-
PDF
- Split View
-
Views
-
Cite
Cite
Jeanette Seyfarth, Peter Garred, Hans O. Madsen, The ‘involution’ of mannose-binding lectin, Human Molecular Genetics, Volume 14, Issue 19, 1 October 2005, Pages 2859–2869, https://doi.org/10.1093/hmg/ddi318
Close - Share Icon Share
Abstract
Mannose-binding lectin (MBL) acts as a serum opsonin in innate immune defense and induces complement activation by the lectin pathway. In humans, low levels of functional serum MBL are caused by the dominant action of three single nucleotide substitutions in exon 1 that disrupt the glycine-rich backbone structure of the protein. The presence of common MBL variant alleles is associated with both infectious and autoimmune diseases. Conversely, it has also been suggested that MBL variants are maintained because of selective advantages for the host. In man, the MBL genetic system comprises one functional gene (MBL2) and one expressed pseudogene (MBL1P1), whereas the lower primate, the rhesus monkey resembles rodents with two functional MBL genes. We have investigated the molecular mechanisms behind the evolutionary loss of MBL expression from lower primates to man, including silencing of the MBL1P1 gene and the generation of MBL2 variant structural alleles and promoter polymorphisms leading to the present human MBL2 haplotypes. We present data showing that the MBL1P1 gene has been repeatedly hit throughout evolution and silenced eventually by mutations in the glycine residues of the collagen-like region. Our results indicate that the MBL1P1 gene has been selectively turned off during evolution through the same molecular mechanisms causing the MBL2 variant alleles in man, suggesting an evolutionary selection for low-producing MBL genes.
DDBJ/EMBL/GenBank accession nos AY970670–AY970685
INTRODUCTION
Mannose-binding lectin (MBL) initiates the lectin pathway of complement activation in primary immune defense by binding to conserved carbohydrate structures (e.g. N-acetylglucosamine) displayed on a variety of microorganisms. The protein may also directly serve as an opsonin, enhancing phagocytosis by binding to cell-surface receptors present on phagocytic cells (1,2).
In serum, MBL is found in complex with MBL-associated serine proteases, MASP-1, MASP-2 and MASP-3, and a non-enzymatic protein MAp19 (3,4). Upon binding of MBL to the microbial surface, MASP-2 is activated and cleaves C4 and C2 through the lectin pathway. The roles of MASP-1 and MAp19, which is an alternative spliced form of MASP-2, have not yet been fully elucidated (5–8). MASP-3, an alternative spliced form of MASP-1, has been suggested as a modulator of the lectin pathway, by performing competitive inhibition of MASP-2 binding to MBL and inhibition of complement activation by MBL-associated MASP-2 (9).
The human MBL protein is encoded by four exons. The N-terminal part of the protein encoded by exon 1 includes three cysteine residues and the first part of the collagen-like domain containing the characteristic Gly-X-Y repeated sequence. Exon 2 encodes the rest of the collagenous domain and a neck region is encoded by exon 3, whereas a carbohydrate recognition domain (CRD) of the protein is encoded by exon 4 (10,11). MBL has an oligomeric structure of two to six subunits. Each subunit comprises three identical 25 kDa polypeptide chains in a triple-helix structure (12). The higher oligomerization of the protein is mediated by the N-terminal part of the protein and is necessary for the function of MBL and the interaction with the MASPs (13,14).
Structural mutations in the form of three single base substitutions positioned in exon 1 of the human MBL2 gene, at codon 52 (Arg to Cys, allele D), codon 54 (Gly to Asp, allele B) and codon 57 (Gly to Glu, allele C), independently reduce the level of functional serum MBL by disrupting the collagenous structure of the protein (15–17). Furthermore, several nucleotide substitutions in the promoter region of the MBL2 gene at position −550 (H/L polymorphism), −221 (X/Y polymorphism) and −427, −349, −336, del (−324 to −329), −70 and +4 (P/Q polymorphisms) affect the MBL serum concentration. Both the frequency of structural mutations and the promoter polymorphisms that are in strong linkage disequilibrium vary among ethnic groups resulting in seven major haplotypes: HYPA, LYQA, LYPA, LXPA, LYPB, LYQC and HYPD. Differences in the distribution of these haplotypes are the major cause of interracial variations in MBL serum levels. Both HYPA and LYQA are high-producing haplotypes, LYPA intermediate-producing haplotype and LXPA low-producing haplotype, whereas LYPB, LYQC and HYPD are defective haplotypes (18,19).
Genotypes causing low levels of MBL have been found associated with both infectious and autoimmune diseases, for recent review refer Turner and Hamvas (20). Because of the high frequencies of these variant alleles in many populations, it has been suggested that MBL structural variants have been preserved because of selective advantages for the host, implying that this may be a balanced genetic system, in analogy to the Hbs allele in malaria (21). One theory suggests that heterozygosity for MBL variant alleles may confer protection against intracellular parasites that use opsonization via complement as a route of gaining cellular entrance (22). Another theory suggests that the MBL mediated route of complement activation may, in some circumstances, be detrimental for the host (15). However, neither of these theories are mutually exclusive. The selective advantage theory is supported by the presence of high frequencies of independently derived defective haplotypes both in African (LYQC; frequency: 0.24) and in South American (LYPB; frequencies: 0.42–0.46) populations (19). Moreover, the presence of common alleles both in the promoter region and in the coding region of the MBL2 gene decreasing the level of MBL in the blood may also be an indicator of such a dualism of the MBL function.
