icon Share
Abstract
Mycoplasma hominis is a commensal genitourinary tract organism that can cause infections outside the genitourinary tract. We investigated a cluster of M. hominis surgical site infections in patients who underwent spine surgery, all associated with amniotic tissue linked to a common donor.
Laboratory tests of tissue product from the donor, including culture, quantitative real-time polymerase chain reaction (qPCR), and whole-genome sequencing were performed. Use of this amniotic tissue product was reviewed. A multistate investigation to identify additional cases and locate any unused products was conducted.
Twenty-seven tissue product vials from a donor were distributed to facilities in 7 states; at least 20 vials from this donor were used in 14 patients. Of these, 4 of 14 (29%) developed surgical site infections, including 2 M. hominis infections. Mycoplasma hominis was detected by culture and qPCR in 2 unused vials from the donor. Sequencing indicated >99% similarity between patient and unopened vial isolates. For 5 of 27 (19%) vials, the final disposition could not be confirmed.
Mycoplasma hominis was transmitted through amniotic tissue from a single donor to 2 recipients. Current routine donor screening and product testing does not detect all potential pathogens. Clinicians should be aware that M. hominis can cause surgical site infections, and may not be detected by routine clinical cultures. The lack of a standardized system to track tissue products in healthcare facilities limits the ability of public health agencies to respond to outbreaks and investigate other adverse events associated with these products.
Mycoplasma hominis is a commensal bacteria colonizing the genitourinary tract in healthy women [1–4] and is found in the respiratory tract [5]. Though usually commensal, it has been associated with genitourinary infections such as pelvic inflammatory disease, bacterial vaginosis [6, 7], and pregnancy-related infections [8–10]. Extragenital infections have been reported [11–13].
Mycoplasma hominis–related surgical site infections have been reported after genitourinary surgery resulting from catheter or surgical instrumentation–related contiguous or hematogenous spread [14]. Surgical site infections due to M. hominis have occurred following other types of surgeries, including spinal surgery [15, 16] and mediastinitis following sternotomy [17]. The infection has been transmitted via solid organ transplantation [18]. While vascular tissue allograft transmission has been described [19], M. hominis transmission through tissues recovered from the genitourinary tract has not been reported, though this is plausible.
CASE REPORT
In September 2015, 4 patients developed surgical site infections 1–3 weeks after spine surgery performed at a single acute care hospital in Ohio. The infections were characterized by fever, erythema, wound discharge, or pain at the surgical site. In the first patient, no pathogen was identified and in the second Staphylococcus epidermidis was identified from wound cultures. Culture of wound material from the third and fourth infected patients revealed colonies on nonselective media that appeared to be consistent with M. hominis. These isolates were sent to a clinical laboratory (Focus Diagnostics) where M. hominis was confirmed using 16S ribosomal DNA (rDNA) sequencing. Both patients were treated with doxycycline for 8–12 weeks; symptoms resolved without recurrence. No other M. hominis infections were reported at this facility during this time.
In 2015, the hospital began an investigation evaluating commonalities between the 4 patients and all patients who had spinal surgery. All 4 had received a tissue allograft made from human amnion recovered from the same donor. A review of other patients who had received human amnion products did not reveal adverse events.
In October 2015, the Ohio Department of Health requested assistance from the Centers for Disease Control and Prevention (CDC) to investigate these surgical site infections. CDC’s objectives were to assist in determining whether the infections were transmitted through the amniotic tissue product, to identify additional recipients who received the product, and to recommend interventions to prevent future transmission.
METHODS
Epidemiologic Investigation and Product Tracking
The clinical course, diagnostic evaluation, and microbiologic data from the 4 patients with surgical site infections were reviewed. The tissue processor was contacted to determine donor-related clinical and epidemiologic information, product procurement, processing, microbiologic testing, and distribution details. An implant card is routinely sent by the tissue processor with each vial. Facilities are requested to voluntarily return the completed card following product use. Information collected includes details on type and date of procedure, facility, healthcare provider, and patient demographics. The card does not contain fields to identify implant card completion date or to specify whether an adverse event occurred, but includes an open field where additional information can be included at the respondent’s discretion. These cards are prestamped by the tissue processor with lot number and serial code, unique to the individual donor and vial, respectively. The roster of facilities that received the product and any completed implant cards were obtained from the tissue processor and reviewed.
