-
PDF
- Split View
-
Views
-
Cite
Cite
Uwe Zeymer, Ralf Zahn, Rudolf Schiele, Wolfgang Jansen, Ernst Girth, Anselm Gitt, Karlheinz Seidl, Rolf Schröder, Steffen Schneider, Jochen Senges, Early eptifibatide improves TIMI 3 patency before primary percutaneous coronary intervention for acute ST elevation myocardial infarction: results of the randomized integrilin in acute myocardial infarction (INTAMI) pilot trial, European Heart Journal, Volume 26, Issue 19, October 2005, Pages 1971–1977, https://doi.org/10.1093/eurheartj/ehi293
Close - Share Icon Share
Abstract
Aims Adjunctive therapy with glycoprotein IIb/IIIa inhibitors has been shown to reduce ischaemic complications and improve clinical outcome in patients with primary percutaneous coronary intervention (PCI) for acute ST elevation myocardial infarction. Little is known about the use of eptifibatide in this setting.
Methods and results One hundred and two patients with ST elevation myocardial infarction <12 h scheduled for primary percutaneous intervention were randomly assigned to early eptifibatide given in the emergency room (early) or optional eptifibatide at the time of PCI (late or no). Primary endpoint was the patency of the infarct vessel before PCI. Patients in the early group received their first eptifibatide bolus, a mean of 45 min before angiography. TIMI 3 patency before PCI was observed in 34% in the early group and 10% in late or no group (P=0.01). The incidence of complete ST resolution 1 h after PCI was 61% in early group and 66% in the late or no group, respectively (P=n.s.). There were no significant differences in the rates of TIMI 3 flow after PCI, death, reinfarction, stroke, and major bleeding complications until day 30.
Conclusion In this pilot trial, double bolus eptifibatide given in the emergency room improved TIMI 3 grade flow of the infarct-related coronary artery before PCI. These results should be confirmed in a larger trial and whether this advantage translates into an improvement in clinical outcome should be tested in a trial with primary clinical endpoints.
Introduction
Acute ST elevation myocardial infarction (STEMI) is generally caused by a thrombotic occlusion of a coronary artery. The primary aim of early therapy is a fast and complete reperfusion of the infarcted myocardium.1,2 This can be achieved by either fibrinolytic therapy or primary percutaneous coronary intervention (PCI). In randomized comparisons, primary PCI is superior to fibrinolysis regarding rates of recanalization of the infarct vessel, preservation of left ventricular, and reduction in the rate of reinfarctions.3,4 Consequently, the in-hospital mortality is lower in patients undergoing primary PCI and it is the preferred reperfusion strategy if performed by skilled investigators. Nevertheless, primary PCI does not always result in a successful myocardial reperfusion despite successful restoration of blood flow in the epicardial infarct-related artery.5–8 Adjunctive therapy with the platelet glycoprotein (GP) IIb/IIIa receptor blocker abciximab improved myocardial reperfusion, left ventricular function, and clinical outcome.9–11 Furthermore, it could be shown that pre-treatment with GP IIb/IIIa inhibitors can result in patency rates of up to 30–40% prior to PCI.12–18 This technically facilitates the intervention and could result in increased TIMI 3 rates and improved microcirculation after PCI. The findings of PAMI and other studies suggest that clinical outcome is improved in patients with TIMI 3 flow before PCI.19 These observations and the result of the PACT study20 suggest an additional benefit from a completely patent infarct vessel before PCI. Distal embolization and impairment of microvascular flow have been well recognized as major problems in PCI.21 Therefore, therapies sought to improve microvascular flow in patients with AMI are under intense clinical investigation.22 In the ESPRIT trial, eptifibatide, a synthetic specific GP IIb/IIIa inhibitor, reduced the rate of thrombotic complications in patients with non-urgent stent implantation.23 This reduction is similar to the reduction of events observed in EPISTENT with abciximab.24 Therefore, we sought to evaluate whether the pre-treatment with eptifibatide improves the TIMI 3 patency rate of the infarct vessel prior to primary PCI compared with placebo and facilitates this intervention.
Methods
Study design
Integrilin in acute myocardial infarction (INTAMI) was a randomized open comparison of early eptifibatide, heparin, and aspirin vs. heparin, aspirin, and optional eptifibatide during PCI in patients with STEMI <12 h scheduled for primary PCI. The study was approved by the Ethics Committee of the Landesärztekammer Rheinland-Pfalz in Mainz, and written informed consent was obtained before randomization.
Patient population
Patients were eligible for inclusion if they were >18 years and presented with an acute STEMI defined with angina or equivalent symptoms >30 min, ST elevation >2 leadings (>2 mm precordial lead, >1 mm limb lead) or ST depression >1 mm precordial lead in posterior MI, new or presumed new LBBB, and PCI were planned. The main exclusion criteria were fibrinolytic therapy within 24 h before randomization, oral anticoagulation with an INR >2, platelets <100 000 or known haemorrhagic diathesis, stroke or TIA within 30 days, evidence of an active gastrointestinal or urogenital bleeding, major surgery within 6 weeks, history of allergic reaction to eptifibatide, severe renal or hepatic insufficiency, contraindication to coronary angiography, severe concomitant disease with life expectation <1 year.
Study procedures
Randomization was stratified by centre in blocks of 10 patients and done with blinded envelopes. After hospitalization and diagnostic confirmation of myocardial infarction, all patients received aspirin (50 mg i.v.) and heparin (5.000 U i.v., immediately followed by infusion 1.000 U/h—target aPTT 50–70 s). Patients randomized to eptifibatide received a double bolus of 180 µg/kg (10 min interval) followed by infusion of 2.0 µg/kg/min >12–24 h as early as possible.
Catheter evaluation and PCI with possible stent implantation were done according to the local guidelines but within 3 h after admission. The continuation of unfractionated heparin post-angiography or procedure was discouraged, but ultimately left to the discretion of the investigator. Clopidogrel was started after PCI with stent, with a loading dose of 300 mg and maintained with a dose of 75 mg daily for at least 30 days.
