-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Horton, Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture, Journal of Experimental Botany, Volume 51, Issue suppl_1, February 2000, Pages 475–485, https://doi.org/10.1093/jexbot/51.suppl_1.475
Close - Share Icon Share
Abstract
The prospects for genetic manipulation of photosynthesis are assessed with an emphasis on the biochemical and morphological aspects of light capture. The connection between different parts of the photosynthetic process is considered together with the influence of environmental factors, development and acclimation, and metabolic regulation. The sites of real and potential photosynthetic losses are identified, using tropical rice as a case study. The important interaction between photosynthetic capacity, acclimation to the light environment, nitrogen accumulation and canopy architecture are discussed. The possibility of genetic intervention to increase both biomass accumulation and improve nitrogen economy simultaneously are considered. Finally, the numerous procedures for genetic manipulation of light harvesting are also discussed, with a view to improving radiation‐use efficiency in crops.
Introduction
In order to meet the demand for food from the growing world population there will have to be significant increases in yield of the major crops grown in developing countries. In rice, for example, an estimated 50% yield increase is needed by the year 2030. Increasing the maximum yield potential is viewed as an important, if not vital, part of any strategy for achieving this increase in yield (Khush and Peng, 1996). Since the harvest index for many crops, such as rice, is approaching a ceiling value, an increase in yield potential will have to involve an increase in crop biomass, i.e. there will have to be more net photosynthesis (Cassman, 1994; Ying et al., 1998a; Mann, 1999a, b). This may be achieved by an increase in Leaf Area Index (LAI) or an increase in net photosynthesis per unit leaf area. Since LAI is generally already high in most crops, the increased assimilate production must come from improved photosynthesis.
The approach to achieving this goal is to bring about an increase in photosynthesis at the leaf level. With appropriate crop management, this increase could then be integrated into an increase in carbon gain at the canopy level and an increase in yield. In this article, some prospects for bringing about such an increase in leaf photosynthesis are assessed, using tropical rice as an example. Emphasis will be placed upon the biochemical and morphological features that help determine the efficiency of radiation use. In particular, the possibility will be considered whether modification of the acclimation of photosynthesis to changes in light intensity, and the related changes to canopy structure and nitrogen acquisition, is a useful strategy for increasing crop photosynthesis.
Photosynthetic complexity
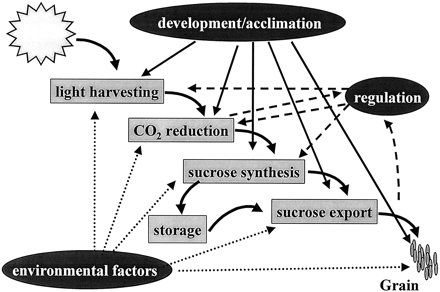
The first question is: what parts of the photosynthetic process could be modified to enhance the amount of carbon gained? This is not an easy question to answer. The problem lies not just in the complexity of the processes that connect light absorption and capture through to carbon dioxide fixation and carbohydrate accumulation, but the interactions that occur between the component subsystems (Fig. 1). Thus, regulatory mechanisms link each of the components, achieving balance and serving to control concentrations of intermediates (Horton, 1985a, b, 1994). The principal regulatory mechanisms are found at the input, where the plant has mechanisms which control the excitation energy in the pigment systems, and at the output, where partitioning between storage carbohydrate and sucrose export to the sink tissues is finely controlled. In between a plethora of regulated enzymes are found. Mathematical models have been constructed to help in this task (Poolman et al., 2000), but their application to real crops in the field is problematical. This metabolic pathway could be analysed mathematically. However, the fact that it also has to include other layers of control, by environmental factors and by changes arizing from development and acclimation, make this task virtually impossible to do in a way that is useful to the plant physiologist or plant breeder.
Faced with this problem, it is imperative that a new approach is found (Horton, 1994). This approach should include the following:
(1) Development of appropriate experimental methods to analyse photosynthetic performance under field conditions so that the sites of limitation to photosynthesis under particular conditions can be identified.
(2) Incorporation of this methodology into analyses of a range of genotypes to explore genetic variation, as well as to test new mutant collections.
(3) Use of genetic manipulation to modulate the capacity of different photosynthetic components and to explore these effects on whole plant performance.
Photosynthesis—from light harvesting to grain production. A flow diagram to indicate the principal subsystems that connect to convert sunlight into crop yield. The influence of internal regulatory mechanisms (dashed lines), environmental factors (dotted lines) and development/acclimation alter both the rate and capacity of the material and energy flux through the whole system.
Identification of photosynthetic losses
The maximum potential photosynthesis defines a theoretical limit that is unlikely to be achieved under field conditions because of a range of internal and external factors. Therefore, another way to analyse limitations in photosynthesis is to attempt to assess where significant losses of potential photosynthesis might be. Such an approach has several advantages. Firstly, it need not distinguish between aspects of photosynthetic capacity (i.e. Pmax) and those of operational photosynthesis (i.e. the extent to which this capacity is attained, or photosynthetic efficiency). This is convenient since the distinction is in many cases fairly arbitrary. Secondly, it allows more readily the identification of particular environmental conditions or developmental stages when losses occur; e.g. photosynthetic losses may occur especially during a particular time of the day, or during a particular stage of leaf development. Also, although not a rigorously quantitative concept, the measurement of a loss immediately indicates how much could be gained by its elimination.
A recent study of field‐grown rice provides an example of the first two steps in this approach. During crop development in the dry season in the Philippines, the following aspects of photosynthesis have been measured or assessed: light absorption with respect to leaf orientation, leaf temperature, chlorophyll fluorescence, gas exchange using IRGA, and content per unit leaf area of pigment, carbohydrate and Rubisco. This study was informative, not just from the new details that were gained about the physiology of the rice crop, but in that it exposed many of the problems of extrapolating from a laboratory‐based study or theory to the field. During this study, a series of sites for potential photosynthetic losses were identified (Murchie et al., 1999; Table 1), some of which are discussed below.
