-
PDF
- Split View
-
Views
-
Cite
Cite
W. Kyle Simmons, Alex Martin, Lawrence W. Barsalou, Pictures of Appetizing Foods Activate Gustatory Cortices for Taste and Reward, Cerebral Cortex, Volume 15, Issue 10, October 2005, Pages 1602–1608, https://doi.org/10.1093/cercor/bhi038
Close - Share Icon Share
Abstract
Increasing research indicates that concepts are represented as distributed circuits of property information across the brain's modality-specific areas. The current study examines the distributed representation of an important but under-explored category, foods. Participants viewed pictures of appetizing foods (along with pictures of locations for comparison) during event-related fMRI. Compared to location pictures, food pictures activated the right insula/operculum and the left orbitofrontal cortex, both gustatory processing areas. Food pictures also activated regions of visual cortex that represent object shape. Together these areas contribute to a distributed neural circuit that represents food knowledge. Not only does this circuit become active during the tasting of actual foods, it also becomes active while viewing food pictures. Via the process of pattern completion, food pictures activate gustatory regions of the circuit to produce conceptual inferences about taste. Consistent with theories that ground knowledge in the modalities, these inferences arise as reenactments of modality-specific processing.
Introduction
How are concepts for everyday objects represented in the brain? Based on accumulating lesion and neuroimaging evidence, an object concept is represented as a distributed circuit of property representations across the brain's modality-specific areas (Martin, 2001; Martin and Chao, 2001; Thompson-Schill, 2003). On encountering a physical object, relevant modalities represent it during perception and action. As the object is processed, association areas partially capture property information on these modalities, so that this information can later be reactivated during conceptual processing, when the object is absent (Damasio and Damasio, 1994; Simmons and Barsalou, 2003). Although these conceptual reenactments share important commonalties with mental imagery, there are also important differences. Mental imagery typically results from deliberate attempts to construct conscious vivid images in working memory. In contrast, the perceptual reenactments that underlie conceptual processing often appear to lie outside awareness, resulting instead from relatively automatic and implicit processes. Of primary interest, these reenactments occur in responses to words and other symbols, and play central roles in the representation of conceptual knowledge (Barsalou, 1999, 2003a,b; Barsalou et al., 2003a,b).
The category of tools illustrates the distribution of property representations across modality-specific brain areas. When people use a hammer, a distributed set of brain areas becomes active to represent the hammer's properties, including its visual form (ventral occipitotemporal cortex), the physical actions used to manipulate it (ventral premotor cortex and intraparietal sulcus), and the visual motion that results (middle temporal gyrus) (Beauchamp et al., 2002; Chao et al., 1999; Chao and Martin, 2000; Damasio et al., 2001; Grafton et al., 1997; Handy et al., 2003; Johnson-Frey, 2004; Martin et al., 1995; Perani et al., 1995). As just described, the brain's association areas capture this distributed set of modality-specific states for later conceptual use. On subsequent occasions, when no hammers are present, reenactments of these states represent hammers conceptually (e.g. during language comprehension and thought).
In the experiment reported here, we explored the distributed property account for the category of foods. Foods constitute a central category for humans, not only in perception and action, but in higher cognition (Ross and Murphy, 1999). Previous research on food concepts has addressed the visual properties of fruits and vegetables, relative to the visual properties of other object categories (McRae and Cree, 2002). Here, we focus instead on the tastes of high-caloric, high-fat processed foods, such as cheeseburgers and cookies (see Fig. 1). We focus on taste properties because the tastes of foods are at least as important as their visual appearances. We focus on processed foods because they are central to the modern diet and because they are associated with strong gustatory and appetitive responses that underlie how people select and consume them.
If a distributed circuit of property information represents food knowledge, then viewing a food picture should not only activate brain areas that represent visual properties of the pictured food, but should also activate brain areas that represent how the food is likely to taste and how rewarding it would be to eat. Once one part of the distributed circuit becomes active by viewing the picture, the remainder should become active via the conceptual inference process of pattern completion across the circuit. Given the central role that such inferences play in normal food selection and consumption, it is essential to understand their bases in the brain. Furthermore, given the extensiveness of eating disorders, obesity and other food-related problems, it is important to understand how people generate taste and reward inferences to the broad array of food representations available in modern culture.