ST-segment resolution evaluation
Twelve-lead ECGs were recorded at baseline and 60 min (45–75 min) after completion of PCI. The sum of the ST-segment elevation was measured by a central core laboratory without knowledge of treatment assignment as previously described.25,26 As in previous studies, complete resolution was defined as resolution of the initial sum of ST-segment elevation ≥70%. Partial resolution was defined as ST resolution <70–30%, whereas no resolution was defined as ST resolution <30%. In addition, the mean extent of ST resolution was calculated and the rate of patients with ≥50% ST resolution was evaluated.
Angiographic analysis
Angiograms were evaluated centrally and blinded. Patency of the infarct-related artery was evaluated centrally and blinded according to the TIMI criteria.27 The myocardial perfusion grade was assessed as described following the criteria of the TIMI group.28
Endpoints
The primary endpoint was the TIMI 3 patency of the infarct-related coronary artery before PCI. Secondary endpoints were TIMI patency following PCI, ST resolution 60 min after PCI, all-cause death, reinfarction, urgent revascularization, stroke (haemorrhagic, non-haemorrhagic), and severe bleeding complications.
Reinfarction was defined by recurrent signs and symptoms of ischaemia at rest accompanied by new or recurrent ST-segment elevations of ≥0.1 mV in at least two contiguous leads lasting ≥30 min and/or enzyme/biochemical evidence of reinfarction: re-elevation of CK-MB or troponin to above the upper limit of normal and increased by ≥50% over the previous value.
Severe bleeding complications were defined as bleeds that lead to haemodynamic compromise requiring intervention (e.g. blood or fluid replacement, inotropic support, surgical repair) or life-threatening or fatal bleeds, and intracerebral bleedings. Urgent revascularization was defined as recurrent chest pain despite optimized medical therapy leading to an unscheduled revascularization procedure. Stroke was diagnosed based on an imaging study and an expert neurologist opinion. Clinical follow-up for all secondary endpoints was done for 30 days after the index event.
Troponin T and creatinine kinase (CK) levels were obtained on admission. CK values were obtained every 8–12 h during the first 48 h and the peak level was recorded.
Statistics
Descriptive statistics was generated for baseline and clinical demographics, treatment variables, and outcomes. The continuous variables were assessed by the Wilcoxon rank sum test and the values were presented as medians and quartiles. Comparisons between the groups were done with Fisher's exact tests. A P-value of <0.05 was considered significant. The analysis was performed on an intention-to-treat basis. The test for the primary endpoint was two-sided and not adjusted for multiple testing. This was done with the Fisher's exact test after dichotomizing the TIMI patency rates. All other comparisons were performed two-sided and the P-values have to be interpreted in a descriptive sense.
Results
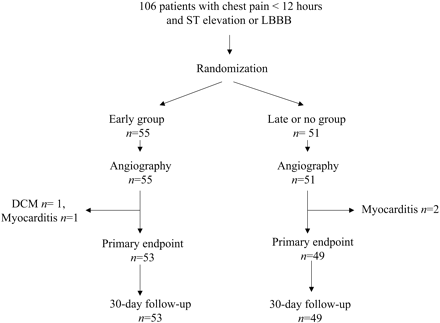
Between October 2002 and April 2004, a total 106 patients were enrolled in three centres into the INTAMI trial. Among these patients, four fulfilled the inclusion criteria, but had acute perimyocarditis (n=3) and dilated cardiomyopathy (n=1) diagnosed by the acute coronary angiography. These patients were excluded from further analysis (Figure 1).
The clinical baseline data of the remaining 102 patients were well balanced between the early eptifibatide group (n=53) and the late or no eptifibatide group (n=49) (Table 1). All patients in the early group and none in the late or no group received double bolus administration of eptifibatide before angiography.
Angiographic findings
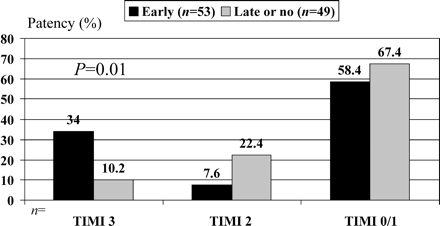
The angiography was performed in all 102 patients. The median time interval between administration of the first eptifibatide bolus and angiograhy was 45 min (Table 2). The angiographic findings are summarized in Table 2. The incidence of a TIMI flow grade 3 of the infarct-related coronary artery before PCI was significantly (P=0.01) higher in the early eptifibatide group (18/53=34%, 95% CI 22.7–47.4%) compared with the late or no eptifibatide group (5/49=10.2%, 95% CI 5.4–20.3%) (Figure 2). In addition, there was a non-significant trend (P=0.06) towards a better patency (TIMI 2/3 flow) of 41.6 (22/53, 95% CI 29.3–54.9%) vs. 32.6% (16/49, 95% CI 21.2–46.6%) and a lower incidence of visible thrombus in the early group. TIMI myocardial perfusion grade 3 was observed in 15/53 (28.3%) and 4/49 (8.2%) (P=0.01) before PCI and 27/52 (51.9%) and 20/47 (42.5%) (P=0.42) after the completion of PCI in the early and late or no groups, respectively.