Photosynthetic losses in rice in the field
Description of the processes (Fig. 1) where losses in photosynthesis may occur in field‐grown rice growing in the tropics. The causes, mechanism and conditions under which the losses may occur are given. Each of these has been demonstrated for rice in field experiments, whereas those with (?) indicate only the possible conditions of loss. See text for further details.
| Process | Cause of loss | Cause and | Conditions | Possible remedy for |
| mechanism | under which | improvement | ||
| loss occurs | ||||
| Light harvesting | Poor absorption | Erect upper leaves | Large solar | Altered canopy |
| angle at midday | structure | |||
| Light saturation | Limitation on Pmax | High irradiance | Improved acclimation | |
| to increase Pmax | ||||
| Down‐regulation | Slow relaxation of | Fluctuating | Decreased and/or | |
| non‐photochemical | light intensity | altered qE | ||
| quenching | ||||
| Electron transport | Photoinhibition | Damage to PSII | Severe stress | Improved |
| reaction centre | conditions, | photoprotection | ||
| older leaves? | ||||
| C assimilation | Photorespiration | Rubisco oxygenase | High leaf | Improved Rubisco; C4 |
| temperature | photosynthesis | |||
| Decline in Pmax | Stomatal | Mid‐morning | Altered stomatal | |
| closure/feedback | in high | responses/carbohydrate | ||
| inhibition | irradiance | metabolism | ||
| Leaf senescence | During grain filling? | Delayed leaf | ||
| senescence | ||||
| Partitioning | Accumulation of | Poor remobilization of | During grain filling? | Altered internal |
| stem carbohydrate | resources | metabolite signalling | ||
| Respiration | Loss of fixed | High LAI with | Mature canopy; | Decreased respiration |
| carbon | inefficient lower | high night | capacity; improved N | |
| leaves | economy in lower | |||
| temperature | leaves to reduce LAI |
| Process | Cause of loss | Cause and | Conditions | Possible remedy for |
| mechanism | under which | improvement | ||
| loss occurs | ||||
| Light harvesting | Poor absorption | Erect upper leaves | Large solar | Altered canopy |
| angle at midday | structure | |||
| Light saturation | Limitation on Pmax | High irradiance | Improved acclimation | |
| to increase Pmax | ||||
| Down‐regulation | Slow relaxation of | Fluctuating | Decreased and/or | |
| non‐photochemical | light intensity | altered qE | ||
| quenching | ||||
| Electron transport | Photoinhibition | Damage to PSII | Severe stress | Improved |
| reaction centre | conditions, | photoprotection | ||
| older leaves? | ||||
| C assimilation | Photorespiration | Rubisco oxygenase | High leaf | Improved Rubisco; C4 |
| temperature | photosynthesis | |||
| Decline in Pmax | Stomatal | Mid‐morning | Altered stomatal | |
| closure/feedback | in high | responses/carbohydrate | ||
| inhibition | irradiance | metabolism | ||
| Leaf senescence | During grain filling? | Delayed leaf | ||
| senescence | ||||
| Partitioning | Accumulation of | Poor remobilization of | During grain filling? | Altered internal |
| stem carbohydrate | resources | metabolite signalling | ||
| Respiration | Loss of fixed | High LAI with | Mature canopy; | Decreased respiration |
| carbon | inefficient lower | high night | capacity; improved N | |
| leaves | economy in lower | |||
| temperature | leaves to reduce LAI |
Photosynthetic losses in rice in the field
Description of the processes (Fig. 1) where losses in photosynthesis may occur in field‐grown rice growing in the tropics. The causes, mechanism and conditions under which the losses may occur are given. Each of these has been demonstrated for rice in field experiments, whereas those with (?) indicate only the possible conditions of loss. See text for further details.
| Process | Cause of loss | Cause and | Conditions | Possible remedy for |
| mechanism | under which | improvement | ||
| loss occurs | ||||
| Light harvesting | Poor absorption | Erect upper leaves | Large solar | Altered canopy |
| angle at midday | structure | |||
| Light saturation | Limitation on Pmax | High irradiance | Improved acclimation | |
| to increase Pmax | ||||
| Down‐regulation | Slow relaxation of | Fluctuating | Decreased and/or | |
| non‐photochemical | light intensity | altered qE | ||
| quenching | ||||
| Electron transport | Photoinhibition | Damage to PSII | Severe stress | Improved |
| reaction centre | conditions, | photoprotection | ||
| older leaves? | ||||
| C assimilation | Photorespiration | Rubisco oxygenase | High leaf | Improved Rubisco; C4 |
| temperature | photosynthesis | |||
| Decline in Pmax | Stomatal | Mid‐morning | Altered stomatal | |
| closure/feedback | in high | responses/carbohydrate | ||
| inhibition | irradiance | metabolism | ||
| Leaf senescence | During grain filling? | Delayed leaf | ||
| senescence | ||||
| Partitioning | Accumulation of | Poor remobilization of | During grain filling? | Altered internal |
| stem carbohydrate | resources | metabolite signalling | ||
| Respiration | Loss of fixed | High LAI with | Mature canopy; | Decreased respiration |
| carbon | inefficient lower | high night | capacity; improved N | |
| leaves | economy in lower | |||
| temperature | leaves to reduce LAI |
| Process | Cause of loss | Cause and | Conditions | Possible remedy for |
| mechanism | under which | improvement | ||
| loss occurs | ||||
| Light harvesting | Poor absorption | Erect upper leaves | Large solar | Altered canopy |
| angle at midday | structure | |||
| Light saturation | Limitation on Pmax | High irradiance | Improved acclimation | |
| to increase Pmax | ||||
| Down‐regulation | Slow relaxation of | Fluctuating | Decreased and/or | |
| non‐photochemical | light intensity | altered qE | ||
| quenching | ||||
| Electron transport | Photoinhibition | Damage to PSII | Severe stress | Improved |
| reaction centre | conditions, | photoprotection | ||
| older leaves? | ||||
| C assimilation | Photorespiration | Rubisco oxygenase | High leaf | Improved Rubisco; C4 |
| temperature | photosynthesis | |||
| Decline in Pmax | Stomatal | Mid‐morning | Altered stomatal | |
| closure/feedback | in high | responses/carbohydrate | ||
| inhibition | irradiance | metabolism | ||
| Leaf senescence | During grain filling? | Delayed leaf | ||
| senescence | ||||
| Partitioning | Accumulation of | Poor remobilization of | During grain filling? | Altered internal |
| stem carbohydrate | resources | metabolite signalling | ||
| Respiration | Loss of fixed | High LAI with | Mature canopy; | Decreased respiration |
| carbon | inefficient lower | high night | capacity; improved N | |
| leaves | economy in lower | |||
| temperature | leaves to reduce LAI |
Canopy structure and leaf orientation
The New Plant Type (NPT) rice varieties are characterized by erect leaves. The breeders' rationale for this morphological trait is that it allows greater penetration of light to the lower leaves, thereby optimizing canopy photosynthesis (Duncan, 1971). In NPT rice the erect leaf orientation and the relatively small number of tillers provides a more open canopy compared to an Indica variety such as IR72. This type of canopy structure complicates the assessment of leaf photosynthesis as required for genetic improvement. Strategies to improve photosynthesis by the leaf have to take into account the resulting complex, heterogeneous distribution of light and photosynthetic activity in the crop canopy.