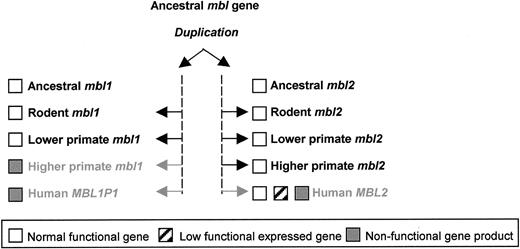
Ancient MBL1 and MBL2 are believed to divagate from a common ancestral MBL gene by gene duplication (23). In mice, MBL is encoded by two distinct functional genes mbl1 and mbl2, also known as mbl-a and mbl-c, that are positioned on different chromosomes 14 and 19, respectively (24), unlike the two human analogs that are closely positioned on chromosome 10 (10,25). Both murine MBLs form higher oligomeric structures and activate complement (26,27). MBL-A has been described to possess a pro-inflammatory function (28), but whether MBL-A and MBL-C possess complementary or diverse functions still remains to be elucidated. MBL-1 and MBL-2 have been detected in sera from the rhesus monkey, whereas MBL is only represented by MBL-2 in chimpanzee and in man (29). In man, MBL1 (MBL1P1) has been characterized as an expressed pseudogene (25). In addition to two nonsense mutations in exon 3 and exon 4, a splicing defect has been observed that leads to the preservation of intron 1 and consequently a pre-termination of the protein.
We have investigated the molecular mechanisms behind the evolutionary loss of MBL-1 expression from the lower primates to man and simultaneously examined the evolutionary events responsible for the established present-day human MBL2 haplotypes.
Our study indicates that both of the human MBL genes have been exposed to mutations resulting in a primary dominant reduction in protein expression and secondary in silencing of the gene, mainly through the action of mutations in the essential glycine codons of the collagene-like repeats and that these mutations are independently arisen in both genes, i.e. not by a gene-conversion event. The silencing of the MBL1 gene and the dominating action of the structural mutations combined with down-regulating mutations in the regulatory parts of MBL2 suggests that there has been an evolutionary selection for low levels of MBL.
Finally, we present the discovery of a human MBL1 transcript without the splicing defect described for the pseudogene, indicating that the glycine substitutions are the primary events responsible for the silencing of the gene. Furthermore, we present structural data on recombinant protein derived from the spliced transcript, and with the intention to examine the potential functions of a prehistoric human MBL-1 protein, we introduced functional analyses on recombinant baboon MBL-1.
RESULTS
Evolution of the MBL2 gene
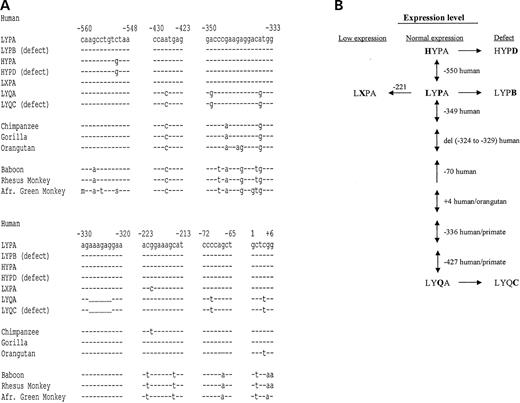
Human and primate DNA sequences containing promoter polymorphisms and structural mutations of the MBL2 gene are available in GenBank (GenBank accession nos AY970679–AY970685). Selected promoter regions of the MBL2 gene are shown in Figure 1A, where the seven human haplotypes are compared with the primate sequences. From this sequence information, we propose that the evolution of the MBL2 gene has originated from an ancestral haplotype as outlined in Figure 1B.
Six polymorphisms positioned at −427, −349, −336, del (−329→−324), −70 and +4 distinguish the human LYP haplotype from the LYQ haplotype. Among these are the LYQ haplotype variants positioned at −427 and −336 also shared by all the analyzed primate species, and the +4 variant present in the orangutan, whereas the remaining variants [−349, del (−324→−329) and –70] are exclusively found in the human LYQ promoter sequence. The −550 H variant is distinctively found in the human HYP promoter sequence, whereas the −221 position constituting a G to C nucleotide substitution in the human LXP/LYP haplotype and also it is affected in the chimpanzee, but in this case by a G to T substitution.
The human MBL2 promoter polymorphisms, encompassing both high- and low-expressing haplotypes, seem thereby to have evolved from an ancestral haplotype arisen in the evolutionary gap between the high-expressing LYQA haplotype and the intermediate-expressing LYPA haplotype.
Evolution of the MBL1P1 pseudogene
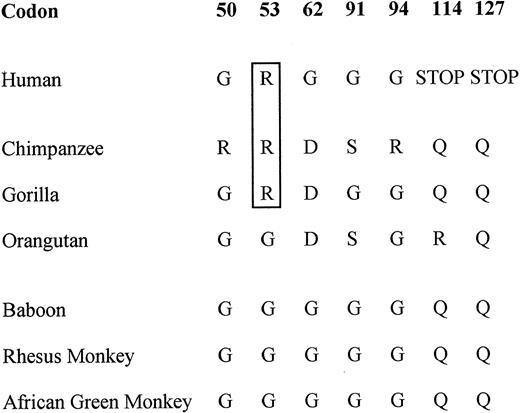
The MBL1P1 coding sequences of humans and primates are available in GenBank (GenBank accession nos AY970670–AY970678). As seen from the selected regions shown in Figure 2, the glycine coding sequence has been repeatedly targeted for mutations both in humans and in the higher primates.
The human MBL1P1 pseudogene does in addition to the previously described intron 1 splicing defect (25) and the two nonsense mutations positioned in exon 3 and exon 4 also possess a codon 53 Gly to Arg substitution. This substitution most likely disrupts the collagenous backbone structure of the protein because of its analogy to the human MBL2 codon 54 Gly to Asp substitution. The MBL1P1 codon 53 substitution was also found among the most closely related primates, the chimpanzee and the gorilla. In addition, the chimpanzee included a codon 50 Gly to Arg substitution and both the chimpanzee and the gorilla contained a codon 62 Gly to Asp substitution. In codon 91, both the chimpanzee and orangutan sequences contained a Gly to Ser substitution and in codon 94, the chimpanzee included yet another Gly to Arg substitution. In codons 114 and 127, a Gln present in all the primates was changed to stop codons in humans. However, the exon 3 stop codon at position 114 was polymorphic and only encoded in some of the individuals in the Danish and Mozambique populations, whereas present in all the analyzed individuals of the South American population, suggesting that this is a late event in the pseudogene evolution (Submitted to dbSNP).
