-
PDF
- Split View
-
Views
-
Cite
Cite
Enrrico Bloise, Sky K. Feuer, Paolo F. Rinaudo, Comparative intrauterine development and placental function of ART concepti: implications for human reproductive medicine and animal breeding, Human Reproduction Update, Volume 20, Issue 6, November/December 2014, Pages 822–839, https://doi.org/10.1093/humupd/dmu032
Close - Share Icon Share
The number of children conceived using assisted reproductive technologies (ART) has reached >5 million worldwide and continues to increase. Although the great majority of ART children are healthy, many reports suggest a forthcoming risk of metabolic complications, which is further supported by the Developmental Origins of Health and Disease hypothesis of suboptimal embryo/fetal conditions predisposing adult cardiometabolic pathologies. Accumulating evidence suggests that fetal and placental growth kinetics are important features predicting post-natal health, but the relationship between ART and intrauterine growth has not been systematically reviewed.
Relevant studies describing fetoplacental intrauterine phenotypes of concepti generated by in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI) and somatic cell nuclear transfer (SCNT) in the mouse, bovine and human were comprehensively researched using PubMed and Google Scholar. Intrauterine growth plots were created from tabular formatted data available in selected reports.
ART pregnancies display minor but noticeable alterations in fetal and placental growth curves across mammalian species. In all species, there is evidence of fetal growth restriction in the earlier stages of pregnancy, followed by significant increases in placental size and accelerated fetal growth toward the end of gestation. However, there is a species-specific effect of ART on birthweights, that additionally vary in a culture condition-, strain-, and/or stage at transfer-specific manner. We discuss the potential mechanisms that underlie these changes, and how they are affected by specific components of ART procedures.
ART may promote measurable alterations to intrauterine growth trajectory and placental function. Key findings include evidence that birthweight is not a reliable marker of fetal stress, and that increases in embryo manipulation result in more deviant fetal growth curves. Because growth kinetics in early life are particularly relevant to adult metabolic physiology, we advise more rigorous assessment of fetal growth and placental function in human ART pregnancies, as well as continued follow-up of ART offspring throughout post-natal life. Finally, strategies to minimize embryo manipulations should be adopted whenever possible.
Introduction
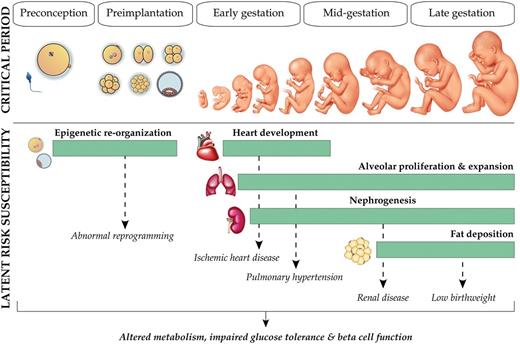
Critical period of developmental sensitivity. Schematic illustrating the windows of human pregnancy most susceptible for influencing the development of components of metabolic syndrome later in life. Metabolic stress, including defects in glucose handling, is a common outcome of stress in utero and is less dependent on the specific timing of the stress occurrence. In addition to increased vulnerability during organogenesis, the preimplantation period is a time of extensive epigenetic remodeling and therefore may be particularly susceptible to stress.
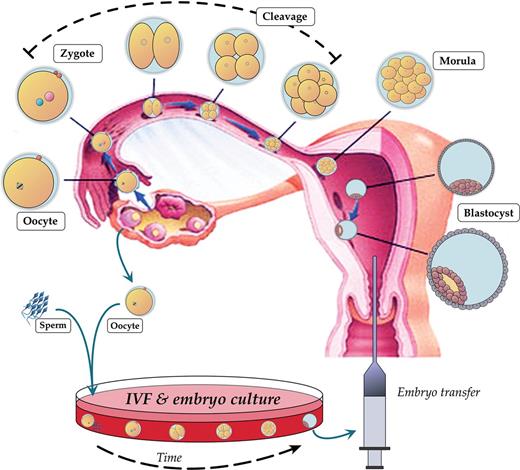
Preimplantation development and IVF. Overview of the stages of preimplantation embryo development from ovulation and fertilization of the oocyte in the oviduct, through cleavage divisions and embryo polarization, to blastocyst formation and implantation into the uterus. In vitro, gametes are isolated for co-incubation, and fertilized zygotes are cultured 3–5 days prior to transfer back into the uterus.
In the following paragraphs, evidence from murine, bovine and finally human data is used to comprehensively describe the intrauterine environment and related biological alterations of ART concepti. Because it is problematic to tease out whether ART-associated adverse outcomes are secondary to the ART procedures or to parental factors, animal models have been especially useful in elucidating intrauterine growth and placentation changes associated with ART. Mouse and bovine models of IVF exhibit intrauterine growth restriction (IUGR) in early pregnancy, followed by accelerated fetal growth rates from mid- to late gestation, correlated with increased placental growth. Changes in fetal and placental growth phenotype are additionally influenced by ART procedure and preimplantation embryo culture conditions, suggesting that more severe embryo manipulations can induce greater abberancies in conceptus phenotype. Bovine studies additionally show a very clear and unique phenotype associated with ART, the so-called ‘Large Offspring Syndrome’ (LOS) (Sinclair et al., 2000; Farin et al., 2006); a condition also observed in the sheep (Young et al., 1998). It is important to emphasize the existence of morphological, temporal and metabolic differences in early embryo development among mammalian species (Prather and First, 1988; Richardson et al., 1997). Therefore, caution is advised when extrapolating the significance of these studies to human ART, as animal models may provide imprecise views of the demands of human development.
Methods
To systematically review the intrauterine phenotype of embryos and fetuses generated by IVF, intracytoplasmic sperm injection (ICSI) and somatic cell nuclear transfer (SCNT), PubMed and Google Scholar were used to identify relevant studies in the mouse, bovine and human. The search strategy comprised a combination of the following terms: IVF, ICSI, SCNT, mouse, bovine/ruminant, human, endometrium, maternal recognition of pregnancy, embryo, fetal growth, placenta, ultrasound, oxygen tension, transcriptome, proteomics, abortion and epigenetics. The intrauterine growth trajectory plots were created from data available in the format of tables in selected reports (cited when pertinent), and statistical significance is displayed as described in the original reports. Only literature written in English was included, without restriction on year of publication. We discuss the current knowledge of ART-induced effects on fetal intrauterine growth trajectory and placental growth, function and physiology, including potential molecular mechanisms underlying the altered phenotypes observed following assisted conception.
ART and Recognition of Pregnancy and Miscarriage
Much attention has been devoted toward minimizing low pregnancy rates, fetal demise and other adverse perinatal outcomes in ART pregnancies. However, it is difficult to compare pregnancy rates and the incidence of miscarriage in IVF versus spontaneously conceived pregnancies, making animal models particularly valuable for these analyses (Table I). In the mouse, IVF is linked with increased abortion rates by embryonic day E12.5 (Delle Piane et al., 2010), a gestational age marked by both the establishment of the chorioallantoic placenta and the onset of fetal dependence upon placental uptake of nutrients from maternal circulation (Malassiné et al., 2003). This study described significantly increased abortion rates for IVF embryos grown under suboptimal conditions (26.89%, Whitten's culture medium and 20% oxygen tension), compared with in vivo embryos (7.09%) or in vivo-generated blastocysts transferred to surrogate dams (flushed blastocyst control group, 7.28% abortion rates). IVF embryos produced in optimized conditions (using K simplex optimized medium (KSOM) with amino acids and 5% oxygen) also had higher abortion rates (17.00%), although this was not significantly different from in vivo or flushed blastocyst controls (Delle Piane et al., 2010). Interestingly, implantation rates were not different among the four groups. Hemkemeyer et al. similarly did not find significant implantation changes between ART mice cultured in different conditions (Hemkemeyer et al., 2014). This indicates that preimplantation culture conditions can affect mouse embryo survival after the implantation period, which may be related to placentation processes or failed maternal recognition of pregnancy.
Fetal loss/miscarriage rates in assisted reproductive technology (ART) concepti.
| Species . | Method of conception . | Medium . | Gestational age . | Miscarriage, abortion rates % (∼) . | References . |
|---|---|---|---|---|---|
| Mouse | IVF | KAA | E12.5 | 17 | Delle Piane et al. (2010) |
| IVF | Whitten's | E12.5 | 27 | ||
| In vivo-flushed blastocyst | – | E12.5 | 7 | ||
| In vivo | – | E12.5 | 7 | ||
| Bovine | IVF | Medium-199 | Term | 55 | Kruip and Daas (1997) |
| SCNT | – | Term | 75 | Pace et al. (2002) and Watanabe and Nagai (2011) | |
| In vivo | – | Term | 5 | King et al. (1985) and Farin et al. (2006) | |
| Human | IVF/ICSI | – | – | 15/22 | Gunby et al. (2010), Gunby et al. (2011) and Tummers et al. (2003) |
| Freshly transferred ICSI | – | – | 19 | Aytoz et al. (1999) | |
| Frozen-thawed transferred ICSI | – | – | 26 | Aytoz et al. (1999) | |
| Spontaneous conception | – | – | 12-24 | Jurkovic et al. (2013) |
| Species . | Method of conception . | Medium . | Gestational age . | Miscarriage, abortion rates % (∼) . | References . |
|---|---|---|---|---|---|
| Mouse | IVF | KAA | E12.5 | 17 | Delle Piane et al. (2010) |
| IVF | Whitten's | E12.5 | 27 | ||
| In vivo-flushed blastocyst | – | E12.5 | 7 | ||
| In vivo | – | E12.5 | 7 | ||
| Bovine | IVF | Medium-199 | Term | 55 | Kruip and Daas (1997) |
| SCNT | – | Term | 75 | Pace et al. (2002) and Watanabe and Nagai (2011) | |
| In vivo | – | Term | 5 | King et al. (1985) and Farin et al. (2006) | |
| Human | IVF/ICSI | – | – | 15/22 | Gunby et al. (2010), Gunby et al. (2011) and Tummers et al. (2003) |
| Freshly transferred ICSI | – | – | 19 | Aytoz et al. (1999) | |
| Frozen-thawed transferred ICSI | – | – | 26 | Aytoz et al. (1999) | |
| Spontaneous conception | – | – | 12-24 | Jurkovic et al. (2013) |
E, embryonic day; SCNT, somatic cell nuclear transfer.