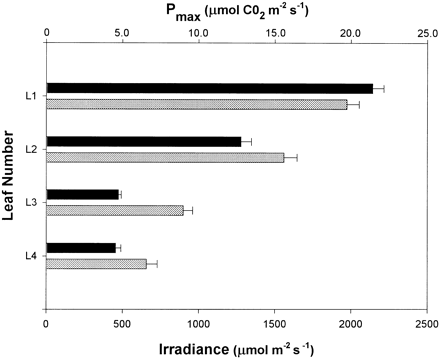
Since the capacity for photosynthesis is dependent on the light intensity during growth, partially shaded lower leaves almost certainly have different contents of photosynthetic components from their upper leaves. Their Pmax values do not represent the true ceiling Pmax of that leaf, but a value that has been down‐regulated as part of long‐term acclimation response. In fact, measurements taken in the field show that there was a remarkably close relationship between the penetration of light into the canopy and the Pmax of the four leaves of the rice plant (Fig. 2). The decline in Pmax was more exaggerated than expected for a pure acclimation response (Table 2), where a 10‐fold decline in irradiance induced by artificial shading only depressed the Pmax of leaf 1 by less than 50%. Clearly, there is also a developmental aspect to the decline in Pmax of the lower leaves.
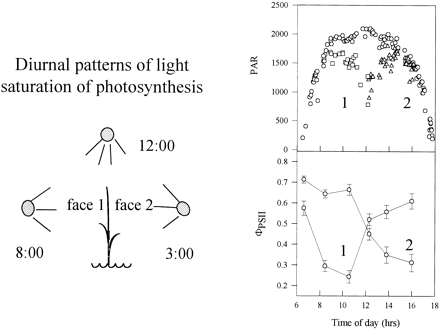
A second feature of the interaction between the rice canopy and light is the interaction between leaf orientation and the solar angle (Murchie et al., 1999). This may produce dramatic effects which are near impossible to duplicate away from the field, and which may be specific to a particular geographical location or climatic condition. In the Philippines, under clear skies, this led to a characteristic diurnal pattern of irradiance, with one surface of a leaf receiving a maximum at around 10 am and the other surface at around 3 pm (Fig. 3). At midday, when the solar irradiation was at a peak, the amount of light absorbed by the upper vertical leaves was small for both surfaces. Such transients are not observed in cloudy skies, prevalent in the tropical wet season, when the solar radiation is both low and diffuse.
For lower leaves, the incident irradiance tended to reach a peak at midday since they are not directly exposed to radiation at the low solar angles in the morning and afternoon. Lower leaves may be exposed to average light intensities of over 500 μmol m−2 s−1 at midday (Fig. 2). To estimate the contribution that these leaves make to whole plant photosynthesis the third and fourth leaves were removed. Despite the relatively low Pmax values for these leaves, their removal resulted in a 45% decline in midday photosynthetic rate. Thus the structure of the rice canopy has two effects: light saturation of upper leaves is minimized and lower leaves receive sufficient light to drive photosynthesis at rates that make a significant contribution to total daily photosynthesis. The end result is that during a diurnal cycle a rather constant rate of whole plant photosynthesis may be achieved.
Light penetration and photosynthetic capacity of a rice canopy. Shown are values for Pmax (black) and irradiance (grey) determined for the four leaves of the rice plant. Pmax values are the maximum for those leaves. Irradiance was recorded at midday at the height of the middle of each leaf.
Diurnal patterns of light saturation of photosynthesis in the upper (flag) leaf of rice. On the left a diagrammatic representation of how the two faces of the erect rice leaf are exposed to peak irradiances in the morning and afternoon. On the right are data obtained: top, recordings of PAR for face 1 (squares), face 2 (triangles) and maximum irradiance (circles). Bottom: data for the parameter ϕPSII obtained from Chl fluorescence measurements for each leaf face. A maximum value of aproximately 0.8 indicates a leaf operating with maximum quantum efficiency. A light‐saturated leaf gives a value of aproximately 0.2 (redrawn from Murchie et al., 1999).
Effect of growth irradiance on photosynthetic characteristics of field‐grown rice
For this experiment, New Plant Type rice plants were grown in the field and exposed to differential shading for 4 weeks. The irradiance values are the approximate mean peak irradiances at midday. Pmax, Chl content and xanthophyll cycle contents were determined for upper leaves (as described by Murchie et al., 1999). The Rubisco content was determined following polyacrylamide gel electrophoresis of soluble protein, and quantified by densitometry of the Coomassie stained gel, relative to Rubisco standards.
| Irradiance | Pmax | Chl content | Rubisco content | Xanthophyll cycle |
| (μmol m−2 s−1) | (μmol CO2 m−2 s−1) | (μg cm−2) | (g m−2) | (% total car) |
| 200 | 15.1±2.4 | 52.1±0.2 | 3.20±0.56 | 20.3±0.4 |
| 800 | 21.1±1.1 | 48.5±0.2 | 4.38±0.55 | 23.3±1.0 |
| 2000 | 24.8±0.9 | 40.1±0.2 | 7.41±1.02 | 36.1±8.0 |
| Irradiance | Pmax | Chl content | Rubisco content | Xanthophyll cycle |
| (μmol m−2 s−1) | (μmol CO2 m−2 s−1) | (μg cm−2) | (g m−2) | (% total car) |
| 200 | 15.1±2.4 | 52.1±0.2 | 3.20±0.56 | 20.3±0.4 |
| 800 | 21.1±1.1 | 48.5±0.2 | 4.38±0.55 | 23.3±1.0 |
| 2000 | 24.8±0.9 | 40.1±0.2 | 7.41±1.02 | 36.1±8.0 |
Effect of growth irradiance on photosynthetic characteristics of field‐grown rice
For this experiment, New Plant Type rice plants were grown in the field and exposed to differential shading for 4 weeks. The irradiance values are the approximate mean peak irradiances at midday. Pmax, Chl content and xanthophyll cycle contents were determined for upper leaves (as described by Murchie et al., 1999). The Rubisco content was determined following polyacrylamide gel electrophoresis of soluble protein, and quantified by densitometry of the Coomassie stained gel, relative to Rubisco standards.