None of the more distant primates including the baboon, the rhesus monkey and the African green monkey had any of these glycine substitutions. However, except for the rhesus monkey (29), it is not known whether the MBL1 gene is functional in these species but due to the high sequence homology, this is most likely the case.
The spliced human MBL1P1 transcript
DNA sequencing of a PCR product amplified with primers positioned in exon 1 and exon 3 of the human MBL1P1 gene revealed the existence of a MBL1P1 transcript without the previously described intron 1 splicing defect but containing the codon 53 Gly to Arg substitution and the exon 3 stop codon. Amplification beyond exon 3 was tested with two different exon 4 anti-sense primers under various conditions without any success. This could indicate that full-length exon 4 (encoding the CRD region) is not included in the naturally occurring MBL1P1 spliced transcript.
Structural analysis of recombinant proteins
On the basis of MBL1 DNA sequencing analysis, the orangutan was found to be the closest human relative of the monkeys, which might possesses a functional MBL-1 protein. However, because no orangutan liver tissue or cDNA library was available, we used a baboon cDNA library with the purpose of making recombinant MBL-1 and investigate which properties of prehistoric MBL-1 could have possessed before it was functionally silenced.
SDS–PAGE, immunoblotting and non-dissociating gel filtration were performed to investigate the oligomeric state of recombinant baboon MBL-1 (rbMBL-1) and spliced human MBL1P1 (rshMBL1P1).
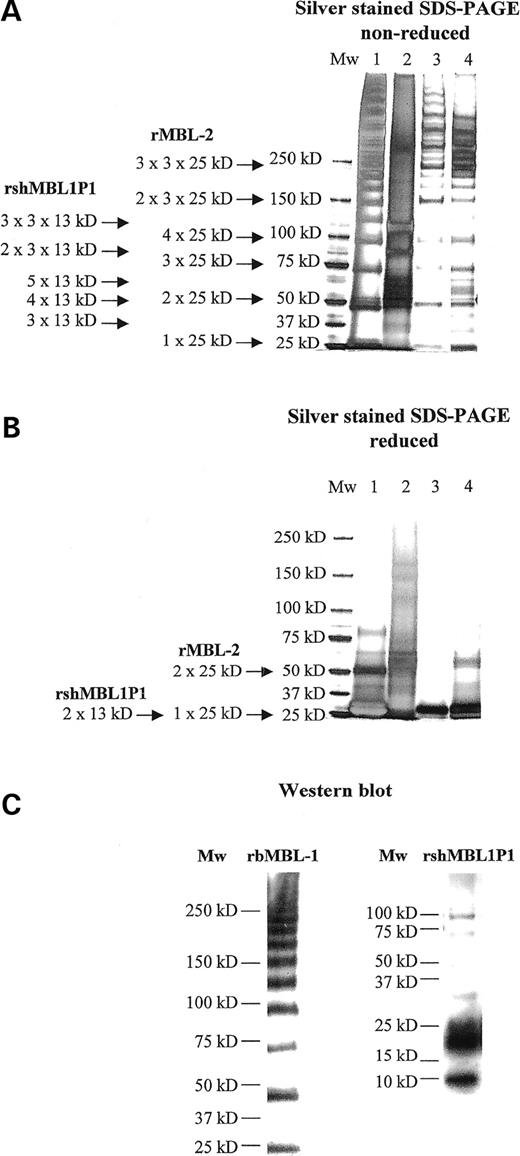
Silver stained SDS–PAGE visualize the non-reduced and reduced denaturated protein structures of rbMBL-1 and rshMBL1P1 in addition to clinical grade MBL-2 [The State Serum Institute (SSI)] and previously characterized human rMBL-2 (32) (Fig. 3A and B). Both rbMBL-1 and rshMBL1P1 form higher oligomeric structures with baboon MBL-1 obtaining a protein structure that resembles the oligomerization pattern described for human wild-type MBL-2. Thus, rbMBL-1 forms structures comprising three polypeptides, but also assembles into several higher oligomeric structures that under reducing conditions in SDS–PAGE generate a single chain unit of ∼25 kDa.
Recombinant spliced human MBL1P1 transcript (sh-MBL1P1) comprises 366 nucleotides encoding a protein of 120 amino acids including a N-terminal region with three cystein residues, a collagen region and a neck region followed by a six repeat His-tag and a stop codon. A single rshMBL1P1 polypeptide has a calculated molecular weight of ∼13 kDa. In theory, rshMBL1P1 is able to form higher oligomeric structures by the formation of a coiled-coil in the neck region followed by cysteine disulfide bonding in the N-terminal region. Suggestive sizes of the oligomerized structures of the human rshMBL1P1 protein under dissociating conditions are shown in Figure 3A and B.
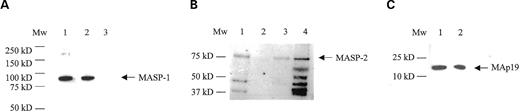
To confirm the specificity of the bands seen by silver stained SDS–PAGE, western blotting of rbMBL-1 and rshMBL1P1 was conducted and probed with either a baboon MBL-1 specific antibody recognizing the baboon MBL1 CRD region or an anti-His antibody recognizing the rshMBL1P1 incorporated His-tag. Identical patterns of protein oligomerization were detected by immunoblotting (Fig. 3C) and silver stained SDS–PAGE (Fig. 3A).