Fetal loss/miscarriage rates in assisted reproductive technology (ART) concepti.
| Species . | Method of conception . | Medium . | Gestational age . | Miscarriage, abortion rates % (∼) . | References . |
|---|---|---|---|---|---|
| Mouse | IVF | KAA | E12.5 | 17 | Delle Piane et al. (2010) |
| IVF | Whitten's | E12.5 | 27 | ||
| In vivo-flushed blastocyst | – | E12.5 | 7 | ||
| In vivo | – | E12.5 | 7 | ||
| Bovine | IVF | Medium-199 | Term | 55 | Kruip and Daas (1997) |
| SCNT | – | Term | 75 | Pace et al. (2002) and Watanabe and Nagai (2011) | |
| In vivo | – | Term | 5 | King et al. (1985) and Farin et al. (2006) | |
| Human | IVF/ICSI | – | – | 15/22 | Gunby et al. (2010), Gunby et al. (2011) and Tummers et al. (2003) |
| Freshly transferred ICSI | – | – | 19 | Aytoz et al. (1999) | |
| Frozen-thawed transferred ICSI | – | – | 26 | Aytoz et al. (1999) | |
| Spontaneous conception | – | – | 12-24 | Jurkovic et al. (2013) |
| Species . | Method of conception . | Medium . | Gestational age . | Miscarriage, abortion rates % (∼) . | References . |
|---|---|---|---|---|---|
| Mouse | IVF | KAA | E12.5 | 17 | Delle Piane et al. (2010) |
| IVF | Whitten's | E12.5 | 27 | ||
| In vivo-flushed blastocyst | – | E12.5 | 7 | ||
| In vivo | – | E12.5 | 7 | ||
| Bovine | IVF | Medium-199 | Term | 55 | Kruip and Daas (1997) |
| SCNT | – | Term | 75 | Pace et al. (2002) and Watanabe and Nagai (2011) | |
| In vivo | – | Term | 5 | King et al. (1985) and Farin et al. (2006) | |
| Human | IVF/ICSI | – | – | 15/22 | Gunby et al. (2010), Gunby et al. (2011) and Tummers et al. (2003) |
| Freshly transferred ICSI | – | – | 19 | Aytoz et al. (1999) | |
| Frozen-thawed transferred ICSI | – | – | 26 | Aytoz et al. (1999) | |
| Spontaneous conception | – | – | 12-24 | Jurkovic et al. (2013) |
E, embryonic day; SCNT, somatic cell nuclear transfer.
Bovine models also demonstrate significant loss of in vitro-generated pregnancies, predominantly during the earliest stages of gestation. In fact, the greatest risk of spontaneous abortion occurs during embryogenesis (extending from conception to the completion of organogenesis, Day 1 to Day 42) rather than the fetal period (completion of organogenesis to parturition, Day 42 to Day 280) or neonatal period (parturition through Day 28 of extra-uterine life) (Farin et al., 2001, 2006). A 55% loss rate has been described for in vitro-produced (IVP) embryos at term (Kruip and Daas, 1997) compared with the in vivo incidence of <5% (King et al., 1985; Farin et al., 2006); a separate study reported a 45% loss rate at Day 14 (McMillan et al., 1997), consistent with the bovine maternal recognition of pregnancy window (Mamo et al., 2011). SCNT-derived pregnancies are also susceptible to embryo/fetal demise, with reported cumulative embryonic loss reaching 25% around gestational Day 30, increasing to 50% by Day 39, and totaling ∼75% by the end of gestation (Pace et al., 2002). Similar cumulative percentages of SCNT pregnancy losses have been independently corroborated (Watanabe and Nagai, 2011). Importantly, increased embryonic and fetal loss ratios vary according to culture media, and ART protocol used (described below) (Farin et al., 2006).
Overall, whether spontaneous abortion is higher in human ART pregnancies is not well established (Tummers et al., 2003), and there is unfortunately a lack of comprehensive studies evaluating the relationship between ART and abortion rates which control for maternal age, BMI, and ethnicity, among other factors. In the general population, spontaneous abortions occur in an estimated 12–24% of reproductive-aged women conceiving naturally (Jurkovic et al., 2013). The Canadian ART Register reported pregnancy losses of 14.9–15.4% in ART conceptions (IVF and ICSI) in the year 2006–2007 (Gunby et al., 2010, 2011). A retrospective study of 1597 clinical pregnancies conceived by IVF and ICSI showed a 21.7% global incidence of spontaneous abortion in singleton ART pregnancies (Tummers et al., 2003), and another group reported a slightly increased risk of spontaneous abortion for ART pregnancies over natural conception (relative risk of 1.20, 95% Confidence Interval 1.03–1.46) (Wang et al., 2004). Aytoz and colleagues evaluated the obstetric outcome for ART pregnancies derived from fresh or cryopreserved embryos generated by IVF or ICSI. Abortion rates were comparable between fresh and frozen IVF embryos (13.1%), whereas freshly transferred ICSI embryos showed increased abortion rates (18.6%) and frozen-thawed ICSI embryos the highest (26.0%, P < 0.05) (Aytoz et al., 1999). Although certain ART conditions are linked with significant increases in miscarriage, overall these numbers are similar to the estimated rates for the naturally conceiving population and an effect of ART on abortion rate is not definitive (Jurkovic et al., 2013).
Chromosomal abnormalities in developing embryos are the most well-described cause for spontaneous abortion (Brown, 2008), and there is little evidence linking ART procedures with chromosomal irregularities in developing concepti. Analysis comparing cytogenetic profiles of abortuses from 133 ART patients (IVF and ICSI) to 144 samples from naturally conceiving subfertile couples revealed similar levels of karyotype defects between ART (63.2%) and spontaneously conceived miscarriages (71.5%). Furthermore, the incidence of aneuploidy did not differ between IVF (54.5%) and ICSI (61.5%) (Bettio et al., 2008). Conversely, other groups have reported both significant increases or no changes in the number of cytogenetic anomalies for ICSI versus IVF miscarriages, although these studies are caveated by small sample size (Bettio et al., 2008).
In summary, the incidence of miscarriage does not appear noticeably dissimilar between naturally conceived and ART pregnancies; however more studies are warranted to confirm this.
Effects of ART on Intrauterine Growth Trajectory
Fetal needs across pregnancy are met by the placenta, a temporary endocrine gland that transfers oxygen and nutrients from the mother to the fetus. Placental interface structure and function is adjusted by the fetus throughout pregnancy, in response to changes in maternal ecology and resource availability (Rutherford et al, 2009). These adaptations are required to provide the best developmental scenario possible to properly coordinate fetal growth and development. Indeed, placental defects are a frequent cause of abnormal fetal development and pregnancy failure (Hill et al., 2000; Norwitz 2006). Therefore, placental developmental competence is integrally linked to developmental stress of the fetus.
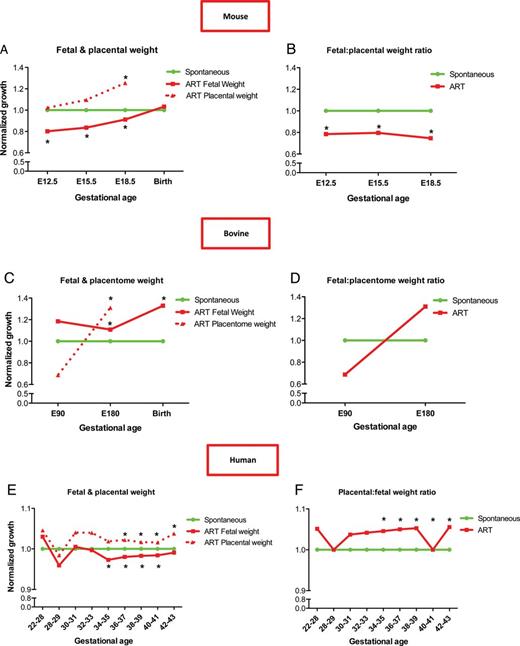
Intrauterine growth restriction, placentomegaly and accelerated fetal growth in mammalian ART pregnancy. Mean fetal and placental ART weights relative to spontaneously conceived concepti, with corresponding placental:fetal weight ratios in mouse (A and B), bovine (C and D) and human (E and F) pregnancies. Adapted from mouse: Delle Piane et al. (2010) and Bloise et al. (2012); bovine: Bertolini et al. (2004); human: Haavaldsen et al. (2012). *P < 0.05. E, embryonic day. Gestational age is days for mouse (A,B) and bovine (C,D) and weeks for humans (E,F).
