-
PDF
- Split View
-
Views
-
Cite
Cite
Erin L. Shelley, Daniel T. Blumstein, The evolution of vocal alarm communication in rodents, Behavioral Ecology, Volume 16, Issue 1, Jan./Feb. 2005, Pages 169–177, https://doi.org/10.1093/beheco/arh148
Close - Share Icon Share
Abstract
On encountering a predator, many species emit potentially risky vocalizations known as alarm calls. We evaluated the relative importance of two adaptive hypotheses on the evolution of calling: (1) communicating to predators, which may function by deterring pursuit and hence increasing individual survival, and (2) an alternative nepotistic hypothesis for alarm calling whereby callers obtain direct and indirect fitness by warning relatives. Focusing on 209 species of rodents, we found significant associations between diurnality and alarm calling, living socially and alarm calling, and diurnality and sociality. Diurnality, however, accounted for nearly three times as much variation in whether or not a species alarm called than did sociality. Phylogenetic tests revealed that the evolution of diurnality preceded the evolution of alarm calling, and that the evolutions of diurnality and sociality were unrelated. Our results are consistent with the hypothesis that alarm communication evolved to communicate to predators. If so, then nepotistic benefits, although important for the maintenance of alarm calling in some rodents, may be relatively less important in its evolution.
Why animals emit potentially risky alarm calls has puzzled evolutionary biologists for decades (Klump and Shalter, 1984; Maynard Smith, 1965), and understanding the adaptive utility of alarm communication has been influential in explaining the evolution of social behavior through kin selection (Keller and Reeve, 2002). Since Sherman's (1977) and Dunford's (1977) classic studies that demonstrated nepotistic benefits from calling in ground squirrels, others have found evidence that animals obtain both direct and indirect fitness (Brown, 1987) by emitting potentially risky alarm vocalizations (Blumstein et al., 1997; Hoogland, 1995; Schwagmeyer, 1980). It is therefore surprising that the evolutionary origin of alarm calling has remained virtually unexplored.
The methods of studying current adaptive utility are, theoretically, straightforward and involve correlative and experimental components: (1) hypothesize an adaptive function, (2) search for correlations between variation in trait expression and evolutionary fitness, and (3) manipulate trait expression to demonstrate causality (Tinbergen, 1963; Tinbergen et al., 1962). Demonstrating the initial conditions favoring the evolution of a trait are, however, explicitly correlative. Evolutionary questions, such as these, are studied by using the comparative method in which the distribution of traits among many species is identified and hypotheses about coevolution are formally tested (Brooks and McLennan, 1991; Harvey and Pagel, 1991; Maddison and Maddison, 2001). Evolution is cumulative, and the conditions favoring the initial evolution of a trait and its subsequent maintenance need not be identical (Darwin, 1859; Reeve and Sherman, 1993). The challenge to understanding the initial evolutionary function is to generate hypotheses and variables suitable for comparative analysis that test complementary adaptive hypotheses. There are two major nonexclusive hypotheses to explain the current adaptive utility of alarm calling: communicating to predators to discourage pursuit (Hasson, 1991), and communicating to conspecifics to warn them about danger (Maynard Smith, 1965, Zuberbühler et al., 1999).
Abundant evidence suggests that animals reduce risk to themselves when emitting alarm signals (Blumstein, 1999; Hasson, 1991) and, under certain circumstances, do not produce alarm signals because by doing so they would make themselves even more vulnerable to predators (Caro, 1995). Because hunting success often requires an element of surprise, individuals may alarm call to alert the potential predator that it has been detected. Alarm signals may also transmit information to the predator about the caller's physical ability to elude capture or defend itself (Fitzgibbon and Fanshawe, 1988). By deterring a predator's attack, a calling individual gains personal fitness benefits. If we assume that alarm calling evolved to communicate to predators, we would expect that calling individuals would try to minimize their risk while calling. One way to do so would be to vocalize only when it is possible to locate and track predators visually, because visual predator detection may more accurately assess the risk of predation (Lima, 1988a,b). Because there is evidence in several species that prey visually assess the relative risk of predation and only call when they are not subjected to excessive risk (Blumstein and Armitage, 1997; Wolff, 1980), we would predict alarm calling species to be diurnal, and alarm calling to be rare or absent in nocturnal species, which are constrained in their ability to reliably assess and manage predation risk.
The intrinsic risk of alarm calling may also be offset by the potential for nepotistic benefits (Sherman, 1977; 1980). Instead of, or in addition to, communicating to the predator, a caller may warn conspecifics about danger. If relatives flee to safety in response to a call, the caller gains inclusive fitness through their survival. If alarm calling evolved via nepotism, we would expect to see social species (particularly those living near kin) more likely to call than are nonsocial species.
Rodents provide an excellent model system in which to study the evolution of alarm calling because they vary in the degrees to which they are vocal, diurnal, and social. We summarized these data for 209 species of rodents from 24 different families, and used both nonphylogentic and phylogenetic tests to investigate the associations between the evolution of diurnality, living socially, and alarm calling.
METHODS
Developing the comparative data set
To sample all rodents, including those that are known to alarm call and those that are not, species account numbers one through 702 in Mammalian Species (references are available in Appendix and Neotropical Rainforest Mammals (Emmons, 1997) were examined. We defined alarm calling as noises, usually loud, emitted when a predator was detected. For most of the 209 species included in our final analyses, our references specified that animals emitted alarm calls in the presence of predators. When we had good descriptions of the sounds a particular species made in a variety of contexts and there was no mention of alarm calls, or the sources specifically stated that the species was not known to alarm call, we classified it as not alarm calling. When we had little or ambiguous data regarding the sounds a species made, we considered it unknown and removed it from the data set. “Fear screams” or other defensive noises (e.g., tooth chattering) were not considered alarm calling. Species for which we were unsure of the context or those species described as making noises when held by a human were not categorized as alarm calling. Data about activity patterns, sociality, vocalizations, and the context in which vocalizations were emitted were summarized. A variety of guides and reviews (references are available as in the Appendix) described additional species of rodents that emit alarm calls, as well as data to fill in gaps about activity patterns, sociality, and vocalizations. Experts were consulted (see Acknowledgments) to provide additional data on several species.
We conducted two complementary analyses. First, for our data set of 209 species, those reported to be active at least 50% of the day were scored as diurnal; those active mostly at night, as nocturnal. Second, we modified our definition of diurnality. In our strict definition, we classified a species as diurnal if it was never reported to be active at night. This reduced our sample size to 156 species. Species likely to be found near kin, because either they lived in family groups or they lived in colonies or foraged in aggregations were scored as social. Animals reported only to be found solitarily or in pairs, or those reported to be territorial and noncolonial, were scored as not social. By defining sociality this way, we classify as social species such as solitary, but colonial, ground squirrels (Spermophilus spp.) that have been the subject of many studies of the adaptive significance of calling, and we classify as nonsocial species such as muskrats (Ondatra zibethicus) and North and South American porcupines (Erithizon dorsatum and Coendou spp.) that inevitably include species with some degree of maternal care.