PCI results
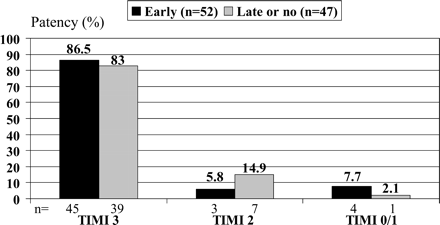
A PCI was performed in 46 (87%) and 46 (94%) of patients in the early and late or no group, respectively. The reasons for not performing PCI were TIMI 3 flow of the infarct vessel and an only moderate or peripheral stenosis in six patients in the early group and one patient in the late or no group, emergency bypass surgery in two patients in the late or no group, and an occluded vessel not suitable for PCI in one patient in the early group, respectively. Eptifibatide during PCI was given to 42 (86%) patients in the late or no group, in three patients in a bail out situation. Eptifibatide in the late or no group was started after the diagnostic procedure before (n=30) or during PCI (n=12). A stent (Boston Express) was implanted in 73 and 74% of patients with PCI in two groups. Emergency coronary artery bypass surgery for severe three-vessel disease was performed in one and two patients in the early and late or no group, respectively. The final TIMI flow grade in patients with and without PCI excluding the patients scheduled for emergency bypass surgery and the patient without PCI attempt is shown in Figure 3.
ST resolution analysis
ST-segment resolution analysis in patients with ECG in the pre-specified time window of 45–75 min after PCI could be performed in 44 and 34 patients in the early and late or no groups, respectively. In Table 3, we included patients seven and eight with evaluable ECG obtained earlier (<45 min) after PCI. Reasons to exclude patients from this analysis were poor quality of the baseline ECG or ECG 2, ECG 2 not obtained, and idioventricular rhythm in ECG 2.
The mean sum of ST-segment elevation at baseline was 7.8 and 8.7 mm in the early and late or no group, respectively. There was no difference in ST resolution between the two groups (Table 3).
In-hospital course and 30 days follow-up
The median CK increased to 899 and 1308 U/L in the early and late or no group, respectively (P=0.4). The eptifibatide infusion was maintained for a median of 16 h in both groups. The concomitant medication during the hospital stay is shown in Table 4.
The total mortality until day 30 was 3.9% and two patients died in both groups. No reinfarction was observed in the late or no group, whereas three reinfarctions occurred in the early group. One was caused by a persistent dissection of the origin of left descending artery and another by occlusion of a coronary artery different from the infarct vessel at the index event because of a heparin induced thrombocytopenia type II on day 7. The clinical events occurring until day 30 are summarized in Table 5.
Discussion
This is the first randomized study to evaluate the effect of early administration of the GP IIb/IIIa inhibitor eptifibatide on patency of the infarct vessel before planned primary PCI in patients with acute ST elevation myocardial infarction. The main finding of this pilot study is that early eptifibatide therapy administered at mean 45 min before angiography seems to improve the rate of pre-interventional TIMI 3 flow.
Previous experience with eptifibatide in STEMI
In a small non-randomized study, eptifibatide given 51 min before angiography was associated with a TIMI 3 flow in the infarct vessel of 33.3% and TIMI 2/3 patency in 56.7%.29 This was higher than the 10% TIMI 3 flow rate in patients from a historical control group from the same institution. In our study, the TIMI 3 patency of 34% was comparable with this report, and was significantly higher than the 11% TIMI 3 patency in the patients receiving just aspirin and heparin before angiography. Therefore, eptifibatide seems to be associated with an ∼20% increase in TIMI 3 flow rate before primary PCI. It has to be noted that the numbers in these studies were small, but the results were comparable. Double bolus administration of eptifibatide induces a rapid and stable level of platelet inhibition within 10–15 min.30 One potential limitation of the earlier administration of GP IIb/IIIa inhibitors could be increased bleeding, but in our trial, no significant differences were observed with respect to major bleeding complications. These results are consistent with the observations in trials using abciximab or tirofiban. However, the number of patients in our study was limited, therefore a potential harmful effect of the early administration cannot be fully excluded.
Myocardial reperfusion and clinical events
The initial advantage of early eptifibatide was not associated with a better myocardial reperfusion indicated by similar extents of ST-segment resolution in both groups. It might well be that eptifibatide has to be administered even earlier in the pre-hospital phase to be beneficial in this respect. Another reason might be that the 20% advantage in TIMI 3 patency before PCI might be not relevant for ST resolution 1 h after completion of the intervention, when TIMI 3 patency was not different between the two groups. However, the extent of ST resolution seen in our study is comparable or even larger when compared with previous reports.7,31–33 Two-third in both groups had >70% ST resolution and >80% of the patients had >50% ST resolution, cut-off points which were associated with an improved clinical outcome in previous studies and, therefore, used in clinical trials. Our results indicate that adjunctive treatment with eptifibatide in primary PCI might improve myocardial reperfusion, which is of utmost importance for in-hospital and long-term outcome.
The 30 days mortality in our trial was low, and there was no difference between the groups with respect to death, reinfarction, and the need for target vessel revascularization. However, our trial was not designed or powered to detect differences in clinical endpoints.
Experience with other GP IIb/IIIa inhibitors in primary PCI
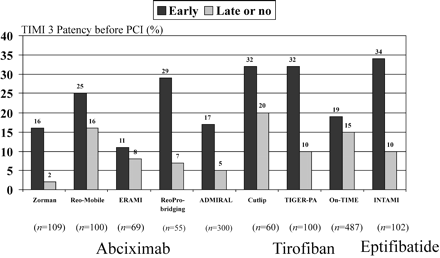
So far, no direct randomized comparison of different GP IIb/IIIa inhibitors in patients with STEMI exists. Pharmacodynamical studies have shown that the level of platelet inhibition seen with abciximab and the double bolus regimen of eptifibatide might be superior to that achieved with tirofiban.34 In a meta-analysis of six randomized trials performed with abciximab or tirofiban, early administration of GP IIb/IIIa inhibitors significantly improved both patency (41.7 vs. 29.8%) and TIMI 3 flow (23 vs. 12%) of the infarct-related coronary artery before planned primary PCI. There was a non-significant reduction of mortality from 4.7 to 3.4% with early when compared with late GP IIb/IIIa inhibitions.35 The rates of TIMI 3 patency before primary PCI in these six randomized trials, two additional trials,10,12 and our study are shown in Figure 4. There was no heterogeneity between the trials. Therefore, it is uncertain if significant differences exist between the three available intravenous GP IIb/IIIa inhibitors when given before planned primary PCI.