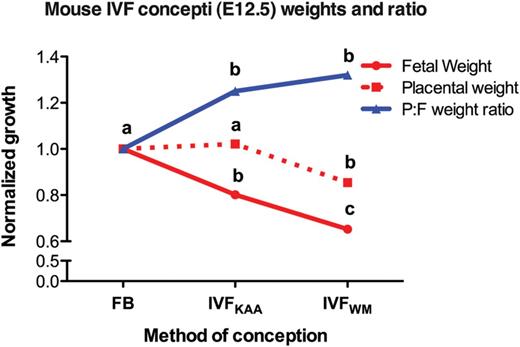
Increased IVF stress exacerbates fetoplacental phenotype. Mouse E12.5 mean fetal weight (red line), placental weight (red dotted line), and corresponding placental:fetal (P:F) weight ratio (blue line) of concepti conceived by IVF and cultured either under optimized conditions (KSOM medium with amino acids under 5% O2, IVFKAA) or suboptimal culture conditions (Whitten's medium under 20% O2, IVFWM), normalized to in vivo-derived blastocysts transferred to surrogate damns (flushed blastocyst control, FB). Different superscripts indicate statistical significance between spontaneously conceived pregnancies and IVF concepti produced in different media as shown in the original reports (P < 0.05). Adapted from Delle Piane et al. (2010).
The unique fetal growth velocities observed in ART models may be related to adaptations in placental phenotype. In the IVF concepti described above, placentae became progressively larger and near term were 25.4% heavier than controls (Delle Piane et al., 2010; Bloise et al., 2012). These embryos were further characterized by a significantly decreased F:P weight ratio at E12.5, E15.5 and E18.5 (Fig. 3). This indicates that placental phenotype is highly responsive to conditions in vitro. Placentomegaly has additionally been observed at E18.5 for both IVF and ICSI inbred B6D2F1 embryos cultured in CZB medium and transferred at the 2-cell stage (Collier et al., 2009). Therefore, placental enlargement is a common outcome in late pregnancy following ART in mice—regardless of the form of ART applied—and a reasonable mechanism by which IVF embryos can accelerate intrauterine growth to reach normal birthweights. Interestingly, no major differences in fetal or placental growth occurred with transfer of blastocysts derived from zygotes flushed out of the uterus after natural mating (Hemkemeyer et al., 2014). The fact that zygote culture is not associated with abnormal intrauterine growth, whereas IVF is, would indicate that the actual fertilization process and/or initial 24 h period in vitro is the sensitive window responsible for altering development, although this hypothesis requires further evaluation.
Fetal growth restriction has also been associated with ART in ruminant models, with IVF-conceived embryos, fetuses, placentae, and offspring differing greatly in morphology and developmental competence compared with those derived in vivo (Drost, 2007). Length of ovine IVF concepti was statistically reduced on E22 (Grazul-Bilska et al., 2013) and E24 (Ptak et al., 2013) (of a ∼147 day gestation). Similarly, in the bovine, fetal ultrasonographic morphometry detected reduced size for in vitro-produced concepti between E37 and E58, but these calves exhibited higher birthweights than in vivo controls at the end of the 285-day gestation period (Bertolini et al., 2002). Notably, IVF F:P ratios differed according to the culture medium used at E70 (Miles et al., 2005), consistent with the mid-pregnancy findings in mouse models (Delle Piane et al., 2010). This reflects a biphasic growth velocity in which the first trimester is marked by limited fetal growth, and subsequently accelerates to yield higher birthweight calves (e.g. the bovine LOS phenotype) (Fig. 3).
In humans, low birthweight is a well-described complication of ART pregnancy (Walker et al., 2000; Rinaudo and Lamb, 2008; Haavaldsen et al., 2012), although few studies have investigated placental size. An extensive analysis in the Norwegian population compared placental and newborn weights in 536 567 singleton pregnancies, including 4557 conceived by IVF, 3192 through ICSI, and 88 pregnancies by the combined use of IVF and ICSI or other unspecified ART procedures (Haavaldsen et al., 2012). As previously reported, ART singletons had significantly lower birthweights than spontaneously conceived (SC) controls (3451.0 g compared with 3560.6 g, respectively), but were additionally characterized by significantly increased placentae (678.9 g versus 673.0 g) and F:P ratio (0.20 for ART versus 0.19 in SC). Changes in birthweight, placental size and F:P weight ratio remained significant regardless of the form of ART, as well as through subsequent adjustment for length of gestation, sex, parity, maternal age and pregnancy complications. It is however worth noting that the statistical significance of these findings may be related to the appreciably large size of the control group, as the impact of ART on birthweight and placental size totaled just 3 and 0.9%, respectively. No differences were found in F:P ratio between IVF and ICSI concepti. An important feature of this study is that the data for placental and fetal weights and ratios were averaged from individual gestational ages categorized as 22–27 weeks, 28–29, 30–31, 32–33, 34–35, 36–37, 38–39, 40–41, and 42–43 weeks of age at birth. Analysis by each week of pregnancy demonstrated that ART birthweights are lower for babies born at 34–41 weeks of gestation. However, birthweights in ART-conceived pregnancies were no longer different in fetuses born after 42 weeks, which could possibly reflect intrauterine catch-up growth in post-term pregnancies (Fig. 3).
In summary, there is a species-specific effect of ART on intrauterine growth rates. Birthweight in mouse IVF concepti is variably smaller or larger than controls in a culture condition-, strain- and/or stage at transfer-specific manner, whereas embryo manipulation in bovine models is associated with higher birthweights (Bertolini et al., 2002, 2004; Farin et al., 2006). In all of these species, fetal catch-up growth is apparent by mid-pregnancy and continues until birth, which may be related to adaptations in placental phenotype, including placentomegaly. In humans, IVF gestations are marked by lower birthweights and increased placental size, and may too show evidence of accelerated intrauterine growth in later stages of gestation (Walker et al., 2000; Rinaudo and Lamb, 2008; Haavaldsen et al., 2012). Low birthweight is an established marker of intrauterine stress and has been linked to coronary heart disease, hypertension and hyperlipidemia, even if within the spectrum of ‘normal’ birthweight (de Boo and Harding, 2006; Meas, 2010). Moreover, a relationship between low birthweight and altered glucose and insulin metabolism in adulthood has been described (de Boo and Harding, 2006; Rinaudo and Wang, 2012). Both impaired fetal growth and accelerated perinatal growth velocity are appreciable signs of fetal stress, and the DOHaD hypothesis emphasizes these features as susceptibility markers of adult disease (Meas, 2010). In fact, many animal studies have demonstrated a strong association between prenatal growth deficit, compensatory growth and increased susceptibility to cardiometabolic defects in adulthood (Armitage et al., 2004). It is difficult to uncouple whether the risk for metabolic syndrome in adult life originates in embryo/fetal adaptations to suboptimal environments during critical periods of development, or instead in the ensuing accelerated growth, or both. Jimenez-Chillaron and colleagues reported that prevention of post-natal catch-up growth in low birthweight mice reversed adult phenotypes of glucose intolerance and diabetes (Jimenez-Chillaron et al., 2006), suggesting that growth kinetics in early life are particularly relevant to adult metabolic physiology. The reasons for altered fetal and placental growth in ART pregnancies are unclear and could be secondary to the underlying infertility affecting people using ART procedures (Romundstad et al., 2008). However, animal data, in which subfertility is not a variable, point to a specific effect of the technology used (for a review see Feuer and Rinaudo, 2012). The data additionally demonstrate that birthweight—a supposed marker of fetal growth trajectory—may not be a reliable indicator of fetal stress (Feuer et al., 2013).
Morphology and Growth of ART Placentae
The evidence that preimplantation embryo manipulation in vitro alters normal F:P weight ratio is indicative of fetal exposure to intrauterine stress. To ensure sufficient support of fetal growth and prevention of fetal demise, adaptive placentomegaly is a reasonable mechanism underlying the intrauterine catch-up growth observed in mouse ART concepti. Histological analyses of mouse IVF placentae at E12.5 (Delle Piane et al., 2010) and E18.5 (Raunig et al., 2011a; Bloise et al., 2012) did not reveal any differences in gross morphology. There were no changes in labyrinth or spongiotrophoblast cell number, in spite of the marked placentomegaly present by E18.5 (Raunig et al., 2011a; Bloise et al., 2012). At both time points, the two layers in IVF placentae displayed significantly more Ki67-positive cells, marking an increase in cellular proliferation. This suggests that placental overgrowth observed in term ART pregnancies is secondary to increased proliferation detectable at mid- to late gestation.
It is also possible that hyperproliferation of labyrinth and spongiotrophoblast cells (Delle Piane et al., 2010; Bloise et al., 2012) is a compensatory response to inflammation and oxidative stress. Mouse ICSI and IVF placentae are marked by increased interleukin (IL)-6 levels, indicative of placental inflammation; complementarily, ICSI placentae exhibit impaired activities of the antioxidants superoxide dismutase, thioredoxin reductase, xanthine oxidase, catalase, glutathione-S-transferase, glutathione peroxidase and glutathione reductase (Raunig et al., 2011b). Abnormal antioxidant defense networks were also observed in fetal livers. Because local inflammation and oxidative stress can trigger cell death (apoptosis), changes in oxidative stress and mechanisms of reactive oxygen species disposal may contribute to the histological phenotypes observed in ART concepti. It is therefore tempting to speculate that increased proliferation is a compensatory response to oxidative stress-induced apoptosis in ART placentae, in order to prevent fetal demise. In fact, the same report showed increased placental apoptosis after IVF and ICSI (Raunig et al., 2011b), although a separate study did not observe any changes in apoptosis levels following IVF and embryo culture (Bloise et al., 2012). These divergent results may be related to the sensitivity of the techniques or conditions of embryo culture and transfer (discussed below).