Additional case finding was attempted in collaboration with state health departments where tissue was distributed. Facilities that received the product from this donation were requested to provide information on whether the vials were used, discarded, or remained in inventory, and whether recipient patients experienced adverse events including infections. Respondents were asked to describe procedures and systems implemented to track human-derived tissue product use within their facilities. We attempted to match both the lot number and serial code for each vial to confirm disposition.
Laboratory Testing
Two unopened vials of product from the donor and any available clinical isolates from infected patients’ surgical sites (n = 1) were obtained from the facility reporting the M. hominis infections for further testing. This included culture and quantitative real-time polymerase chain reaction (qPCR) performed at the University of Alabama at Birmingham Diagnostic Mycoplasma Laboratory to detect M. hominis in the tissue product vials and whole-genome sequencing (WGS) performed at CDC to compare isolates from the tissue products to those from the ill recipient.
Cultures were performed by inoculating fluid from the 2 unopened samples into 0.9 mL of 10B and SP4 broth and performing 3 ten-fold serial dilutions. A 20-µL aliquot of each dilution was plated onto A8 and SP4 agar. Broths were examined twice daily for color change from ammonia production and agar plates were examined daily under a stereomicroscope for appearance of “fried egg” colonies of approximately 100 µm in diameter, typical of M. hominis, which usually appears after 24–48 hours of incubation. DNA was isolated from the original samples and from the Mycoplasma species isolated in culture using the proteinase K method [20]. The qPCR assay was performed using the Roche LightCycler 480 (Roche Diagnostics, Indianapolis, Indiana).
Isolates from each source were cultured for subsequent WGS analysis. Eighty-milliliter cultures of each isolate, confirmed as M. hominis by qPCR verification, were incubated for 96 hours to achieve sufficient quantities for WGS. DNA was extracted for WGS using the MasterPure DNA purification kit (EpiCentre, Madison, Wisconsin). DNA concentration was quantified using the Qubit dsDNA HS Assay Kit (Thermo Fisher, Waltham, Massachusetts).
WGS was performed using the Pacific Biosciences RS II genome analyzer (Pacific Biosciences, Menlo Park, California). The PacBio DNA Sequencing Kit 4.0 version 2, PacBio DNA/Polymerase Binding Kit P6 version 2, PacBio RSII SMRT Cells 8Pac version 3, and MagBead Kit version 3 were all used for sequencing. Optimization of 2 manufacturer-supported protocols was performed to increase sequencing yields. Supplementary File A has a detailed description of sequencing library prep performed for this study.
Genomic sequences were constructed with the hierarchical genome assembly process (HGAP3) assembler [21]. The MAFFT program was used to construct a whole-genome alignment to identify single-nucleotide polymorphisms (SNPs) [22]. Sites containing “N” or gaps were excluded from the analysis. Variant sites were used to construct a core SNP tree with RAxML [23]. The average nucleotide identity score was calculated at an intra- and interlevel comparison with M. hominis Sprott [24].
RESULTS
Epidemiologic Investigation and Product Tracking
The amniotic tissue product was obtained from a live, voluntary, non-remunerated donor with no significant past medical history, during cesarean delivery. Per manufacturer protocol, the amnion is processed with blunt force dissection, cleaned with saline, cut and dispensed into vials, cryopreserved, and used as an injectable material. The product is not sterilized and is described by the manufacturer to act as a biologic structural matrix for wound healing and for minimizing pain in surgical wounds, tendonitis, and plantar fasciitis.
The donor underwent medical history and behavioral risk assessment, which emphasized genetic diseases and high-risk sexual behaviors, in addition to infectious diseases screening, including human immunodeficiency virus, hepatitis B and C, syphilis, and chlamydia. All results were negative. The donor had no signs of infection at the time of delivery. The amnion was processed aseptically and tested for microorganisms by aerobic and anaerobic culture at several points during production. No microorganisms were identified on these cultures that were incubated for 2 weeks. No media or procedures designed to ensure Mycoplasma species detection were included as part of the testing procedures.