Nepotistic benefits from calling could have evolved or be maintained by the benefits from warning vulnerable offspring during a period of parental care (Dawkins, 1979; Hamilton, 1964a,b). However, in some squirrels (Swaisgood et al., 1999), mothers direct antipredator behavior and vocalizations specifically toward predators during the time when they care for vulnerable and unresponsive offspring. Species reported to alarm call in the presence of a predator or other disturbances were scored as alarm calling, whereas species were scored as not alarm calling if there were vocalization data, but alarm calling was not reported.
Developing the phylogeny
By using the phylogeny outlined in McKenna and Bell (1997), which updates that of Simpson (1945), we classified to genus the 209 species for which we had complete and unambiguous data. This resolved the majority of genera that were represented by only one or two species. Those genera with more than two species remained as unresolved polytomies; many were subsequently resolved by using species-level phylogenetic hypotheses proposed in various studies. Typically, only a single additional phylogeny was sufficient to resolve the species within a genus. The following criteria were applied, in this order, to resolve any genus-level polytomies: (1) molecular hypotheses were used over morphological hypotheses; (2) more recent hypotheses were used over less recent hypotheses; and (3) all else being equal, hypotheses constructed by using parsimony methods were given priority, with consensus between equally parsimonious trees being the most desired. We also explored the effect that two recent molecular phylogenies of holarctic ground squirrels (Harrison et al., 2003; Herron et al., 2004) had on our main results by modifying our tree where appropriate and rerunning all analyses.
Details on phylogeny development
1. Sciurus: Two molecular hypotheses (Oshida and Masuda, 2000), using parsimony and likelihood methods. Both hypotheses follow the same structure, but the likelihood method further resolves a polytomy of four species.
2. Marmota: Two molecular hypotheses using parsimony (Kruckenhauser et al., 1999) and then likelihood methods (which further resolves a polytomy of three of the species; Steppan et al., 1999).
3. Spermophilus: Molecular hypothesis using parsimony methods (Kruckenhauser et al., 1999) and a compiled hypothesis (Blumstein and Armitage, 1998).
4. Cynomys: Compiled hypothesis (Blumstein and Armitage, 1998).
5. Tamias: Molecular hypothesis using strict consensus methods (Piaggio and Spicer, 2001).
6. Neotoma: Two molecular hypotheses using strict consensus methods (Hayes and Harrison, 1992) and parsimony methods (Shipley et al., 1990).
7. Peromyscus: Molecular hypothesis using parsimony methods (Hogan et al., 1997).
8. Reithrodontomys: Two molecular hypotheses using parsimony methods (Bell et al., 2001) and a phenogram (Nelson et al., 1984).
9. Microtus: Two molecular hypotheses using consensus and likelihood methods (Conroy and Cook, 2000). Both hypotheses follow the same structure, but the likelihood methods further resolve a large polytomy.
10. Gerbillurus: Molecular hypothesis using parsimony methods (Qumsiyeh et al., 1991).
11. Rattus: Molecular hypothesis using UPGMA methods (Baverstock et al., 1986).
12. Notomys: Morphological hypothesis using parsimony methods (Watts et al., 1992).
13. Geomys: Two molecular hypotheses using consensus and neighbor-joining methods (Jolley et al., 2000). Both hypotheses follow the same structure, but the joining-joining methods further resolve a large polytomy.
14. Pappogeomys: Redrawn morphological hypothesis (DeWalt et al., 1993; Russell, 1968).
15. Perognathus: Molecular hypothesis using parsimony methods (Riddle, 1995).
16. Chaetodipus: Molecular hypothesis using likelihood methods (Riddle et al., 2000).
17. Dipodomys: Morphological phenogram. For the resolution of most of the species within Dipodomys spp., we chose to use a morphologically based hypothesis (Carrasco, 2000) over a molecularly based hypothesis (Mantooth et al., 2000) from the same year because the morphological tree was more complete and included many more species from this genus. Nonetheless, both sources agreed on the placement of all but one of the species of Dipodomys.
18. Ctenomys: Molecular hypothesis using parsimony methods (Slamovits et al., 2001).
19. Proechimys: Molecular and morphological hypothesis grouping two species separate from the others (Gardner and Emmons, 1984).
Testing the comparative hypotheses
Fisher's Exact tests and logistic regression analyses were used to determine if the presence or absence of alarm calling was influenced by activity pattern or sociality. P values < .05 were considered significant, and p values from .05–.10 were marginally significant. Because such a species-based analysis cannot identify the directionality of trait evolution, and such an analysis is potentially confounded because it does not account for phylogenetic nonindependence between closely related species (Harvey and Pagel, 1991), two phylogenetically based analyses (run with our phylogeny and a one modified based on data reported in Herron et al., 2004) were also used to study the evolution of alarm calling in rodents.
The concentrated changes test (Maddison, 1990) was used to determine the likelihood that the evolution of alarm calling was concentrated on portions of the phylogenetic tree in which diurnal species or in species likely to be found near kin were present. The test requires a fully resolved phylogeny, hence the random resolve option in MacClade version 4.03 (Maddison and Maddison, 2001) was used to resolve any remaining polytomies. The three dichotomous traits were optimized onto the resolved tree to reconstruct the ancestor states for each. In some instances, strict parsimony was not able to fully resolve the reconstructions. Thus, ACCTRAN and DELTRAN algorithms were applied to each character tree, resulting in a total of six ancestor state reconstructions. The ACCTRAN algorithm accelerates changes in traits toward the root of the tree, maximizing early gains and forcing early subsequent reversals. The DELTRAN algorithm, on the other hand, delays changes in traits away from the root, thus maximizing parallel changes (Maddison and Maddison, 2001).
The large number of species prevented the use of the concentrated changes test for calculating the exact probability of trait distributions. Instead, the “actual changes” simulation option was used for 10,000 replicates to estimate p values for each reconstruction. Also, to account for incorrect resolutions of the ancestor state, simulations were run with the “either ancestral” option selected. To minimize the possibility of falsely interpreting results as significant, a conservative approach was maintained in two ways. First, a Bonferroni correction was applied to the critical p value for hypotheses tested by using both ACCTRAN and DELTRAN reconstructions (new p critical .05/2 = .025). Second, fewer and as many gains in the distinguished character, as well as fewer than, as many, or more losses in the distinguished character than actually counted in our analyses, were used when calculating the p value in MacClade.
Although the concentrated changes test allowed comparisons between the distributions of two traits on a phylogenetic tree (Swofford and Maddison, 1987), the contingent states test (Sillén-Tullberg, 1993) allowed the use of the phylogenetic reconstruction of characters to ask whether the transition in one character from zero to one or from one to zero, or the lack of a transition, is equally likely to occur under either state of another character. Thus, it indicated the likelihood that the evolutionary origin of a given trait preceded the evolution of another trait. The main assumption involved is that each branch has an equal probability of state transition. To understand the directionality of the evolution of the three traits, a series of pair-wise contingent states tests using CoSta version 1.03 (Lindenfors, 1999) were performed for each reconstruction. p values less than the Bonferroni-corrected .025 were interpreted as significant, and p < .05 as marginally significant.
