-
PDF
- Split View
-
Views
-
Cite
Cite
Scott Cassie, Igor Koturbash, Darryl Hudson, Mike Baker, Yaroslav Ilnytskyy, Rocio Rodriguez-Juarez, Edgar Weber, Olga Kovalchuk, Novel retinoblastoma binding protein RBBP9 modulates sex-specific radiation responses in vivo, Carcinogenesis, Volume 27, Issue 3, March 2006, Pages 465–474, https://doi.org/10.1093/carcin/bgi261
Close - Share Icon Share
Abstract
Retinoblastoma (RB) tumor suppressor is a key regulator of apoptosis, a central mediator of the proliferative block induced by ionizing radiation (IR) and a binding target for a variety of proteins that regulate its activity. One of the recently discovered and the least investigated of these is the novel Rb-binding protein RBBP9/BOG. We studied the effects of acute and chronic low dose radiation (LDR) exposure on the induction of RBBP9 and RB signaling pathway in vivo in mouse spleen and found that RBBP9 played a pivotal role in IR responses in vivo . We observed that chronic LDR exposure led to a significant increase of RBBP9 expression in males and a significant decrease in females. Elevated RBBP9 expression in males was paralleled by a pronounced dephosphorylation of RB and a significant drop of PCNA and cyclin A expression. On the contrary, chronic exposure in females led to decreased levels of RBBP9 and increased levels of hyperphosphorylated RB (ppRB) in spleen. Decreased levels of ppRB in spleen of chronically exposed males were correlated with strongly elevated apoptotic rates. In females, the radiation-induced increase of apoptotic index was much less pronounced. Quite surprisingly, the observed sex-specific signaling changes did not result in the sex-specificity of cellular proliferation. The molecular mechanisms and possible repercussions of the radiation-induced sex differences in cellular proliferation and apoptosis are discussed.
Introduction
Ionizing radiation (IR) is a well-known DNA damaging agent and human carcinogen. Inability to build up a well-ordered response to radiation-induced DNA damage can result in the accumulation of genome defects and may contribute to malignant transformation ( 1 , 2 ). Following IR exposure, an efficient and robust cell-specific program of either cell cycle arrest or apoptosis is initialized. The coordination of such complex cellular processes is highly regulated and involves DNA damage detection, signaling and activation of the key effector proteins. Central to the radiation-induced DNA damage responses in mammalian cells is retinoblastoma (RB) tumor suppressor, which regulates both cell growth and apoptosis ( 1 – 7 ).
RB is a central mediator of the proliferative block induced by IR ( 8 , 9 ). It is indispensable for orchestrating a proper G 1 checkpoint, S-phase progression as well as G 2 checkpoint maintenance in response to DNA damage ( 2 , 8 – 13 ). There is also increasing evidence that RB has the potential to protect cells from apoptotic cell death. The data comes from studies of Rb −/− cells that show increased levels of the cell death and are profoundly sensitive to apoptosis inducing agents ( 7 , 13 ).
RB is a binding target for a variety of proteins ( 14 ). Thus, identification of the factors that interact, interfere with and/or control the function of the RB protein is crucial for understanding radiation-induced cell cycle control and carcinogenesis.
One of the most recently discovered and the least studied RB binding proteins is RBBP9/BOG. It shares some homology with other RB-binding proteins and contains the RB-binding motif LXCXE ( 14 ). The overexpression of RBBP9/BOG was shown to lead to malignant transformation of RLE cells. Furthermore, this protein was overexpressed in a variety of tumors, including several leukemias ( 14 ).
We have recently shown that this novel protein may play a pivotal role in IR responses in vivo ( 15 ). In our previous studies of murine skeletal muscle tissue, RBBP9 expression was strongly affected by whole-body low dose radiation (LDR) exposure, but to a very different extent in males and females ( 15 ). The findings were later confirmed in liver and spleen (O. Kovalchuk, unpublished data). These studies provide the first experimental evidence of sex differences in RB-binding proteins.
We hypothesized that sex differences in RBBP9/BOG expression may lead to changes in the expression and/or activation of RB protein and thus affect the downstream proteins regulating cellular proliferation and apoptosis in vivo .
It is well established that the sensitivity of cells to radiation depends on their origin ( 9 , 16 ). Spleen, a pivotal part of hematopoietic system is an important radiation target organ. RB is known to play a central role in the radiation response of splenocytes in vivo ( 9 ).
The vast majority of studies of the radiation responses in vivo used rather high doses within the range of 5–10 Gy, which were often sufficient to induce the animal's death ( 16 – 18 ). Recently though, the sub-lethal low dose of 1 Gy was also proven to induce profound RB signaling and apoptotic changes in mouse spleen ( 9 ). Coincidentally, in our previous studies, the most pronounced sex differences in RBBP9/BOG expression were observed upon LDR exposure of 0.5 Gy ( 15 ).
In the present study, we investigated the effect of LDR exposure (0.5 Gy) on the expression of RBBP9/BOG, ppRB as well as RB/E2F target proteins in vivo in spleens of male and female mice. In parallel, we studied the degrees of radiation-induced cell proliferation and apoptosis in spleen. We report that LDR exposure led to pronounced sex-specific changes in the expression of RBBP9. Moreover, RBBP9 modulates RB phosphorylation in vivo in a sex-specific manner, leading to sex differences in the expression of RB/E2F target proteins PCNA and cyclin A and in apoptotic rates. Contrarily, no sex differences were observed in the levels of cell proliferation.