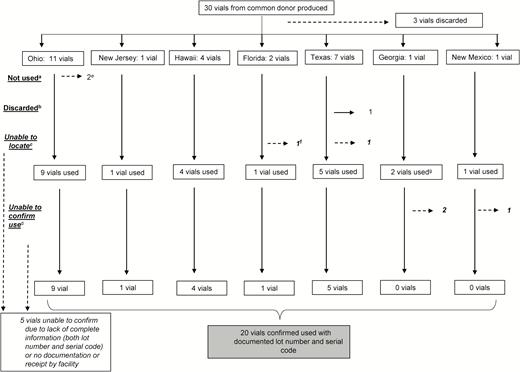
Thirty product vials from this index donation were produced (Figure 1). Three (3/30 [10%]) were retained by the manufacturer and discarded. Twenty-seven (27/30 [90%]) vials along with implant cards were distributed to 7 states. Facilities receiving the product included acute care hospitals, freestanding surgical centers, and outpatient facilities that provided primary care, alternative medicine, or cosmetic procedures. Twenty-five (25/27 [93%]) cards were returned to the manufacturer.
Amniotic tissue product distribution and use by state, Mycoplasma hominis outbreak investigation, 2015. aNot used: facility did not use but retained vials. bDiscarded: facility did not use and did not retain vials. cFacility unable to confirm vial had been received or implanted in a patient. dFor these vials, we were only able to find documentation that they were the correct lot number. We were unable to confirm serial code due to lack of documentation in medical record. eThese 2 vials were tested at the University of Alabama at Birmingham. fPer manufacturer report, facility in Florida received 2 vials but facility reported receiving and using only 1 vial. gPer manufacturer report, facility in Georgia only received 1 vial but facility reported receiving and using 2 vials.
The contents of 20 (20/27 [74%]) vials were confirmed as implanted in 14 patients during 17 separate procedures and categorized as “used.” Of the remaining 7 vials, 2 were unopened and 1 vial was discarded by a facility for unknown reasons prior to the investigation. For 3 vials, the disposition could not be determined as the receiving facility only documented lot number but not serial code of used vials. One facility was unable to confirm receipt of any vials, though the manufacturer reported distribution.
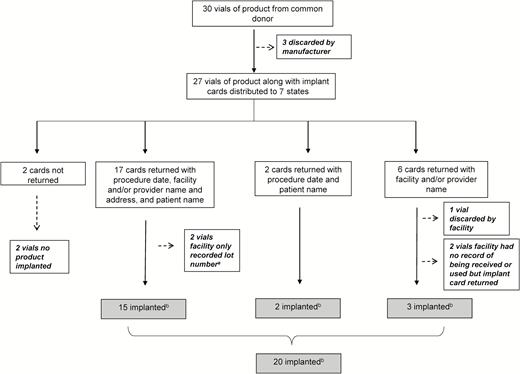
Of the 27 distributed vials, 25 had implant cards returned to the manufacturer with any information (Figure 2). The 2 cards not returned corresponded to 2 vials whose contents were not implanted. Three vials had implant cards returned but the product was not actually implanted in patients according to available records. Of those vials with implant cards returned to the manufacturer, 20 vials’ contents were confirmed as implanted in patients. Thirteen individual patients were identified from 19 (13/19 [68%]) implant cards containing patient identifiers.
Amniotic tissue product implant cards returned to manufacturer, Mycoplasma hominis outbreak investigation, 2015. aFor these vials, we were only able to find documentation that the lot number matched the implant card. We were unable to confirm that serial code matched implant card due to lack of documentation in medical record. bFor these vials, we confirmed both the lot number and serial code of vial used matched the lot number and serial code on implant card.
Twenty vials’ contents with this donor’s lot number and a serial code matching those reported by the manufacturer were implanted in 14 patients. However, the contents of 23 vials with the donor’s lot number (without a confirmed serial code) were implanted in 17 different patients during 20 different procedures (Table 1; Figure 2). The product was most commonly used during spine surgeries (8/20 [40%]), followed by joint injections (4/20 [20%]). In 13 (13/20 [65%]) procedures, the product was mixed with bone marrow aspirate before use, including in the 2 patients who developed M. hominis infections.