Facilitated PCI
Although there is general agreement that primary PCI is the preferred method of reperfusion in patients with STEMI, when it can be performed timely and by experienced operators, the strategy remains limited by the lack of interventional facilities in most medical centres, even in the western countries. In addition, there is always a certain time delay before PCI restores patency of the infarct-related artery in which the necrosis of the myocardium goes on. The rationale behind pre-treatment with fibrinolytics, GP IIb/IIIa inhibitors or both before PCI, the so-called facilitated PCI, is that by providing early reperfusion, myocardium can be salvaged before complete mechanical restoration can be achieved.36 However, so far no study has shown a large improvement in myocardial perfusion before PCI with facilitated PCI. Clearly, the intervention is easier to perform with an already patent infarct vessel.37 Three larger clinical trials (ASSENT-4 PCI, CARESS, and FINESSE) are currently investigating this concept with various regimens.38 The advantage of GP IIb/IIIa inhibitors seems to be the lower rate of intra-cerebral bleeding complications compared with fibrinolysis alone or in combination with GP IIb/IIIa inhibitors. In contrast, the rates of epicardial and myocardial reperfusion prior to PCI are higher with fibrinolysis. It might well be that for different patient groups and time delays to PCI, different strategies are preferable, e.g. fibrinolysis for longer delays until PCI and GP IIb/IIIa inhibitors for shorter delays from presentation until primary PCI, and patients with increased risk for intra-cerebral bleedings. As mentioned before, the study was underpowered to show any significant differences in clinical events. Clearly, normal flow in the infarct-related artery is desirable before primary PCI to facilitate this intervention and to salvage myocardium. However, whether the 20% advantage in TIMI 3 flow associated with the early eptifibatide administration leads to an improvement in clinical outcome needs to be addressed in a larger trial with primary clinical endpoints.
Limitations
This was a pilot study without statistical power calculations regarding the primary and secondary endpoints. Especially it was not powered to detect any differences in the clinical endpoints. Our study was not double-blind and placebo-controlled. However, the evaluation of the efficacy endpoints TIMI patency and ST resolution were made blinded. Owing to the limited number of patients in our study, a type I alpha error for the primary endpoint TIMI patency of the infarct vessel before PCI cannot be excluded. In addition, the safety of the early eptifibatide strategy with respect to bleeding complications can only be assessed in a large clinical study.
Conclusion
In this pilot trial, double bolus eptifibatide given in the emergency room improved TIMI 3 grade flow of the infarct-related coronary artery before PCI. These results should be confirmed in a larger trial and whether this advantage translates into an improvement in clinical outcome should be tested in a trial with primary clinical endpoints.
Acknowledgement
This study was supported by a grant from ESSEX Pharma GmbH, Munich, Germany.
Figure 1 Flow diagram about study patients included in the study and the endpoint analysis.
Figure 2 TIMI flow grade of the infarct-related coronary artery at angiography before planned primary PCI.
Figure 3 Final TIMI flow grade of the infarct-related coronary artery in patients without emergency bypass surgery.
Figure 4 Comparison of the TIMI 3 flow rates before primary PCI in patients treated with early and late or no GP IIb/IIIa inhibitors in nine randomized trials. (Zorman et al.,16 Reo-Mobile,17 ERAMI,18 Reo-Bridging,12 ADMIRAL,10 Cutlip,15 TIGER-PA,14 On-TIME,13 INTAMI.39)
Baseline characteristics of the two study groups
| . | Early (n=53) . | Late or no (n=49) . | P-value . |
|---|---|---|---|
| Demographics | |||
| Mean age (years) | 61+13 | 61+11 | 0.8 |
| Male sex (%) | 79 | 66 | 0.12 |
| Presenting characteristics | |||
| Body mass index | 29+7 | 28+6 | 0.8 |
| Pulse (b.p.m.) | 80+21 | 74+17 | 0.2 |
| Systolic blood pressure (mmHg) | 133+22 | 136+26 | 0.6 |
| Diastolic blood pressure (mmHg) | 77+13 | 79+17 | 0.5 |
| Anterior infarct location (%) | 43 | 39 | 0.6 |
| Troponin T positive on admission (%) | 21 | 28 | 0.8 |
| Killip class >1 (%) | 16 | 14 | 0.9 |
| Medical history | |||
| Smoker | 51% | 41% | 0.3 |
| Hyperlipidaemia | 55% | 67% | 0.2 |
| Diabetes mellitus | 15% | 22% | 0.3 |
| Prior myocardial infarction | 12% | 16% | 0.4 |
| Prior PCI | 4% | 18% | 0.02 |
| Prior CABG | 0% | 2% | 0.3 |
| Prior angina | 19% | 25% | 0.5 |
| . | Early (n=53) . | Late or no (n=49) . | P-value . |