Materials and methods
Model
In this study we used an in vivo mouse model to study sex differences in protein expression and phosphorylation in spleen following radiation exposure. The mouse model is widely used, well characterized and generally accepted for studies of radiation-induced changes in vivo ( 9 ). Handling and care of animals was in strict accordance with the recommendations of the Canadian Council for Animal Care and Use. The procedures have been approved by the University of Lethbridge Animal Welfare Committee.
Irradiation of animals
In the course of the first study 24 C57BL/6 mice (12 males and 12 females, Charles River, ON, Canada) were divided into three groups: control group, acutely exposed group and group subjected to chronic exposure for 10 days. All animals were 45 days old at the start of the experiment, had comparable body weights and were housed in a virus-free facility. Animals were monitored daily by a trained technician. No signs of pain or distress were observed during the course of the experiment. Mice from the ‘chronic/repetitive’ group were exposed to whole-body irradiation of 50 cGy applied as 5 cGy of X-rays per day (0.2 cGy/s) for 10 days to mimic chronic repetitive exposure. The ‘acute’ group of mice was irradiated with 50 cGy (0.2 cGy/s) of X-rays only once, on the 10th day of treatment of the ‘chronic’ group. Control mice were ‘mock’ treated. All animals were killed 3 h after the last treatment on day 10. Based on our previous data, expression of various genes and proteins peaks at 2–3 h after exposure ( 15 ). Thus, in order to be able to see these anticipated changes, we have chosen 3 h post-exposure as the killing time. To re-confirm the data we conducted three additional experiments according to the same experimental scheme—the second experiment using 24 C57BL/6 mice (12 males and 12 females) and, later, the third and the forth experiments using 18 C57BL/6 each (9 males and 9 females). All experiments yielded congruent data.
Spleen tissue was sampled immediately following killing and divided into three equal parts. One part was frozen using liquid nitrogen and stored at −80°C until processed for protein analysis. The other part was fixed in 4% buffered formalin, processed, embedded in paraffin wax and further used for histological and immunohistochemical analysis. The third part was used for flow cytometric detection of apoptosis.
Western immunobloting
Western immunoblotting for RBBP9, total RB, unphosphorylated RB, p-RB Ser 780, p-RB Ser 795, p-RB Ser 807/811, PCNA, cyclin A, cyclin E, cyclin D1, cyclin D3 and cyclin B1 were conducted using spleen tissue. Tissue samples were sonicated in 0.4–0.8 ml of ice-chilled 1% SDS and boiled for 10 min. Small aliquots (10 μl) of homogenate were reserved for protein determination using protein assay reagents from Bio-Rad (Hercules, CA). Equal amounts of proteins (20 μg) were separated by SDS–PAGE in slab gels of 8 or 12% polyacrylamide, made in duplicates, and transferred to PVDF membranes (Amersham, Baie d'Urfé, Québec). Membranes were incubated with antibodies against. RBBP9/BOG (1:1000; BD Biosciences, Mississauga, ON), total RB (1:1000, BD Biosciences), under-phosphorylated RB (1:500, BD Biosciences), (p-RB Ser 780, p-RB Ser 795, p-RB Ser807/811, 1:1000; Cell Signalling, Beverly, MA), PCNA (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), cyclin A (1:1000 Lab Vision/Neomarkers, Fremont, CA), cyclin D1, cyclin D3, cyclin B1 (1:1000, Cell Signaling) and cyclin E (1:200, Lab Vision/Neomarkers). Antibody binding was revealed by incubation with horseradish peroxidase-conjugated secondary antibodies (Amersham) and the ECL Plus immunoblotting detection system (Amersham). Chemiluminescence was detected by Biomax MR films (Eastman Kodak, New Haven, CT). Unaltered PVDF membranes were stained with Coomassie Blue (Bio-Rad, Hercules) and the intensity of the Mr 50 000 protein band was assessed as a loading control. Signals were quantified using NIH ImageJ 1.63 Software and normalized to both GAPDH and the Mr 50 000 protein band, which gave consistent results (values relative to Mr 50 000 are plotted).
Proliferation analysis—Ki67 and PCNA immunohistochemistry
Automated staining for Ki67 was conducted at the Lethbridge Regional Hospital Central Laboratory in accordance with the standard developed protocols and using tonsil tissue as a positive control for the stain quality. In brief, upon the citrate buffer epitope retrieval slides were rinsed and subjected to serum blocking to prevent non-specific binding of immunoglobulin. Sections then were incubated with mouse anti-human Ki67 (Novocastra, Newcastle, UK), rinsed and subjected to peroxidase blocking. Following the peroxidase blocking the slides were incubated with the secondary biotinylated antibody, subjected to HRP-Streptavidin detection and counterstained with hematoxylin. Cells positive for Ki67 presented as red/pink, whereas negative cells stained blue. Tonsil tissue served as a positive control. For another independent experiment an automated staining for Ki67 was conducted by the Calgary Central Lab Services using anti-human Ki67 antibody (DakoCytomation, Carpinteria, CA). Importantly, both experiments yielded congruent results.
An automated staining for PCNA was conducted by the Calgary Central Lab Services using the primary anti-PCNA antibody (Novocastra). Upon the secondary antibody application, HRP-Streptavidin detection and counterstaining with hematoxylin cells positive for PCNA stained brown/purple, whereas negative cells stained blue. Tonsil tissue served as a positive control.
Analysis of apoptosis—H&E stain
Paraffin embedding, sectioning and staining were conducted at Central Vet Labs, Edmonton, AB. Spleen sections (4 μm thick) were cut from paraffin, de-waxed and stained with haematoxylin and eosin at Central Vet Labs using standard protocol. Apoptosis was assessed by well-defined morphological characteristics including chromatin condensation, cell shrinkage and formation of apoptotic bodies as recommended by Wyllie et al. ( 19 ). Apoptosis was quantified by enumerating apoptotic cells in at least 20 high power fields. Apoptosis is presented as the mean number of apoptotic cells ± standard error of the mean per high power field.