Amniotic Tissue Product Use and Adverse Events for Patients Who Received Vials From the Donor’s Lot—Mycoplasma hominis Outbreak Investigation, 2015
| Procedures/Patients . | No. of Vials Used . | Product Mixed With Bone Marrow . | Adverse Event/Symptoms . | Treatment . | Microorganisms . |
|---|---|---|---|---|---|
| Spine surgeriesa | |||||
| Patient A | 1 | Yes | Pain, subcutaneous seroma | Cefazolin | No growth |
| Patient Bb | 1 | Yes | None | NA | NA |
| Patient Bb | 1 | Yes | None | NA | NA |
| Patient C | 1 | Yes | Fever, pain, superficial fluid collection | Vancomycin, clindamycin | Staphylococcus epidermidis |
| Patient D | 1 | Yes | Fever, pain | Ceftriaxone, doxycycline | Staphylococcus haemolyticus, Mycoplasma hominis, Propionibacterium acnes |
| Patient E | 1 | Yes | Fever, erythema, serosanguineous drainage | Vancomycin, ceftriaxone, doxycycline | Mycoplasma hominis, Propionibacterium acnes |
| Patient F | 1 | Yes | None | NA | NA |
| Patient G | 1 | Yes | None | NA | NA |
| Knee surgeryc | |||||
| Patient Hd | 1 | Yes | None | NA | NA |
| Patient Id | 1 | Yes | None | NA | NA |
| Patient J | 1 | Yes | None | NA | NA |
| Foot surgerye | |||||
| Patient Kf | 1 | No | None | NA | NA |
| Intraarticular injections | |||||
| Patient Hd | 1 | No | None | NA | NA |
| Patient Id | 1 | No | None | NA | NA |
| Patient L | 1 | Yes | None | NA | NA |
| Patient M | 1 | Yes | None | NA | NA |
| Ultrasound-guided injection of neuroma | |||||
| Patient N | 1 | No | None | NA | NA |
| Soft tissue or subcutaneous injections | |||||
| Patient O | 4 | No | None | NA | NA |
| Patient Pf | 1 | No | None | NA | NA |
| Patient Qf | 1 | No | None | NA | NA |
| Procedures/Patients . | No. of Vials Used . | Product Mixed With Bone Marrow . | Adverse Event/Symptoms . | Treatment . | Microorganisms . |
|---|---|---|---|---|---|
| Spine surgeriesa | |||||
| Patient A | 1 | Yes | Pain, subcutaneous seroma | Cefazolin | No growth |
| Patient Bb | 1 | Yes | None | NA | NA |
| Patient Bb | 1 | Yes | None | NA | NA |
| Patient C | 1 | Yes | Fever, pain, superficial fluid collection | Vancomycin, clindamycin | Staphylococcus epidermidis |
| Patient D | 1 | Yes | Fever, pain | Ceftriaxone, doxycycline | Staphylococcus haemolyticus, Mycoplasma hominis, Propionibacterium acnes |
| Patient E | 1 | Yes | Fever, erythema, serosanguineous drainage | Vancomycin, ceftriaxone, doxycycline | Mycoplasma hominis, Propionibacterium acnes |
| Patient F | 1 | Yes | None | NA | NA |
| Patient G | 1 | Yes | None | NA | NA |
| Knee surgeryc | |||||
| Patient Hd | 1 | Yes | None | NA | NA |
| Patient Id | 1 | Yes | None | NA | NA |
| Patient J | 1 | Yes | None | NA | NA |
| Foot surgerye | |||||
| Patient Kf | 1 | No | None | NA | NA |
| Intraarticular injections | |||||
| Patient Hd | 1 | No | None | NA | NA |
| Patient Id | 1 | No | None | NA | NA |
| Patient L | 1 | Yes | None | NA | NA |
| Patient M | 1 | Yes | None | NA | NA |
| Ultrasound-guided injection of neuroma | |||||
| Patient N | 1 | No | None | NA | NA |
| Soft tissue or subcutaneous injections | |||||
| Patient O | 4 | No | None | NA | NA |
| Patient Pf | 1 | No | None | NA | NA |
| Patient Qf | 1 | No | None | NA | NA |
There were 14 patients with confirmed lot number and serial code, and 3 patients with confirmed lot number only.