RESULTS
Using Fisher's Exact tests, we found significant associations between diurnality and alarm calling, living socially and alarm calling, and diurnality and sociality (Table 1). Diurnality, however, accounted for three to six times as much variation in whether or not a species alarm called than did sociality (Table 2). Logistic regression analyses also allowed us to study the independent influence of diurnality and demonstrated that both diurnality and sociality may explain significant variation in whether or not a species alarm called. However, being diurnal was relatively more important in explaining the extant pattern of alarm calling in rodents (Table 2), a finding that became even stronger when we used a more strict definition of diurnal.
Associations between diurnality and alarm calling, sociality and alarm calling, and diurnality and sociality
| . | Alarm call . | . | . | Alarm call . | . | . | Social . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diurnal . | No . | Yes . | Social . | No . | Yes . | Diurnal . | No . | Yes . | ||||||||
| “Liberal” definition of diurnality* | ||||||||||||||||
| No | 55 | 25 | No | 46 | 40 | No | 47 | 33 | ||||||||
| Yes | 18 | 111 | Yes | 27 | 96 | Yes | 39 | 90 | ||||||||
| “Strict” definition of diurnality† | ||||||||||||||||
| No | 37 | 15 | No | 27 | 36 | No | 32 | 20 | ||||||||
| Yes | 5 | 99 | Yes | 15 | 78 | Yes | 31 | 73 | ||||||||
| . | Alarm call . | . | . | Alarm call . | . | . | Social . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diurnal . | No . | Yes . | Social . | No . | Yes . | Diurnal . | No . | Yes . | ||||||||
| “Liberal” definition of diurnality* | ||||||||||||||||
| No | 55 | 25 | No | 46 | 40 | No | 47 | 33 | ||||||||
| Yes | 18 | 111 | Yes | 27 | 96 | Yes | 39 | 90 | ||||||||
| “Strict” definition of diurnality† | ||||||||||||||||
| No | 37 | 15 | No | 27 | 36 | No | 32 | 20 | ||||||||
| Yes | 5 | 99 | Yes | 15 | 78 | Yes | 31 | 73 | ||||||||
All p-values < 0.0001;
all p-values < 0.0004.
Associations between diurnality and alarm calling, sociality and alarm calling, and diurnality and sociality
| . | Alarm call . | . | . | Alarm call . | . | . | Social . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diurnal . | No . | Yes . | Social . | No . | Yes . | Diurnal . | No . | Yes . | ||||||||
| “Liberal” definition of diurnality* | ||||||||||||||||
| No | 55 | 25 | No | 46 | 40 | No | 47 | 33 | ||||||||
| Yes | 18 | 111 | Yes | 27 | 96 | Yes | 39 | 90 | ||||||||
| “Strict” definition of diurnality† | ||||||||||||||||
| No | 37 | 15 | No | 27 | 36 | No | 32 | 20 | ||||||||
| Yes | 5 | 99 | Yes | 15 | 78 | Yes | 31 | 73 | ||||||||
| . | Alarm call . | . | . | Alarm call . | . | . | Social . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diurnal . | No . | Yes . | Social . | No . | Yes . | Diurnal . | No . | Yes . | ||||||||
| “Liberal” definition of diurnality* | ||||||||||||||||
| No | 55 | 25 | No | 46 | 40 | No | 47 | 33 | ||||||||
| Yes | 18 | 111 | Yes | 27 | 96 | Yes | 39 | 90 | ||||||||
| “Strict” definition of diurnality† | ||||||||||||||||
| No | 37 | 15 | No | 27 | 36 | No | 32 | 20 | ||||||||
| Yes | 5 | 99 | Yes | 15 | 78 | Yes | 31 | 73 | ||||||||
All p-values < 0.0001;
all p-values < 0.0004.
Results of four logistic regression analyses conducted on species values
| . | Independent variable(s) . | . | . | |||
|---|---|---|---|---|---|---|
| Dependent variable . | Diurnal p . | Social p . | R2 . | |||
| “Liberal” definition of diurnality | ||||||
| Alarm call | <.0001 | — | .247 | |||
| Alarm call | — | <.0001 | .082 | |||
| Alarm call | <.0001 | .0031 | .280 | |||
| Social | <.0001 | — | .059 | |||
| “Strict” definition of diurnality | ||||||
| Alarm call | <.0001 | — | .436 | |||
| Alarm call | — | .0002 | .074 | |||
| Alarm call | <.0001 | .149 | .447 | |||
| Social | .0001 | — | .069 | |||
| . | Independent variable(s) . | . | . | |||
|---|---|---|---|---|---|---|
| Dependent variable . | Diurnal p . | Social p . | R2 . | |||
| “Liberal” definition of diurnality | ||||||
| Alarm call | <.0001 | — | .247 | |||
| Alarm call | — | <.0001 | .082 | |||
| Alarm call | <.0001 | .0031 | .280 | |||
| Social | <.0001 | — | .059 | |||
| “Strict” definition of diurnality | ||||||
| Alarm call | <.0001 | — | .436 | |||
| Alarm call | — | .0002 | .074 | |||
| Alarm call | <.0001 | .149 | .447 | |||
| Social | .0001 | — | .069 | |||
Results of four logistic regression analyses conducted on species values
| . | Independent variable(s) . | . | . | |||
|---|---|---|---|---|---|---|
| Dependent variable . | Diurnal p . | Social p . | R2 . | |||
| “Liberal” definition of diurnality | ||||||
| Alarm call | <.0001 | — | .247 | |||
| Alarm call | — | <.0001 | .082 | |||
| Alarm call | <.0001 | .0031 | .280 | |||
| Social | <.0001 | — | .059 | |||
| “Strict” definition of diurnality | ||||||
| Alarm call | <.0001 | — | .436 | |||
| Alarm call | — | .0002 | .074 | |||
| Alarm call | <.0001 | .149 | .447 | |||
| Social | .0001 | — | .069 | |||
| . | Independent variable(s) . | . | . | |||
|---|---|---|---|---|---|---|
| Dependent variable . | Diurnal p . | Social p . | R2 . | |||
| “Liberal” definition of diurnality | ||||||
| Alarm call | <.0001 | — | .247 | |||
| Alarm call | — | <.0001 | .082 | |||
| Alarm call | <.0001 | .0031 | .280 | |||
| Social | <.0001 | — | .059 | |||
| “Strict” definition of diurnality | ||||||
| Alarm call | <.0001 | — | .436 | |||
| Alarm call | — | .0002 | .074 | |||
| Alarm call | <.0001 | .149 | .447 | |||
| Social | .0001 | — | .069 | |||
In both data sets (i.e., the 209 species versus 156 species data sets), using the concentrated changes test, we found that both ACCTRAN (p < .001) and DELTRAN (p < .0001) reconstructions revealed significantly more species evolving alarm calling on branches of the tree exhibiting diurnality than would be expected by chance. Alarm calling was also significantly more likely to have evolved on branches of the tree exhibiting sociality (ACCTRAN, p < .0001; DELTRAN, p < .0001). In addition, a significantly greater number of social species was concentrated on branches of the tree characterized by diurnality (ACCTRAN, p < .0001; DELTRAN, p < .0001).