Analysis of apoptosis and cell cycle by flow cytometry
For the flow cytometric analysis of apoptosis mouse splenocytes were prepared by gently grinding the spleen tissue in RPMI 1640 medium through the 70 μM filter. Lysis of the red blood cells was conducted using the Red Blood Cells Lysis Buffer (BD Biosciences, San Jose, CA) according to the manufacture's instructions, and the resulting splenocytes suspension was analyzed using the APO-DIRECT™ and Annexin V—FITC™ assays (BD Biosciences) according to the manufacturer's instructions. The APO-DIRECTTM assay was used to analyze the late apoptosis stages that involve DNA fragmentation. This assay was also used for the analysis of cell cycle distribution according to the manufacturer's instructions.
The Annexin V—FITC™ assay, which detects relocation of membrane phosphatidyl serine from the intracellular surface to the extracellular surface of the membranes, was used to detect the early apoptotic events. The flow cytometric analysis was conducted at the University of Calgary Flow Cytometry facility.
Statistical analysis
The main statistical procedures were described by Sokal and Rohlf ( 20 ). Statistical treatment and plotting of the results were performed using the JMP5, Sigma Plot and Excel for Windows XP software. The results were presented as mean values ± standard error.
Results
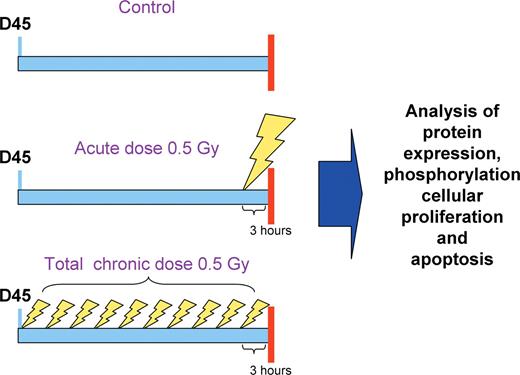
In the course of the study C57BL/6 mice (males and females of comparable weight) were divided into three groups: control group, acutely exposed group and chronic/repetitive group. Animals from the repetitive ‘chronic’ group were exposed to whole-body irradiation of 0.05 Gy of X-rays per day for 10 days. The ‘acute’ group of mice was exposed to 0.5 Gy of X-rays only once—coinciding with day 10 of treatment in the ‘chronic’ group ( Figure 1 ). Western blotting was performed to quantify changes in the expression levels of proteins of interest and/or their phosphorylation state.
Experimental scheme. In the course of the study experimental animals (males and females of comparable body weight) were divided into three groups: control group, acutely exposed group and group subjected to chronic exposure for 10 days. Mice from the ‘chronic/repetitive’ group were exposed to whole-body irradiation of 50 cGy applied as 5 cGy of X-rays per day (0.2 cGy/s) for 10 days to mimic chronic repetitive exposure. The ‘acute’ group of mice were irradiated with 50 cGy (0.2 cGy/s) of X-rays only once, on the 10th day of treatment of the ‘chronic’ group. Control mice were ‘mock’ treated. All animals were killed 3 h after the last treatment on day 10.
Sex differences in radiation-induced protein expression and phosphorylation in spleen
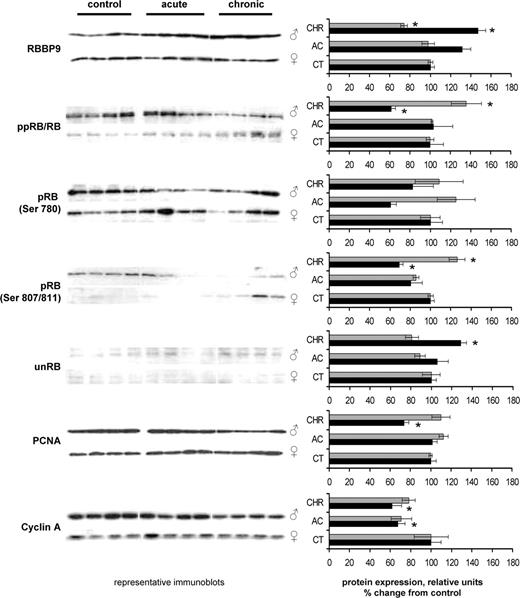
Analysis revealed very significant sex differences in the expression of RBBP9 in murine spleen in vivo . RBBP9 was significantly 1.5-fold ( P < 0.05) up-regulated in males following chronic radiation exposure. Contrarily, a 1.5-fold ( P < 0.05) down-regulation was observed in chronically exposed females ( Figure 2 ). Interestingly, the RBBP9 expression changes were inversely correlated with the levels of RB phosphorylation. Indeed, in chronically exposed male spleen the levels of hyperphosphorylated RB (ppRB) significantly decreased by 39% ( P < 0.05) as compared with untreated controls. In contrast, in female spleen, chronic exposure to 0.5 Gy led to a significant 35% ( P < 0.05) increase in ppRB.
Radiation-induced changes in expression of RBBP9, RB, pRB Ser 780, pRB Ser 870/811, unRB, PCNA and cyclin A in spleens of female and male mice. Lysates from spleen tissue were subjected to immunoblotting using antibodies against RBBP9, total RB, pRB Ser 780, pRB Ser 870/811, unphosphorylated RB, PCNA and cyclin A. Protein levels relative to those of control animals are shown as the mean ± SEM; asterisk indicates P < 0.05, Student's t -test. Black bars: males, gray bars: females. CT: control, AC-acutely exposed animals, CHR: chronically exposed animals. All sample loading was normalized to protein content. Representative western blots from four independent experiments are shown.