Abbreviation: NA, not applicable.
aIncludes lumbar decompression, spinal fusion/spinal instrumentation, posterior lumbar interbody fusion.
bReceived product during 2 separate procedures on different dates.
cIncludes total knee replacement, meniscectomies.
dReceived product during 2 separate procedures on same date.
eIncludes open reduction internal fixation metatarsal.
fThree patients with confirmed lot number only.
Amniotic Tissue Product Use and Adverse Events for Patients Who Received Vials From the Donor’s Lot—Mycoplasma hominis Outbreak Investigation, 2015
| Procedures/Patients . | No. of Vials Used . | Product Mixed With Bone Marrow . | Adverse Event/Symptoms . | Treatment . | Microorganisms . |
|---|---|---|---|---|---|
| Spine surgeriesa | |||||
| Patient A | 1 | Yes | Pain, subcutaneous seroma | Cefazolin | No growth |
| Patient Bb | 1 | Yes | None | NA | NA |
| Patient Bb | 1 | Yes | None | NA | NA |
| Patient C | 1 | Yes | Fever, pain, superficial fluid collection | Vancomycin, clindamycin | Staphylococcus epidermidis |
| Patient D | 1 | Yes | Fever, pain | Ceftriaxone, doxycycline | Staphylococcus haemolyticus, Mycoplasma hominis, Propionibacterium acnes |
| Patient E | 1 | Yes | Fever, erythema, serosanguineous drainage | Vancomycin, ceftriaxone, doxycycline | Mycoplasma hominis, Propionibacterium acnes |
| Patient F | 1 | Yes | None | NA | NA |
| Patient G | 1 | Yes | None | NA | NA |
| Knee surgeryc | |||||
| Patient Hd | 1 | Yes | None | NA | NA |
| Patient Id | 1 | Yes | None | NA | NA |
| Patient J | 1 | Yes | None | NA | NA |
| Foot surgerye | |||||
| Patient Kf | 1 | No | None | NA | NA |
| Intraarticular injections | |||||
| Patient Hd | 1 | No | None | NA | NA |
| Patient Id | 1 | No | None | NA | NA |
| Patient L | 1 | Yes | None | NA | NA |
| Patient M | 1 | Yes | None | NA | NA |
| Ultrasound-guided injection of neuroma | |||||
| Patient N | 1 | No | None | NA | NA |
| Soft tissue or subcutaneous injections | |||||
| Patient O | 4 | No | None | NA | NA |
| Patient Pf | 1 | No | None | NA | NA |
| Patient Qf | 1 | No | None | NA | NA |
| Procedures/Patients . | No. of Vials Used . | Product Mixed With Bone Marrow . | Adverse Event/Symptoms . | Treatment . | Microorganisms . |
|---|---|---|---|---|---|
| Spine surgeriesa | |||||
| Patient A | 1 | Yes | Pain, subcutaneous seroma | Cefazolin | No growth |
| Patient Bb | 1 | Yes | None | NA | NA |
| Patient Bb | 1 | Yes | None | NA | NA |
| Patient C | 1 | Yes | Fever, pain, superficial fluid collection | Vancomycin, clindamycin | Staphylococcus epidermidis |
| Patient D | 1 | Yes | Fever, pain | Ceftriaxone, doxycycline | Staphylococcus haemolyticus, Mycoplasma hominis, Propionibacterium acnes |
| Patient E | 1 | Yes | Fever, erythema, serosanguineous drainage | Vancomycin, ceftriaxone, doxycycline | Mycoplasma hominis, Propionibacterium acnes |
| Patient F | 1 | Yes | None | NA | NA |
| Patient G | 1 | Yes | None | NA | NA |
| Knee surgeryc | |||||
| Patient Hd | 1 | Yes | None | NA | NA |
| Patient Id | 1 | Yes | None | NA | NA |
| Patient J | 1 | Yes | None | NA | NA |
| Foot surgerye | |||||
| Patient Kf | 1 | No | None | NA | NA |
| Intraarticular injections | |||||
| Patient Hd | 1 | No | None | NA | NA |
| Patient Id | 1 | No | None | NA | NA |
| Patient L | 1 | Yes | None | NA | NA |
| Patient M | 1 | Yes | None | NA | NA |
| Ultrasound-guided injection of neuroma | |||||
| Patient N | 1 | No | None | NA | NA |
| Soft tissue or subcutaneous injections | |||||
| Patient O | 4 | No | None | NA | NA |
| Patient Pf | 1 | No | None | NA | NA |
| Patient Qf | 1 | No | None | NA | NA |
There were 14 patients with confirmed lot number and serial code, and 3 patients with confirmed lot number only.