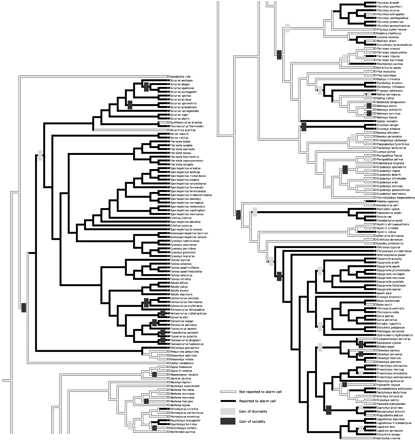
The contingent states test allowed us to identify the directionality of this pattern (Table 3). In both ACCTRAN (Figure 1) and DELTRAN reconstructions, diurnality preceded the evolution of alarm calling. When we used ACCTRAN reconstructions, sociality did not precede the evolution of alarm calling. In contrast, DELTRAN reconstructions revealed a tendency for sociality to precede the evolution of alarm calling. For both ACCTRAN and DELTRAN reconstructions of character states, species that were diurnal were not more likely to evolve sociality.
Results of contingent states test
| “Liberal” definition of diurnality . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Dependent variable state . | . | . | . | . | . | |||||||
| . | . | ACCTRAN . | . | . | DELTRAN . | . | . | |||||||
| . | . | Alarm call . | . | . | Alarm call . | . | . | |||||||
| Independent variable state . | . | 0->0 . | 0->1 . | p . | 0->0 . | 0->1 . | p . | |||||||
| Diurnal | No | 107 | 8 | .0247 | 117 | 9 | .0018 | |||||||
| Yes | 28 | 8 | 30 | 11 | ||||||||||
| Social | No | 87 | 6 | .0549 | 91 | 7 | .0290 | |||||||
| Yes | 48 | 10 | 56 | 13 | ||||||||||
| Social | Social | |||||||||||||
| 0->0 | 0->1 | p | 0->0 | 0->1 | p | |||||||||
| Diurnal | No | 88 | 11 | .8202 | 90 | 10 | .6321 | |||||||
| Yes | 73 | 11 | 65 | 10 | ||||||||||
| “Liberal” definition of diurnality . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Dependent variable state . | . | . | . | . | . | |||||||
| . | . | ACCTRAN . | . | . | DELTRAN . | . | . | |||||||
| . | . | Alarm call . | . | . | Alarm call . | . | . | |||||||
| Independent variable state . | . | 0->0 . | 0->1 . | p . | 0->0 . | 0->1 . | p . | |||||||
| Diurnal | No | 107 | 8 | .0247 | 117 | 9 | .0018 | |||||||
| Yes | 28 | 8 | 30 | 11 | ||||||||||
| Social | No | 87 | 6 | .0549 | 91 | 7 | .0290 | |||||||
| Yes | 48 | 10 | 56 | 13 | ||||||||||
| Social | Social | |||||||||||||
| 0->0 | 0->1 | p | 0->0 | 0->1 | p | |||||||||
| Diurnal | No | 88 | 11 | .8202 | 90 | 10 | .6321 | |||||||
| Yes | 73 | 11 | 65 | 10 | ||||||||||
| “Strict” definition of diurnality . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Dependent variable state . | . | . | . | . | . | |||||||
| . | . | ACCTRAN . | . | . | DELTRAN . | . | . | |||||||
| . | . | Alarm call . | . | . | Alarm call . | . | . | |||||||
| Independent variable state . | . | 0->0 . | 0->1 . | p . | 0->0 . | 0->1 . | p . | |||||||
| Diurnal | No | 185 | 3 | <.0001 | 179 | 2 | .0014 | |||||||
| Yes | 36 | 8 | 30 | 5 | ||||||||||
| Social | No | 77 | 6 | .207 | 79 | 5 | .112 | |||||||
| Yes | 144 | 5 | 130 | 2 | ||||||||||
| Social (strict parsimony) | ||||||||||||||
| 0->0 | 0->1 | p | ||||||||||||
| Diurnal | No | 57 | 10 | .1420 | ||||||||||
| Yes | 50 | 3 | ||||||||||||
| “Strict” definition of diurnality . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Dependent variable state . | . | . | . | . | . | |||||||
| . | . | ACCTRAN . | . | . | DELTRAN . | . | . | |||||||
| . | . | Alarm call . | . | . | Alarm call . | . | . | |||||||
| Independent variable state . | . | 0->0 . | 0->1 . | p . | 0->0 . | 0->1 . | p . | |||||||
| Diurnal | No | 185 | 3 | <.0001 | 179 | 2 | .0014 | |||||||
| Yes | 36 | 8 | 30 | 5 | ||||||||||
| Social | No | 77 | 6 | .207 | 79 | 5 | .112 | |||||||
| Yes | 144 | 5 | 130 | 2 | ||||||||||
| Social (strict parsimony) | ||||||||||||||
| 0->0 | 0->1 | p | ||||||||||||
| Diurnal | No | 57 | 10 | .1420 | ||||||||||
| Yes | 50 | 3 | ||||||||||||
p values significant after a Bonferroni correction are in bold. All tests were two-tailed.