| Irradiance | Pmax | Chl content | Rubisco content | Xanthophyll cycle |
| (μmol m−2 s−1) | (μmol CO2 m−2 s−1) | (μg cm−2) | (g m−2) | (% total car) |
| 200 | 15.1±2.4 | 52.1±0.2 | 3.20±0.56 | 20.3±0.4 |
| 800 | 21.1±1.1 | 48.5±0.2 | 4.38±0.55 | 23.3±1.0 |
| 2000 | 24.8±0.9 | 40.1±0.2 | 7.41±1.02 | 36.1±8.0 |
| Irradiance | Pmax | Chl content | Rubisco content | Xanthophyll cycle |
| (μmol m−2 s−1) | (μmol CO2 m−2 s−1) | (μg cm−2) | (g m−2) | (% total car) |
| 200 | 15.1±2.4 | 52.1±0.2 | 3.20±0.56 | 20.3±0.4 |
| 800 | 21.1±1.1 | 48.5±0.2 | 4.38±0.55 | 23.3±1.0 |
| 2000 | 24.8±0.9 | 40.1±0.2 | 7.41±1.02 | 36.1±8.0 |
Light saturation
Despite the erect leaf posture, photosynthesis of rice leaves is still light saturated for several hours of the day. This was reflected in recorded values of ϕPSII of approximately 0.2 in mid‐morning (Fig. 3). Light saturation represents an important photosynthetic loss and could contribute significantly to a decline in radiation conversion efficiency.
Photosynthesis of field‐grown plants saturated at aproximately 1500 μmol m−2 s−1, well below the peak growth light and, consequently, field quantum efficiencies drop to low values for large parts of the day (Fig. 3). In previous studies on other plant species in the laboratory, it has been demonstrated that acclimation to irradiance results in high quantum efficiency being maintained at the growth irradiance (Walters and Horton, 1994; Walters et al., 1999). In contrast, in rice in the field, acclimation to the maximum growth irradiance has not fully occurred. This observation suggests that acclimation itself may be subject to light saturation and that this ceiling is reached under field conditions. This was confirmed by experiments in which plants were shaded in the field; a 60% shade for several weeks only marginally depressed the Pmax below that obtained for unshaded plants (Table 2). Plants fully acclimated to high light intensity would not be expected to show elevated photoprotective responses since they can utilize a high proportion of the absorbed light for photosynthesis. The clear photoprotective responses to excess light shown by the rice plants grown at high light—a decrease in Chl content and an increase in the content of xanthophyll cycle carotenoids (Table 2; Murchie and Horton, 1998)—are therefore consistent with the saturation of photosynthetic acclimation.
As Pmax does not increase sufficiently when plants are grown in full sunlight they suffer the effects of absorbing excess light energy; light saturation of photosynthesis resulting in a decline in radiation conversion efficiency, and a potential for photoinhibition. This failure of Pmax to acclimate fully to the tropical growth irradiance raises the important question as to what determines this ceiling for the acclimation of Pmax. On the one hand there may be some kind of ‘physical’ limit to Pmax that has been reached, for example, perhaps the maximum concentration of Rubisco in the chloroplast has been attained. On the other hand the acclimation limit could arise because of a developmental limitation to the dynamic range of acclimation that prevents the potential Pmax being attained.
Sunflecks
A further complexity is that the light environment of the lower leaves is highly heterogeneous, with light penetration not only being attenuated by shading but also giving rise to sunflecks (Pearcy et al., 1996). Photosynthetic activity in lower leaves of a rice crop is largely unexplored, and the key question is whether sunflecks are exploited for carbon gain. This is determined by two features of the photosynthetic apparatus: firstly, the photosynthetic capacity; secondly, the kinetic properties of the enzymes whose activity needs to be increased to accommodate the increased flux. The first depends upon the extent to which photosynthetic acclimation has tuned the system to the peak light intensity or to the average. In the rice crop, the Pmax of lower leaves is significantly less that that determined for upper leaves (Fig. 3), lower than that expected for an acclimation response to the light environment, suggesting that sunflecks will saturate photosynthesis in these leaves. However, it is impossible to generalize, and it is likely that the type of response will be determined by the canopy structure as well as by the intensity, direction and duration of sunflecks.
Leaf temperature
In the field experiments, it was found that leaf temperature reached 40 °C for much of the day. At this temperature significant photosynthetic losses arise from increased photorespiration. Pmax measured in the field at ambient and saturating CO2 were consistent with an approximate 40% loss due to photorespiration (Leegood and Edwards, 1996). These levels of photorespiratory losses that have been frequently reported for C3 crops cultivated under tropical conditions have given rise to the desire to introduce C4‐like features into tropical rice (Mann, 1999b).
Diurnal depressions in photosynthesis
Midday depression of photosynthesis is a common occurrence. In NPT rice, the depression was observed not at midday but during mid‐morning (Murchie et al., 1999). This result links this phenomenon to peak irradiance rather than a diurnal cycle. The speed at which it occurs does not suggest that it arises because of feedback inhibition though build‐up of photosynthate (Winder et al., 1998). However, the limitation is real, and results in an estimated loss of 30% daily photosynthesis. A clearer understanding of this phenomenon is urgently required. It is of interest that the depression in photosynthesis in rice varieties shows genetic variation, and a smaller midday depression was correlated with an increase in yield (Black et al., 1995). One possibility is that the depression in photosynthesis arises from an ‘inappropriate’ closure of stomata.
Photoinhibition
Young leaves, even those uppermost in the canopy did not suffer chronic photoinhibition at peak irradiance, and any decrease in photochemical efficiency was transient, and reversed as the irradiance declined again (Murchie et al., 1999). Examination of kinetics of recovery from potentially photoinhibitory conditions indicated that the dark‐adapted Fv/Fm was suppressed over a lag period, but these decreases were relatively small and diminished in line with the change in irradiance.