We presented pictures of food and non-food entities (location pictures) to subjects undergoing event-related fMRI and predicted that a distributed circuit of brain areas would become active to represent the visual and gustatory properties of the pictured foods. Regarding the visual properties of foods, a large literature demonstrates that ventral temporal regions underlie the representation of objects' visual form properties (Ishai et al., 1999, 2000). Thus, we expected regions of the inferior temporal and fusiform gyri to respond to the distinctive visual properties of the pictured foods. Analogously, location pictures should activate parahippocampal gyrus, given that this region responds to the visual-spatial properties characteristic of buildings and landmarks (Aguirre et al., 1998; Epstein and Kanwisher, 1998; Epstein et al., 1999).
Most importantly, the current study attempted to demonstrate that pictures of visual objects, in this case foods, can produce taste inferences. If the distributed account of concept representation is correct, then multiple modality-specific regions should become active when people represent foods conceptually. Not only should visual areas become active to represent a food's unique visual properties, gustatory areas should become active to represent how the food tastes. Once people access knowledge for a pictured food, an inference is produced about how it tastes. Even though people are not actually tasting the food, their gustatory system becomes active to represent this inference.
Specifically, we predicted that simply viewing pictures of appetizing foods (relative to locations) should activate two brain regions that commonly respond to actual taste stimuli in psychophysical neuroimaging studies (Francis et al., 1999; de Araujo et al., 2003a,b; O'Doherty et al., 2001b). The first area, a region in the insula/operculum, is known to represent how foods actually taste (Rolls et al., 1988; Rolls and Scott, 2003; Scott et al., 1986). The second area, a region in orbitofrontal cortex (OFC), is known to represent the reward values of tastes (Gottfried et al., 2003; Rolls et al., 1989). Here we demonstrate that simply viewing pictures of processed foods activates both brain regions in much the same way that taste stimulants do in psychophysical studies.
Materials and Methods
Subjects
Nine right-handed, native-English-speaking volunteers from the Emory University community participated in the scanning study (six female and three male; age range, 18–45 years). All participants completed a health questionnaire prior to scanning and none of the participants indicated a history of neurological problems. In accordance with protocols prescribed by Emory University's Institutional Review Board, all participants read and signed an informed consent document describing the procedures and possible risks.
Sixteen native-English-speaking volunteers from the Emory community participated in the stimulus selection study (ten female and six male; age 19–46 years). None of these volunteers participated in the later brain imaging experiment. As with the imaging participants, all participants read and signed an informed consent document describing the procedures and possible risks in accordance with protocols prescribed by Emory University's Institutional Review Board.
Experimental Design
Before beginning the brain imaging phase of the study, 32 types of foods and 35 types of locations were selected as candidate materials. The foods (e.g. cheeseburger, spaghetti, cookie, etc.) in the list were chosen because they are all encountered frequently in American society. In addition, only processed foods that are relatively high in fat and calories were used. No fruits or vegetables were included. The locations (e.g. house, mall, school, etc.) in the list were chosen because they are all types of places that participants in the study might visit frequently.
The foods and locations were equated for familiarity by having volunteers (none of whom participated in the brain imaging experiment) provide familiarity ratings for the 35 types of locations, and 32 types of foods. Ratings were made on a 1–7 scale, with 1 indicating that a type of food or location was completely unfamiliar and 7 indicating that it was extremely familiar. Based on these ratings, 15 food and 15 location types were selected such that no reliable familiarity differences existed between the two groups of stimuli. Between six and ten pictures for each type of food and location were then collected.
A group of 16 participants viewed all 259 pictures and rated each for how typical it was of its respective food or location type. Ratings were made on a 1–7 scale, with 1 indicating that a picture was not at all typical of its food or location type and 7 indicating that it was very typical. For each type of food or location, the three most typical pictures were selected for use in the imaging study, thus yielding a total of 90 picture stimuli (45 foods, 45 locations) equated for typicality. All of the food and location pictures depicted non-unique entities that would not be individually recognizable to the participants. Finally, 23 location pictures and 22 food pictures were randomly selected to create phase-scrambled images that were presented during scanning as filler items (see Fig. 1).