Bovine ART is also characterized by placental overgrowth. In vitro-derived concepti were supported by placentomes with increased diameter and decreased thickness compared with controls (Bertolini et al., 2002). This phenotype was present in early pregnancy (E72 to E93) and maintained to term, at which point the longer, thinner placentomes additionally displayed larger surface areas due to the presence of giant cotyledon structures, which were correlated with the LOS. In another report, no alterations in placental morphometry or stereological parameters were described for third trimester IVF concepti (E229) relative to artificially inseminated (AI) animals (Constant et al., 2006). Conversely, SCNT placentomes displayed increased volume density of fetal connective tissue and decreased volume density of the maternal epithelium, demonstrating that the growth of the maternal and fetal components of the placentomes were not similar (Constant et al., 2006).
Sufficient invasion into the uterine epithelium and appropriate vascularity are essential components of placental function, as the maternal:fetal interface is the basis for all communication and fetal support. It follows that without proper vascularization, placental growth and activity may be impaired, leading to a compromised fetal environment and the inability to meet the evolving demands of a growing fetus. Microarray analysis of E50 IVF- and SCNT-derived bovine placentae compared with artificial insemination-derived control placentae identified 58 genes commonly misexpressed in the two experimental groups. These genes were enriched for involvement in organogenesis, extracellular structure and matrix organization, as well as the development, regulation, and maintenance of blood vessels and vasculature (Salilew-Wondim et al., 2013). Another particularly relevant study identified changes in placental expression of vascular endothelial growth factor (VEGF)-A and basic fibroblast growth factor—two major angiogenic factors controlling placental vascularization—in SCNT bovine placentae at term (Campos et al., 2010). Other reported alterations in blood vessel development included decreases in both blood vessel density and levels of Vegf mRNA in cotyledonary tissue from IVF bovine concepti (Miles et al., 2005), an effect occurring in a culture medium-specific manner. Desensitized VEGF signaling could underline the impaired blood vessel development, since it has been demonstrated that placentomes from cloned animals are less responsive to VEGF treatments (Sousa et al., 2012). Further, impaired cotyledonary vasculogenesis and angiogenesis during earlier stages of pregnancy could have detrimental effects on subsequent placental development and maturation (Meegdes et al., 1988). For example, elevated expression of Hif-1α (hypoxia-inducible factor) in SCNT-derived maternal and chorioallantoic tissues reflects generalized hypoxic conditions, suggesting an insufficient maternal blood supply (Hoffert-Goeres et al., 2007).
Increased placental size and thickness have also been described in human ART pregnancies, independent of ART procedure type, infertility factor, pregnancy complications, perinatal outcome, or fetal weight (Daniel et al., 1999). Other histopathological discrepancies include a significantly higher incidence of villous edema and micro calcifications (Lalosević et al., 2003), increased thickness and a higher prevalence of hematomas (Joy et al., 2012), which lead to greater risk of perinatal complications (Elchalal et al., 2000). There is a greater frequency of abnormal cord insertion (Daniel et al., 1999), which has previously been related to ART-associated IUGR (odds ratio: 3.69) (Cai et al., 2006). A comparative study of placental blood barrier, fetal capillaries, villous stroma, cytotrophoblast and syncytiotrophoblast substructure by transmission-electron microscopy in eight ART and fifteen spontaneously conceived term placentae identified several ultrastructural changes (Zhang et al., 2011). ART placentae showed degenerative alterations to terminal villi (predominantly in the syncytiotrophoblast lineage), including thicker placental barrier, fewer apical microvilli and a greater number of multiple vacuoles. These abnormalities at the maternal:fetal interface could reflect impaired establishment of the placental blood barrier, a hypothesis further supported by proteomic analyses identifying changes in membrane trafficking, cytoskeletal integrity, metabolism, the stress response, and nucleic acid processing in IVF and ICSI placentae (Zhang et al., 2008). Subsequent microarray analysis further distinguished ART samples by abnormal transmembrane transport, metabolism, oxidative stress, immune response and cell differentiation gene expression (Zhang et al., 2010).
In summary, it is clear that ART procedures impact morphology and growth of ART placentae in different species. The extent to which these changes are present varies according to the species investigated and methodologies applied. Whether these differences play a causal role in the fetal growth alterations or are secondary to other ART-induced modifications remains to be elucidated. Table II summarizes the placental phenotypes described in ART concepti.
Placental phenotype and molecular alterations described in ART concepti.
| Species . | ART . | Placental phenotype . | Molecular alterations . | References . |
|---|---|---|---|---|
| Mouse | IVF | ⇑ proliferation at labyrinth and junctional zones; ⇑ number of apoptotic cells | Abnormal antioxidant defense network; Alterations in placental transcriptome; Hypomethylation at the H19 locus | Doherty et al. (2000), Mann et al. (2004), Delle-Pianne et al (2010), Faunque et al. (2010), Raunig et al. (2011a, b) and Bloise et al. (2012) |
| ICSI | ⇑ number of apoptotic cells | Abnormal antioxidant defense network | ||
| Bovine | IVF | Longer and thinner placentomes during early pregnancy (E72 to E93); Larger cotyledonary surface and giant cotyledon structures at term; Heavier placentomes at E180 and ⇓ blood vessels density | ⇓ levels of cotyledonary Vegf mRNA | Bertolini et al. (2002), Bertolini et al. (2004) and Miles et al. (2005) |
| SCNT | Reduced number of placentomes; edematous and/or hemorrhagic changes; ⇑ volume density of fetal connective tissues; ⇓ volume density of maternal epithelium | Alterations in placental transcriptome; ⇑ caruncular and chorio-allantoic Hif1α mRNA expression; Desensitization of VEGF signaling in placentomes | Chavatte-Palmer et al. (2002), Hoffert-Goeres et al. (2007), Chavatte-Palmer et al. (2012), Sousa et al. (2012) and Salilew-Wondim et al. (2013) | |
| Human | IVF | ⇑ placental thickness; ⇑ incidence of hematomas and villous edema; ⇑ incidence of micro calcifications | Hypomethylation at the H19 locus | Lalosević et al. (2003), Joy et al. (2012) and Nelissen et al. (2013) |
| Species . | ART . | Placental phenotype . | Molecular alterations . | References . |
|---|---|---|---|---|
| Mouse | IVF | ⇑ proliferation at labyrinth and junctional zones; ⇑ number of apoptotic cells | Abnormal antioxidant defense network; Alterations in placental transcriptome; Hypomethylation at the H19 locus | Doherty et al. (2000), Mann et al. (2004), Delle-Pianne et al (2010), Faunque et al. (2010), Raunig et al. (2011a, b) and Bloise et al. (2012) |
| ICSI | ⇑ number of apoptotic cells | Abnormal antioxidant defense network | ||
| Bovine | IVF | Longer and thinner placentomes during early pregnancy (E72 to E93); Larger cotyledonary surface and giant cotyledon structures at term; Heavier placentomes at E180 and ⇓ blood vessels density | ⇓ levels of cotyledonary Vegf mRNA | Bertolini et al. (2002), Bertolini et al. (2004) and Miles et al. (2005) |
| SCNT | Reduced number of placentomes; edematous and/or hemorrhagic changes; ⇑ volume density of fetal connective tissues; ⇓ volume density of maternal epithelium | Alterations in placental transcriptome; ⇑ caruncular and chorio-allantoic Hif1α mRNA expression; Desensitization of VEGF signaling in placentomes | Chavatte-Palmer et al. (2002), Hoffert-Goeres et al. (2007), Chavatte-Palmer et al. (2012), Sousa et al. (2012) and Salilew-Wondim et al. (2013) | |
| Human | IVF | ⇑ placental thickness; ⇑ incidence of hematomas and villous edema; ⇑ incidence of micro calcifications | Hypomethylation at the H19 locus | Lalosević et al. (2003), Joy et al. (2012) and Nelissen et al. (2013) |
VEGF, vascular endothelial growth factor; Hif-1α, hypoxia-inducible factor 1-alpha.
Placental phenotype and molecular alterations described in ART concepti.