In older leaves, when a significant proportion adopted a more horizontal orientation, photoinhibition was stronger, and did not recover so readily as in younger erect leaves when the irradiance declined. However, again, the decline in Fv/Fm did not persist, and recovery was complete overnight. It is hard to argue that in an irrigated crop, in a tropical environment as found at IRRI, that photoinhibition represents a major source of photosynthetic loss. Equally, however, under these conditions acclimation to high light had saturated in terms of the irradiance‐dependent increase in Pmax and xanthophyll cycle pool size (Table 2), and could be considered just at the threshold of significant light stress. Therefore, in more extreme conditions, where light intensities are higher and sustained for longer periods, and where water management is perhaps less comprehensive than at IRRI, photoinhibition could become a problem. Certainly, the fact that rice under favourable conditions is so close to light stress indicates that many other crops in more marginal habitats probably suffer considerable photosynthetic losses through photoinhibition.
Nitrogen economy
Optimizing N‐use efficiency is a top priority for crop improvement. It is not possible to deal with this topic comprehensively here. However, it is clear that nitrogen economy and photosynthesis are inextricably linked since Rubisco accounts for a large fraction of leaf N. Two ideologies have dominated thinking on this point. The first is that photosynthesis per unit N should be enhanced, i.e. reducing the amount of Rubisco but maintaining photosynthetic capacity. Linked to this idea is the desire to improve the efficiency of Rubisco by reducing Rubisco oxygenase activity (Mann, 1999b). The second, in contrast, suggests that the way to increase Pmax is to increase leaf N since, over a wide range of N fertilization, photosynthesis correlates positively with leaf N (Peng et al., 1995).
Both of these ignore the key role of leaf N as a reservoir of remobilizable N that is needed to sustain grain yield (Ying et al., 1998b). Therefore, a strategy that results in lower leaf N may in fact limit crop yield under some conditions, particularly those designed to give high yields. Improving photosynthetic efficiency should perhaps be separated from the question of nitrogen use efficiency. Eliminating photorespiratory losses should be accompanied by a maintenance of Rubisco content not a diminution. Similarly, a strategy to increase leaf N should also be viewed as a worthwhile aim in its own right regardless of whether it could lead to an increase in photosynthetic capacity, since Rubisco N may well be essential as a mobilizable N store.
Rubisco contents of rice plants vary considerably, and do not always correlate with Pmax. The content of Rubisco in New Plant Type rice are extremely high, the values in Table 2 are 3–5 times higher than in an Indica variety, IR72. Despite this, photosynthetic rates are little different between the two varieties. The higher levels of Rubisco supports the notion of it being a N store and the genetic variation in Rubisco content may provide an approach to uncovering the genes controlling its synthesis and breakdown.
It has been argued that the provision of N for the developing plant and, particularly, grain production is a principal factor in determining canopy architecture (Sinclair and Sheehy, 1999). In order to accumulate enough N, a large leaf area index is needed. So that these extra leaves contribute a net carbon gain rather than being parasitic, the canopy has to be open to allow light penetration, hence erect leaves are needed. However, this may not be a perfect strategy. It is well known that increased N application to a crop does result in an increase in total N in the canopy (Peng et al., 1994; Ying et al., 1998b), but this is invariably associated with a high leaf area index and the associated high respiratory costs of leaves with low photosynthetic rates. The strategy is also weakened by the developmental/acclimation responses described above that actually lower the Rubisco content in these leaves (in the experiment shown in Fig. 2 Rubisco contents of leaf 4 were only 30% of those measured in leaf 1). Thus, these leaves do not represent an efficient (N/unit energy cost) store of N. The problem that has to be overcome, is how to enhance N per unit leaf area. As will be suggested below, a solution to these dilemmas is to intervene in the mechanism that regulates the amount of leaf Rubisco. If this could be achieved, a major rethink may be required for the design of canopy architecture.
What determines Pmax?
As stated at the beginning, there is no single answer to this question. Even for a single crop species, the answer depends upon what environmental conditions, what developmental state or even what times of day are being considered. Generally, it seems the capacity of several processes share the control of photosynthesis at light saturation, including Rubisco content and electron transport capacity. This indicates concerted control of expression of a variety of photosynthetic genes. In turn, this expression is tuned to the environmental conditions and developmental state.
Pmax is clearly limited by photorespiratory losses, and an advocated strategy for crop improvement in C3 plant is the introduction of C4‐like features that can lead to elevation of local CO2 concentration. Alternatively, introduction of a more efficient Rubisco could give rise to the same effect (Mann, 1999b). One complication, even if the genetic engineering could be carried out successfully, is still whether limitations elsewhere in the system would be approached before the full benefit was realized. Acclimation, such as that frequently observed to elevated CO2, may bring about a depression in Pmax.
Some targets for genetic manipulation
Source and sink
Most discussion of the whole plant factors limiting crop yield focuses on whether the source or the sink is the limiting factor (Egli, 1998). Clearly, as evident from Fig. 1, it is incorrect to consider source and sink operating independently. Indeed, it is suggested that the concepts of source and sink are redundant terms in the context of considering regulation and limitation of the biochemical processes that determine crop yield. Not only do regulation mechanisms ensure that all parts are balanced, but there is control during development. For example, seed number, an important yield component and an index of sink strength, is determined by net photosynthesis during the reproductive phase (Egli, 1998). More fundamentally, source and sink are rather arbitrary divisions in a continuous process of linked biochemical reactions (Fig. 1); as with any such pathway, limitation is distributed along this continuum, and discussion of ‘source and sink limitation’ is as flawed as the consideration of ‘rate‐limiting steps’ in biochemistry.
It is not possible to assess here the potential of all possible targets for genetic manipulation to bring about marked and sustainable increases in crop yield. Indeed, the concept developed above suggests that this will require manipulation of several steps. For rice, there is clearly great promise in manipulation of the pathways leading to starch deposition in the developing grain, or in increasing the transport capacity of sucrose through to the developing spikelet. However, here, only aspects of manipulation of chloroplast photosynthesis will be considered.