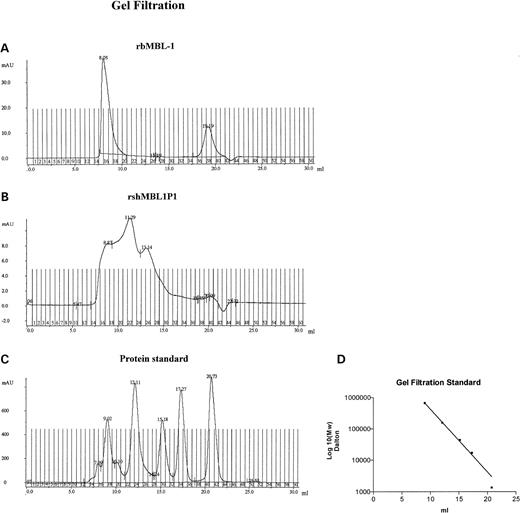
By non-dissociating gel filtration (Fig. 4), rbMBL-1 eluted in one major peak corresponding to a molecular weight of ∼750 kDa and a minor peak probably representing denaturated protein. rshMBL1P1 eluted in three major peaks corresponding to molecular weights of ∼650, 250 and 75 kDa. By coating the eluted fractions on microtiter plates, the protein specificity of the peaks obtained by gel filtration was verified in ELISA using antibodies specific for either baboon MBL-1 or spliced human MBL1P1 (Biosource). Although these antibodies generated a high background in ELISA, ∼50% elevated absorbance was detected in the major peak fractions when compared with background (data not shown).
Functional analysis of rbMBL-1
The binding of human MASP-1 and MASP-2 to rbMBL-1 was investigated by an MASP interaction assay. Recombinant baboon MBL-1 (rbMBL-1) was able to interact with both MASP-1 and MASP-2 from human serum as shown by the western blots in Figure 5A and B, respectively. In Figure 5A, MASP-1 interaction is seen as a band of ∼85 kDa. A similar band pattern was observed when rbMBL-1 was replaced with normal human serum that includes functional MBL-2. No interaction was observed when the empty transfected vector construct pEDdC was used. Directed against the N-terminal part of MASP-2, the MASP-2 antibody recognized both MASP-2 and MAp19. MASP-2 and MAp19 interactions with rbMBL-1 were seen as bands of ∼69 kDa (Fig. 5B) and 19 kDa (Fig. 5C), respectively. Similar banding patterns of MASP-2 interaction were detected with both human serum and purified clinical grade MBL-2 (a kind gift from Dr Claus Koch, SSI), which contain associated MASP-2. No interaction was observed when incubated with the empty transfected vector construct pEDdC.
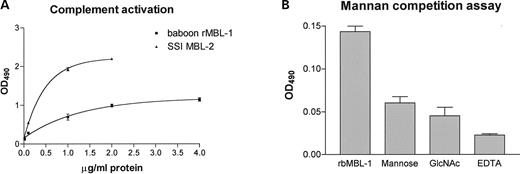
Upon binding to mannan-coated surfaces, rbMBL-1 was capable of activating complement and was shown to be a Ca2+-dependent protein with binding specificities towards both N-acetylglucosamine and mannose as measured by EDTA inhibition of complement activity and decrease in complement activity by mannan competition. Thus, rbMBL-1 seems to possess properties similar to human MBL-2, but does not activate complement as strongly as purified MBL-2. The graphic representation of complement activation and inhibition are shown in Figure 6A and B, respectively.
DISCUSSION
Previously, we described the MBL2 haplotypes that have been identified in humans. These seven haplotypes were found to constitute a balanced interplay between structural and regulatory polymorphisms (18,19). In the present study, DNA sequencing of the human and primate MBL2 promoter and coding regions clearly indicated that the established present-day human MBL2 haplotypes were developed from an ancient MBL2 haplotype, derived in the evolutionary gap between the high-expressing LYQA haplotype and the intermediate-expressing LYPA haplotype, thereby still supporting the theory that human MBL2 has evolved from a high-expressing haplotype into low-expressing and defective haplotypes (Fig. 1). It is yet unknown whether there are human haplotypes that like the primates are intermediates between the LYQA and the LYPA haplotypes, but such haplotypes still have not been reported because they are probably lost during evolution. Recently similar findings were reported relating the oldest haploptype to LYQA and LYPA (30).
The MBL genes, MBL1P1 and MBL2, are the most likely products of a gene-duplication event (23). Upon prehistoric gene duplication, MBL1 and MBL2 were probably functionally redundant, placing both under a selection pressure to reduce their joint activity to that of a single ancestral gene. In the classical model of gene duplication, one of the copies will degenerate to a pseudogene, typically by premature stop codons and frameshift mutations that prevent their expression, whereas the other copy is placed under purifying selection. Alternatively, the sub-functionalization model proposes that gene copies acquire independent complementary loss-of-function mutations, such that both genes are required to produce the functions obtained by the single ancestral gene (31). Although speculative, the presence of polymorphisms in the regulatory region of MBL2 (and perhaps also MBL1) and mutations specifically in the Gly encoding sequences of both genes favor this model for an early outcome of MBL1 and MBL2 in gene duplication. Figure 7 illustrates the evolutionary path of MBL1 and MBL2 from gene duplication to subsequent gene silencing.
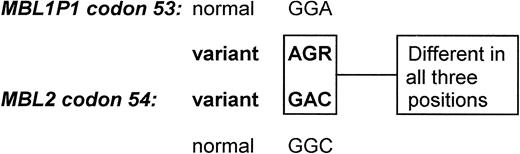
The finding of a correctly spliced human MBL1P1 transcript and the presence of several glycine substitutions in the coding sequence of the chimpanzee MBL1 indicate that this specific way of change has been the primary event in MBL1 silencing, which is also supported by the similar glycine substitutions found in MBL2. The mutations leading to the substitution of glycine residues are probably introduced by specific but independent events. The most likely introduction of an analog mutation in two analog genes is through a gene-conversion event. In case of the human MBL1P1 and MBL2 genes, the sequences of the mutated homologous codons (codons 53 and 54, respectively) differ in all three nucleotide positions (Fig. 8), which make it very unlikely that gene conversion is the responsible event.
Assessing the mutational frequency of the individual amino acid positions present in the chimpanzee Gly-X-Y repeats further support that the glycine residues of the collagen-like region represent the primary targets in MBL1 gene silencing. Although not significant, higher prevalence of Gly substitutions (5/19 repeats) is found when compared with substitutions in the X (1/19 repeats) and Y (3/18 repeats) positions of the collagene-like repeat structure.