Abbreviation: NA, not applicable.
aIncludes lumbar decompression, spinal fusion/spinal instrumentation, posterior lumbar interbody fusion.
bReceived product during 2 separate procedures on different dates.
cIncludes total knee replacement, meniscectomies.
dReceived product during 2 separate procedures on same date.
eIncludes open reduction internal fixation metatarsal.
fThree patients with confirmed lot number only.
No implant cards contained reports of adverse events including those for patients with known infections. There was no date on the cards to indicate when the card was completed. It is unknown if these cards were returned prior to the development of adverse events. After contacting all facilities, the 4 surgical site infections in Ohio were the only identified adverse events (4/17 [24%]) and thus no other M. hominis infections were identified in recipients of tissue product from this donor.
There was no systematic mechanism in place for tracking or searching for use of tissue products at any facilities involved in the investigation. In most facilities, the lot number and serial code were recorded in an individual patient medical record, but this practice was not uniformly applied.
Laboratory Testing
Two unopened vials from the donor underwent testing culture and qPCR. “Fried egg”–appearing colonies consistent with M. hominis were identified on agar. DNA isolated from both the thawed samples as well as the colonies isolated on agar was positive for M. hominis by qPCR. In addition to M. hominis, both vials were culture positive for Ureaplasma species, identified by typical colonial morphology and urease production on A8 agar [25]. These organisms grown in culture were identified as Ureaplasma parvum by qPCR [26]. Both vials were also PCR positive for U. parvum when tested directly.
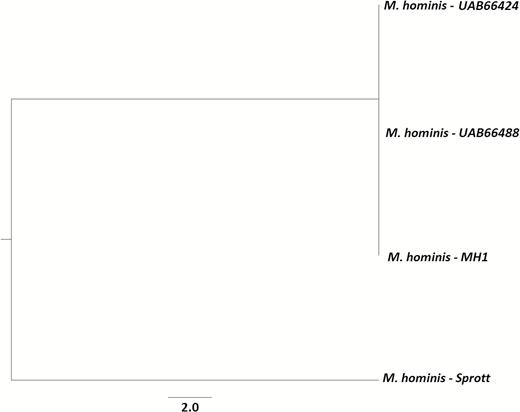
The recovered isolate from an ill patient was frozen in transport media at –70°C at a clinical reference laboratory. This isolate and the M. hominis isolates from the University of Alabama were transported to CDC for WGS. The isolates from both unopened vials were compared to the patient isolate, with resulting genome similarities of 99.87% and 99.86%. Figure 3 depicts the genetic relatedness between the isolates sequenced in this investigation and a National Center for Biotechnology Information reference genome.
Phylogenetic tree of newly sequenced Mycoplasma hominis isolates, along with the outgroup isolate M. hominis Sprott. Phylogenetic tree was inferred with RAxML using 231227 core variant single-nucleotide polymorphisms with a core genome size of 61%. Tree was visualized using the FigTree application.
DISCUSSION
This investigation describes the first documented transmission of M. hominis from human amniotic tissue causing surgical site infections, although M. hominis transmission has been reported via solid organs and vascular graft tissues [18, 19]. In these reports, M. hominis has caused allograft-related sepsis, infected hematoma, and respiratory infections.