Delaying leaf senescence
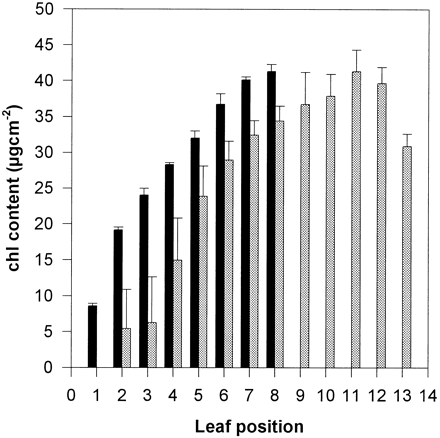
Increasing the duration of photosynthesis offers an opportunity of increasing the total amount of carbon fixed by a crop. Delaying leaf senescence has therefore become a prime target for crop improvement (Thomas and Howarth, 2000). Leaf senescence is a complex developmental process that is under hormonal control as well as being affected by internal metabolic (N and C status) and environmental (irradiance) factors (Thomas and Stoddart, 1980; Smart, 1994). A variety of ‘stay‐green’ phenotypes have been described, although there is no clearly demonstrated influence on yield. Genetic manipulation of the synthesis of cytokinin has resulted in a delay of leaf senescence and an increase in growth rate for tobacco (Gan and Amasino, 1995). Delay of leaf senescence in tomato by antisense suppression of ethylene biosynthesis has been observed (John et al., 1995). However, in this case there was no observable effect on biomass and, in fact, retention of the lower older leaves resulted in a restriction on appearance of new leaves (Fig. 4). It is argued, therefore, that a strategy for photosynthetic improvement through increasing leaf longevity has to be interfaced with an approach which, at the same time, optimizes N economy during this critical phase of crop maturation.
Wild‐type (grey) and a transformed tomato plant (black) showing delayed senescence as a result of expression of an antisense ACC oxidase gene (John et al., 1995). Note the retention of Chl in the older lower leaves in the transformed plant, but the decrease in number of leaves. Leaf position 1 is the oldest and 13 the youngest.
Increasing Pmax
The Pmax of the leaf of any plant species is highly variable and determined by environmental factors during growth and by internal metabolic/developmental factors. Therefore, it is unlikely that a true Pmax is ever recorded. The question is: how much scope is there for increasing Pmax if the mechanisms that regulate it can be identified? An important experiment has been carried out using tomato; plants were allowed to senesce and then the upper leaves were removed: this resulted not only in regreening of the senescent leaves, but moreover, the Pmax attained was significantly higher that that previously found in these leaves at full maturity (Table 3). This graphically demonstrates that there is a capacity for higher Pmax if normal developmental controls are disrupted. In fact if Pmax and Chl content are measured during tomato leaf development peak values are found only for a relatively brief period before decline begins (Fig. 4), again consistent with the notion that a ceiling Pmax is rarely attained. Similarly, in rice, photosynthetic capacity of fully expanded uppermost leaves declines markedly, well before flowering, and correlating with a decrease in leaf N content (Peng et al., 1998)
Increasing Pmax is unlikely to be brought about by altering the expression of a single photosynthetic gene; rather, intervention into the processes that control Pmax in response to environmental or metabolic cues represents the most useful strategy. The challenge will be to bring about such a Pmax elevation in all upper leaves, such that, for cereals, on a per tiller basis there is an overall increase in the capacity of carbon supply to the grain.
Regreening of senescent leaves of tomato
Pmax was determined using IRGA in air at 25 °C for a fully expanded leaf and a senescent leaf on 10‐week‐old tomato plants. The regreened leaf was obtained by removing the upper leaves leaving just two greenish‐yellow leaflets on the plant, and regreening followed for 12 d. Data are means of four different plants.
| Leaf | Pmax | Chl content |
| (μmol CO2 m−2 s−1) | (μg cm−2) | |
| Fully expanded leaf | 18.1±2.3 | 35.6±1.1 |
| Senescent leaf | 8.4±0.8 | 10.6±0.7 |
| Regreened leaf | 24.6±1.5 | 50.7±3.0 |
| Leaf | Pmax | Chl content |
| (μmol CO2 m−2 s−1) | (μg cm−2) | |
| Fully expanded leaf | 18.1±2.3 | 35.6±1.1 |
| Senescent leaf | 8.4±0.8 | 10.6±0.7 |
| Regreened leaf | 24.6±1.5 | 50.7±3.0 |
Regreening of senescent leaves of tomato
Pmax was determined using IRGA in air at 25 °C for a fully expanded leaf and a senescent leaf on 10‐week‐old tomato plants. The regreened leaf was obtained by removing the upper leaves leaving just two greenish‐yellow leaflets on the plant, and regreening followed for 12 d. Data are means of four different plants.
| Leaf | Pmax | Chl content |
| (μmol CO2 m−2 s−1) | (μg cm−2) | |
| Fully expanded leaf | 18.1±2.3 | 35.6±1.1 |
| Senescent leaf | 8.4±0.8 | 10.6±0.7 |
| Regreened leaf | 24.6±1.5 | 50.7±3.0 |
| Leaf | Pmax | Chl content |
| (μmol CO2 m−2 s−1) | (μg cm−2) | |
| Fully expanded leaf | 18.1±2.3 | 35.6±1.1 |
| Senescent leaf | 8.4±0.8 | 10.6±0.7 |
| Regreened leaf | 24.6±1.5 | 50.7±3.0 |
Disrupting acclimation in lower leaves
In rice, the contribution of lower leaves to photosynthesis is significant even though their Pmax is considerably less than the upper leaves. This decrease in Pmax appears to result, at least in part, from the light acclimation of photosynthesis. What would be the purpose of disrupting this acclimation process? An answer might lie in the N economy of the plant. If N‐supply to the developing grain is limiting, a greater reserve of remobilizable N in the lower leaves could overcome this limitation. Therefore, disruption of that part of photosynthetic acclimation that results in a decline in Rubisco content could maintain N storage in these leaves. As discussed above, in certain NPT varieties of rice, Rubisco contents are much higher than needed for photosynthesis, suggesting that this is an achievable objective. Thus, an important objective for future research is to identify the genetic basis of Rubisco content, and to determine how its synthesis/degradation may be uncoupled from those mechanisms that determine photosynthetic activity.
Light harvesting and non‐photochemical quenching
The light‐harvesting pigment–protein complexes are the primary contacts with sunlight. Their importance in the photosynthetic process is exemplified by the presence of regulatory mechanisms which adjust the delivery of light energy to the electron transport system so that light input and metabolic demand are co‐ordinated (Horton, 1985a, b, 1989). It should be asked whether the regulatory mechanisms are optimized for the favourable conditions encountered in many agricultural systems. Conversely, since the function of these mechanisms is to provide protection from photoinhibition, it should be considered whether photosynthetic performance might be improved under stress conditions by manipulation of the light‐harvesting proteins.