During scanning, participants viewed food, location and scrambled pictures. For each picture, participants used a response pad to provide yes/no judgments as to whether it was the same or different as the preceding picture. The pictures were presented in the center of the screen for 2 s each. Interspersed among picture presentations were variable (‘jittered’) interstimulus intervals (mean = 5.7 s, range = 2–20 s) that were included to optimize estimation of the event-related fMRI response. During these interstimulus intervals, participants saw a fixation cross presented in the center of the screen. Participants were instructed that when they saw the fixation cross they should continue attending to the screen and prepare for the next picture presentation.
Prior to beginning data collection, participants performed an abbreviated practice run to insure that they understood the task instructions. Functional data were collected in three scanning runs. The trial lists for the three runs were counterbalanced across participants. During each run, participants saw 16 food and 16 location pictures. Fifteen picture presentations from each category were novel pictures, while one picture was repeated to maintain the participants' attention to the picture repetition detection task. In other words, one location picture and one food picture was repeated in each scanning run. Across the three scanning runs, each subject saw three food picture repetitions and three location picture repetitions. Subjects were told in advance that repeated stimuli would occur in each run. Knowing this and given that the repeated stimuli occurred infrequently, this task requires subjects to pay close attention to each picture presentation to insure that they did not miss a repetition trial. The data from the repetition trials in each run were not analyzed given that they were only included to ensure that participants remained attentive to the task. Subjects were highly accurate at repetition detection (Mean correct = 98.8%, SD = 0.94). Each 5 min 8 s run consisted of 4 min 48 s of the repetition detection task, followed by an additional 20 s rest period.
Image Acquisition and Analysis
Pictures were back-projected onto a screen located at the head of the scanner and were viewed through a mirror mounted on the head coil. Stimulus presentation and response collection was controlled using Presentation software (v. 0.70, www.neurobs.com).
In each of the three imaging runs, 154 gradient echo recalled MR volumes depicting BOLD contrast were collected with a 3 T Siemens Trio scanner. Each volume consisted of 34 contiguous, 2 mm thick slices in the axial plane (TE = 30 ms, TR = 2000 ms, flip angle = 90°, FOV = 192 mm2, 64×64 matrix). Voxel size at acquisition was 3 × 3 × 2 mm, but was 3 × 3 × 3 mm after spatial normalization.
Prior to statistical analyses, image preprocessing was conducted in SPM99 (Wellcome Department of Neurology, UK, http://www.fil.ion.ucl.ac.uk). To reduce motion-related signal changes between volumes, each participant's scans were realigned and resliced using sinc interpolation. Volumes were then normalized to a template EPI scan and finally smoothed in the axial plane using a 6 mm isotropic Gaussian kernel.
Subsequent statistical analyses were also conducted using SPM99. First, individual subjects' data were analyzed using multiple regression. For each subject, event-related changes in neural activity were modeled using a finite impulse response model corresponding to picture stimuli presentation and convolved to the standard SPM hemodynamic response function. Interstimulus fixation periods having variable durations served as the signal baseline. Global effects were removed by proportional scaling and the data were low-pass filtered. Condition effects at the subject level were then assessed with orthogonal contrasts comparing neural activity for food and location pictures. These contrast images, one for each participant, were then analyzed in a second-level random effects analysis of the foods–locations and locations–food contrasts using one sample t-tests. A statistical significance threshold of P < 0.005 (uncorrected for multiple comparisons) and a spatial extent threshold of at least seven contiguous voxels (corresponding to P < 0.05 uncorrected) was used in the random effects analyses.
There are at least two reasons why the use of uncorrected P-values in the present study is warranted. First, the activations reported here were identified using random effects analyses which take into account both within- and between-subjects variance. Not only does this allow the results to be generalized to the population from which subjects were drawn, but it also makes the analyses inherently robust statistically. Secondly, based on much previous research reported in the literature (see Introduction and Discussion), we started with a priori hypotheses that the insula/operculum and OFC would be active in the food–location contrasts. Additionally, given that both food and location pictures depicted common objects, both conditions should activate regions in the ventral temporal cortex known to represent objects' visual form properties. More specifically, however, we predicted that the fusiform/parahippocampal gyrus would be active in the locations–foods contrasts. To be reported here as significant, any other areas of activity would need to be active at the P < 0.05 level with correction for multiple comparisons. No other areas reached this level of statistical significance.