Results of contingent states test
| “Liberal” definition of diurnality . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Dependent variable state . | . | . | . | . | . | |||||||
| . | . | ACCTRAN . | . | . | DELTRAN . | . | . | |||||||
| . | . | Alarm call . | . | . | Alarm call . | . | . | |||||||
| Independent variable state . | . | 0->0 . | 0->1 . | p . | 0->0 . | 0->1 . | p . | |||||||
| Diurnal | No | 107 | 8 | .0247 | 117 | 9 | .0018 | |||||||
| Yes | 28 | 8 | 30 | 11 | ||||||||||
| Social | No | 87 | 6 | .0549 | 91 | 7 | .0290 | |||||||
| Yes | 48 | 10 | 56 | 13 | ||||||||||
| Social | Social | |||||||||||||
| 0->0 | 0->1 | p | 0->0 | 0->1 | p | |||||||||
| Diurnal | No | 88 | 11 | .8202 | 90 | 10 | .6321 | |||||||
| Yes | 73 | 11 | 65 | 10 | ||||||||||
| “Liberal” definition of diurnality . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Dependent variable state . | . | . | . | . | . | |||||||
| . | . | ACCTRAN . | . | . | DELTRAN . | . | . | |||||||
| . | . | Alarm call . | . | . | Alarm call . | . | . | |||||||
| Independent variable state . | . | 0->0 . | 0->1 . | p . | 0->0 . | 0->1 . | p . | |||||||
| Diurnal | No | 107 | 8 | .0247 | 117 | 9 | .0018 | |||||||
| Yes | 28 | 8 | 30 | 11 | ||||||||||
| Social | No | 87 | 6 | .0549 | 91 | 7 | .0290 | |||||||
| Yes | 48 | 10 | 56 | 13 | ||||||||||
| Social | Social | |||||||||||||
| 0->0 | 0->1 | p | 0->0 | 0->1 | p | |||||||||
| Diurnal | No | 88 | 11 | .8202 | 90 | 10 | .6321 | |||||||
| Yes | 73 | 11 | 65 | 10 | ||||||||||
| “Strict” definition of diurnality . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Dependent variable state . | . | . | . | . | . | |||||||
| . | . | ACCTRAN . | . | . | DELTRAN . | . | . | |||||||
| . | . | Alarm call . | . | . | Alarm call . | . | . | |||||||
| Independent variable state . | . | 0->0 . | 0->1 . | p . | 0->0 . | 0->1 . | p . | |||||||
| Diurnal | No | 185 | 3 | <.0001 | 179 | 2 | .0014 | |||||||
| Yes | 36 | 8 | 30 | 5 | ||||||||||
| Social | No | 77 | 6 | .207 | 79 | 5 | .112 | |||||||
| Yes | 144 | 5 | 130 | 2 | ||||||||||
| Social (strict parsimony) | ||||||||||||||
| 0->0 | 0->1 | p | ||||||||||||
| Diurnal | No | 57 | 10 | .1420 | ||||||||||
| Yes | 50 | 3 | ||||||||||||
| “Strict” definition of diurnality . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Dependent variable state . | . | . | . | . | . | |||||||
| . | . | ACCTRAN . | . | . | DELTRAN . | . | . | |||||||
| . | . | Alarm call . | . | . | Alarm call . | . | . | |||||||
| Independent variable state . | . | 0->0 . | 0->1 . | p . | 0->0 . | 0->1 . | p . | |||||||
| Diurnal | No | 185 | 3 | <.0001 | 179 | 2 | .0014 | |||||||
| Yes | 36 | 8 | 30 | 5 | ||||||||||
| Social | No | 77 | 6 | .207 | 79 | 5 | .112 | |||||||
| Yes | 144 | 5 | 130 | 2 | ||||||||||
| Social (strict parsimony) | ||||||||||||||
| 0->0 | 0->1 | p | ||||||||||||
| Diurnal | No | 57 | 10 | .1420 | ||||||||||
| Yes | 50 | 3 | ||||||||||||
p values significant after a Bonferroni correction are in bold. All tests were two-tailed.
ACCTRAN reconstruction of the evolution of vocal alarm communication in 209 rodent species (ACCTRAN resolution; black squares and bars indicate alarm calling present, white squares and bars indicate alarm calling absent). Reconstructions (ACCTRAN) of the evolutionary origins of diurnality (light bars) and sociality (dark bars) are overlaid on the alarm calling phylogeny that is formally evaluated using the contingent states tests (results reported in Table 3).
DISCUSSION
The results of our analyses are consistent with the hypothesis that the evolution of diurnality preceded the evolution of alarm calling in rodents. Overall, sociality also appeared to be associated with the evolution of alarm calling; however, the results of the contingent states tests suggested that species that had evolved sociality were, at most, only marginally more likely to evolve alarm calling.
Although both diurnal and nocturnal animals have sensory capabilities that function well during their respective periods of activity, vision is a key modality to accurately assess and dynamically track predation risk (Lima, 1988a,b). Because these tests all indicated that the evolution of diurnality preceded the evolution of alarm calling, and because prior evidence that prey alarm call only when there is sufficient light to detect and track predators (Blumstein and Armitage, 1997; Wolff, 1980), we suggest that alarm calling may have evolved as a means of communicating to predators. If information about a caller's fitness and state of alertness is transmitted to a potential predator through alarm calling, the caller is likely to discourage pursuit and increase the chance of the its survival (Blumstein, 1999; Cresswell, 1994; Fitzgibbon and Fanshawe, 1988; Hasson, 1991). Such detection signaling may be the original function of alarm communication in rodents.
In certain species, alarm calling may also have independently evolved to communicate to conspecifics to warn them of danger. In these cases, the inherent risk of alarm calling may be offset by the potential for nepotistic fitness benefits (Dunford, 1977; Sherman, 1977). Our results cannot exclude the hypothesis that the evolution of calling to warn vulnerable offspring (see Blumstein et al., 1997) was possible only once the safety associated with diurnality evolved. However, if the primary function of calling was to warn vulnerable offspring, we would expect that virtually all diurnal species should alarm call because all have vulnerable offspring at some point of their lives. That they do not suggests, to us, that something else might be important. In addition, paternal care in mammals is not widespread, but males of many species alarm call. Available data do not permit a detailed analysis of sex differences in alarm calling, but the observation that males of nonsocial species call (Burke da Silva et al., 1994) may provide additional evidence against the necessity of a nepotistic origin of calling.
Within this phylogenetic hypothesis, we see certain species that have evolved diurnality, alarm calling, and sociality but subsequently reverted back to a nocturnal lifestyle while retaining alarm calling. Although the initial evolution of alarm calling in these species may have required the evolution of a diurnal activity pattern, alarm calling may subsequently be maintained by the current adaptive utility of social, nepotistic benefits.
The nonphylogenetic analyses and the phylogenetically based concentrated changes test both indicate an association between sociality and diurnality, but they do not specifically test for directionality. The contingent states test, however, reveals that diurnality did not directly lead to the evolution of sociality. This finding eliminates a potential indirect pathway for the evolution of alarm calling; species that evolved diurnality were not more likely to evolve sociality and then, subsequently, evolve alarm calling. Instead, the evolution of diurnality appears to be predominantly and independently responsible for evolution of alarm calling in rodents. Thus, although there are two conceivable pathways to the evolution of alarm calling, surprisingly, given the importance of its adaptive utility, sociality may be of secondary importance in rodents.
APPENDIX
Anderson S, Woods CA, Morgan GS, Oliver WLR, 1983. Geocapromys brownii. Mammal Sp 201:1–5.
Best TL, 1988. Dipodomys spectabilis. Mammal Sp 311:1–10.
Best TL, 1991. Dipodomys nitratoides. Mammal Sp 381:1–7.
Best TL, 1995. Sciurus deppei. Mammal Sp 505:1–5.
Best TL, 1995. Sciurus variegatoides. Mammal Sp 500:1–6.
Best TL, 1995. Spermophilus adocetus. Mammal Sp 504:1–4.
Best TL, 1995. Spermophilus mohavensis. Mammal Sp 509:1–7.