Several phosphorylation sites are known to affect the functional activity of the RB protein. Among those are Ser807/811, Ser 795 and Ser780. We checked the phosphorylation status of these sites and noted that the most prominent sex differences were observed in the phosphorylation of Ser807/811 ( Figure 2 ). In parallel, we checked the levels of the unphosphorylated RB, using the monoclonal antibody against it. Congruently, we found that chronic radiation exposure led to a significant 30% reduction ( P < 0.05) of unphosphorylated RB (unRB) in female spleen and a significant 34% elevation ( P < 0.05) in the levels of unRB in male spleen.
Sex differences in radiation-induced expression of RB/E2F targets
Unphosphorylated/active RB interacts with E2F and plays a pivotal role in the regulation of the expression of a variety of genes involved in cell cycle progression. Phosphorylation of RB renders it inactive and confers its inability to assemble transcription repression complexes with E2F over a variety of promoters including PCNA, cyclin A, cyclin E, cdc2 and others ( 1 , 2 , 4 ). The observed sex differences in RB phosphorylation status prompted us to evaluate the radiation-induced changes in the expression of PCNA and cyclin A in spleens of male and female mice using western immunoblotting. We found that in male spleen, PCNA and cyclin A were significantly ( P < 0.05) repressed upon chronic irradiation, whereas in females no such repression was observed ( Figure 2 ). Western immunoblotting data were in consensus with the data obtained by real-time PCR. Real-time PCR data revealed that radiation exposure resulted in significant decrease in the levels transcripts of the RB/E2F target genes PCNA, cyclin A as well as DHFR in male spleen (data not shown). These observations promoted us to further investigate the changes in the rates of cellular proliferation and apoptosis in vivo .
Radiation induced cellular proliferation and apoptosis in vivo
RB is known to regulate both cell growth and apoptosis and is a central mediator of the radiation-induced proliferative block and apoptotic response ( 1 , 2 – 6 , 8 , 9 ). We thus attempted to correlate the observed RB phosphorylation and the RB-driven PCNA and cyclin A changes with the rates of cellular proliferation and apoptosis in male and female spleen upon LDR exposure.
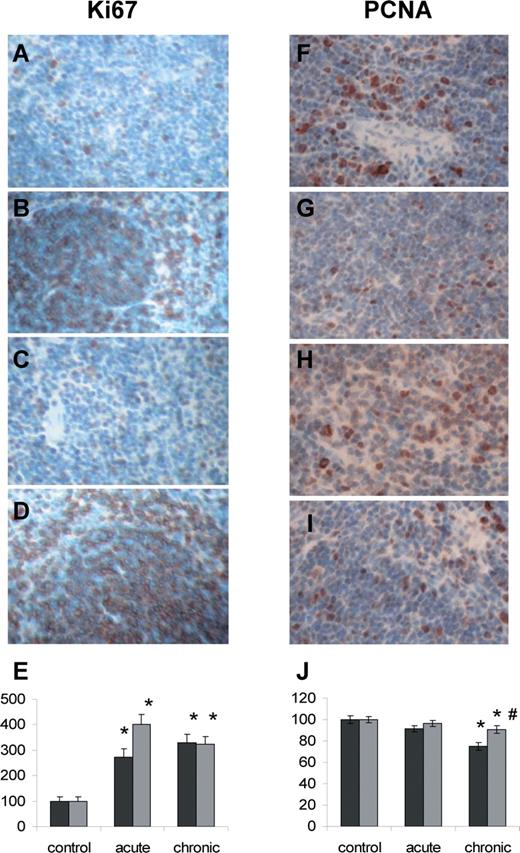
Quite surprisingly, we noted that in both sexes radiation exposure led to a significant increase in cellular proliferation, as studied by Ki67 immunohistochemistry. Ki67 is a nuclear protein, which is expressed in proliferating cells. It is expressed during late G 1 , S, G 2 and M phases of the cell cycle, whereas cells in the G 0 (quiescent) phase are negative for this protein ( 21 ). We found that acute and chronic LDR exposure led to 3- to 4-fold increase in the number of Ki67 positive cells in male and female spleen as compared with un-irradiated controls ( Figure 3A–E ).
Radiation-induced changes in the cellular proliferation levels monitored by Ki67 (A–E) and PCNA (F–J) immunohistochemistry. ( A ) Control male; ( B ) chronically exposed male; ( C ) control female; ( D ) chronically exposed female; ( E ) Ki67 proliferation index in control and exposed male an female spleen; data are shown as the mean ± SEM; * P < 0.05, Student's t -test, as compared to control; ( F ) control male; ( G ) chronically exposed male; ( H ) control female; ( I ) chronically exposed female; ( J ) PCNA index in control and exposed male and female spleen; data are shown as the mean ± SEM; * P < 0.05, Student's t -test, as compared to control; #P < 0.05, Student's t -test, as compared to males. Black bars–males, grey bars–females.
Having seen the decrease in the levels of PCNA and cyclin A proteins and the unexpected increase in the cellular proliferation monitored by Ki67 immunohistochemistry we decided to use another cellular proliferation marker to understand the nature of this apparent discrepancy. We, thereafter, analyzed the levels of cellular proliferation by PCNA immunohistochemistry. We found that the data of PCNA immunohistochemistry analysis correlated with the PCNA expression levels previously monitored by the western immunoblotting. We found a significant 25% decrease ( P < 0.05) in the number of PCNA positive cells in spleen of chronically exposed males. A slight ∼10% decrease in PCNA positive cell was also noted in chronically exposed females, but it was much less pronounced than in males ( Figure 3F–J ).