Results
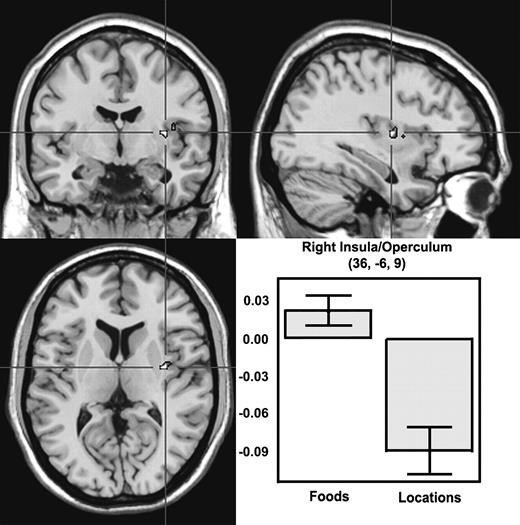
Viewing food pictures for two s in a simple picture-matching task activated gustatory cortex. Specifically, food pictures, relative to location pictures, activated a region of the right insula/operculum, an area that psychophysical research has shown represents the tastes of foods (extent threshold, P = 0.004; see Table 1 and Fig. 2). Importantly, this region was not only significantly more active for food pictures than for location pictures, but it was also reliably activated relative to the fixation baseline (one-tailed, P = 0.033).
Viewing food pictures elicits activity in insula/operculum. A high-resolution anatomical scan showing activity in right insula/operculum associated with viewing pictures of food items. The bar graph displays the average percent signal change in the right insula/operculum cluster for all nine subjects during a period between 4 and 14 s post-stimulus. The y-axis indicates percent signal change relative to signal baseline, with error bars representing ± 1 SEM of the subjects. The data shown in the bar graph were obtained in the random effects contrast of foods > locations with P < 0.005.
Regions showing differential responses to food and location pictures
| Contrast . | Side/location . | MNI coordinates . | . | . | Peak T . | P . | ||
|---|---|---|---|---|---|---|---|---|
. | . | x . | y . | z . | . | . | ||
| Foods > locations | R insula | 36 | −6 | 9 | 5.92 | <0.001 | ||
| L OFC | −21 | 33 | −18 | 6.60 | <0.001 | |||
| L OFC/anterior cingulate | −18 | 45 | −6 | 5.08 | <0.001a | |||
| R inferior temporal gyrus | 48 | −45 | −12 | 5.05 | <0.001 | |||
| R inferior temporal gyrus | 48 | −66 | −9 | 5.99 | <0.001 | |||
| L fusiform | −48 | −60 | −18 | 4.69 | 0.001 | |||
| Locations > foods | L fusiform | −21 | −39 | −12 | 14.50 | <0.001 | ||
| R fusiform | 27 | −42 | −15 | 9.61 | <0.001 | |||
| Contrast . | Side/location . | MNI coordinates . | . | . | Peak T . | P . | ||
|---|---|---|---|---|---|---|---|---|
. | . | x . | y . | z . | . | . | ||
| Foods > locations | R insula | 36 | −6 | 9 | 5.92 | <0.001 | ||
| L OFC | −21 | 33 | −18 | 6.60 | <0.001 | |||
| L OFC/anterior cingulate | −18 | 45 | −6 | 5.08 | <0.001a | |||
| R inferior temporal gyrus | 48 | −45 | −12 | 5.05 | <0.001 | |||
| R inferior temporal gyrus | 48 | −66 | −9 | 5.99 | <0.001 | |||
| L fusiform | −48 | −60 | −18 | 4.69 | 0.001 | |||
| Locations > foods | L fusiform | −21 | −39 | −12 | 14.50 | <0.001 | ||
| R fusiform | 27 | −42 | −15 | 9.61 | <0.001 | |||
L, left; R, right.