The light‐harvesting system of PSII (LHCII) comprises the products of the six Lhcb genes (Lhcb1–6) that are assembled into four types of complexes known as LHCIIa, LHCIIb, LHCIIc, and LHCIId. LHCIIb is a trimeric complex binding approximately 60% of PSII chlorophyll. LHCIIa, LHCIIc and LHCIId are monomeric complexes more widely known as CP29, CP26, and CP24, respectively (Jansson, 1994; Peter and Thornber, 1991). In addition, there are a number of LHCII‐related proteins associated with PSII (Jansson, 1999).
For plants in limiting irradiance, this light‐harvesting system allows photosynthesis to function with maximum efficiency. However, in high light, there is a profound change in the function of the light‐harvesting system (Horton et al., 1996)—there is now excess excitation energy, which is converted to heat, a process monitored by the (non‐photochemical) quenching of chlorophyll fluorescence. Non‐photochemical quenching, which results from the combined effects of the increase in ΔpH and the de‐epoxidation of the carotenoid violaxanthin to form zeaxanthin, is referred to as qE. Both these factors would appear to affect one or more of these polypeptides (Horton et al., 1996). Therefore, different conformational states are envisaged (Horton et al., 1991): an unprotonated form that binds violaxanthin and is efficient in light harvesting, and a second form that is protonated, binds zeaxanthin and efficiently converts excitation energy into heat.
There is considerable variation in the capacity and kinetics of non‐photochemical quenching between species (Ruban et al., 1993; Johnson et al., 1993). In general, species tolerant to stress conditions show larger and faster development of qE upon illumination with excess light. Therefore two strategies for crop improvement emerge:
(1) For crops growing in marginal habitats an enhanced capacity for qE is desirable.
(2) For crops growing in favourable conditions qE should be reduced or eliminated to remove the regulatory costs—these include photosynthetic losses arizing from the mis‐match between the relaxation kinetics of qE and transitions in light intensity (see above).
Progress towards achieving these objectives is now possible because of the increased understanding of qE that has been obtained from physiological and biochemical studies. There are now a number of different genetic strategies available for the genetic manipulation of light harvesting (Table 4). The identification of Arabidopsis mutants with an altered capacity for qE may lead to identification of the genes which should be targeted for manipulation of crop plants. Mutants deficient in qE have been found, and one of these is defective in violaxanthin de‐epoxidase (Nyogi et al., 1997). A similar, but less drastic end‐point might be reached by expression of an antisense violaxanthin de‐epoxidase gene. So far, there are no reports of gene manipulations leading to an increase in qE capacity. Recently it has been shown that a mutant that does not form qE, but which has normal xanthophyll cycle activity, is missing the LHCII‐related proteins encoded by the PsbS gene (Li et al., 2000).
Other strategies for gene manipulation include the use of antisense expression to reduce the content per PSII reaction centre of the minor LHCII complexes CP29 and CP26 (Horton et al., 1999). Although expression of antisense Lhcb1 did not have any effect on the amount of the main LHCIIb complex (Flachmann and Kühlbrandt, 1995), a >90% reduction in the content of Lhca4 protein (a light‐harvesting apoprotein of PSI) by expression of an antisense Lhca4 gene has been reported (Zhang et al., 1997). It is anticipated that an antisense approach will be successful in reducing the contents of CP29 and CP26 (S Jansson, personal communication). These complexes appear to play an important role in qE (Walters et al., 1994; Jahns and Schweig, 1995) and their manipulation is expected to alter the characteristics of regulation of light harvesting.
One problem with this strategy is that elimination of these proteins might well disrupt the PSII macrostructure, leading to a constitutive reduction in quantum yield. A key question will be whether other Lhcb proteins will be inserted into the macrocomplex, allowing basic functions to occur, but with altered regulatory characteristics. In the longer term, a more desirable strategy is to obtain plants with genetic knockouts of Lhcb genes, which can then be complemented by expression of introduced Lhcb genes with alterations at key regulatory sites. Biochemical studies are already describing the sites involved in proton (Walters et al., 1996; Pesaresi et al., 1997) and xanthophyll binding to Lhcb proteins (Sandonà et al., 1998), and with this knowledge ‘designer’ Lhcb proteins may be expressed in plants. Whether it will be possible to enhance qE capacity by merely overexpressing any of the Lhc genes involved in qE remains to be established.
Strategies for genetic investigations of light harvesting
Description of the different approaches that allow the genetic manipulation of light harvesting—these involve either alteration of the pigment composition, leading indirectly to alteration in Lhc protein content, direct effects on the protein content or selection of functional alteration.
| Component | Methodology | Reference |
| Chlorophyll | Random mutagenesis (pale green phenotype) | (Thornber and Highkin, 1974) |
| (Simpson et al., 1985) | ||
| Antisense expression | (Härtel and Grim, 1998); | |
| (Härtel et al., 1998) | ||
| Carotenoid | Random mutagenesis (Hplc screening) | (Pogson et al., 1996, 1998) |
| Expression of novel genes | (Misawa et al., 1994) | |
| (Davison et al., 1999) | ||
| Antisense expression | (Davison et al., 1999) | |
| Lhc proteins | Random mutagenesis (pale green phenotypes) | (Misawa et al., 1994) |
| (Davison et al., 1999) | ||
| Antisense expression | (Flachmann and Kühlbrandt, 1995) | |
| (Zhang et al., 1997) | ||
| (Horton et al., 1999) | ||
| Identification of Lhc gene disruptions in mutant populations | None reported | |
| NPQ (qE) | Random mutagenesis (Chl fluorescence screening) | (Nyogi et al., 1997) |
| (Nyogi, 1999) |
| Component | Methodology | Reference |
| Chlorophyll | Random mutagenesis (pale green phenotype) | (Thornber and Highkin, 1974) |
| (Simpson et al., 1985) | ||
| Antisense expression | (Härtel and Grim, 1998); | |
| (Härtel et al., 1998) | ||
| Carotenoid | Random mutagenesis (Hplc screening) | (Pogson et al., 1996, 1998) |
| Expression of novel genes | (Misawa et al., 1994) | |
| (Davison et al., 1999) | ||
| Antisense expression | (Davison et al., 1999) | |
| Lhc proteins | Random mutagenesis (pale green phenotypes) | (Misawa et al., 1994) |
| (Davison et al., 1999) | ||
| Antisense expression | (Flachmann and Kühlbrandt, 1995) | |
| (Zhang et al., 1997) | ||
| (Horton et al., 1999) | ||
| Identification of Lhc gene disruptions in mutant populations | None reported | |
| NPQ (qE) | Random mutagenesis (Chl fluorescence screening) | (Nyogi et al., 1997) |
| (Nyogi, 1999) |
Strategies for genetic investigations of light harvesting
Description of the different approaches that allow the genetic manipulation of light harvesting—these involve either alteration of the pigment composition, leading indirectly to alteration in Lhc protein content, direct effects on the protein content or selection of functional alteration.