|---|---|---|---|
| Demographics | |||
| Mean age (years) | 61+13 | 61+11 | 0.8 |
| Male sex (%) | 79 | 66 | 0.12 |
| Presenting characteristics | |||
| Body mass index | 29+7 | 28+6 | 0.8 |
| Pulse (b.p.m.) | 80+21 | 74+17 | 0.2 |
| Systolic blood pressure (mmHg) | 133+22 | 136+26 | 0.6 |
| Diastolic blood pressure (mmHg) | 77+13 | 79+17 | 0.5 |
| Anterior infarct location (%) | 43 | 39 | 0.6 |
| Troponin T positive on admission (%) | 21 | 28 | 0.8 |
| Killip class >1 (%) | 16 | 14 | 0.9 |
| Medical history | |||
| Smoker | 51% | 41% | 0.3 |
| Hyperlipidaemia | 55% | 67% | 0.2 |
| Diabetes mellitus | 15% | 22% | 0.3 |
| Prior myocardial infarction | 12% | 16% | 0.4 |
| Prior PCI | 4% | 18% | 0.02 |
| Prior CABG | 0% | 2% | 0.3 |
| Prior angina | 19% | 25% | 0.5 |
Baseline characteristics of the two study groups
| . | Early (n=53) . | Late or no (n=49) . | P-value . |
|---|---|---|---|
| Demographics | |||
| Mean age (years) | 61+13 | 61+11 | 0.8 |
| Male sex (%) | 79 | 66 | 0.12 |
| Presenting characteristics | |||
| Body mass index | 29+7 | 28+6 | 0.8 |
| Pulse (b.p.m.) | 80+21 | 74+17 | 0.2 |
| Systolic blood pressure (mmHg) | 133+22 | 136+26 | 0.6 |
| Diastolic blood pressure (mmHg) | 77+13 | 79+17 | 0.5 |
| Anterior infarct location (%) | 43 | 39 | 0.6 |
| Troponin T positive on admission (%) | 21 | 28 | 0.8 |
| Killip class >1 (%) | 16 | 14 | 0.9 |
| Medical history | |||
| Smoker | 51% | 41% | 0.3 |
| Hyperlipidaemia | 55% | 67% | 0.2 |
| Diabetes mellitus | 15% | 22% | 0.3 |
| Prior myocardial infarction | 12% | 16% | 0.4 |
| Prior PCI | 4% | 18% | 0.02 |
| Prior CABG | 0% | 2% | 0.3 |
| Prior angina | 19% | 25% | 0.5 |
| . | Early (n=53) . | Late or no (n=49) . | P-value . |
|---|---|---|---|
| Demographics | |||
| Mean age (years) | 61+13 | 61+11 | 0.8 |
| Male sex (%) | 79 | 66 | 0.12 |
| Presenting characteristics | |||
| Body mass index | 29+7 | 28+6 | 0.8 |
| Pulse (b.p.m.) | 80+21 | 74+17 | 0.2 |
| Systolic blood pressure (mmHg) | 133+22 | 136+26 | 0.6 |
| Diastolic blood pressure (mmHg) | 77+13 | 79+17 | 0.5 |
| Anterior infarct location (%) | 43 | 39 | 0.6 |
| Troponin T positive on admission (%) | 21 | 28 | 0.8 |
| Killip class >1 (%) | 16 | 14 | 0.9 |
| Medical history | |||
| Smoker | 51% | 41% | 0.3 |
| Hyperlipidaemia | 55% | 67% | 0.2 |
| Diabetes mellitus | 15% | 22% | 0.3 |
| Prior myocardial infarction | 12% | 16% | 0.4 |
| Prior PCI | 4% | 18% | 0.02 |
| Prior CABG | 0% | 2% | 0.3 |
| Prior angina | 19% | 25% | 0.5 |
Angiographic findings before PCI
| . | Early (n=53) (%) . | Late or no (n=49) (%) . | P-value . |
|---|---|---|---|
| Infarct vessel | |||
| Left main | 2 (3.8) | 1 (2.0) | 0.6 |
| LAD | 19 (35.9) | 18 (36.7) | 0.9 |
| CX | 8 (15.1) | 8 (16.3) | 0.9 |
| RCA | 24 (45.3) | 21 (42.9) | 0.8 |
| Bypass graft | 0 | 1 (2.0) | 0.3 |
| Diseased vessels | |||
| 1 | 17 (32.1) | 12 (24.5) | |
| 2 | 22 (41.5) | 16 (32.7) | 0.3 |
| 3 | 14 (26.4) | 21 (42.9) | |
| Visible thrombus or fresh occlusion before PCI | 30 (57.7) | 34 (70.8) | 0.1 |
| . | Early (n=53) (%) . | Late or no (n=49) (%) . | P-value . |
|---|---|---|---|
| Infarct vessel | |||
| Left main | 2 (3.8) | 1 (2.0) | 0.6 |
| LAD | 19 (35.9) | 18 (36.7) | 0.9 |
| CX | 8 (15.1) | 8 (16.3) | 0.9 |
| RCA | 24 (45.3) | 21 (42.9) | 0.8 |
| Bypass graft | 0 | 1 (2.0) | 0.3 |
| Diseased vessels | |||
| 1 | 17 (32.1) | 12 (24.5) | |
| 2 | 22 (41.5) | 16 (32.7) | 0.3 |
| 3 | 14 (26.4) | 21 (42.9) | |
| Visible thrombus or fresh occlusion before PCI | 30 (57.7) | 34 (70.8) | 0.1 |
LAD, left anterior descending artery; CX, circumflex artery; RCA, right coronary artery.
Angiographic findings before PCI
| . | Early (n=53) (%) . | Late or no (n=49) (%) . | P-value . |
|---|---|---|---|
| Infarct vessel | |||
| Left main | 2 (3.8) | 1 (2.0) | 0.6 |
| LAD | 19 (35.9) | 18 (36.7) | 0.9 |
| CX | 8 (15.1) | 8 (16.3) | 0.9 |
| RCA | 24 (45.3) | 21 (42.9) | 0.8 |
| Bypass graft | 0 | 1 (2.0) | 0.3 |
| Diseased vessels | |||
| 1 | 17 (32.1) | 12 (24.5) | |
| 2 | 22 (41.5) | 16 (32.7) | 0.3 |
| 3 | 14 (26.4) | 21 (42.9) | |
| Visible thrombus or fresh occlusion before PCI | 30 (57.7) | 34 (70.8) | 0.1 |
| . | Early (n=53) (%) . | Late or no (n=49) (%) . | P-value . |
|---|---|---|---|
| Infarct vessel | |||
| Left main | 2 (3.8) | 1 (2.0) | 0.6 |
| LAD | 19 (35.9) | 18 (36.7) | 0.9 |
| CX | 8 (15.1) | 8 (16.3) | 0.9 |
| RCA | 24 (45.3) | 21 (42.9) | 0.8 |
| Bypass graft | 0 | 1 (2.0) | 0.3 |
| Diseased vessels | |||
| 1 | 17 (32.1) | 12 (24.5) | |
| 2 | 22 (41.5) | 16 (32.7) | 0.3 |
| 3 | 14 (26.4) | 21 (42.9) | |
| Visible thrombus or fresh occlusion before PCI | 30 (57.7) | 34 (70.8) | 0.1 |
LAD, left anterior descending artery; CX, circumflex artery; RCA, right coronary artery.