We decided to get further insight into the reasons for the differences in the results of the two methods used for cellular proliferation analysis. Overall, Ki67 staining reveals all the cells that are not in G 0 stage. PCNA, in contrast, is specific to the cells in S-phase. We thus hypothesized that the increase in the numbers of the Ki67 positive cells may be due to the G 1 block and/or increased entry into G 1 .
With this in mind we conducted the analysis of the cell cycle distribution in the splenocytes of the control and exposed mice using propidium iodine (PI) staining flow cytometry. We found that radiation-induced G 1 arrest was more pronounced in males than in females. We also found that the amount of cells in S-phase decreased in chronically exposed animals, particularly—in males, which correlated with the PCNA down-regulation in males (data not shown).
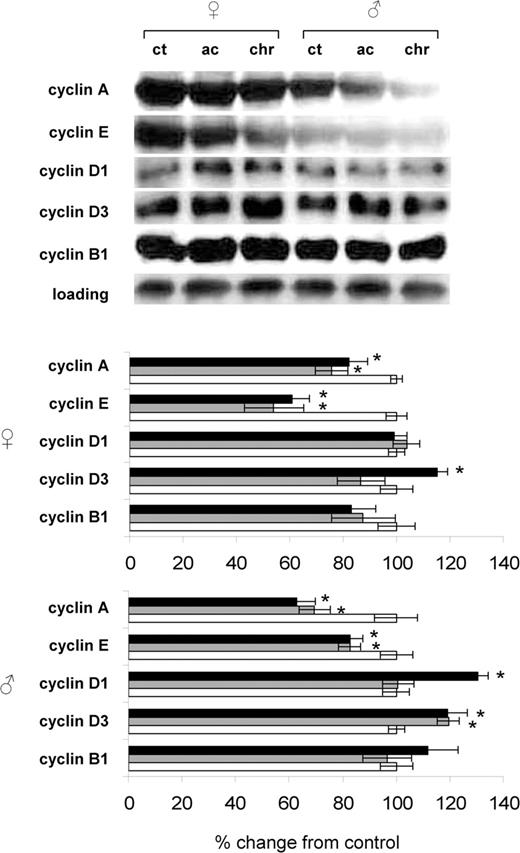
In parallel, we analyzed the expression of the cyclins operating in G 1 , S and G 2 phases by western immunoblotting. These were cyclins D1, D3 and E (for G 1 or early S) and cyclin B1 (for G 2 ). In parallel, we repeated the analysis of the cyclin A expression ( Figure 4 ).
Radiation-induced changes in expression of cyclins in spleens of female and male mice. Lysates from spleen tissue were subjected to immunoblotting using antibodies against cyclin A, cyclin E, cyclin D1, cyclin D3 and cyclin B1. Protein levels relative to those of control animals are shown as the mean ± SEM; asterisk indicates P < 0.05, Student's t -test. While bars: control animals, gray bars: acutely exposure animals, black bars: chronically exposed animals. All sample loading was normalized to protein content. Representative western blots from two independent experiments are shown.
We noted that radiation exposure led to significant reduction of the cyclin A expression in both sexes, which was more pronounced in males. Indeed, chronic radiation exposure led to a significant 18% reduction in cyclin A levels in female spleen and to a more prominent 38% reduction ( P < 0.05) in male spleen as compared with respective controls. Cyclin E expression was also decreased in both sexes. Chronic radiation exposure profoundly affected the expression of cyclins D1 and D3. In spleens of chronically exposed male mice expression of cyclin D1 was significantly 30% elevated ( P < 0.05). Contrarily, no cyclin D1 expression changes were observed in spleens of female mice. Cyclin D3 expression was up-regulated by chronic radiation exposure in both sexes. The up-regulation of cyclin D3 was slightly more pronounced in males ( Figure 4 ).
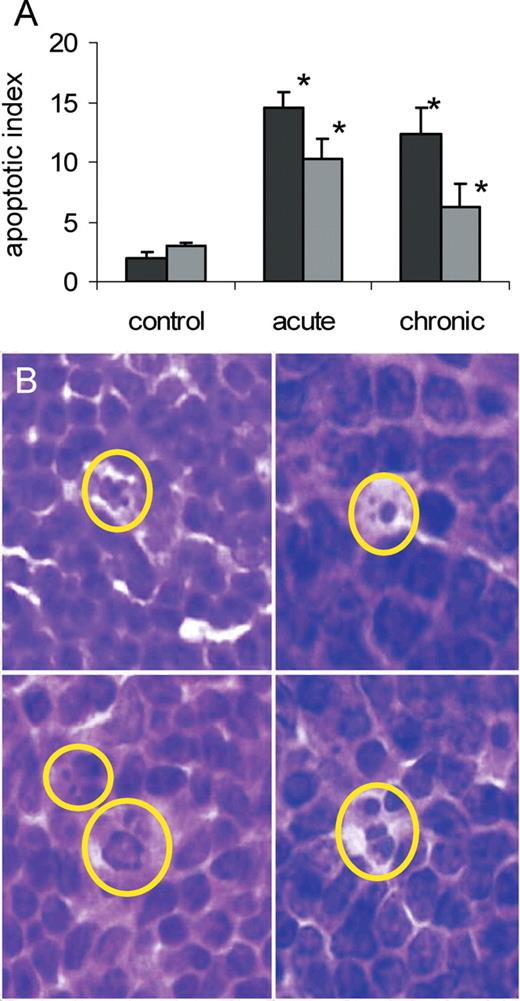
In parallel we performed apoptosis analysis in the splenic tissue of control and exposed male and female mice. Apoptosis was assessed on morphological characteristics, such as chromatin condensation, cell shrinkage and formation of apoptotic bodies ( 9 ) ( Figure 4 ). We found an induction of apoptosis in splenocytes of male and female mice upon acute as well as chronic LDR exposure ( Figure 5 ). Consistent with the previous studies, the highest levels of apoptosis were observed in the white pulp ( 9 ). The apoptosis induction was more pronounced in male spleen, where acute and chronic exposure led to significant ( P < 0.05) 7.3-fold and 6.2-fold increase of apoptosis, respectively. In female spleen, the effect was less pronounced, causing a 3.4-fold and 2.1-fold increase ( P < 0.05) in apoptotic cells upon acute and chronic exposure, respectively.