While this region was significantly active for food pictures relative to location pictures, it was not reliably active relative to the fixation baseline.
Regions showing differential responses to food and location pictures
| Contrast . | Side/location . | MNI coordinates . | . | . | Peak T . | P . | ||
|---|---|---|---|---|---|---|---|---|
. | . | x . | y . | z . | . | . | ||
| Foods > locations | R insula | 36 | −6 | 9 | 5.92 | <0.001 | ||
| L OFC | −21 | 33 | −18 | 6.60 | <0.001 | |||
| L OFC/anterior cingulate | −18 | 45 | −6 | 5.08 | <0.001a | |||
| R inferior temporal gyrus | 48 | −45 | −12 | 5.05 | <0.001 | |||
| R inferior temporal gyrus | 48 | −66 | −9 | 5.99 | <0.001 | |||
| L fusiform | −48 | −60 | −18 | 4.69 | 0.001 | |||
| Locations > foods | L fusiform | −21 | −39 | −12 | 14.50 | <0.001 | ||
| R fusiform | 27 | −42 | −15 | 9.61 | <0.001 | |||
| Contrast . | Side/location . | MNI coordinates . | . | . | Peak T . | P . | ||
|---|---|---|---|---|---|---|---|---|
. | . | x . | y . | z . | . | . | ||
| Foods > locations | R insula | 36 | −6 | 9 | 5.92 | <0.001 | ||
| L OFC | −21 | 33 | −18 | 6.60 | <0.001 | |||
| L OFC/anterior cingulate | −18 | 45 | −6 | 5.08 | <0.001a | |||
| R inferior temporal gyrus | 48 | −45 | −12 | 5.05 | <0.001 | |||
| R inferior temporal gyrus | 48 | −66 | −9 | 5.99 | <0.001 | |||
| L fusiform | −48 | −60 | −18 | 4.69 | 0.001 | |||
| Locations > foods | L fusiform | −21 | −39 | −12 | 14.50 | <0.001 | ||
| R fusiform | 27 | −42 | −15 | 9.61 | <0.001 | |||
L, left; R, right.
While this region was significantly active for food pictures relative to location pictures, it was not reliably active relative to the fixation baseline.
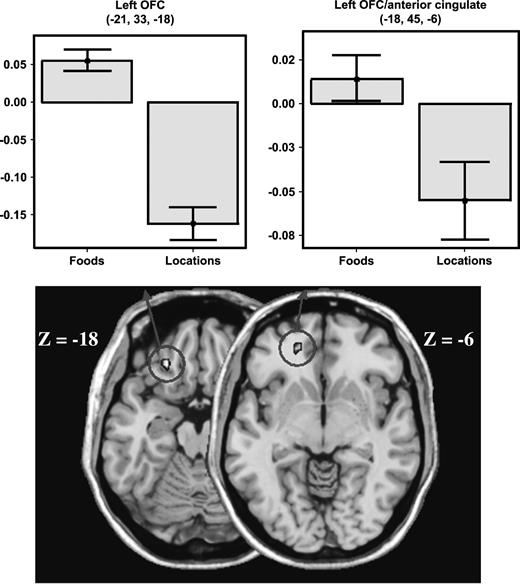
In addition, food pictures, relative to location pictures, activated two regions in the left OFC that psychophysical research has shown represents the reward values of tastes. One of these regions was located in the lateral portion of the OFC (extent threshold, P = 0.05; Fig. 3); the other, located more superiorly, stretched into the anterior aspect of the cingulate cortex (extent threshold, P = 0.01). While the lateral OFC region was reliably activated relative to the fixation baseline (P < 0.001), the more superior OFC/anterior cingulate region was not (one-tailed, P = 0.155).
Viewing pictures of foods elicits activity in left OFC. A high-resolution anatomical scan showing activity in left OFC associated with viewing pictures of food items. The bar graph on the left displays the average percent signal change in the left OFC for all nine subjects during a period between 4 and 14 s post-stimulus. The bar graph on the right displays the average percentage signal change in the left OFC/anterior cingulate cluster for all nine subjects during a period between 4 and 14 s post-stimulus. The y-axis indicates percent signal change relative to signal baseline, with error bars representing ±1 SEM of the subjects. The data shown in the bar graphs were obtained in the random effects contrast of foods > locations with P < 0.005.