Genitourinary tract tissues are at risk of being contaminated by colonizing microorganisms, such as M. hominis and U. parvum. However, the donor screening, product treatment, and testing employed by the manufacturers of this product cannot detect all microorganisms, including M. hominis. Tissue products are variably treated with antibiotic washes, radiation, and other processes that may not kill certain microorganisms. Many tissues, including those described here, are not terminally sterilized to preserve vascularity and other properties. In addition, because of the unique traits of M. hominis, standard aerobic and anaerobic culture techniques used to identify the etiology of surgical site infections will not ensure identification of the organism. Providers should be aware of these risks and consider the risk-benefit tradeoff of using these tissues.
In the United States, approximately 2000000 tissue allografts are made annually from >30000 donors [27]. More than 100 grafts can be manufactured from a single donor and distributed both within the United States and abroad [28]. However, as is highlighted here, the lack of standardized reporting or tissue traceability in US healthcare facilities and difficulty in tracking tissues from an infected donor to potentially many recipients poses a significant challenge when investigating transplant-transmitted infections. This is not limited to amniotic material but also includes musculoskeletal, cardiovascular, skin, and ocular grafts, which are routinely used in a multitude of medical procedures and clinical settings. When donor-derived disease transmission occurs, there is often no tracking system in the healthcare facility, precluding the prompt identification of at-risk recipients and delaying the implementation of timely interventions that may prevent further morbidity or mortality. In contrast to the common coding and nomenclature system used in the United States for blood and blood products [29], individual tissue banks can decide arbitrarily how to name tissue grafts, without adhering to a common standard. These names can be proprietary, resulting in an identical product produced by separate tissue banks having different names. This may complicate efforts to conduct public health case finding and issuance of public health advisory notifications when an infection or adverse event is related to a specific tissue product.
Additionally, in the United States, unlike with blood and organs for which oversight extends into the facilities performing the transfusion or transplant, there are no regulatory mandates for standardized systems for tracking tissues once they leave the manufacturer [28, 30]. The US Food and Drug Administration’s regulatory authority over tissue products is limited to tissue recovery, processing, storage, labeling, packaging, and distribution as well as donor screening and testing, and does not extend to facilities where these are used until an event becomes a public health concern.
The present investigation shows that even if implant cards, which are intended to serve a notification role, are returned at a high rate, the information provided can be scant, with missing and/or inaccurately recorded information. For example, 1 vial confirmed as discarded and not used in a patient had a completed implant card returned. Other studies have found that healthcare facilities provide limited or incomplete information to the manufacturers after tissue implantation [30–32]. An additional limitation of these cards is related to the timing of completion relative to graft use in the recipient. Even in the cards returned for recipients with known M. hominis infections, no adverse event was reported, possibly due to timing of the card return.
The findings of this investigation are subject to the following limitations. Scant information on several of the implant cards and no systematic process to track tissues at the healthcare facilities forced a reliance on provider recollection to locate vials in question. We did not start contacting individual facilities until after testing of the unopened product was completed. Therefore, patients received this product many months prior to providers being notified of potential M. hominis infections. In the present investigation, M. hominis was only identified in 2 patients who received the product. It is possible that other patients were infected with M. hominis but were missed. The patients with M. hominis infections also grew other organisms and it is possible that these accounted for their symptoms. Mycoplasma hominis pathogenicity is thought to be low; in 1 review of extragenital infections, most patients recovered without antibiotic therapy or with drainage alone [14]. Additionally, patients could have been treated with antibiotics for prophylaxis or unrelated infections. It is important to note that because Mycoplasma species are small, fastidious organisms lacking a cell wall, they are often undetected using standard techniques [33, 34]. In a previous organ transplant transmission, M. hominis infection in a recipient was confirmed only by fluorescent antibody testing of the lung allograft [18]. Given the difficulty of isolating M. hominis using standard culture techniques, qPCR and other methods may be warranted.
In summary, M. hominis infection can be transmitted via amniotic tissue products, resulting in surgical site infections. Mycoplasma hominis and related organisms are difficult to culture using standard techniques. Data on outcomes after use of these amniotic tissue products are lacking. Recognition of tissue-transmitted infections and identification of other at-risk recipients are hindered by the lack of standardized tissue tracking systems in the United States, particularly in healthcare facilities.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The use of trade names is for identification purposes only and does not constitute endorsement by the CDC or the Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References





Comments