| Species . | ART . | Placental phenotype . | Molecular alterations . | References . |
|---|---|---|---|---|
| Mouse | IVF | ⇑ proliferation at labyrinth and junctional zones; ⇑ number of apoptotic cells | Abnormal antioxidant defense network; Alterations in placental transcriptome; Hypomethylation at the H19 locus | Doherty et al. (2000), Mann et al. (2004), Delle-Pianne et al (2010), Faunque et al. (2010), Raunig et al. (2011a, b) and Bloise et al. (2012) |
| ICSI | ⇑ number of apoptotic cells | Abnormal antioxidant defense network | ||
| Bovine | IVF | Longer and thinner placentomes during early pregnancy (E72 to E93); Larger cotyledonary surface and giant cotyledon structures at term; Heavier placentomes at E180 and ⇓ blood vessels density | ⇓ levels of cotyledonary Vegf mRNA | Bertolini et al. (2002), Bertolini et al. (2004) and Miles et al. (2005) |
| SCNT | Reduced number of placentomes; edematous and/or hemorrhagic changes; ⇑ volume density of fetal connective tissues; ⇓ volume density of maternal epithelium | Alterations in placental transcriptome; ⇑ caruncular and chorio-allantoic Hif1α mRNA expression; Desensitization of VEGF signaling in placentomes | Chavatte-Palmer et al. (2002), Hoffert-Goeres et al. (2007), Chavatte-Palmer et al. (2012), Sousa et al. (2012) and Salilew-Wondim et al. (2013) | |
| Human | IVF | ⇑ placental thickness; ⇑ incidence of hematomas and villous edema; ⇑ incidence of micro calcifications | Hypomethylation at the H19 locus | Lalosević et al. (2003), Joy et al. (2012) and Nelissen et al. (2013) |
| Species . | ART . | Placental phenotype . | Molecular alterations . | References . |
|---|---|---|---|---|
| Mouse | IVF | ⇑ proliferation at labyrinth and junctional zones; ⇑ number of apoptotic cells | Abnormal antioxidant defense network; Alterations in placental transcriptome; Hypomethylation at the H19 locus | Doherty et al. (2000), Mann et al. (2004), Delle-Pianne et al (2010), Faunque et al. (2010), Raunig et al. (2011a, b) and Bloise et al. (2012) |
| ICSI | ⇑ number of apoptotic cells | Abnormal antioxidant defense network | ||
| Bovine | IVF | Longer and thinner placentomes during early pregnancy (E72 to E93); Larger cotyledonary surface and giant cotyledon structures at term; Heavier placentomes at E180 and ⇓ blood vessels density | ⇓ levels of cotyledonary Vegf mRNA | Bertolini et al. (2002), Bertolini et al. (2004) and Miles et al. (2005) |
| SCNT | Reduced number of placentomes; edematous and/or hemorrhagic changes; ⇑ volume density of fetal connective tissues; ⇓ volume density of maternal epithelium | Alterations in placental transcriptome; ⇑ caruncular and chorio-allantoic Hif1α mRNA expression; Desensitization of VEGF signaling in placentomes | Chavatte-Palmer et al. (2002), Hoffert-Goeres et al. (2007), Chavatte-Palmer et al. (2012), Sousa et al. (2012) and Salilew-Wondim et al. (2013) | |
| Human | IVF | ⇑ placental thickness; ⇑ incidence of hematomas and villous edema; ⇑ incidence of micro calcifications | Hypomethylation at the H19 locus | Lalosević et al. (2003), Joy et al. (2012) and Nelissen et al. (2013) |
VEGF, vascular endothelial growth factor; Hif-1α, hypoxia-inducible factor 1-alpha.
Changes to Placental Nutrient Transport
Critical to proper placental function in support of fetal growth is nutrient transport capacity. Accordingly, altered conceptus growth velocity and weight in ART pregnancies may be linked to changes in placental transport efficiency, leading to impaired support of fetal growth (Table III). Insufficient nourishment would precipitate IUGR of the fetus, as well as compensatory placentomegaly. Placental function and nutrient transport were recently explored in a study monitoring fetal accumulation of glucose and neutral amino acids in IVF mouse embryos. Maternal intrajugular injection of 14C-methyl aminoisobutyric acid (14C-MeAIB), a sytem A amino acid transporter substrate (SNAT: see below), revealed a 58.1% decrease in placental transport for the IVF group compared with controls (P < 0.05), with a corresponding 36% decrease in fetal accumulation of MeAIB (P < 0.05). (Bloise et al., 2012). No changes were observed in placental glucose transport following maternal intrajugular injection of 14C-methyl-D-glucose between IVF and control mice (24.8% lower radiolabeled glucose in IVF placentae and 3% lower in IVF fetuses, not significant).
Summary of placental nutrient transport in ART concepti.
| Species . | ART . | Fetal:placental weight ratio . | Placental nutrient transport . | Transporters expression . | References . |
|---|---|---|---|---|---|
| Mouse | IVF | ⇓ as of E12.5 | ⇓ neutral amino acid Transport at E18.5; No change in glucose transport | ⇓Glut1, Glut3/GLUT3, Snat2 and Snat3 mRNA | Delle-Pianne et al. (2010) and Bloise et al. (2012) |
| Bovine | IVF | ⇓ at gestational days E70 and E180. | ⇓ fetal plasma glucose concentrations at E90; ⇑ plasma fructose concentrations soon after birth | ⇔ Glut1 and Glut3 mRNA expression | Bertolini et al. (2002) and Bertolini et al. (2004) |
| SCNT | ⇓ after E220 | ⇔ fetal glucose concentrations; ⇓ allantoic fluid glucose and fructose levels | ⇑ Glut1 and Glut3 mRNA expression | Hirayama et al. (2011) and Chavatte-Palmer et al. (2012). | |
| Human | IVF/ICSI | ⇓ as of 34th week of gestation | – | ⇑ SNAT1 mRNA expression | Haavaldsen et al. (2012) and Zhang et al. (2010) |
| Species . | ART . | Fetal:placental weight ratio . | Placental nutrient transport . | Transporters expression . | References . |
|---|---|---|---|---|---|
| Mouse | IVF | ⇓ as of E12.5 | ⇓ neutral amino acid Transport at E18.5; No change in glucose transport | ⇓Glut1, Glut3/GLUT3, Snat2 and Snat3 mRNA | Delle-Pianne et al. (2010) and Bloise et al. (2012) |
| Bovine | IVF | ⇓ at gestational days E70 and E180. | ⇓ fetal plasma glucose concentrations at E90; ⇑ plasma fructose concentrations soon after birth | ⇔ Glut1 and Glut3 mRNA expression | Bertolini et al. (2002) and Bertolini et al. (2004) |
| SCNT | ⇓ after E220 | ⇔ fetal glucose concentrations; ⇓ allantoic fluid glucose and fructose levels | ⇑ Glut1 and Glut3 mRNA expression | Hirayama et al. (2011) and Chavatte-Palmer et al. (2012). | |
| Human | IVF/ICSI | ⇓ as of 34th week of gestation | – | ⇑ SNAT1 mRNA expression | Haavaldsen et al. (2012) and Zhang et al. (2010) |
SNAT, sytem A amino acid transporter substrate.
Summary of placental nutrient transport in ART concepti.
| Species . | ART . | Fetal:placental weight ratio . | Placental nutrient transport . | Transporters expression . | References . |
|---|---|---|---|---|---|
| Mouse | IVF | ⇓ as of E12.5 | ⇓ neutral amino acid Transport at E18.5; No change in glucose transport | ⇓Glut1, Glut3/GLUT3, Snat2 and Snat3 mRNA | Delle-Pianne et al. (2010) and Bloise et al. (2012) |
| Bovine | IVF | ⇓ at gestational days E70 and E180. | ⇓ fetal plasma glucose concentrations at E90; ⇑ plasma fructose concentrations soon after birth | ⇔ Glut1 and Glut3 mRNA expression | Bertolini et al. (2002) and Bertolini et al. (2004) |
| SCNT | ⇓ after E220 | ⇔ fetal glucose concentrations; ⇓ allantoic fluid glucose and fructose levels | ⇑ Glut1 and Glut3 mRNA expression | Hirayama et al. (2011) and Chavatte-Palmer et al. (2012). | |
| Human | IVF/ICSI | ⇓ as of 34th week of gestation | – | ⇑ SNAT1 mRNA expression | Haavaldsen et al. (2012) and Zhang et al. (2010) |
| Species . | ART . | Fetal:placental weight ratio . | Placental nutrient transport . | Transporters expression . | References . |
|---|---|---|---|---|---|
| Mouse | IVF | ⇓ as of E12.5 | ⇓ neutral amino acid Transport at E18.5; No change in glucose transport | ⇓Glut1, Glut3/GLUT3, Snat2 and Snat3 mRNA | Delle-Pianne et al. (2010) and Bloise et al. (2012) |
| Bovine | IVF | ⇓ at gestational days E70 and E180. | ⇓ fetal plasma glucose concentrations at E90; ⇑ plasma fructose concentrations soon after birth | ⇔ Glut1 and Glut3 mRNA expression | Bertolini et al. (2002) and Bertolini et al. (2004) |
| SCNT | ⇓ after E220 | ⇔ fetal glucose concentrations; ⇓ allantoic fluid glucose and fructose levels | ⇑ Glut1 and Glut3 mRNA expression | Hirayama et al. (2011) and Chavatte-Palmer et al. (2012). | |
| Human | IVF/ICSI | ⇓ as of 34th week of gestation | – | ⇑ SNAT1 mRNA expression | Haavaldsen et al. (2012) and Zhang et al. (2010) |
SNAT, sytem A amino acid transporter substrate.
Changes to SNATs have been broadly correlated with pathologically growth-restricted fetuses (Mahendran et al., 1993; Glazier et al., 1997; Harrington et al., 1999; Jansson et al., 2002). Impaired SNAT activity has been observed in placentae from IUGR-complicated pregnancies (Jansson et al., 2002). The expression and activity of SNAT transporters are involved in modifying net placental nutrient transport capacity in coordination with placental size (Coan et al., 2008). SNATs are regulated by glucocorticoids (Audette et al., 2010, 2011), hypoxic conditions (Nelson et al., 2003) and nutrition (Jones et al., 2009), indicating their ability to transduce a myriad of environmental signals and facilitate adaptations in placental nutrient transport ability appropriately. In mice generated by IVF, Snat2 and Snat4 mRNA levels were down-regulated in whole placentae at embryonic day E18.5, which may contribute to the impaired transport of neutral amino acids observed in IVF concepti (Bloise et al., 2012).