| Component | Methodology | Reference |
| Chlorophyll | Random mutagenesis (pale green phenotype) | (Thornber and Highkin, 1974) |
| (Simpson et al., 1985) | ||
| Antisense expression | (Härtel and Grim, 1998); | |
| (Härtel et al., 1998) | ||
| Carotenoid | Random mutagenesis (Hplc screening) | (Pogson et al., 1996, 1998) |
| Expression of novel genes | (Misawa et al., 1994) | |
| (Davison et al., 1999) | ||
| Antisense expression | (Davison et al., 1999) | |
| Lhc proteins | Random mutagenesis (pale green phenotypes) | (Misawa et al., 1994) |
| (Davison et al., 1999) | ||
| Antisense expression | (Flachmann and Kühlbrandt, 1995) | |
| (Zhang et al., 1997) | ||
| (Horton et al., 1999) | ||
| Identification of Lhc gene disruptions in mutant populations | None reported | |
| NPQ (qE) | Random mutagenesis (Chl fluorescence screening) | (Nyogi et al., 1997) |
| (Nyogi, 1999) |
| Component | Methodology | Reference |
| Chlorophyll | Random mutagenesis (pale green phenotype) | (Thornber and Highkin, 1974) |
| (Simpson et al., 1985) | ||
| Antisense expression | (Härtel and Grim, 1998); | |
| (Härtel et al., 1998) | ||
| Carotenoid | Random mutagenesis (Hplc screening) | (Pogson et al., 1996, 1998) |
| Expression of novel genes | (Misawa et al., 1994) | |
| (Davison et al., 1999) | ||
| Antisense expression | (Davison et al., 1999) | |
| Lhc proteins | Random mutagenesis (pale green phenotypes) | (Misawa et al., 1994) |
| (Davison et al., 1999) | ||
| Antisense expression | (Flachmann and Kühlbrandt, 1995) | |
| (Zhang et al., 1997) | ||
| (Horton et al., 1999) | ||
| Identification of Lhc gene disruptions in mutant populations | None reported | |
| NPQ (qE) | Random mutagenesis (Chl fluorescence screening) | (Nyogi et al., 1997) |
| (Nyogi, 1999) |
Conclusions
That increasing crop yield requires an increase in photosynthesis is now recognized, and genetic manipulation of photosynthetic processes has become a key target for crop improvement (Mann, 1999a, b). However, whilst current knowledge of the component reactions are for the most part well understood, their integration into the whole plant process is not. Without extreme good fortune, manipulation of photosynthesis to increase yield is at present an unlikely prospect for the near future. Solutions to the problem of increasing crop yield will come only with increased understanding of the underlying processes at the molecular level, and the integration of this knowledge to understand how a plant works. A central conclusion that has emerged from the recent studies outlined in this article is the need to understand the complex interactions between canopy architecture and the biochemistry of the photosynthetic apparatus.
Fax: +44 114 222 2787. E‐mail:yp.horton@sheffield.ac.uk
I am grateful to numerous colleagues and collaborators for their contribution to the work described in this article: Erik Murchie and Stella Hubbart for obtaining the data on rice and providing insights into the life of the rice crop; John Bennett, Shaobing Peng and John Sheehy at IRRI for guidance and many stimulating discussions; Vanessa Rushton for providing the data on tomato leaf senescence; Alexander Ruban for several years of collaborative investigation of qE; Stefan Jansson for collaboration in the genetic manipulation of light harvesting; and Robin Walters for many discussions about photosynthetic acclimation.
References
Black CC, Tu Z‐P, Counce PA, Yao P‐F, Angelov MN.
Cassman KG.
Davison PA, Buckley R, Hunter CN, Horton P.
Flachmann R, Kuhlbrandt W.
Gan S, Amasino RM.
Genty B, Briantais J‐M, Baker NR.
Hartel H, Reinhardt I, Grimm B.
Hartel H, Grimm B.
Horton P.
Horton P.
Horton P.
Horton P.
Horton P, Benson S, Ruban AV, Jansson S, Ganetag U, Andersson J, Gustafsson P.
Horton P, Ruban AV, Rees D, Pascal AA, Noctor G, Young AJ.
Horton P, Ruban AV, Walters RG.
Jahns P, Schweig S.
Jansson S.
Jansson S.
John I, Drake R, Farrell A, Cooper W, Lee P, Horton P, Grierson D.
Johnson GN, Young AJ, Scholes JD, Horton P.
Khush GS, Peng S.
Leegood RC, Edwards G.
Li X‐P, Bjorkman O, Shils C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK.
Misawa N, Masamoto K, Hori T, Ohtani T, Boger P, Sandmann G.
Murchie EH, Chen Y‐z, Hubbart S, Peng S, Horton P.
Murchie EH, Horton P.
Nyogi K.
Nyogi KK, Björkman O, Grossman AR.
Pearcy RW, Krall JP, Sassenrath‐Cole GF.
Peng S, Cassman KG, Kropff MJ.
Peng S, Khush GS, Cassman KG.
Peng S, Lza RC, Khush GS, Sanico AL, Visperas RM, Garcia FV.
Pesaresi P, Sandonà D, Giuffra E, Bassi R.
Peter GF, Thornber P.
Pogson B, McDonald KA, Truong M, Britton G, DellaPenna D.
Pogson BJ, Nyogi K, Björkman O, DellaPenna D.
Ruban AV, Young AJ, Horton, P.
Sandonà D, Croce R, Pagano A, Crimi M, Bassi R.
Simpson DJ, Machold O, Hoyer‐Hansen G, von Wettstein D.
Thornber JP, Highkin HR.
Walters RG, Horton P.
Walters RG, Rogers JJM, Shephard F, Horton P.
Walters RG, Ruban AV, Horton P.
Walters RG, Ruban AV, Horton P.
Winder TL, Sun J, Okita TW, Edwards GE.
Ying J, Peng S, He Q, Yang H, Yang C, Visperas RM, Cassman KG.
Ying J, Peng S, Yang G, Zhou N, Visperas RM, Cassman KG.




Comments