Best TL, Burt SL, Bartig JL, 1994. Tamias quadrivittatus. Mammal Sp 466:1–7.
Best TL, Granai NJ, 1994. Tamias obscurus. Mammal Sp 472:1–6.
Best TL, Hildreth NJ, Jones C, 1989. Dipodomys deserti. Mammal Sp 339:1–8.
Best TL, Skupski MP, 1994. Perognathus flavus. Mammal Sp 471:1–10.
Best TL, Titus AS, Caesar K, Lewis CL, 1990. Ammospermophilus harrisii. Mammal Sp 366:1–7.
Best TL, Titus AS, Lewis CL, Caesar K, 1990. Ammospermophilus nelsoni. Mammal Sp 367:1–7.
Birkenholz DE, 1972. Neofiber alleni. Mamm Sp 15:1–4.
Branch LC, Villareal D, Fowler GS, 1994. Factors influencing population dynamics of the plains viscacha (Lagostomus maximus, Mammalia, Chinchillidae) in scrub habitat of central Argentina. J Zool Lond 232:383–395.
Brand LR, 1976. The vocal repertoire of chipmunks (genus Eutamias) in California. Anim Behav 24:319–335.
Braun JK, Mares MA, 1989. Neotoma micropus. Mammal Sp 330:1–9.
Bronner G, Gordon S, Meester J, 1988. Otomys irroratus. Mammal Sp 308:1–6.
Brooks RJ, Banks EM, 1973. Behavioural biology of the collard lemming (Dicrostonyx groenlandians [Trail]): an analysis of acoustic communication. Anim Behav Monogr 6:1–83.
Brudzynski SM, 2001. Pharmacological and behavioral characteristics of 22 kHz alarm calls in rats. Neurosci Biobehav Rev 25:611–617.
Campos CM, Tognelli MF, Ojeda RA, 2001. Dolichotis patagonum. Mammal Sp 652:1–5.
Carraway LN, Verts BJ, 1991. Neotoma fuscipes. Mammal Sp 386:1–10.
Carraway LN, Verts BJ, 1993. Aplodontia rufa. Mammal Sp 431:1–10.
Carraway LN, Verts BJ, 1994. Sciurus griseus. Mammal Sp 474:1–7.
Ceballos-G. G, Wilson DE, 1985. Cynomys mexicanus. Mammal Sp 248:1–3.
Cervantes FA, Sosa VJ, Martinez J, Gonzalez RM, Dowler RC, 1993. Pappogeomys tylorhinus. Mammal Sp 433:1–4.
Chapman JA, Feldhamer GA, 1982. Wild mammals of North America: biology, management, and economics. Baltimore, Maryland: Johns Hopkins University Press; 1147.
Clark TW, Hoffmann RS, Nadler CF, 1971. Cynomys leucurus. Mammal Sp 7:1–4.
Clawson RG, Clawson JA, Best TL, 1994. Tamias alpinus. Mammal Sp 461:1–6.
Clawson RG, Clawson JA, Best TL, 1994. Tamias quadrimaculatus. Mammal Sp 469:1–6.
Conroy CJ, Cook JA, 1999. Microtus xanthognathus. Mammal Sp 627:1–5.
Cornely JE, Baker RJ, 1986. Neotoma mexicana. Mammal Sp 262:1–7.
Daly M, Daly S, 1975. Socio-ecology of Saharan gerbils, especially Meriones libycus. Mammalia 39:289–311.
Davidow-Henry BR, Jones JKJ, Hollander RR, 1989. Cratogeomys castanops. Mammal Sp 338:1–6.
de Graaff G, 1981. The rodents of Southern Africa: notes on their identification, distribution, ecology and taxonomy. Durban: Butterworths.
Diaz GB, Ojeda RA, Gallardo MH, Giannoni SM, 2000. Tympanoctomys barrerae. Mammal Sp 646:1–4.
Dolan PG, Carter DC, 1977. Glaucomys volans. Mammal Sp 78:1–6.
Ebensperger LA, 1998. Sociality in rodents: the New World fossorial hystricognaths as study models. Rev Chil Hist Nat 71:65–77.
Eisenberg JF, 1974. The function and motivational basis of Hystricomorph vocalizations. Symp Zool Soc London 34:211–247.
Eisenberg JF, 1989. Mammals of the neotropics, Vol. 1: the northern neotropics: Panama, Colombia, Venezuela, Guyana, Suriname, French Guiana. Chicago: University of Chicago Press.
Eisenberg JF, Redford KH, 1999. Mammals of the neotropics, Vol. 3: the central neotropics: Ecuador, Peru, Bolivia, Brazil. Chicago: University of Chicago Press.
Elliott CL, Flinders JT, 1991. Spermophilus columbianus. Mammal Sp 372:1–9.
Emmons LH, 1978. Sound communication among African rainforest squirrels. Z Tierpsychol 47:1–49.
Ernest KA, Mares MA, 1987. Spermophilus tereticaudus. Mammal Sp 274:1–9.
Eshelman BD, Cameron GN, 1987. Baiomys taylori. Mammal Sp 285:1–7.
Eshelman BD, Sonnemann CS, 2000. Spermophilus armatus. Mammal Sp 637:1–6.
Francescoli G, 2001. Vocal signals from Ctenomys pearsoni pups. Acta Theriol 46:327–330.
Francescoli G, 2002. Geographic variation in vocal signals of Ctenomys pearsoni. Acta Theriol 47:35–44.
Frase BA, Hoffmann RS, 1980. Marmota flaviventris. Mammal Sp 135:1–8.
Fulk GW, 1976. Notes on the activity, reproduction, and social behavior of Octodon degus. J Mammal 57:495–505.
Gannon WL, 1988. Zapus trinotatus. Mammal Sp 315:1–5.
Gannon WL, Lawlor TE, 1989. Variation of the chip vocalization of three species of Townsend chipmunks (Genus Eutamias). J Mammal 70:740–753.
Garrison TE, Best TL, 1990. Dipodomys ordii. Mammal Sp 353:1–10.
Gharaibeh BM, Jones C, 1996. Myosciurus pumilio. Mammal Sp 523:1–3.
Giacalone J, Wells N, Willis G, 1987. Observations on Syntheosciurus brochus (Sciuridae) in Volcan Poas National Park, Costa Rica. J Mammal 68:145–147.
Gulotta EF, 1971. Meriones unguiculatus. Mammal Sp 3:1–5.
Haltenorth T, Diller H, 1980. A field guide to the mammals of Africa including Madagascar. London: Collins.
Hayssen V, 1991. Dipodomys microps. Mammal Sp 389:1–9.
Hodgdon HE, Larson JS, 1973. Some sexual differences in behaviour within a colony of marked beavers (Castor canadensis). Anim Behav 21:147–152.
Hoffmeister DF, 1986. Mammals of Arizona. Tucson, Arizona: University of Arizona Press.