Because only one individual of each monkey species was analyzed, the actual frequency of homozygous and heterozygous MBL1 and MBL2 substitutions could not be estimated, however, as several monkey species were investigated, this does not interfere with our results on MBL2 haplotype origin and the overall high frequency of glycine substitutions present in the higher primates.
The effect of these glycine substitutions on MBL-1 protein function is unknown. Because all of these substitutions are situated in the collagenous backbone of the protein, probably, they all result in low-molecular weight proteins with profound reduced avidity for ligands and reduced capacity to activate complement as shown in the case of the human MBL-2 analog that was recently produced recombinantly with naturally occurring glycine substitutions in codons 54 and 57 and artificial glycine substitutions introduced in codons 51 and 60 (32).
The splicing defect and the introduced stop codons in MBL1P1 are probably secondary events in MBL1 silencing towards pseudogene degeneration, which is indicated by the discovery of a correctly spliced MBL1P1 transcript and by the absence of an exon 3 stop codon in some of the analyzed individuals.
Transcriptionally active and expressed pseudogenes have been reported (33,34). Furthermore, some pseudogenes have been suggested to play a role in the regulation of their derived genes, as recently described for the murine makorin1-p1 pseudogene that was shown to have a biological function in regulating the expression of its normal counterpart makorin1 by competitive interaction at either the RNA or the DNA level (35,36). Whether the MBL1P1 pseudogene or the truncated spliced MBL1P1 transcript has a function or is a remnant of the evolution remains unknown. We find that the MBL1P1 pseudogene is transcribed abundantly in a most tissues (data not shown), which most likely indicates that control of transcription has been lost because of lack of function of the gene. A similar broad transcription pattern has been described for the human 𝛉-defensin pseudogene that contains a premature stop codon in humans and in higher primates but is intact and tissue specifically transcribed in the rhesus monkey (37). The spliced MBL1P1 transcript was detected upon 40 cycles of PCR amplification suggesting that the mRNA is present in very low amounts. Action by the nonsense-mediated mRNA decay pathway (38), targeting premature mRNA for rapid decay, might explain the low abundance of this transcript that include a premature stop codon in exon 3 as well as the inability to amplify transcripts containing the exon 4 stop codon. The recombinant protein encoding the N-terminal part, the collagen-like region and a neck region seem to assemble into some higher oligomeric forms. Future studies will show whether the recombinant produced protein is able to interact with the MASPs and potentially exert a competitive function in vivo, although the presence of a MBL1P1 codon 53 Gly mutation, which is analogous to the MBL2 codon 54 Gly mutation, disagree with MASP interaction.
With purpose to examine the potential functions of an ancient human MBL-1 protein, we produced recombinant baboon MBL-1 and investigated the basic structural and functional characteristics. The recombinant protein was shown to obtain overall characteristics similar to human MBL-2 by exhibiting Ca2+-dependent binding specificity towards mannan, mannose and N-acetylglucosamine, assembled into higher oligomeric structures and was able to interact with both MASP-1 and MASP-2 from human serum and activate complement. Gel filtration was used to estimate the oligomeric state of the recombinant proteins under non-dissociating conditions and both rbMBL-1 and rshMBL1P1 was shown to assemble into higher oligomeric forms, however, the exact molecular weight of these structures awaits further investigation by sucrose density gradient centrifugation and mass spectrometry.
Although the study on recombinant baboon MBL-1 does not resolve whether MBL-1 and MBL-2 obtain complementary or diverse effector functions in pathogen recognition, their similar structure and function as well as silencing pattern suggest that the proteins prehistorically were functionally redundant.
In a recent study on MBL2 evolution in primates (39), high conservation of the molecule was found in all non-human primates rejecting the hypothesis that a positive selection pressure has acted on MBL by direct interaction with different pathogens during evolution. In contrast, a second study (30) suggested that MBL2 had been under recent selective pressure. Evolutionary duplication, diversification and selection have been described for several genes, including the β-defensin genes (40,41) and the olfactory receptor (OR) genes (42). Positive selection of the β-defensin genes probably occurred in response to pathogen evolution, although reduced chemosensory dependence promoted the purifying selection that has acted on the human OR gene repertoire, increasing the number of OR pseudogenes from 28–36% in primate species to >60% in man. In the present study, we included the MBL1P1 pseudogene in our investigations. We found that glycine specific substitutions, probably introduced by specific but independent events, have occurred not only in human MBL2 but also in MBL1 throughout evolution and most likely before pseudogene degeneration, indicating that selective pressure has acted on these genes.
In conclusion, our data indicate that MBL1 and MBL2 have been selectively silenced by the same molecular mechanisms; ultimately down-regulating MBL levels in present-day man. This is consistent with the hypothesis that a selective advantage of low MBL levels has evolved during evolution.
MATERIALS AND METHODS
Subjects, samples and cell lines
DNA was prepared from human blood samples collected from individuals originating from Denmark, Mozambique and South America as previously described (19). DNA from 10 unselected individuals in each population was used for the analysis of the MBL1P1 gene. One individual of each monkey species was analyzed. Primate DNA was extracted from commercially available ATCC cell lines CRL-1854, CRL-1609, CRL-6315, CRL-1850, CRL-1495, CCL-70 and CRL-1688.
The CHO DG44 cell line was a kind gift from Professor Lawrence Chasin, Columbia University, New York (43). The pEDdC expression vector was kindly supplied by The Genetics Institute, Cambridge (44,45). All media and supplements were purchased from Sigma-Aldrich. Oligonucleotides were purchased from DNA technology, Denmark.
Amplification and sequencing
Four parallel PCR reactions were made for each of the amplifications with the PCR primers listed in Supplementary Material, Table S1. Standard PCR reactions were performed by 35 cycles of amplification at 58°C annealing temperature and included Platinum Taq DNA-polymerase (Invitrogen) and ∼0.2 µg DNA. Identical PCR products were pooled and sequenced by internal priming under standard conditions using the BigDye terminator v3.0 cycle sequencing ready reaction mix (Applied Biosystems). Sequencing products were purified with Genoprep magnetic streptavidin beads (Genovision), and the DNA sequencing was performed using the ABI3100 Genetic Analyzer (Applied Biosystems).