ST-segment resolution (STR) 30–75 min after completion of PCI
| . | Early (n=51) (%) . | Late or no (n=42) . | P-value . |
|---|---|---|---|
| No STR (<30%) | 8 (15.7) | 4 (9.6) | |
| Partial STR (30–69%) | 12 (23.5) | 10 (23.8) | 0.7 |
| Complete STR (≥70%) | 29 (60.8) | 21 (66.6) | |
| ≥50% STR | 41 (80.4) | 34 (80.9) | 0.9 |
| Median STR | 76.7 | 75.3 | 0.9 |
| . | Early (n=51) (%) . | Late or no (n=42) . | P-value . |
|---|---|---|---|
| No STR (<30%) | 8 (15.7) | 4 (9.6) | |
| Partial STR (30–69%) | 12 (23.5) | 10 (23.8) | 0.7 |
| Complete STR (≥70%) | 29 (60.8) | 21 (66.6) | |
| ≥50% STR | 41 (80.4) | 34 (80.9) | 0.9 |
| Median STR | 76.7 | 75.3 | 0.9 |
ST-segment resolution (STR) 30–75 min after completion of PCI
| . | Early (n=51) (%) . | Late or no (n=42) . | P-value . |
|---|---|---|---|
| No STR (<30%) | 8 (15.7) | 4 (9.6) | |
| Partial STR (30–69%) | 12 (23.5) | 10 (23.8) | 0.7 |
| Complete STR (≥70%) | 29 (60.8) | 21 (66.6) | |
| ≥50% STR | 41 (80.4) | 34 (80.9) | 0.9 |
| Median STR | 76.7 | 75.3 | 0.9 |
| . | Early (n=51) (%) . | Late or no (n=42) . | P-value . |
|---|---|---|---|
| No STR (<30%) | 8 (15.7) | 4 (9.6) | |
| Partial STR (30–69%) | 12 (23.5) | 10 (23.8) | 0.7 |
| Complete STR (≥70%) | 29 (60.8) | 21 (66.6) | |
| ≥50% STR | 41 (80.4) | 34 (80.9) | 0.9 |
| Median STR | 76.7 | 75.3 | 0.9 |
Concomitant therapy until discharge
| Therapy . | Early (n=53) (%) . | Late or no (n=49) (%) . | P-value . |
|---|---|---|---|
| Stent during primary PCI | 73 | 74 | 0.8 |
| Aspirin | 98 | 96 | 0.5 |
| Clopidogrel | 94 | 98 | 0.4 |
| Unfractionated heparin | 87 | 88 | 0.9 |
| Low molecular weight heparin | 81 | 81 | 0.9 |
| Beta-blockers | 87 | 94 | 0.2 |
| ACE- inhibitors/ARBs | 91 | 88 | 0.6 |
| Statins | 91 | 94 | 0.5 |
| Therapy . | Early (n=53) (%) . | Late or no (n=49) (%) . | P-value . |
|---|---|---|---|
| Stent during primary PCI | 73 | 74 | 0.8 |
| Aspirin | 98 | 96 | 0.5 |
| Clopidogrel | 94 | 98 | 0.4 |
| Unfractionated heparin | 87 | 88 | 0.9 |
| Low molecular weight heparin | 81 | 81 | 0.9 |
| Beta-blockers | 87 | 94 | 0.2 |
| ACE- inhibitors/ARBs | 91 | 88 | 0.6 |
| Statins | 91 | 94 | 0.5 |
ACE, angiotensin converting enzyme; ARB, angiotensinogen receptor blocker.
Concomitant therapy until discharge
| Therapy . | Early (n=53) (%) . | Late or no (n=49) (%) . | P-value . |
|---|---|---|---|
| Stent during primary PCI | 73 | 74 | 0.8 |
| Aspirin | 98 | 96 | 0.5 |
| Clopidogrel | 94 | 98 | 0.4 |
| Unfractionated heparin | 87 | 88 | 0.9 |
| Low molecular weight heparin | 81 | 81 | 0.9 |
| Beta-blockers | 87 | 94 | 0.2 |
| ACE- inhibitors/ARBs | 91 | 88 | 0.6 |
| Statins | 91 | 94 | 0.5 |
| Therapy . | Early (n=53) (%) . | Late or no (n=49) (%) . | P-value . |
|---|---|---|---|
| Stent during primary PCI | 73 | 74 | 0.8 |
| Aspirin | 98 | 96 | 0.5 |
| Clopidogrel | 94 | 98 | 0.4 |
| Unfractionated heparin | 87 | 88 | 0.9 |
| Low molecular weight heparin | 81 | 81 | 0.9 |
| Beta-blockers | 87 | 94 | 0.2 |
| ACE- inhibitors/ARBs | 91 | 88 | 0.6 |
| Statins | 91 | 94 | 0.5 |
ACE, angiotensin converting enzyme; ARB, angiotensinogen receptor blocker.