Amino acids not only provide substrates for protein synthesis, but also serve as metabolic energy sources during fetal development. In fact, it is estimated that upwards of 20–40% of the total energy supplied to the fetal/placental unit is derived from amino acids (Bauer et al., 1998; Cleal and Lewis, 2008). Moreover, the quantity and composition of amino acids supplied to the fetus play an important role in determining fetal growth, as fetuses have specific and evolving metabolic requirements for certain amino acids across gestation (Cleal and Lewis, 2008). The reduced availability of amino acids in ART placentae and subsequent decrease in amino acid delivery to the fetus could lead to reprogramming of fetal metabolism, including adaptations toward the utilization of different metabolic pathways to better support fetal growth. However, the molecular mechanisms underlying these metabolic changes and reduced amino acid transport are unknown and could be diverse (Seckl and Holmes, 2007).
Changes in steroid efflux have also been described in ART pregnancies. Collier and co-workers showed that IVF- and ICSI-derived placentae displayed different activities of the steroid metabolizing enzymes UDP-glucuronosyltransferase and sulfotransferase, as well as the steroid regenerating enzymes β-glucuronidase and aryl sulfatase. These changes were correlated with higher levels of the steroid metabolites androstane-3α-17β-diol glucuronide and dehydroepiandrosterone sulfate in fetal blood from IVF and ICSI pregnancies (Collier et al., 2009). In another study, the same group reported diminished cholesterol levels in IVF and ICSI placentae (Raunig et al., 2011a). Interestingly, there were no differences in cholesterol concentration in the fetal compartment. Because cholesterol is vital to proper embryo patterning and development (Raunig et al., 2011a), it is possible that placental/fetal adaptations favor cholesterol accumulation in the fetus at the forfeit of cholesterol deposition in the placenta; alternatively, the placental changes may be compensated for through increased fetal synthesis of cholesterol de novo.
Because fetal glucose production is minimal, fetal glucose supply depends almost exclusively on the placenta. Investigation in bovine models has revealed evidence of abnormal intrauterine carbohydrate metabolism following ART. At E90, IVF concepti were growth-restricted with significantly reduced fetal glucose, but by E180 IVF fetuses outweighed controls (with parallel increases in placentome size) and glucose levels were restored (Bertolini et al., 2004). Interestingly, there were no changes in IVF placentome expression of the glucose transporters Glut1 or Glut3. Consistent with the larger placental phenotype in mice, this suggests that changes in placental weight might contribute to the restored glucose concentrations. Comparatively, SCNT-derived fetuses did not display different plasmatic glucose levels at term relative to in vivo-derived controls, but did exhibit up-regulated caruncular Glut1 and Glut3 expression (Hirayama et al., 2011), indicating that the relationship between mRNA expression and metabolite concentration in the bovine placenta is complex. Further, fructose concentrations were increased in E180 IVF fetal plasma. It is therefore possible that in conjunction with increased placentome growth, alternative carbohydrates may be exploited to combat early pregnancy fetal IUGR, leading to restored fetal glucose levels, accelerated fetal growth and larger offspring outcome. (Bertolini et al., 2004).
Unfortunately, very little is known about placental nutrient transport in human ART pregnancies. Microarray analysis of IVF human placentae at term identified an over two-fold increase in mRNA expression of the SNAT 1 neutral amino acid transporter (Solute carrier family 38, member 1 – SLC38A1) compared with spontaneously conceived pregnancies (Zhang et al., 2010). Increased placental SLC38A1 amino acid transporter expression may be an important adjustment toward ensuring that ART concepti meet their nutrient requirements across pregnancy, although this remains to be determined. Further investigation is warranted in order to clarify whether placental nutrient transport is misregulated in human ART-derived pregnancies.
Preimplantation Determinants of Intrauterine Growth: How ART Procedures May Affect Development
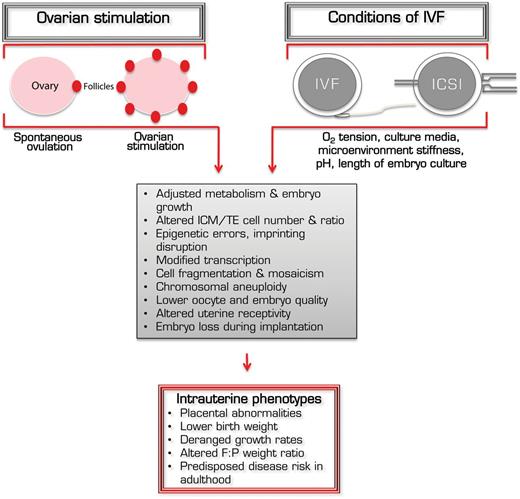
Mechanisms by which ART procedures affect acute and long-term growth. Both ovarian stimulation with exogenous gonadotrophins as well as assorted components of IVF and embryo culture conditions, including oxygen tension, culture medium composition, culture substrate rigidity, pH and the duration of embryo culture, can affect oocyte and embryo developmental competence. This can lead to epigenetic errors, cell mosaicism and ploidy defects, while also inducing adjustments to embryo metabolism, transcription and proliferation. Cumulatively, these acute outcomes can influence intrauterine growth kinetics, leading to the fetal and placental phenotypes observed in ART offspring, and possibly subsequent increased risk of metabolic disease in adulthood. ICM, inner cell mass; TE, trophectoderm.
Blastocyst cell number and developmental competence
The developmental potential of the blastocyst predicts post-implantation capacity, and ART has a well-described effect on blastocyst formation, viability and cell number. Embryo culture generally introduces an 18–24 lag in blastocyst development, and various components of embryo culture become key determinants of embryonic developmental competence and ART success rates (Rinaudo and Schultz, 2004; Rinaudo et al., 2006). As culture conditions better recapitulate the endogenous environment, blastocyst rates increase, predisposing higher pregnancy and live birth rates. For example, the oviduct and uterine environments are stratified by pH approximately >7.5 and <7.1, respectively, with slight variations across different mammalian species (Feuer and Rinaudo, 2012). Lowering the culture medium pH from 7.3 after 48 h of culture to 7.15 improved blastocyst outcome over embryos maintained at one pH (Hentemann et al., 2011). What is more, even small variations in culture conditions exert differential effects on development, as exemplified by a comprehensive analysis of the individual effects of 13 different human IVF culture protocols on mouse blastocyst development and proliferation by Schwarzer and colleagues (Schwarzer et al., 2012). They observed condition-specific consequences on blastocyst and fetal developmental rates, cell numbers, gene expression signatures, and litter sizes, indicating that particular elements of culture have measured and defined outcomes for cell proliferation, lineage specification and embryo viability.
It is well appreciated that blastocyst proliferation and cell lineage pool size impact fetal development and viability, with decreased cell number correlating with impaired fetal competence. Mouse blastocysts comprising fewer cells have reduced implantation rates and litter sizes, and yield an increased number of abortive sites (Delle Piane et al., 2010; Bloise et al., 2012). Moreover, reduced blastocyst cell number signifies fewer progenitors contributing to the fetal and extraembryonic derivative lineages (ICM and trophectoderm [TE] cells, respectively), which may lead to several deleterious consequences for fetal growth. Studies in mouse embryos have shown that artificial reduction of preimplantation embryo cell number can significantly delay gastrulation, morphogenesis and somite segmentation (Power and Tam, 1993). This rescheduling of early post-implantation events may precede the accelerated compensatory intrauterine growth observed in later stages of ART pregnancies. Decreases in blastocyst cell number after in vitro culture of bovine embryos is additionally correlated with impaired developmental potential, which may precede the LOS phenotype (Van Soom et al., 1997). A recent study in humans demonstrated that TE morphologic grade (based in part off cell number) is a stronger predictor of successful ART outcome than ICM grade; this may be related to optimal ploidy or improved blastocyst-endometrium communication (Hill et al., 2013).
Because ICM and TE cells have extraordinarily different fates, behaviors, morphologies and functions, many ART-induced changes may be masked by analyses of whole embryos. Microarray profiling of ICM and TE transcriptional signatures have revealed significant differential expression between the two lineages for pathways of differentiation and pluripotency, cell signaling, molecular transport, transcriptional regulation, and metabolism in mice (Giritharan et al., 2012), bovines (Ozawa et al., 2012), and humans (Adjaye et al., 2005). Although very few studies have investigated the transcriptional changes specific to ICM or TE cells following ART, embryos produced by IVF (mice) and SCNT (bovine) both show drastically reduced differential expression between ICM and TE lineages, specifically for genes involved in development and pluripotency (Fujii et al., 2010; Giritharan et al., 2012). Detailed analysis of the individual cell fates in mouse embryos revealed an impact of IVF on ICM cell pluripotency, phospholipid metabolism, liptoxicity and urea cycle gene expression signatures (Giritharan et al., 2012). Comparably, IVF largely down-regulated TE expression of genes functioning in solute transport and placentation. Loss of gene signatures distinguishing ICM versus TE segregation as well as differentiation and placentation processes would contribute to the altered lineage ratios and fetoplacental growth abnormalities observed in utero. In fact, is has been suggested that alterations to TE gene expression could be in part responsible for altered placental size found in human ICSI-derived pregnancies (Haavaldsen et al., 2012).
The sensitivities of ICM:TE number and overall blastocyst viability to the individual effects of specific embryo culture components, including culture medium composition, oxygen tension and microenvironment stiffness/rigidity, have been the subject of numerous studies. Outstandingly, more severe culture conditions yield more deviant phenotypes (Rinaudo et al., 2006; Kolahi et al., 2012; Schwarzer et al., 2012).
Oxygen tension
In the mammalian reproductive tract, oxygen concentrations change from ∼8% at the time of fertilization in the oviduct, to ∼1–2% in the uterus (Bavister, 2004). However, this gradient is abolished with embryo culture, and several reports have argued the harmful effects of higher oxygen concentrations on preimplantation development.