Discovery of a correctly sh-MBL1P1
Amplification of the human MBL1P1 pseudogene (primer 5′-CCTGTGCAGATACCCAGAAGACCTG-3′and 5′-TGGTCCAGTTCTGATCTCAGGCTCTAT-3′) from a panel of different human tissue cDNAs (Clontech) revealed a weak product with smaller molecular weight size in addition to the expected unspliced product. This product, appearing upon 40 cycles of amplification under the PCR conditions described earlier, was detected in cDNA from both liver and lung tissues. After agarose gel separation and purification using the QiaExII gel extraction kit (Qiagen), the product was TA cloned into the pCR 2.1-TOPO vector and subsequently DNA sequenced with vector primers as described earlier.
rMBL1 expression constructs
sh-MBL1P1 was amplified from liver cDNA (Clontech) by a sense primer introducing an XbaI sequence for cloning purpose 5′-GGTTCTAGAGCCATGTTCCTGTTTCCATCATTCCC-3′, and an anti-sense primer that in addition to XbaI contained a sequence encoding a C-terminal His6-tag 5′-AGGTCTAGACTAATGGTGATGGTGATGATGTAGCTTTCTCTCTAAGTTGGC-3′. The PCR product, obtained upon 40 cycles of amplification as described earlier, was TA cloned into the pCR 2.1-TOPO vector, excised by XbaI restriction digest and subsequently ligated into the XbaI linearized pEDdC expression vector. DNA sequencing using biotinylated pEDdC vector primers (5′-TGTGGCAGGCTTGAGATC-3′ and 5′-CTCGTCAAGAAGACAGGG-3′) verified the expression construct. Baboon MBL1 was amplified from a baboon cDNA library (Stratagene) with sense primer 5′-TTTGTTCTAGATTATGTTCCTGTTTCCGTCATTCCCTGTC-3′ and an anti-sense primer 5′-TTAAATCTAGATCAGGCAGGAAACTCACAGACGGC-3′ both introducing XbaI restriction sites for cloning purpose. The PCR was performed with 32 cycles of amplification and the expression construct prepared and verified as described earlier.
Production and purification of recombinant protein
The dihydrofolatereductase deficient Chinese hamster ovary cell line CHO DG44 was used for stable transfection using the pEDdC expression vector. 2×106 confluent cells were co-transfected with 8 µg expression vector construct and 0.2 µg pSVneo plasmid containing a neomycin selectable marker (G418) (Stratagene) using 20 µl lipofectamine 2000 reagent according to the manufacturer's protocol (Invitrogen). Cultivation, selection and gene amplification steps were performed as recently described (32). Stable transfectants were grown to a very high cell density before MTX-selection media were replaced. Media containing the recombinant protein of interest were centrifuged 5 min. at 4000g to remove cell debris and was stored at −80°C before purification. An aliquot of 100 ml media containing baboon MBL-1 was incubated ON at 4°C with 5 ml mannan-agarose bead suspension (Sigma-Aldrich) in 100 ml binding buffer containing 50 mm Tris–HCl pH 7.5, 150 mm NaCl and 10 mm CaCl2 pH 7.8. The suspension was collected on a column (Bio-Rad), washed with 200 ml binding buffer and the protein eluted from the beads by adding 4×5 ml elution buffer containing 50 mm Tris–HCl pH 7.5, 150 mm NaCl and 10 mm EDTA pH 8.0. The elutes were pooled and dialyzed (spectra pore membrane, MWCO: 10 000, Pierce) against 10 mm Tris–HCl pH 7.5, 150 mm NaCl containing 5 mm CaCl2. The protein was concentrated on Centricon Plus-20 (MWCO: 10 000) spin columns as described by the manufacturer (Milipore). Media containing spliced human MBL1P1 were incubated ON at 4°C with Ni-NTA superflow beads and was similarly processed using buffers recommended by the supplier (Qiagen). Elute was dialyzed against 10 mm Tris–HCl pH 7.5, 150 mm NaCl containing 5 mm CaCl2 and the protein was concentrated (MWCO: 5000). Approximately 1.5 ml of each recombinant protein was obtained with concentrations ranging from 250 to 500 µg/ml.
Gel filtration
Gel filtration was conducted using the Äkta purifier 900 (Pharmacia Biotech). A 0.1 mg purified rbMBL-1 or rhsMBL1P1 was fractionated on a Superdex 200 column (300×10 mm2 and flow rate 0.5 ml/min) equilibrated in buffer containing 10 mm Tris and 150 mm NaCl pH 7.4. The void volume of the column was by the Blue dextran 2000 standard (Amersham Biosciences) determined to 7.73 ml. The Bio-Rad gel filtration standard (Bio-Rad) was used to estimate the molecular weight of the recombinant proteins.
SDS–PAGE and immunoblotting
Recombinant proteins were analyzed by SDS–PAGE using NuPAGE 4–12% bis-Tris gels and MES running buffer or 3–8% Tris acetate gels and Tris acetate running buffer (NOVEX). Proteins were detected by silver staining (Silver Express, Novex) or by western blotting using Hybond ECL nitrocellulose membranes (Amersham Biosciences) and NuPAGE transfer buffer. Blots were probed using either a polyclonal baboon MBL-1 antibody (Biosource) or an anti-His-HRP antibody (Qiagen) recognizing the rshMBL1P1 incorporated His6-tag. Immunoblots were developed using West Femto Chemiluminescent Substrate (Pierce). Molecular sizes of the proteins were estimated from the Precision or Precision Plus Protein standards (Bio-Rad).