Clinical events until day 30
| Event . | Early (n=53) (%) . | Late or no (n=49) . | P-value . |
|---|---|---|---|
| Death | 2 (3.8) | 2 (4.1%) | 0.9 |
| Reinfarction | 3 (5.7) | 0 | 0.09 |
| Repeat target vessel revascularization | 2 (3.8) | 1 (2.0) | 0.6 |
| PCI different vessel | 4 (7.6) | 4 (8.0%) | 0.9 |
| Coronary artery bypass grafting Emergency CABG | 3 (5.7) | 5 (10.2) | 0.4 |
| 1/3 (1.9) | 2/5 (4.0%) | ||
| Stroke | 0 | 0 | — |
| Severe bleeding complication | 2 (3.8) | 2 (4.1%) | 0.9 |
| Thrombocytopenia <100.000 | 1 (2.0) | 1 (2.0%) | 0.3 |
| Event . | Early (n=53) (%) . | Late or no (n=49) . | P-value . |
|---|---|---|---|
| Death | 2 (3.8) | 2 (4.1%) | 0.9 |
| Reinfarction | 3 (5.7) | 0 | 0.09 |
| Repeat target vessel revascularization | 2 (3.8) | 1 (2.0) | 0.6 |
| PCI different vessel | 4 (7.6) | 4 (8.0%) | 0.9 |
| Coronary artery bypass grafting Emergency CABG | 3 (5.7) | 5 (10.2) | 0.4 |
| 1/3 (1.9) | 2/5 (4.0%) | ||
| Stroke | 0 | 0 | — |
| Severe bleeding complication | 2 (3.8) | 2 (4.1%) | 0.9 |
| Thrombocytopenia <100.000 | 1 (2.0) | 1 (2.0%) | 0.3 |
Clinical events until day 30
| Event . | Early (n=53) (%) . | Late or no (n=49) . | P-value . |
|---|---|---|---|
| Death | 2 (3.8) | 2 (4.1%) | 0.9 |
| Reinfarction | 3 (5.7) | 0 | 0.09 |
| Repeat target vessel revascularization | 2 (3.8) | 1 (2.0) | 0.6 |
| PCI different vessel | 4 (7.6) | 4 (8.0%) | 0.9 |
| Coronary artery bypass grafting Emergency CABG | 3 (5.7) | 5 (10.2) | 0.4 |
| 1/3 (1.9) | 2/5 (4.0%) | ||
| Stroke | 0 | 0 | — |
| Severe bleeding complication | 2 (3.8) | 2 (4.1%) | 0.9 |
| Thrombocytopenia <100.000 | 1 (2.0) | 1 (2.0%) | 0.3 |
| Event . | Early (n=53) (%) . | Late or no (n=49) . | P-value . |
|---|---|---|---|
| Death | 2 (3.8) | 2 (4.1%) | 0.9 |
| Reinfarction | 3 (5.7) | 0 | 0.09 |
| Repeat target vessel revascularization | 2 (3.8) | 1 (2.0) | 0.6 |
| PCI different vessel | 4 (7.6) | 4 (8.0%) | 0.9 |
| Coronary artery bypass grafting Emergency CABG | 3 (5.7) | 5 (10.2) | 0.4 |
| 1/3 (1.9) | 2/5 (4.0%) | ||
| Stroke | 0 | 0 | — |
| Severe bleeding complication | 2 (3.8) | 2 (4.1%) | 0.9 |
| Thrombocytopenia <100.000 | 1 (2.0) | 1 (2.0%) | 0.3 |
References
Simes RJ, Topol EJ, Holmes DR Jr, White HD, Rutsch WR, Vahanian A, Simoons ML, Morris D, Betriu A, Califf RM. Link between the angiographic substudy and mortality outcomes in a large randomised trial of myocardial reperfusion: importance of early and complete infarct artery perfusion.
Anderson JL, Karagounis LA, Califf RM. Metaanalysis of five reported studies on the relation of early coronary patency grades with mortality and outcomes after acute myocardial infarction.
Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials.
GUSTO IIb Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction.
Claeys MJ, Bosmans J, Veenstra L, Jorens P, De Raedt H, Vrints CJ. Determinants and prognostic implications of persistent ST-segment elevation after primary angioplasty for acute myocardial infarction.
Santoro GM, Valenti R, Buonamici P, Bolognese L, Cerisano G, Moschi G, Trapani M, Anoniucci D, Fazzini PF. Relation between ST-segment changes and myocardial perfusion evaluated by myocardial contrast echocardiography in patients with acute myocardial infarction treated with direct angioplasty.
McLaughlin MG, Stone GW, Aymong E, Gardner G, Mehran R, Lansky AJ, Grines CL, Tcheng JE, Cox DA, Stuckey T, Garcia E, Guagliumi G, Turco M, Josephson ME, Zimetbaum P. Prognostic utility of comparative methods for assessment of ST-segment resolution after primary angioplasty for acute myocardial infarction.
Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Carroll JD, Rutherford BD, Lansky AJ. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction.
Neumann FJ, Blasini R, Schmitt C, Alt E, Dirschinger J, Gawaz M, Kastrati A, Schomig A. Effect of glycoprotein IIb/IIIa receptor blockade on recovery of coronary flow and left ventricular function after the placement of coronary-artery stents in acute myocardial infarction.
Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, Boulenc JM, Morice MC, Maillard L, Pansieri M, Choussat R, Pinton P. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction.
Antoniucci D, Rodriguez A, Hempel A, Valenti R, Migliorini A, Vigo F, Parodi G, Fernandez-Pereiva C, Moschi G, Bartorelli A, Santoro GM, Bolognese L, Colombo A. A Randomized trial comparing primary infarct stenting with or without abciximab in acute myocardial infarction.
Gyöngyösi M, Domanovits H, Benzer W, Haugk M, Heinisch B, Sodeck G, Hodl R, Gual G, Bonner G, Wojta J, Laggner A, Glogar D, Huber K. Use of abciximab prior to primary angioplasty in STEMI results in early recanalization of the infarct-related artery and improved tissue reperfusion — results of the Austrian multicentre randomized ReoPro-BRIDGING study.
van't Hoff AWJ, Ernst N, de Boer MJ, de Winter R, Boersma E, Bunt T, Petronio S, Marcel Gosselink AT, Jap W, Hollak F, Hoorntje JC, Suryapranata H, Dambrink JH, Zijlstra F. Facilitation of primary angioplasty by early start of a glycoprotein 2b/3a inhibitor: results of the On-TIME trial.