Culture of mouse zygotes in 20% (atmospheric) compared with 5% oxygen levels significantly reduced the number of embryos reaching the blastocyst stage, as well as number of ICM cells and total cell number (Rinaudo et al., 2006). Similarly, percentage oxygen during in vitro maturation of oocytes can affect blastocyst rate, TE cell number and apoptosis levels, as well as fetal and placental weights (Banwell et al., 2007). Further, oxygen tension has a pronounced effect on gene expression in blastocysts. Culture of mouse zygotes to blastocysts in optimized media conditions (KSOM supplemented with amino acids) under 5 versus 20% oxygen tensions induced significant changes to 29 and 354 genes, respectively, compared with in vivo-produced embryos. The same experiment using a suboptimal medium, Whitten's medium, affected the expression of 114 genes (5% oxygen) and 1159 genes (20% oxygen), and was additionally associated with decreased blastocyst rates and cell numbers (Rinaudo and Schultz, 2004; Rinaudo et al., 2006). This indicates a synergism between culture medium and oxygen concentration, such that the detrimental effects of suboptimal media are severely compounded by higher oxygen tensions. Moreover, high oxygen during culture was linked with increased abortion rates, decreased fetal and placental weights, and higher F:P ratio (Delle Piane et al., 2010).
The stressful effects of high oxygen have also been shown in bovines. Lowering the oxygen concentration from ambient to 5% correlated with increased blastocyst rates and expression of genes involved in glucose transport, mitochondrial function and integrity, and antioxidant defense (Balasubramanian et al., 2007). In contrast, atmospheric oxygen induced higher transcript levels of oxidative stress genes. This indicates that high oxygen produces a stress response in the embryo, which substantiates the fact that lower oxygen tensions mimic a more physiological preimplantation environment.
A meta-analysis of seven randomized human ART studies associated physiologic oxygen concentrations with greater percentages of blastocyst formation and significantly increased implantation rates (P = 0.006) (Gomes Sobrinho et al., 2011). Other studies not included in this analysis have corroborated higher blastocyst development, implantation, and pregnancy rates for human embryos cultured in low oxygen (Catt and Henmam, 2000; Kea et al., 2007; Meintjes et al., 2009; Waldenström et al., 2009; Kovacic et al., 2010). A separate meta-analysis similarly reported increases in clinical pregnancy, ongoing pregnancy, and live birth rates for 5% compared with 20% culture conditions, with no effect on multiple pregnancy, miscarriage, or congenital anomaly risk (Bontekoe et al., 2010). To date, an effect of oxygen tension on birthweight has not been systematically reviewed. Interestingly, embryos transferred on Day 2 or 3 of development display no changes in fertilization rate (P = 0.54), implantation rate (P = 0.63), or ongoing pregnancy rate (P = 0.19) (discussed below) (Gomes Sobrinho et al., 2011).
Overall, the data described in this section indicate that oxygen tension is a major factor regulating embryo growth in different species. Although some studies comparing the impact of low versus high oxygen concentrations on pregnancy outcome remain controversial, we wish to advise in favor of culturing embryos in a low-oxygen environment when performing ART.
Length of embryo culture
The efficacy and safety of cleavage (Day 3) versus blastocyst (Day 5) stage transfer of human embryos is one of the most discussed controversies in reproductive medicine today. Evidence in the literature demonstrates that clinical pregnancy and live birth rates from Day 5 transfer are superior (Papanikolaou et al., 2006, 2008; Blake et al., 2007), but blastocyst transfer is additionally linked with increased prenatal complications, preterm birth, low Apgar score, respiratory diagnoses, and congenital malformations (Milki et al., 2003; Källén et al., 2010; Maheshwari et al., 2013; Dar et al., 2014). Additionally, duration of embryo culture is a highly significant independent factor determining birthweight in humans: weights are markedly higher following blastocyst transfer (Zhu et al., 2014), and the percentage of babies born large for gestational age nearly doubles for Day 5 compared with Day 2/3 transfer (Makinen et al., 2013).
One explanation for the length of embryo culture effects is the sensitivity of the blastocyst after cell segregation into the ICM and TE lineages. There is a well-defined role for oxygen in trophoblast proliferation and differentiation (reviewed in Red-Horse et al., 2004). The earliest stages of placentation favor hypoxic conditions, which support cytotrophoblast expansion; as these cells invade the uterine epithelium, exposure to higher oxygen levels from the maternal blood supply favors their differentiation (Genbacev et al., 1997). Further, ablation of different oxygen-sensing or -responsive proteins—including von Hippel Lindau or HIF-1β—compromises placental cell fate determination, leading to fetal demise secondary to placental defects (Gnarra et al., 1997; Adelman et al., 2000). A study using trophoblast stem cells derived from TE showed that HIF is specifically involved in modulating epigenetic changes that govern trophoblast differentiation (Adelman et al., 2000). It is possible that culturing embryos in ambient oxygen predisposes to impairments to placentation and subsequent intrauterine growth, whereas the uterine environment abrogates the deleterious effects of high oxygen concentrations in embryos tranferred on Day 2/3. This is further evidenced by an effect of high oxygen exposure (compared with hypoxic conditions, 2% oxygen) exclusively during the morula-blastocyst transition on F:P weight ratio (Feil et al., 2006). It would be interesting to assess viability, cell number, and developmental stress in IVF blastocysts previously transferred as cleavage embryos, to determine if the final stages of preimplantation development in vivo are sufficient to rescue ART-induced changes present earlier.
Rigitidy of the culture substrate
Another important feature of the preimplantation microenvironment is surface stiffness. A conventional polystyrene petri dish (PD) has a measured elasticity of 1 GPa whereas the uterine epithelium has a ∼1kPa stiffness—a difference in rigidity of over 106. Culture of mouse embryos either on polydimethyl-siloxane-coated PDs or fabricated 3D type I collagen gels (both with elasticities of 1kPa) resulted in a significantly greater frequency of development to the 2-cell, blastocyst, and hatching blastocyst stages compared with control PD embryos (Kolahi et al., 2012). Further, 1kPa conditions were associated with increased blastocyst cell number, particularly within the TE lineage. This further indicates that blastocyst developmental potential improves as culture conditions better mimic the female reproductive tract.
Gamete and preimplantation embryo metabolism
The evolution of zygote to blastocyst occurs over a dynamic range of nutrients, oxygen, and growth signals distinguishing the oviduct from uterus. Therefore, the progression between the two microenvironments requires a metabolic plasticity that is achieved through flexible management of glycolytic activity, oxidative phosphorylation, and membrane transport (Gardner and Leese, 1988; Leese, 2012). For example, as the zygote transitions to blastocyst, its metabolic activity preferentially shifts from pyruvate to glucose oxidation, with parallel increases in glucose availability upon entry into the uterus. However, the absence of glucose in culture prevents the characteristic decline of pyruvate uptake after compaction (Brinster, 1965). This adaptability confers acute survival advantages, yet in more stressful or artifical environments it may not be optimal long term.
In the mouse, TE cells generate ∼80% of total blastocyst ATP production and are responsible for 90% of the embryo's amino acid turnover, primarily to fuel the sodium transporter enzymes that facilitate cavitation (Houghton, 2006). It follows that reduced TE cell number would have a profound impact on embryo integrity. Additionally, impaired proliferation can denote metabolic or oxidative stress, prompting the redistribution of cellular resources away from proliferative activities and alternatively toward repair pathways (i.e. the quiet embryo hypothesis) (Leese, 2012). This is evidenced by the observation that embryo culture is associated with an increase in mitochondrial activity, amino acid turnover, and tandem decreased expression of genes involved in shunting glycolytic intermediates toward biosynthetic processes versus the TCA cycle (and ATP syntheis) (Krisher and Prather, 2012; Redel et al., 2012).
In all reports investigating the effects of IVF, ICSI, and embryo culture on blastocyst gene expression, the majority of the transcriptional changes are to genes involved in metabolic processes (Rinaudo and Schultz, 2004; Rinaudo et al., 2006; Giritharan et al., 2007, 2010, 2012; Schwarzer et al., 2012). In particular, studies investigating the effects of culture medium composition on preimplantation development have revealed that growth and cell fate in the early embryo is sensitive to the availability of different metabolic substrates (carbohydrates, amino acids), which can consequentially affect post-implantation development. In fact, the addition of exogenous pyruvate and lactate to the medium during the pronuclear stage (and subsequent manipulation of redox potential) alters birthweights and post-natal growth curves of mouse ART concepti (Banrezes et al., 2011).
Moreover, different forms of exogenous gonadotrophin preparations can impact oocyte and embryo metabolism. Following controlled ovarian stimulation, oocytes progressing to the metaphase II stage exhibited unique amino acid turnover profiles depending on their exposure to recombinant FSH versus hMG (a urinary gonadotrophin) treatments (Hemmings et al., 2013). Because oocyte metabolic state predisposes fertilization and developmental competency (Hemmings et al., 2012), hormone-induced changes to oocyte metabolism could pervasively influence future growth potential. Correspondingly, ovulation induction impairs embryo development to blastocyst and also affects global expression of genes involved in growth and proliferation, including DNA and protein synthesis, transcription, RNA post-transcriptional modification, and other bioenergetic pathways supporting anabolism (Gad et al., 2011).