Viewing food pictures, relative to location pictures, also produced robust activity in ventral occipitotemporal cortex, bilaterally. Two of these areas were located in the right hemisphere; one extending from the inferior occipital gyrus forward into the inferior temporal gyrus (extent threshold, P = 0.02) and the other located more anteriorly in the inferior temporal gyrus (extent threshold, P = 0.035). Additional activity was observed in the left hemisphere, stretching from inferior occipital gyrus into the fusiform and inferior temporal gyri (extent threshold, P = 0.001). In addition to producing significantly more activity than location pictures, food pictures reliably activated each of the ventral temporal areas above the signal baseline (P < 0.0001).
In constrast, and consistent with previous reports (Aguirre et al., 1998; Epstein and Kanwisher, 1998; Epstein et al., 1999), location pictures, relative to food pictures, produced bilateral activity extending from the medial portion of the fusiform gyrus into parahippocampal gyrus (see Table 1). Activity in these regions was not only greater for locations than foods, but was also reliably activated relative to the signal baseline (P < 0.001 for both hemispheres).
Discussion
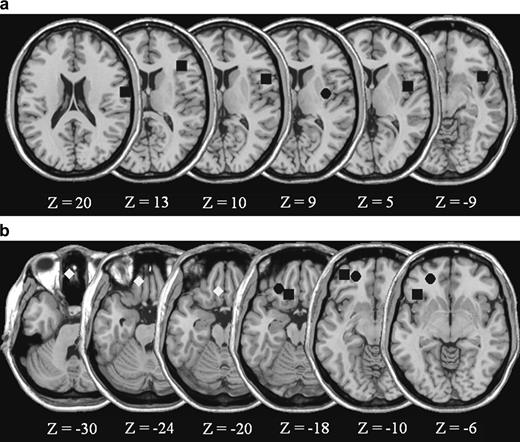
These findings support the hypothesis that a distributed circuit of brain regions represents conceptual knowledge about foods. As Figure 4a,b illustrates, viewing food pictures activated two brain regions that lie in close proximity to gustatory regions active during psychophysical studies of taste perception (Francis et al., 1999; de Araujo et al., 2003a,b; O'Doherty et al., 2001b). As Figure 4a illustrates, food pictures activated the insula/operculum very near regions that become active when people actually taste glucose, sucrose, salt, or umami. As Figure 4b similarly illustrates, food pictures also activate OFC very near regions that become active when people experience taste stimuli directly. The close proximity of the regions active for food pictures to well-established gustatory areas suggests that food pictures automatically activate gustatory areas to produce conceptual inferences about taste properties.
(a) Locations of peak right hemisphere insula/operculum activations reported in taste perception studies. (b) Locations of peak left OFC activations across various tasks. The squares in the insula/operculum at Z = 20 and Z = −9 represent peak activations observed when participants taste sucrose, whereas the square in the lateral OFC at Z = −10 is the peak activation in the area observed to respond to the combination of gustatory and olfactory stimuli, and thus is a likely candidate for being the center of flavor representation (de Araujo et al., 2003). The square in the insula/operculum at Z = 13 indicates an area of common activation when participants tasted either glucose or salt (O'Doherty et al., 2001). The squares in the insula/operculum at Z = 10 and in the OFC at Z = −6 indicate the peak activations observed when participants taste umami (de Araujo et al., 2003). The squares in the insula/operculum at Z = 5 and in the OFC at Z = −18 represent peak activations when participants tasted glucose (Francis et al., 1999). Diamonds in the inferior medial OFC represent peak activations observed when participants receive abstract rewards (O'Doherty et al., 2001). The circle in the OFC at Z = −10 represents peak activation observed when participants verify the taste properties of concepts using strictly linguistic stimuli (Simmons, Pecher, Hamann, Zeelenberg, and Barsalou, under review). Finally, the circles in the insula/operculum at Z = 9 and in the OFC at Z = −18 and Z = −6 indicate the activation peaks observed in the present study when participants viewed food pictures. When necessary, coordinates reported in other studies were converted from Talairach to MNI space.