Hoogland JL, 1996. Cynomys ludovicianus. Mammal Sp 535:1–10.
Hudson WH, 1872. On the habits of the vizcacha (Lagostomus trichodactylus). Proc Zool Soc Lond 1872:822–833.
Intress C, Best TL, 1990. Dipodomys panamintinus. Mammal Sp 354:1–7.
Jackson JE, Branch LC, Villarreal D, 1996. Lagostomus maximus. Mammal Sp 543:1–6.
Jenkins SH, Busher PE, 1979. Castor canadensis. Mammal Sp 120:1–8.
Jenkins SH, Eshelman BD, 1984. Spermophilus beldingi. Mammal Sp 221:1–8.
Johnson DW, Armstrong DM, 1987. Peromyscus crinitus. Mammal Sp 287:1–8.
Kelt DA, 1988. Dipodomys heermanni. Mammal Sp 323:1–7.
Kingdon J, 1974. East African mammals: an atlas of evolution in Africa: hares and rodents. New York: Academic Press.
Kleiman DG, 1974. Patterns of behaviour in hystricomorph rodents. Symp Zool Soc Lond 34:171–209.
Koffler BR, 1972. Meriones crassus. Mammal Sp 9:1–4.
Koprowski JL, 1994. Sciurus niger. Mammal Sp 479:1–9.
Kwiecinski GG, 1998. Marmota monax. Mammal Sp 591:1–8.
Lacher TE, Jr., 1981. The comparative social behavior of Kerodon rupestris and Galea spixii and the evolution of behavior in the Caviidae. Bull Carnegie Mus Nat Hist 17:1–71.
Linzey DW, Packard RL, 1977. Ochrotomys nuttalli. Mammal Sp 75:1–6.
Macdonald D, 1984. The encyclopedia of mammals. New York: Facts on File; 895.
McCarty R, 1975. Onychomys torridus. Mammal Sp 59:1–5.
McCarty R, 1978. Onychomys leucogaster. Mammal Sp 87:1–6.
McGhee ME, Genoways HH, 1978. Liomys pictus. Mammal Sp 83:1–5.
Medway L, 1978. The wild mammals of Malaya (Peninsular Malaysia) and Singapore, 2nd ed. Kuala Lumpur: Oxford University Press.
Merritt JF, 1978. Peromyscus californicus. Mammal Sp 85:1–6.
Michener GR, Koeppl JW, 1985. Spermophilus richardsonii. Mammal Sp 243:1–8.
Mones A, Ojasti J, 1986. Hydrochoerus hydrochaeris. Mammal Sp 264:1–7.
Nagorsen DW, 1987. Marmota vancouverensis. Mammal Sp 270:1–5.
Nash DJ, Seaman RN, 1977. Sciurus aberti. Mammal Sp 80:1–5.
Nikolskii AA, Sukhanova MV, 1992. Situation dependent variations of a call emitted by great gerbil, Rhombomys optimus and by Brandt's vole, Microtus brandti, retreating to burrows. Zool Zh 71:125–132.
Nitikman LZ, 1985. Sciurus granatensis. Mammal Sp 246:1–8.
Nowak RM, 1999. Walker's mammals of the world, 6th ed. Baltimore, Maryland: Johns Hopkins University Press.
Nowak RM, Paradiso JL, 1983. Walker's mammals of the world, 4th ed. Baltimore, Maryland: Johns Hopkins University Press.
Oaks EC, Young PJ, Kirkland GL Jr, Schmidt DF, 1987. Spermophilus variegatus. Mammal Sp 272:1–8.
O'Farrell MJ, Blaustein AR, 1974. Microdipodops megacephalus. Mammal Sp 46:1–3.
O'Shea TJ, 1991. Xerus rutilus. Mammal Sp 370:1–5.
Pardinas UFJ, Galliari CA, 2001. Reithrodon auritus. Mammal Sp 664:1–8.
Paulson DD, 1988. Chaetodipus hispidus. Mammal Sp 320:1–4.
Pepper JW, Baraude SH, Lacey EA, Sherman PW, 1991. Vocalizations of the naked mole-rat. In: The biology of the naked mole-rat (Sherman PW, Jarvis JUM, Alexander RD, eds). Princeton, New Jersey: Princeton University Press; 243–274.
Perez EM, 1992. Agouti paca. Mammal Sp 404:1–7.
Perrin MR, Dempster ER, Downs CT, 1999. Gerbillurus paeba. Mammal Sp 606:1–6.
Pessoa LM, dos Reis SF, 1993. Proechimys dimidiatus. Mammal Sp 441:1–3.
Pessoa LM, dos Reis SF, 1996. Proechimys iheringi. Mammal Sp 536:1–4.
Pessoa LM, dos Reis SF, 2002. Proechimys albispinus. Mammal Sp 693:1–3.
Pizzimenti JJ, Collier GD, 1975. Cynomys parvidens. Mammal Sp 52:1–3.
Pizzimenti JJ, Hoffmann RS, 1973. Cynomys gunnisoni. Mammal Sp 25:1–4.
Randall JA, 1994. Convergences and divergences in communication and social organisation of desert rodents. Aust J Zool 42:405–433.
Randall JA, 2001. Evolution and function of foot drumming as communication in mammals. Am Zool 41:1143–1156.
Randall JA, Rogovin KA, Shier DM, 2000. Antipredator behavior of a social desert rodents: footdrumming and alarm calling in the great gerbil, Rhombomys opiums. Behav Ecol Sociobiol 48:110–118.
Redford KH, Eisenberg JF, 1992. Mammals of the neotropics, Vol. 2: the southern cone. Chicago: University of Chicago Press.
Reich LM, 1981. Microtus pennsylvanicus. Mammal Sp 159:1–8.
Reig OA, 1970. Ecological notes on the fossorial octodont rodent Spalacopus cyanus (Molina). J Mammal 51:592–601.
Rickart EA, 1987. Spermophilus townsendii. Mammal Sp 268:1–6.
Rickart EA, Yensen E, 1991. Spermophilus washingtoni. Mammal Sp 371:1–5.
Roberts TJ, 1977. The mammals of Pakistan. London: Ernest Benn Ltd.
Rood JP, 1972. Ecological and behavioural comparisons of three genera of Argentine cavies. Anim Behav Monogr 5:1–83.
Schleich CE, Busch C, 2002. Juvenile vocalizations of Ctenomys talarum (Rodentia: Octodontidae). Acta Theriol 47:25–33.
Siegel HI, 1985. The hamster: reproduction and behavior. In. New York: Plenum Press; 440.
Smolen MJ, 1981. Microtus pinetorum. Mammal Sp 147:1–7.
Snyder DP, 1982. Tamias striatus. Mammal Sp 168:1–8.
Sokolov VE, Kotenkova EV, Mikailenko AG, 1998. Mus spicilegus. Mammal Sp 592:1–6.
Stalling DT, 1990. Microtus ochrogaster. Mammal Sp 355:1–9.