Functional assays
Complement activation
MaxiSorp microtiter plates (NUNC) were coated ON at 4°C with 0.1 g/l mannan in buffer containing 15 mm Na2CO3 and 35 mm NaHCO3 pH 9.6. Uncoated binding sites were blocked by 1 h incubation at RT with 1 mg/ml bovine serum albumine (BSA) in 10 mm Tris–HCl pH 7.5 and 150 mm NaCl. Plates were washed five times in MBL buffer containing 4.0 mm sodium barbital, 0.15 m NaCl, 2.6 mm CaCl2, 2.12 mm MgCl2 pH 7.4 and 0.05% Tween-20. The wells were incubated ON at 4°C with recombinant protein diluted in MBL buffer, and the washing procedure was repeated. As a source of complement and MASP components, 1% MBL deficient serum (genotype HYPD/HYPD) diluted in 10 mm Tris–HCl, 150 mm NaCl containing 5 mm CaCl2 and 0.05% Tween-20 was added to the wells and incubated 1 h at 37°C. Between subsequent steps, washes were performed five times in MBL buffer. Dilutions were made in the same buffer. Incubation for 1 h at 37°C with a 1:10 000 dilution of rabbit anti-human C4 antibody (DAKO, Denmark) was followed by 1 h incubation at 37°C with a 1:2000 dilution of HRP-linked anti-rabbit Ig antibody (Amersham Biosciences). The reaction was developed using OPD tablets as described by the manufacturer (DAKO) and absorbance was registered at 490 nm using a Dynatech MR5000 ELISA reader.
The sugar binding specificity of the recombinant baboon MBL-1 was assessed through a mannan competition assay made by adding either 100 mm mannose, 100 mmN-acetylglucosamine or 10 mm EDTA to the recombinant protein before incubation in mannan-coated wells. Binding to sugar molecules was interpreted by a decrease in complement activity measured as described earlier.
MASP-binding
MaxiSorp microtiter plates (NUNC) were coated ON at 4°C with 0.1 g/l mannan in buffer containing 15 mm Na2CO3 and 35 mm NaHCO3 pH 9.6. Uncoated binding sites were blocked by 4 h incubation at 4°C with 5% BSA in a binding buffer containing 50 mm Tris–HCl pH 7.5, 150 mm NaCl and 25 mm CaCl2. Plates were washed three times in binding buffer containing 1 mg/ml BSA and recombinant protein was incubated in the wells ON at 4°C. The wash was repeated and the wells were incubated 2 h at 4°C with 10% MBL deficient serum diluted in BSA containing binding buffer. The wells were washed three times in an ice cold BSA containing binding buffer, and bound protein was eluted by adding NuPAGE LDS sample buffer (Novex). The samples were analyzed for MASP-1 and MASP-2 binding by non-reducing SDS–PAGE (4–12% bis-Tris SDS–PAGE in MES running buffer) and western blot probing utilizing either a monoclonal anti-human MASP-1 antibody (2B11, Dr Misao Matsushita, Tokai University, Japan) or a monoclonal anti-human MASP-2 antibody (a kind gift from Dr Claus Koch, SSI, Denmark), followed by incubation with HRP linked anti-mouse Ig antibody (DAKO, Denmark).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG Online.
ACKNOWLEDGEMENTS
We thank Bjarke E. Hansen for his invaluable assistance in the gel filtration experiment. We are grateful for the excellent technical assistance of Ms Bente Frederiksen and Vibeke Weirup. This work was supported by The Novo Nordisk Foundation, The Danish Medical Research Council (No. 9802472), The Copenhagen Hospital Corporation (H:S) and EU grant (QLGI-CT-2001-01039).
Conflict of Interest statement. None declared.
Figure 1. (A) Important polymorphic regions in the upstream regulatory part of the MBL2 gene. (B) Evolution of the MBL2 haplotypes.
Figure 2. Important amino acid substitutions in MBL‐1.
Figure 3. Structural analysis of recombinant proteins. (A) Non-reduced and (B) reduced silver stained SDS–PAGE, respectively. Lane 1: recombinant baboon MBL-1; lane 2: recombinant spliced human MBL1P1; lane 3: recombinant MBL-2 (32); and lane 4: purified MBL-2 (SSI). (C) Western blot analyses of recombinant baboon MBL-1 (rbMBL-1) and recombinant spliced human MBL1P1 (rshMBL1P1), respectively. Samples were run on non-reduced SDS–PAGE and was western blot probed using either an anti-rbMBL-1 specific antibody or a penta-His-HRP antibody directed against the C-terminal rshMBL1P1 incorporated His6-tag.
Figure 4. Gel filtration of recombinant proteins. (A) Recombinant baboon MBL-1, (B) recombinant spliced human MBL1P1, (C) Bio-Rad gel filtration protein standard (peak 1: 670 000 kDa, peak 2: 158 000 kDa, peak 3: 44 000 kDa, peak 4: 17 000 kDa and peak 5: 1350 kDa) and (D) Molecular weight standard curve of Bio-Rad gel filtration protein standard.
Figure 5. MASP interaction with rbMBL-1. Samples were run on non-reducing SDS–PAGE and were western blot probed using either an anti-MASP-1 antibody (A) or an anti-MASP-2 antibody (B and C). (A) MASP-1 interaction shown by a band of ∼83 kDa. Lane 1: recombinant baboon MBL-1, lane 2: human serum pool, lane 3: empty transfected vector pEDdC. (B) MASP-2 interaction shown by a band of ∼75 kDa. Lane 1: recombinant baboon MBL-1, lane 2: empty transfected vector pEDdC, lane 3: human serum pool, lane 4: purified MBL-2 (SSI). (C) MAp19 interaction shown by a band of ∼20 kDa. Lane 1: recombinant baboon MBL-1 and lane 2: recombinant MAp19 diluted 1:100.
Figure 6. Recombinant baboon MBL-1 complement activity and binding specificity. (A) Complement activity of recombinant baboon MBL-1 and purified serum MBL-2 from SSI was measured by C4 deposition and registered by OD490 in ELISA. (B) The sugar binding specificity of recombinant baboon MBL-1 was measured by a decrease in complement activity using a mannan-competition assay. Baboon rMBL-1 was either added directly to mannan-coated wells or pre-incubated with either 100 mm mannose, 100 mm GlcNAc or 10 mm EDTA. The data are based on triplicate experiments.
Figure 7. The evolutionary path of MBL1 and MBL2 from ancestral MBL gene duplication to functional gene silencing.