The two taste areas observed here are associated with different functions in the gustatory system. The insula/operculum receives projections from the ventroposterior medial nucleus of the thalamus (Rolls and Scott, 2003), the main subcortical processing area for gustatory input, and has been associated with taste per se. The OFC, in contrast, receives projections from the insula/operculum (Rolls and Scott, 2003) and has been associated with the reward values of specific tastes. Specifically, electrophysiological studies in monkeys show that the firing rates of neurons in insula/operculum are not modulated by hunger and satiety, suggesting that they represent taste independent of reward (Rolls et al., 1988). Conversely, the firing rates of neurons in OFC are modulated by hunger and satiety, suggesting that they represent the current reward value of tastes (Rolls et al., 1988). Thus, when a monkey is hungry, the firing rate of OFC neurons is high, given that the reward value of food is high. Similarly in humans, greater activation occurs in gustatory OFC before participants are satiated than after (Gottfried et al., 2003).
Taste reward areas are located in a different OFC region than the reward areas for other stimuli (Elliot et al., 2000; O'Doherty et al., 2001a; Rolls, 2000). For example, the caudal OFC responds to olfactory rewards (de Araujo et al., 2003b; Zald and Pardo, 2000; Öngür et al., 2003), whereas the inferior medial OFC responds to abstract rewards (e.g. money) (O'Doherty et al., 2001a). Interestingly, the inferior medial OFC has a markedly different cytoarchitectonic structure than the more lateral aspect of the OFC where taste activations occur (Öngür et al., 2003). Thus, the OFC areas active in the present study appear to represent the reward value of tastes, rather than reward in general. As Rolls (2000, p. 285) notes, ‘it is important to realize that it is not just some general “reward” that is represented in the oritofrontal cortex, but instead a very detailed and information-rich representation of which particular reward or punisher is present’.
Laterality of the Taste Activations
Food pictures activated the right insula/operculum, and the left OFC. Our a priori prediction was that food pictures would activate both regions bilaterally. Examination of the psychophysical taste literature, however, clarifies the laterality of our results. First, consider the insula/operculum. Although many psychophysical taste studies observe bilateral activity in this area, the response is typically stronger and more spatially extensive on the right (Small et al., 1999). This may explain why we only found right insula/operculum activation for food pictures. Indeed, lowering the cluster size threshold in our random effects analysis (but not the P-value threshold) revealed significant activity in a region of the left frontal operculum (−48, 21, 12) that is commonly activate in psychophysical taste studies (Small et al., 1999). Although this cluster of activity was smaller in magnitude and size relative to the activation seen on the right, it suggests that our findings are consistent with the general trend in the psychophysical taste literature for greater insula/operculum activation in the right hemisphere than in the left.
With respect to the OFC, we found significant activations only on the left. It is noteworthy that studies in the psychophysical taste literature are inconsistent with regard to laterality, with bilateral activity reported only in approximately half of the studies. Again, lowering the cluster size threshold (but not the P-value threshold) on the random effects analysis revealed significant activity in the right OFC (15, 45, −3) in nearly the identical location as seen on the left (−18, 45, −6). Perhaps the best explanation, however, for why we observe activity in the left OFC comes from a recent finding by Kringelbach et al. (2003). These researchers identified an area in the left OFC where activity was correlated with subjects' ratings of taste pleasantness. Interestingly, the area they identified is approximately one centimeter from the activity we observed in the lateral OFC. Given that we only showed pictures of highly appetizing foods, it makes sense that we would observe activity very near the left OFC region that tracks taste pleasantness.
Conclusion
The findings reported here indicate that the gustatory system produces taste responses to pictures of foods, not just to actual foods. Other studies have reported similar results. A previous neuroimaging study on pictures of foods found activation in areas near those observed here (insula and OFC), but using a blocked design with fixed-effects analyses (Killgore et al., 2003). Indeed, still other research has found that even words for tastes activate taste areas (Simmons, W.K., Pecher, D., Hamann, S.B., Zeelenberg, R. and Barsalou, L.W., under review; see Fig. 4b). In general, pictures and words appear to activate property inferences for food tastes and rewards, thus grounding conceptual knowledge in modality-specific brain areas.