Steele MA, 1998. Tamiasciurus hudsonicus. Mammal Sp 586:1–9.
Steele MA, 1999. Tamiasciurus douglasii. Mammal Sp 630:1–8.
Streubel DP, Fitzgerald JP, 1978. Spermophilus tridecemlineatus. Mammal Sp 103:1–5.
Stubbe A, Janke S, 1994. Some aspects of social behaviour in the vole Microtus brandti (Radde, 1861). Pol Ecol Stud 20:449–457.
Sutton DA, 1992. Tamias amoenus. Mammal Sp 390:1–8.
Tamura N, Hayashi F, Miyashita K, 1988. Dominance hierarchy and mating bahavior of the Formosan squirrel Callosciurus erythraeus thaiwanensis. J Mammal 69:320–331.
Tognelli MF, Campos CM, Ojeda RA, 2001. Microcavia australis. Mammal Sp 648:1–4.
Torres-Mura JC, Contreras L, 1998. Spalacopus cyanus. Mammal Sp 594:1–5.
Van Den Brink FH, 1968. A field guide to the mammals of Britain and Europe. Boston: Houghton Mifflin Company.
Veal R, Caire W, 1980. Peromyscus eremicus. Mammal Sp 118:1–6.
Veitl S, Begall S, Burda H, 2000. Ecological determinants of vocalisation parameters: the case of the coruro Spalacopus cyanus (Octodontidae), a fossorial social rodent. Bioacoustics 11:129–148.
Verts BJ, Carraway LN, 1987. Thomomys bulbivorus. Mammal Sp 273:1–4.
Verts BJ, Carraway LN, 2002. Neotoma lepida. Mammal Sp 699:1–12.
Verts BJ, Kirkland GL, Jr., 1988. Perognathus parvus. Mammal Sp 318:1–8.
Watts CHS, 1975. Vocalizations of Australian hopping mice (Rodentia, Notomys). J Zool 177:247–263.
Wells NM, Giacalone J, 1985. Syntheosciurus brochus. Mammal Sp 249:1–3.
Wells-Gosling N, Heaney LR, 1984. Glaucomys sabrinus. Mammal Sp 229:1–8.
Whitaker JO, Jr, 1972. Zapus hudsonius. Mammal Sp 11:1–7.
Whitaker JO Jr, Wrigley RE, 1972. Napaeozapus insignis. Mammal Sp 14:1–6.
Whitaker JO Jr, 1996. National Audubon Society field guide to North American mammals. New York: Knopf.
White TG, Albercio MS, 1992. Dinomys branickii. Mammal Sp 410:1–5.
Wiley RW, 1980. Neotoma floridana. Mammal Sp 139:1–7.
Williams DF, Kilburn KS, 1991. Dipodomys ingens. Mammal Sp 377:1–7.
Williams LR, Cameron GN, 1991. Geomys attwateri. Mammal Sp 382:1–5.
Williams SL, 1982. Geomys personatus. Mammal Sp 170:1–5.
Willner GR, Feldhamer GA, Zucker EE, Chapman JA, 1980. Ondatra zibethicus. Mammal Sp 141:1–8.
Wilson DE, Ruff S, 1999. The Smithsonian book of North American mammals. Washington, DC: Smithsonian Institution Press; 750.
Woods CA, 1973. Erethizon dorsatum. Mammal Sp 29:1–6.
Woods CA, Boraker DK, 1975. Octodon degus. Mammal Sp 67:1–5.
Woods CA, Contreras L, Willner-Chapman G, Whidden HP, 1992. Myocastor coypus. Mammal Sp 398:1–8.
Yensen E, Sherman PW, 1997. Spermophilus brunneus. Mammal Sp 560:1–5.
Young CJ, Jones JK, Jr, 1982. Spermophilus mexicanus. Mammal Sp 164:1–4.
Youngman PM, 1975. Mammals of the Yukon Territory. Ottawa: National Museum of Canada.
Zegers DA, 1984. Spermophilus elegans. Mammal Sp 214:1–7.
We thank T. Best, E. Heymann, C. Knogge, and M. Mares for expert help with missing data; J. Hare, P. Narins, P. Nonacs, T. Ord, K. Pollard, M. Zuk, and an anonymous reviewer for comments on previous versions of the manuscript; and the UCLA Division of Life Sciences for partial support though set-up funds to D.T.B.
References
Baverstock PR, Adams M, and Watts HS,
Bell DM, Hamilton MJ, Edwards CW, Wiggins LE, Muñiz Martínez R, Strauss RE, Bradley RD, and Baker RJ,
Blumstein DT,
Blumstein DT, and Armitage KB,
Blumstein DT, and Armitage KB,
Blumstein DT, Steinmetz J, Armitage KB, and Daniel JC,
Brooks DR, and McLennan DA,
Brown JL,
Burke da Silva K, Kramer DL, and Weary, DM,
Carrasco MA,
Conroy CJ, and Cook JA,
Cresswell W,
Darwin C,
DeWalt TS, Sudman PD, Hafner MS, and Davis SK,
Emmons LH,
Fitzgibbon CD, and Fanshawe JH,
Gardner AL, and Emmons LH,
Harrison RG, Bogdanowicz SM, Hoffmann RS, Yensen E, and Sherman PW,
Harvey PH, and Pagel MD,
Hasson O,
Hayes JP, and Harrison RG,
Herron MD, Castoe TA, and Parkinson CL,
Hogan KM, Davis SK, and Greenbaum IF,
Hoogland JL,
Jolley TW, Honeycutt RL, and Bradley RD,
Keller L, and Reeve HK,
Klump GM, and Shalter MD,
Kruckenhauser L, Pinsker W, Haring E, and Arnold W,
Lima SL,
Lima SL,
Maddison DR, and Maddison WP,
Maddison WP,
Mantooth SJ, Jones C, and Bradley RD,
McKenna MC, and Bell SK,
Nelson K, Baker RJ, Shellhammer HS, and Chesser RK,
Oshida T, and Masuda R,
Piaggio AJ, and Spicer GS,
Qumsiyeh MB, Hamilton MJ, Dempster ER, and Baker RJ,
Reeve HK, and Sherman PW,
Riddle BR,
Riddle BR, Hafner DJ, and Alexander LF,
Russell RJ,
Schwagmeyer PL,
Shipley MM, Stangl FB, Jr., Cate RL, and Hood CS,
Sillén-Tullberg B,
Simpson GG,
Slamovits CH, Cook JA, Lessa EP, and Rossi MS,
Steppan SJ, Akhverdyan MR, Lyapunova EA, Fraser DG, Vorontsov NN, Hoffman RS, and Braun MJ,
Swaisgood RR, Owings DH, and Rowe MP,
Swofford DL, and Maddison WP,
Tinbergen N, Broekhuysen GJ, Feekes F, Houghton JCW, Kruuk H, and Szule E,
Watts CHS, Baverstock PR, Burrell J, and Krieg M,