Figure 8. Comparison of MBL1P1 codon 53 and MBL2 codon 54 in man. It is unlikely that the mutations disrupting the Gly-X-Y structures have occurred through an accidental gene conversion between the MBL1P1 and the MBL2 genes as the nucleotides differ between the genes in all three codon positions. R=A or G.
References
Ghiran, I., Barbashov, S.F., Klickstein, L.B., Tas, S.W., Jensenius, J.C. and Nicholson-Weller, A. (
Ogden, C.A., deCathelineau, A., Hoffmann, P.R., Bratton, D., Ghebrehiwet, B., Fadok, V.A. and Henson, P.M. (
Stover, C.M., Thiel, S., Thelen, M., Lynch, N.J., Vorup-Jensen, T., Jensenius, J.C. and Schwaeble, W.J. (
Thiel, S., Petersen, S.V., Vorup-Jensen, T., Matsushita, M., Fujita, T., Stover, C.M., Schwaeble, W.J. and Jensenius, J.C. (
Ambrus, G., Gal, P., Kojima, M., Szilagyi, K., Balczer, J., Antal, J., Graf, L., Laich, A., Moffatt, B.E., Schwaeble, W. et al. (
Hajela, K., Kojima, M., Ambrus, G., Wong, K.H., Moffatt, B.E., Ferluga, J., Hajela, S., Gal, P. and Sim, R.B. (
Rossi, V., Cseh, S., Bally, I., Thielens, N.M., Jensenius, J.C. and Arlaud, G.J. (
Vorup-Jensen, T., Petersen, S.V., Hansen, A.G., Poulsen, K., Schwaeble, W., Sim, R.B., Reid, K.B., Davis, S.J., Thiel, S. and Jensenius, J.C. (
Dahl, M.R., Thiel, S., Matsushita, M., Fujita, T., Willis, A.C., Christensen, T., Vorup-Jensen, T. and Jensenius, J.C. (
Sastry, K., Herman, G.A., Day, L., Deignan, E., Bruns, G., Morton, C.C. and Ezekowitz, R.A. (
Taylor, M.E., Brickell, P.M., Craig, R.K. and Summerfield, J.A. (
Turner, M.W. (
Thielens, N.M., Cseh, S., Thiel, S., Vorup-Jensen, T., Rossi, V., Jensenius, J.C. and Arlaud, G.J. (
Wallis, R. and Drickamer, K. (
Lipscombe, R.J., Sumiya, M., Hill, A.V., Lau, Y.L., Levinsky, R.J., Summerfield, J.A. and Turner, M.W. (
Madsen, H.O., Garred, P., Kurtzhals, J.A., Lamm, L.U., Ryder, L.P., Thiel, S. and Svejgaard, A. (
Sumiya, M., Super, M., Tabona, P., Levinsky, R.J., Arai, T., Turner, M.W. and Summerfield, J.A. (
Madsen, H.O., Garred, P., Thiel, S., Kurtzhals, J.A., Lamm, L.U., Ryder, L.P. and Svejgaard, A. (
Madsen, H.O., Satz, M.L., Hogh, B., Svejgaard, A. and Garred, P. (
Turner, M.W. and Hamvas, R.M. (
Garred, P., Harboe, M., Oettinger, T., Koch, C. and Svejgaard, A. (
Garred, P., Madsen, H.O., Kurtzhals, J.A., Lamm, L.U., Thiel, S., Hey, A.S. and Svejgaard, A. (
Sastry, R., Wang, J.S., Brown, D.C., Ezekowitz, R.A., Tauber, A.I. and Sastry, K.N. (
White, R.A., Dowler, L.L., Adkison, L.R., Ezekowitz, R.A. and Sastry, K.N. (
Guo, N., Mogues, T., Weremowicz, S., Morton, C.C. and Sastry, K.N. (
Hansen, S., Thiel, S., Willis, A., Holmskov, U. and Jensenius, J.C. (
Liu, H., Jensen, L., Hansen, S., Petersen, S.V., Takahashi, K., Ezekowitz, A.B., Hansen, F.D., Jensenius, J.C. and Thiel, S. (
Takahashi, K., Gordon, J., Liu, H., Sastry, K.N., Epstein, J.E., Motwani, M., Laursen, I., Thiel, S., Jensenius, J.C., Carroll, M. et al. (
Mogues, T., Ota, T., Tauber, A.I. and Sastry, K.N. (
Bernig, T., Taylor, J.G., Foster, C.B., Staats, B., Yeager, M. and Chanock, S.J. (
Prince, V.E. and Bryan Pickett, F. (
Larsen, F., Madsen, H.O., Sim, R.B., Koch, C. and Garred, P. (
Balakirev, E.S. and Ayala, F.J. (
Olsen, M.A. and Schechter, L.E. (
Hirotsune, S., Yoshida, N., Chen, A., Garrett, L., Sugiyama, F., Takahashi, S., Yagami, K., Wynshaw-Boris, A. and Yoshiki, A. (
Nguyen, T.X., Cole, A.M. and Lehrer, R.I. (
Singh, G. and Lykke-Andersen, J. (
Verga Falzacappa, M.V., Segat, L., Puppini, B., Amoroso, A. and Crovella, S. (
Antcheva, N., Boniotto, M., Zelezetsky, I., Pacor, S., Verga Falzacappa, M.V., Crovella, S. and Tossi, A. (
Semple, C.A.M., Rolfe, M. and Dorin, J.R. (
Gilas, Y., Man, O., Pääbo, S. and Lancet, D. (
Urlaub, G., Mitchell, P.J., Kas, E., Chasin, L.A., Funanage, V.L., Myoda, T.T. and Hamlin, J. (
Davies, M.V. and Kaufman, R.J. (
- alleles
- mannose binding lectin
- mutation
- recombinant proteins
- polymorphism
- autoimmune diseases
- collagen
- complement activation
- exons
- genes
- glycine
- haplotypes
- macaca mulatta
- nucleotides
- opsonin
- papio
- primates
- pseudogenes
- rodentia
- genetics
- involution
- complement activation, lectin pathway
- sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- host (organism)