In the experiment reported here, taste inferences arose even when subjects performed fast superficial processing of food stimuli. Subjects were required to only assess whether the current picture exactly matched the previous picture, each presented for only 2 s. No categorization or other form of conceptual processing was required. Furthermore, the large majority of trials required the subject to note that the current picture differed from the previous picture, a judgment that could have potentially interfered with making conceptual inferences. In general, the fact that taste inferences were produced under this particular set of task conditions attests to their strength and ubiquity.
Consistent with previous findings, the experiment here indicated that conceptual representations are distributed across the brain areas that underlie their processing in perception and action. Because different categories are associated with different distributions of multimodal properties (McRae and Cree, 2002), different categories rely on different configurations of brain areas for conceptual representation. As reviewed earlier, much work has shown that thinking about tools activates brain areas that process visual form, visual motion, and object manipulation. Analogously, we have shown here that thinking about food activates brain areas that process taste, taste reward and food shape. Thus our findings support the view that the brain areas representing knowledge for a particular category are those typically used to process its physical instances.
Besides having implications for theories of distributed conceptual representation, these findings have implications for various societal issues related to food, such as eating disorders, obesity and advertising. Taste inferences in the gustatory system, as observed here, arise in response to a wide variety of food stimuli in the environment and in the media. In eating disorders and obesity, the perception of foods and food pictures, as well as thoughts of food, may be associated with dysfunctional inferences about taste and reward. Conversely, behavioral, cognitive and pharmacological interventions may, in part, restore the gustatory activity underlying inferences about taste and reward to more normal forms.
This work was supported by NIMH grant 1F31MH070152-01 to K.S. and National Science Foundation grants SBR-9905024 and BCS-0212134 and Emory University research funds to L.W.B.. We are grateful to Melissa Armstrong and Christine Wilson for their assistance in stimulus preparation.
References
Aguirre GK, Zarahan E, D'Esposito M (
Barsalou LW (
Barsalou LW, Niedenthal PM, Barbey A, Ruppert J (
Barsalou LW, Simmons WK, Barbey AK, Wilson CD (
Beauchamp MS, Lee KE, Haxby JV, Martin A (
Chao LL, Martin A (
Chao LL, Haxby JV, Martin A (
Damasio AR, Damasio H (
Damasio H, Grabowski TJ, Tranel D, Ponto LLB, Hichwa RD, Damasio AR (
de Araujo IET, Kringelbach ML, Rolls ET, Hobden P (
de Araujo IET, Rolls ET, Kringelbach ML, McGlone F, Phillips N (
Elliott R, Dolan RJ, Frith CD (
Epstein R, Kanwisher N (
Epstein R, Harris A, Stanley D, Kanwisher N (
Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E (
Gottfried JA, O'Doherty J, Dolan RJ (
Grafton ST, Fadiga L, Arbib MA, Rizzolatti G (
Handy TC, Grafton ST, Shroff NM, Ketay S, Gazzaniga MS (
Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV (
Ishai A, Ungerleider LG, Martin A, Haxby JV (
Killgore WDS, Yount AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA (
Kringelbach ML, O'Doherty J, Rolls ET, Andrews C (
McRae K, Cree GS (
Martin A (
Martin A, Chao LL (
Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG (
O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C (
O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F (
Öngür D, Ferry AT, Price JL (
Perani D, Cappa SF, Bettinardi V, Gorno-Tempini M, Matarrese M, Fazio F (
Rolls ET, Scott TR (
Rolls ET, Scott TR, Sienkiewicz ZJ, Yaxley S (
Rolls ET, Sienkiewicz ZJ, Yaxley S (
Ross BH, Murphy GL (
Scott TR, Yaxley S, Sienkiewicz ZJ, Rolls ET (
Simmons WK, Barsalou LW (
Small D, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M (
Thompson-Schill SL (
Author notes
1Department of Psychology, Emory University, Atlanta, GA 30322, USA and 2Cognitive Neuropsychology Section, Laboratory of Brain and Cognition, National Institute of Mental Health, Bethesda, MD, USA