Radiation-induced apoptosis in murine spleen as monitored by nuclear fragmentation. ( A ) Number of apoptotic cells (apoptotic index) in spleens of male and female mice upon acute and chronic whole-body radiation exposure (0.5 Gy), data are shown as the mean + SEM; asterisk P < 0.05, Student's t -test, as compared with control. ( B ) Examples of apoptosis characterized by nuclear fragmentation, HE stain, spleen white pulp, magnification 100×. Black bars–males, grey bars–females.
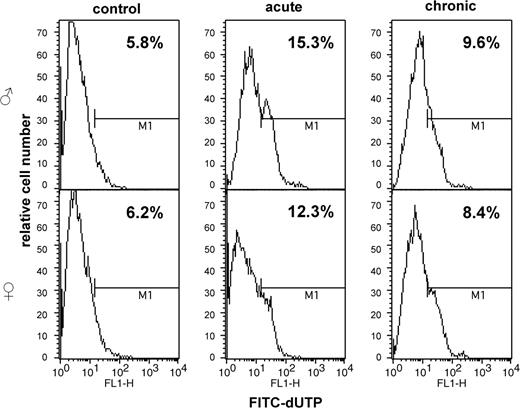
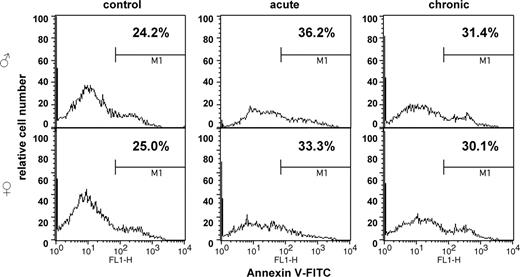
Morphological assessment of apoptosis, though widely used, usually allows the detection of late apoptotic stages and is rather subjective. To re-confirm the data on the induction of apoptosis by LDR in mouse spleen we employed two flow cytometic assays. The TUNEL assay detects the levels of DNA fragmentation and, thus, reveals the late apoptotic stages. The Annexin-V assay detects a translocation of membrane phosphatidyl serine from the intracellular to the extracellular surface of the cellular membrane during the initial apoptotic stages. This allows for a detection of early apoptotic events. When used in combination with PI, Annexin-V labeling allows the detection of a full spectrum of apoptotic events. The levels and trends in apoptotic processes detected by analysis of the cellular morphological characteristics correlated with the results obtained by both APO-TUNEL and Annexin V flow cytometry. These methods confirmed that levels of chronic LDR-induced apoptosis in female spleen were lower than in male spleen, although the sex differences observed by flow cytometric studies were less pronounced ( Figures 6 and 7 ).
APO-DIRECT flow cytometric analysis of radiation-induced apoptosis in murine spleen.
Radiation-induced apoptosis in murine spleen as studied by Annexin V flow cytometry.
Discussion
We used a murine spleen model to analyze the sex differences in the expression of the novel RB-binding protein RBBP9 and molecular repercussions of these differences for theRB-related signaling and consequently, cellular proliferation and apoptosis. The C57BL/6 mouse strain is frequently used to analyze the mechanisms of radiation-induced proliferation and apoptosis ( 9 ). In the present study, we have demonstrated that chronic whole-body LDR exposure led to pronounced sex differences in the expression of novel RB-binding protein RBBP9 as well as in RB phosphorylation and the downstream expression of RB/E2F targets PCNA, cyclin A and DHFR. Our findings also revealed significant sex differences in the levels of radiation-induced apoptosis and intriguing changes in the levels of cellular proliferation.
The main findings of the present study were: (i) chronic LDR led to pronounced sex differences in the expression of RBBP9, namely, up-regulation in males and down-regulation in females; (ii) the LDR-induced changes in RBBP9 expression were linked to the sex-specific changes in the RB phosporylation status; (iii) the sex-specific changes in RB phosphorylation resulted in differences in the expression of RB/E2F targets, especially—PCNA and cyclin A; (iv) the sex-specific RB phosphorylation pattern led to sex-specific differences in apoptotic rates; (v) the sex-specific expression of RB/E2F target proteins did not result in sex-specific rates of cellular proliferation; (vi) LDR exposure led to significant increase in cellular proliferation as monitored by Ki67 immunohistochemistry and (vii) the observed increase of cellular proliferation as monitored by the increased levels of Ki67 positive cells was most probably due to the radiation-induced G 1 arrest.
This is the first report on the very pronounced and reproducible sex differences in the expression of the RBBP9 protein. The RBBP9 protein shares homology with other known RB-binding proteins and contains the RB-binding motif LXCXE ( 14 ). It is able to interact with RB, as previously shown using the yeast two-hybrid system and co-immunoprecipitation ( 14 ).
We found that RBBP9 expression was inversely correlated with RB phosphorylation, suggesting that it may be involved in modulation of the RB phosphorylation status.