Lee DP, Herity NA, Hiatt BL, Fearon WF, Rezaee M, Carter AJ, Huston M, Schreiber D, DiBattiste PM, Yeung AC. Adjunctive platelet glycoprotein IIb/IIIa receptor inhibition with tirofiban before primary angioplasty improves angiographic outcomes. Results of the TIGER-PA pilot trial.
Cutlip DE, Ricciardi MJ, Frederick SL, Ling FS, Carrozza JP Jr, Dua V, Garringer J, Giri S, Caputo RP. Effect of tirofiban before primary angioplasty on initial coronary flow and early ST segment resolution in patients with acute myocardial infarction.
Zorman S, Zorman D, Noc M. Effects of abciximab pretreatment in patients with acute myocardial infarction undergoing primary angioplasty.
Arntz HR, Schröder JF, Pels K, Schwimmbeck P, Witzenbichler B, Schultheiis HP. Prehospital versus periprocedural administration of abciximab in STEMI: early and late results from the randomised REO-Mobile study.
Mesquita Gabriel H, Oliveira J, Canas de Silva P, Marquas da Costa, Franca C, Vagueiro C. Early administration of abciximab bolus in the emergency room improves microperfusion after primary percutaneous coronary intervention, as assessed by TIMI frame count: results of the ERAMI trial.
Stone GW, Cox D, Garcia E, Brodie BR, Morice MC, Griffin J, Mattos L, Lansky AJ, O'Neil WW, Grines CL. Normal flow (TIMI-3) before mechanical reperfusion is an independent determinant of survival in acute myocardial infarction. Analysis from the primary angioplasty in myocardial infarction trials.
Ross AM, Coyne KS, Reiner JS, Greenhouse SW, Fink C, Frey A, Moreyra E, Traboulsi M, Racine N, Riba AL, Thompson MA, Rohrbeck S, Lundergan CF. A randomized trial comparing primary angioplasty with a strategy of short-acting thrombolysis and immediate planned rescue angioplasty in acute myocardial infarction: the PACT trial.
Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease.
Roe MT, Ohman EM, Maas ACP, Christenson RH, Mahaffey KW, Granger CB, Harrington RA, Califf RM, Krucoff MW. Shifting the open-artery hypothesis downstream: the quest for optimal reperfusion.
The ESPRIT Investigators. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomized, placebo-controlled trial.
The EPISTENT Investigators. Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with the use of platelet glycoprotein IIb/IIIa blockade.
Schröder R, Zeymer U, Wegscheider K, Neuhaus KL. Comparison of the predictive value of ST segment elevation resolution 90 and 180 minutes after start of streptokinase in acute myocardial infarction. A substudy of the Hirudin for Improvement of Thrombolysis (HIT)-4 Study.
Zeymer U, Schröder K, Wegscheider K, Senges J, Neuhaus KL, Schroder R. ST resolution in a single electrocardiographic lead: a simple and accurate predictor of cardiac mortality in patients with fibrinolytictherapy for acute ST elevation myoardial infarction.
TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings.
Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, Mc Cabe CH, van De Werf F, Braunwald E. The relationship of the TIMI myocardial perfusion grade to mortality following thrombolytic administration.
Cutlip DE, Cove CJ, Irons D, Kalaria V, Le M, Cronmiller H, Caufield L, Pomerantz RM, Ling FS. Emergency room administration of eptifibatide before primary angioplasty for ST elevation acute myocardial infarction and its effect on baseline coronary flow and procedure outcomes.
Tcheng JE, Strony J, Lorenz TJ, O'Shea JC. ESPRIT in context: pharmacology matters!
van't Hof AWJ, Liem A, de Boer MJ, Zijlstra F. Clinical value of 12-lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction.
Claeys MJ, Bosmans J, Veentrstra L, Jorens P, De Raedt H, Vrints CJ. Determinants and prognostic implications of persistent ST-segment elevation after primary angioplasty for acute myocardial infarction: importances of microvascular reperfusion injury on clinical outcome.
Zeymer U, Schröder R, Machnig T, Neuhaus KL. Primary percutaneous coronary intervention accelerates early myocardial reperfusion in comparison to fibrinolytic therapy in patients with acute myocardial infarction.
Batchelor WB, Tolleson TR, Huang Y, Larsen RL, Mantell RM, Dillard P, Davidian M, Zhang D, Cantor WJ, Sketch MH Jr, Ohman EM, Zidar RM, Harrington RA. Randomized comparison of platelet inhibition with abciximab, tirofiban and eptifibatide during percutaneous coronary intervention in acute coronary syndromes. The COMPARE trial.
Montelascot G, Borentain M, Payot L, Collet JP, Thomas D. Early versus late administration of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention of acute ST-segment elevation myocardial infarction.
Waters RE, Mahaffey KW, Granger C, Roe M. Current perspectives on reperfusion therapy for acute ST-segment elevation myocardial infarction: integrating pharmacologic and mechanical reperfusion strategies.
Zijlstra F, Ernst N, de Boer MJ, Nibbering E, Suryapranata H, Hoorntje JC, Dambrink JH, van't Hof AW, Verheugt FW. Influence of prehospital administration of aspirin and heparin on initial pantency of the infarct-related artery in patients with acute ST elevation myocardial infarction.
Di Mario C, Bolognese L, Maillard L, Dudek D, Gambarati G, Manari A, Guiducci V, Patrizi G, Rusconi LC, Piovaccari G, Hibon AR, Belpomme V, Indolfic C, Olivari Z, Steffanino G, Zmudka K, Airoldi F, Panzarasa R, Flather M, Steg PG. Combined Abciximab Reteplase Stent Study in acute Myocardial infarction (CARESS in AMI).