Imprinted genes and epigenetic changes
As discussed above, IVF and embryo culture introduce significant changes to patterns of gene expression in blastocysts in a condition-specific fashion, and many alternations in transcriptional signatures persist well beyond the ART procedures. For example, culture-specific transcriptional signatures are apparent in placental gene expression (Fauque et al., 2010; Schwarzer et al., 2012). Maintence of ART-induced changes following the preimplantation period suggests the existence of a ‘memory’ of the ART event that impacts subsequent intrauterine and posnatal growth.
It is widely believed that the mechanism of reprogramming is epigenetic in nature. Epigenetic regulation occurs at the DNA level predominantly through the methylation of cytosine bases residing in CpG dinucleotides, or by post-translational modification of histone proteins. These covalent moieties merge into combinatorial signatures that affect chromatin conformation, DNA accessibility, and gene expression through a variety of mechanisms. Importantly, embryogenesis is marked by significant chromatin remodeling in conjunction with cell differentiation, making the preimplantation stages uniquely vulnerable to environmental perturbances (Fig. 1). It is possible that ART procedures induce stochastic changes to epigenetic and transcriptional regulation that become permanently incorporated into programmes of cell fate (Feuer et al., 2013).
It is likely that any epigenetic changes associated with ART occur in a locus-specific fashion. Immunohistochemical analysis of in vivo and IVF mouse embryos did not observe any global changes in the levels or distribution of the histone modifications H4 acetylation (H4ac), H3 lysine 9 trimethylation (H3K9me3), or phosphorylated H3 at serine 10 (H3S10p) across all stages of preimplantation development (zygote to blastocyst) (Huang et al., 2007). However, reduced fetal size in early pregnancy has been correlated to impaired placental expression and activity of the methylation maintenance enzyme DNMT1 in IVP sheep placentae, the dysfunction of which was directly related to fetal growth restriction and miscarriage rates (Ptak et al., 2013). Global versus site-specific methylation levels were not evaluated in the ART- and in vivo-derived placentae.
Investigators have focused extensively on the effects of ART on imprinted genes, which are expressed monoallelically in a parent-of-origin-dependent manner. This is particularly relevant for genes controlling growth and development, as it affords more stringent regulation of gene dosage. Sometimes this parental allele-based expression is preferential only in a specific subset of tissues, notably the placenta (Tunster et al., 2013). Evidence has emerged demonstrating that imprinted genes are vulnerable to sustained methylation aberrancies following ART, and several excellent reviews have been written on the topic (Manipalviratn et al., 2009; Marchesi et al., 2012).
H19 is an imprinted gene expressed exclusively from the maternally-inherited chromosome, and plays a major role in both limiting placental growth, as well as controlling fetal growth via coordinated nutrient transfer from mother to fetus (Fowden et al., 2006). Several reports have shown that embryo culture can alter H19 methylation, leading to its biallelic expression in a subset of mouse embryos generated in vitro. (Doherty et al., 2000; Mann et al., 2004). Importantly, these alterations (i.e. hypomethylation of the H19 paternal allele) persisted after implantation, with placental tissue displaying active expression of the normally silent H19 allele. This indicates that changes in embryo methylation induced by in vitro culture can persist in later stages of intrauterine life. Significant H19 hypomethylation and corresponding increased H19 mRNA expression have also been observed in human ART placentae (5 IVF and 30 ICSI), although this was not correlated with changes in IVF neonatal birthweight, birthweight standard deviation score, or gestational age (Nelissen et al., 2013). However, H19 expression defects have been associated with placentomegaly (Bloise et al., 2012; Keniry et al., 2012). As a result, impairments to imprinting mechanisms (including establishment and maintenance of DNA methylation) could have a direct effect on the unique intrauterine growth velocities observed in IVF concepti. A related hypothesis is that errors in DNA methylation may be responsible for the decreased number of TE cells in IVF preimplantation embryos (Rinaudo and Lamb, 2008).
In addition to embryo culture, the administration of exogenous gonadotrophins for ovarian stimulation can affect DNA methylation status at imprinted genes. A recent evaluation of hormone dose on DNA methylation architecture in mouse blastocysts showed a dose-dependent loss of methylation at the maternally imprinted Snrpn, Peg3, and Kcnq1ot1 loci, and gain of methylation at the normally hypomethylated maternal H19 allele, with higher hormone dosages producing greater methylation aberrancies (Market-Velker et al., 2010). Unexpectedly, gonadotrophin exposure resulted in a loss of H19 methylation at the paternally imprinted allele, a surprising result because ovulation induction is believed to affect genomic imprinting during oocyte development. This suggests an impact of ovarian stimulation on the acquisition and/or maintenance of imprints across preimplantation development. In a follow-up study, the same group concluded that ovulation induction does not affect methylation acquisition at the examined loci, but instead impairs maintenance of these imprints during preimplantation development (Denomme et al., 2011).
In humans, ovarian stimulation has been linked to a gain of H19 methylation and a loss of PEG1 methylation in oocytes, although these results may be confounded by fertility defects in the superovulated ART patients (Sato et al., 2007). A separate report analyzed H19 methylation profiles in oocytes at various developmental stages following in vitro maturation, and a subset of the metaphase II oocytes examined (5 of 20 total) exhibited aberrant methylation signatures (Borghol et al., 2006). Although the specific effects of these changes remain obscure, it is clear that assorted components of ART can induce epigenetic lesions and may compromise the expression of developmentally relevant genes, placental development, fetal growth and viability.
Uterine receptivity
In order to establish the maternofetal crosstalk crucial for a healthy pregnancy, the uterus must accommodate a microenvironment that is both receptive to implantation and supportive of subsequent fetal growth, and the embryo must reciprocate with pregnancy recognition signaling (Bazer et al., 2011). It is becoming increasingly apparent that many pregnancy complications such as pre-eclampsia or preterm delivery—known risks associated with ART—can be traced to abnormalities in implantation and placentation (reviewed in Norwitz, 2006). For example, blastocyst expression of Il-6 helps facilitate blastocyst-uterine crosstalk and may be involved in trophoblast differentiation and invasion (Norwitz 2006); IVF-derived mouse embryos exhibit a 5.7-fold reduction in TE cell expression of Il-6 (Giritharan et al., 2012).
Several studies indicate that controlled ovarian stimulation impairs uterine receptivity (Fauser and Devroey, 2003). Horcajadas and colleagues performed microarray analysis of endometrial biopsies obtained from superovulated and natural cycles to elucidate the impact of exogenous gonadotrophins on the early- to mid-secretory transition (Horcajadas et al., 2008). The pre-receptive and receptive phases were distinguished by two separate patterns of gene expression (218 and 133 genes differentiating the two stages, respectively). Ovulation induction induced a 2-day delay in the transition between the two clusters, demonstrating that ovarian stimulation affects the timing of endometrial maturation with possible consequences for receptivity and implantation (Papanikolaou et al., 2005). Differences in ovarian steroid release can also influence the composition of oviduct fluid. Because variations in nutrient and electrolyte availability impact embryo metabolism and growth, this could impair future fetal developmental competence (Murray et al., 1995).
There is also an effect of gonadotrophin dosage on the length of the implantation window (Simon et al., 2003). The luminal endometrial endothelium is responsible for promoting apposition and attachment of the developing blastocyst exclusively during the implantation period. Blastocyst adhesion to the endometrium is predominantly supported either by the addition of progesterone after prior priming with 17β-estradiol (humans), or alternatively by the presence of 17β-estradiol after appropriate priming with progesterone (rodents). Studies in mice have shown that differential doses of exogenous 17β-estradiol can manipulate the duration of the implantation window: administering low levels extend and maintain the window of receptivity, whereas higher doses can rapidly induce a refractory state (Simon et al., 2003).
This may be related to changes in uterine gene expression. Compared with low levels of exogenous progesterone supplementation, exposure to high concentrations reduced endometrial expression of the progesterone and estrogen receptors in cycling heifers (McNeill et al., 2006). Superovulated animals additionally demonstrated sustainably high progesterone levels through Day 6 following artificial insemination compared with unstimulated animals (Gad et al., 2011). It follows that in addition to influencing receptivity for implantation, gonadotrophin administration may induce other endometrial changes that could subsequently affect placentation or maternal recognition of pregnancy.
Conclusions
In conclusion, there is compelling evidence that ART has significant effects on intrauterine growth trajectory and placental support of fetal growth. This is particularly important because fetal growth restriction followed by accelerated perinatal growth velocity mark both fetal stress and a predisposition for cardiometabolic pathologies in adulthood, as described by the DOHaD hypothesis. It is likely that many components of ART procedures, including embryo culture environment and duration, oxygen tension, and gonadotrophin stimulation, impact fetoplacental development through combined effects on oocyte maturation, cell proliferation and specification, blastocyst viability, and endometrial receptivity for implantation. Together these variables could alter maternal recognition of pregnancy that in turn might lead to abnormal placental and fetal growth kinetics. Because differences in fetal intrauterine growth do exhibit species specificity, this highlights the need for focused research on human ART concepti (whenever ethically possible). Specifically, systematic evaluation of fetal growth and placental physiology across gestation with post-natal follow-up are required in order to determine whether ART in humans produces deleterious changes to intrauterine growth trajectory, placental function and subsequent post-natal health.
Authors' roles
E.B. and P.F.R. conceived and designed the study. E.B., S.K.F. and P.F.R. each contributed to performing the search, analyzing the data and writing the manuscript.
Funding
This work was supported by CNPq/Ciencia sem Fronterias (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasilia, Brazil), grant number: 402343/2012-3 to E.B. This work was also supported by NIH 5T32-DK007418-32 to S.K.F. and NICHD RO1-062803-01A1 to P.F.R.
Conflict of interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research we have reported in this manuscript.