We noted that in male spleen LDR exposure led to a significant down-regulation of RBBP9, pronounced RB activation by dephosphorylation and the subsequent down-regulation of PCNA and cyclin A. No such activation was observed in female spleen, where radiation did not lead to inhibition of the pro-replication proteins and even resulted in a slight increase in PCNA expression. This constitutes an important novel finding.
Interestingly, the previous study by Woitech et al. ( 14 ) has shown that in cultured cells, the co-immunoprecipitated RBBP9/RB complexes do not contain E2F-1, and RBBP9 can displace E2F-1 from E2F-1/RB complexes in vitro . Thus, RBBP9 overexpression could presumably lead to destabilization of RB/E2F complexes and the subsequent lack of suppression of their target proteins leading to increased proliferation rates. The previous study has also indicated that overexpression of RBBP9 indeed led to increased cellular proliferation and oncotransformation ( 14 ).
Our data do not completely support those conclusions. Should the E2F displacement by overexpressed RBBP9, previously reported in RLE cells in vitro , be true in murine spleen in vivo , then we should not have observed the suppression of PCNA and cyclin A upon chronic irradiation in males, where LDR exposure induced pronounced RBBP9 up-regulation and the consequent RB dephosphorylation. Furthermore, in the current study we found that the cellular proliferation as monitored by Ki67 expression was induced upon radiation exposure regardless of the RBBP9 expression status and RB phosphorylation status, which RBBP9 seemed to modulate. On the other hand, we cannot exclude that the unphosphorylated RB could bind E2F1–3, leading to suppression of E2F target genes. In this case RB-induced changes may not depend upon RBBP9. Further studies are clearly needed to understand the biological mechanism and the cellular repercussions of the radiation effects on RB interactions with E2F proteins in vivo.
The mechanistic discrepancies between our data and the previous evidence may also be explained by the fact that in vitro cell lines are not always reminiscent of the in vivo situation, particularly upon whole-body exposure. Furthermore, tissue-specificity of RBBP9 expression may also play a role in its interactions with RB and RB target molecules.
The other important outcome of our study was the fact that the radiation-induced increase in cellular proliferation, as monitored by Ki67 immunohistochemistry, was observed in parallel with a decreased expression of cyclin A andPCNA.
Furthermore, immunohistochemical analysis of proliferation using Ki67 and PCNA as markers also yielded quite opposite results. The discrepancy was the most striking in the chronically exposed male animals where the levels of Ki67 positive cells significantly increased, whereas the levels of PCNA positive cells significantly decreased. The observed decrease in amounts of PCNA-expressing cells in the chronically exposed males correlated with the down-regulation of PCNA expression observed by western immunoblotting.
There may be several explanations to this enigmatic phenomenon. First—the exact regulation of Ki67 expression still has to be uncovered ( 21 – 24 ). Ki67 is a commonly used proliferative marker that is employed to calculate the proliferating index—a ratio of dividing versus non-dividing cells. The cellular concentration of this protein increases in G 1 , S, G 2 and M phases and then falls to zero in resting (G 0 ) cells. Ki67 was shown to harbor DNA-binding properties. Recent studies have suggested that Ki67 is involved in the protein interactions during cell cycle progression, possibly via associations with various phosphorylated proteins ( 22 – 24 ). Notwithstanding, the exact function as well as regulation of expression of Ki67 is still unclear ( 22 – 24 ). PCNA, though, is a well-known S-phase marker. Thus, the decrease in PCNA expression, along with the reduced levels of cyclin A may be indicative of the decrease in the amount of S-phase cells ( 25 ). Indeed, the flow cytometry analysis confirmed a slight decrease in the amount of the S-phase cells, which was more pronounced in males (data not shown).
Along with the S-phase delay/suppression, the newly recruited cells may still be entering G 1 phase as well as progressing through G 2 . Many ‘pro-survival’ proto-oncogenic molecules are known to induce proliferation upon genotoxic stress exposure via stimulation of G 1 entry and/or progression ( 25 ). Along with this, radiation is known to cause the G 1 arrest.
In this study we observed a radiation-induced G 1 arrest, which was more pronounced in males. Furthermore, we found that chronic radiation exposure led to the strong and significant up-regulation of cyclin D1 in male spleen. It also led to the strong upregulation of cyclin D3 in both sexes, which was also more pronounced in the male spleen. This pattern of cyclin D1 and D3 expression correlated with the higher levels of G 1 cells in the spleen cells of chronically exposed male mice. Thus, the elevated levels of G 1 cells may in part explain the increased levels of Ki67 positive ‘proliferating’ cells in spleens of chronically exposed males.
The sex-specificity in the cyclin D1 expression also requires attention in the future. Cyclin D1 expression could be up-regulated via the function of PKB/AKT protooncogene ( 26 – 29 ). Activation of PKB/AKT upon radiation exposure was previously shown ( 29 ). In this study we found a significant 42% increase ( P < 0.05) of PKB/AKT phosphorylation in spleens of chronically exposed males (data not shown). Contrarily, in spleens of acutely and chronically exposed females we observed significantly decreased PKB/AKT phosphorylation (data not shown). One thus could speculate that the proliferation-inhibitory potential of unphosphorylated RB in spleens of chronically exposed males was to some degree counteracted by activation of the cellular protooncogene PKB/AKT and up-regulation of cyclin D1 expression ( 26 – 29 ). Overall, the role of PKB/AKT and its interplay with the RB axis has to be further addressed.
Besides being crucially important for proliferation control, RB plays an important role in regulation of apoptosis and there is growing evidence that RB is a mediator of cell death ( 3 , 9 , 11 ). In the current study, pronounced differences in RB phosphorylation were correlated with the rates of apoptosis. Indeed, increased RBBP9 expression was paralleled by a loss of phosphorylation of RB in chronically exposed males and was associated with a strong and significant increase in apoptosis. It should also be noted that apoptotic levels were the highest in acutely exposed male spleen, even though the RB dephosphorylation trend was statistically insignificant. Apoptosis in female spleen, though increasing upon irradiation, was lower than in male spleen ( P < 0.05). Moreover, the background apoptosis level was slightly lower in males ( P < 0.05), where the background of RBBP9 expression was lower and RB phosphorylation was higher than in females. This observation is in good agreement with previous studies, which have shown that the shift from hyperphosphorylated to hypophosphorylated RB is usually linked to increased apoptotic cell death ( 9 , 30 ). Dephosphorylated RB is thought to assist cell death, most probably owing to the fact that RB dephosphorylation is a necessary step towards its degradation and elimination as a cell-death inhibitor ( 31 ). It was shown that RB dephosphorylation is a precursor for its cleavage by caspases ( 8 ). Interestingly, sex differences in RB phosphorylation in relationship to apoptosis induction were never studied before. The previous studies used either cell lines or mixed groups of animals, where the sex effects, if any, were largely masked. Indeed, radiation was shown to strongly induce apoptosis in C57BL/6 mice, but sex differences were never discussed because of the aforementioned reasons ( 9 ). The molecular mechanisms and cellular repercussions of the sex differences in the aforementioned processes have to be further addressed.
In summary, here we primarily examined the role of RBBP9-RB/E2F axis in cellular proliferation and apoptosis upon radiation exposure in spleen. We cannot exclude that other pathways, that were not a subject of this paper, contribute to the regulation of those vitally important processes in the cells. The contribution of the other pathways to radiation-induced proliferation and apoptosis and any sex differences in their activation have to be further addressed.
Taken together, our data constitute a foundation to analyze whether differences in radiation sensitivity occur via RBBP9-RB interactions as well as the interrelationships with the other molecules. The results obtained using this mouse model system can potentially be extrapolated to other animals, and possibly, even humans. Thus the current study opens the door for further research into the male versus female response to genotoxic stress.
These authors contributed equally to this work.
We are thankful to Dr Ron MacKay and Mrs Karen Kay of the Lethbridge Regional Hospital and to Mrs Tracey Lenek of the Calgary Laboratory Services for help with Ki67 and PCNA immunohistochemistry, to Kristy Kutanzi, Jonathan Loree and Benjamin Thibault for technical assistance and to Igor Kovalchuk for critical reading of the manuscript. We are grateful to Mrs. Laurie Robertson of the University of Calgary Flow Cytometry Core Facility for assistance with the flow cytometric analysis of cell proliferation and apoptosis. We would like to acknowledge the financial support from the University of Lethbridge, NSERC and CIHR to Olga Kovalchuk.
Conflict of Interest Statement : None declared.
References
Chau,B.N. and Wang,J.Y. (
Naderi,S., Hunton,I.C. and Wang,J.Y. (
Bowen,C., Birrer,M. and Gelmann,E.P. (
Sherr,C.J. and McCormick,F. (
Harbour,J.W. and Dean,D.C. (
Wang,J.Y., Naderi,S. and Chen,T.T. (
Wang,J., Guo,K., Wills,K.N. and Walsh,K. (
Harrington,E.A., Bruce,J.L., Harlow,E. and Dyson,N. (
Wallace,M., Coates,P.J., Wright,E.G. and Ball,K.L. (
Brugarolas,J., Moberg,K., Boyd,S.D., Taya,Y., Jacks,T. and Lees,J.A. (
Knudsen,K.E., Booth,D., Naderi,S., Sever-Chroneos,Z., Fribourg,A.F., Hunton,I.C., Feramisco,J.R., Wang,J.Y. and Knudsen,E.S. (
Flatt,P.M., Tang,L.J., Scatena,C.D., Szak,S.T. and Pietenpol,J.A. (
Almasan,A., Yin,Y., Kelly,R.E., Lee,E.Y., Bradley,A., Li,W., Bertino,J.R. and Wahl,G.M. (
Woitach,J.T., Zhang,M., Niu,C.H. and Thorgeirsson,S.S. (
Kovalchuk,O., Ponton,A., Filkowski,J. and Kovalchuk,I. (
Komarova,E.A., Christov,K., Faerman,A.I. and Gudkov,A.V. (
Midgley,C.A., Owens,B., Briscoe,C.V., Thomas,D.B., Lane,D.P. and Hall,P.A. (
Wilson,J.W., Pritchard,D.M., Hickman,J.A. and Potten,C.S. (
Wyllie,A.H., Kerr,J.F. and Currie,A.R. (
Birner,P., Ritzi,M., Musahl,C., Knippers,R., Gerdes,J., Voigtlander,T., Budka,H. and Hainfellner,J.A. (
Callum,D. and Hall,P. (
Scholzen,T. and Gerdes,J. (
Brown,L. and Gatter,K. (
Martinez,J.D., Taylor Parker,M., Fultz,K.E., Ignatenko,N.A. and Gerner,E.W. (
Datta,S.R., Brunet,A. and Greenberg,M.E. (
Brazil,D.P., Yang,Z.Z. and Hemmings,B.A. (
Hanada,M., Feng,J. and Hemmings,B.A. (
Zingg,D., Riesterer,O., Fabbro,D., Glanzmann,C., Bodis,S. and Pruschy,M. (
Wang,H., Grand,R.J., Milner,A.E., Armitage,R.J., Gordon,J. and Gregory,C.D. (
Author notes
Department of Biological Sciences, University of Lethbridge, Lethbridge, Alberta, Canada T1K 3M4 and 1DSM Nutritional Products Ltd, Kaiseraugst CH-4303, Switzerland